The Role of Salivary Biomarkers in Monitoring Oral Health in Patients with Implants and Periodontitis
Abstract
1. Introduction
2. Material and Methods
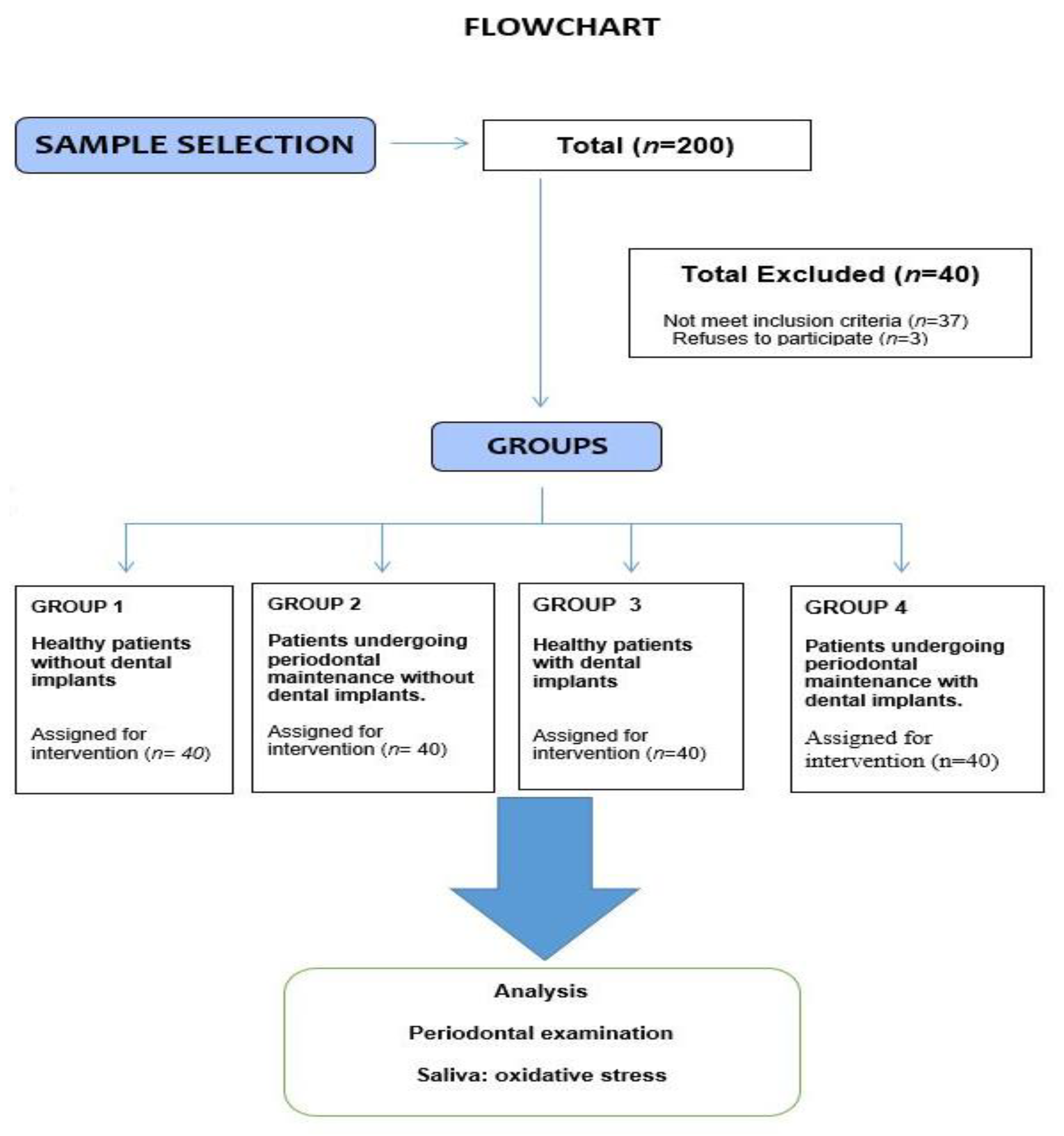
2.1. Sample Selection
- Group 1: Healthy patients without implants (n = 40)
- Group 2: Patients undergoing periodontal maintenance without dental implants (n = 40)
- Group 3: Healthy patients with implants older than six months (n = 40)
- Group 4: Patients undergoing periodontal maintenance with implants older than six months (n = 40)
2.2. Clinical Variables
2.3. Sampling of Saliva
2.4. Measurement of Salivary Oxidative Stress
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elani, H.W.; Starr, J.R.; Da Silva, J.D.; Gallucci, G.O. Trends in Dental Implant Use in the U.S., 1999–2016, and Projections to 2026. J. Dent. Res. 2018, 97, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Slots, J. Primer on Etiology and Treatment of Progressive/Severe Periodontitis: A Systemic Health Perspective. Periodontol. 2020, 83, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.; Al-Ansari, A.; Al-Khalifa, K.; Alhareky, M.; Gaffar, B.; Almas, K. Global Prevalence of Periodontal Disease and Lack of Its Surveillance. Sci. World J. 2020, 2020, 2146160. [Google Scholar] [CrossRef] [PubMed]
- Sczepanik, F.S.C.; Grossi, M.L.; Casati, M.; Goldberg, M.; Glogauer, M.; Fine, N.; Tenenbaum, H.C. Periodontitis Is an Inflammatory Disease of Oxidative Stress: We Should Treat It That Way. Periodontol. 2020, 84, 45–68. [Google Scholar] [CrossRef]
- Waddington, R.J.; Moseley, R.; Embery, G. Reactive Oxygen Species: A Potential Role in the Pathogenesis of Periodontal Diseases. Oral Dis. 2000, 6, 138–151. [Google Scholar] [CrossRef]
- Chapple, I.L.; Matthews, J.B. The Role of Reactive Oxygen and Antioxidant Species in Periodontal Tissue Destruction. Periodontol. 2007, 43, 160–232. [Google Scholar] [CrossRef]
- Wang, Y.; Andrukhov, O.; Rausch-Fan, X. Oxidative Stress and Antioxidant System in Periodontitis. Front. Physiol. 2017, 8, 910. [Google Scholar] [CrossRef]
- Song, Z.; Weigl, P.; Wang, B. Correlations of inflammatory cytokines, oxidative stress markers, and matrix metallo-proteinases in gingival crevicular fluid with peri-implantitis. Eur. J. Inflamm. 2019, 17, 2058739219845542. [Google Scholar] [CrossRef]
- Chen, M.; Cai, W.; Zhao, S.; Shi, L.; Chen, Y.; Li, X.; Sun, X.; Mao, Y.; He, B.; Hou, Y.; et al. Oxidative Stress-Related Biomarkers in Saliva and Gingival Crevicular Fluid Associated with Chronic Peri-odontitis: A Systematic Review and Meta-Analysis. J. Clin. Periodontol. 2019, 46, 608–622. [Google Scholar] [CrossRef]
- Tóthová, L.; Kamodyová, N.; Červenka, T.; Celec, P. Salivary Markers of Oxidative Stress in Oral Diseases. Front. Cell. Infect. Microbiol. 2015, 5, 73. [Google Scholar] [CrossRef]
- Čižmárová, B.; Tomečková, V.; Hubková, B.; Hurajtová, A.; Ohlasová, J.; Birková, A. Salivary Redox Homeostasis in Human Health and Disease. Int. J. Mol. Sci. 2022, 23, 10076. [Google Scholar] [CrossRef] [PubMed]
- Viglianisi, G.; Tartaglia, G.M.; Santonocito, S.; Amato, M.; Polizzi, A.; Mascitti, M.; Isola, G. The Emerging Role of Salivary Oxidative Stress Biomarkers as Prognostic Markers of Periodontitis: New Insights for a Personalized Approach in Dentistry. J. Pers. Med. 2023, 13, 166. [Google Scholar] [CrossRef] [PubMed]
- Baltacioğlu, E.; Akalin, F.A.; Alver, A.; Balaban, F.; Unsal, M.; Karabulut, E. Total Antioxidant Capacity and Su-peroxide Dismutase Activity Levels in Serum and Gingival Crevicular Fluid in Post-Menopausal Women with Chronic Periodontitis. J. Clin. Periodontol. 2006, 33, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Mohideen, K.; Chandrasekar, K.; Ramsridhar, S.; Rajkumar, C.; Ghosh, S.; Dhungel, S. Assessment of Oxidative Stress by the Estimation of Lipid Peroxidation Marker Malondialdehyde (MDA) in Patients with Chronic Periodontitis: A Systematic Review and Meta-Analysis. Int. J. Dent. 2023, 2023, 6014706. [Google Scholar] [CrossRef] [PubMed]
- Baňasová, L.; Kamodyová, N.; Janšáková, K.; Tóthová, Ľ.; Stanko, P.; Turňa, J.; Celec, P. Salivary DNA and markers of oxidative stress in patients with chronic periodontitis. Clin. Oral Investig. 2015, 19, 201–207. [Google Scholar] [CrossRef]
- Tamaki, N.; Tomofuji, T.; Maruyama, T.; Ekuni, D.; Yamanaka, R.; Takeuchi, N.; Yamamoto, T. Relationship between periodontal condition and plasma reactive oxygen metabolites in patients in the maintenance phase of periodontal treatment. J. Periodontol. 2008, 79, 2136–2142. [Google Scholar] [CrossRef] [PubMed]
- Bressan, E.; Ferroni, L.; Gardin, C.; Bellin, G.; Sbricoli, L.; Sivolella, S.; Brunello, G.; Schwartz-Arad, D.; Mijiritsky, E.; Penarrocha, M.; et al. Metal Nanoparticles Released from Dental Implant Surfaces: Potential Contribution to Chronic Inflammation and Peri-Implant Bone Loss. Materials 2019, 12, 2036. [Google Scholar] [CrossRef]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.L. Peri-implantitis. J. Periodontol. 2018, 89 (Suppl. S1), S267–S290. [Google Scholar] [CrossRef]
- Aguirre-Zorzano, L.A.; Estefanía-Fresco, R.; Telletxea, O.; Bravo, M. Prevalence of peri-implant inflammatory disease in patients with a history of periodontal disease who receive supportive periodontal therapy. Clin. Oral Implant. Res. 2015, 26, 1338–1344. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Periodontally compromised vs. periodontally healthy pa-tients and dental implants: A systematic review and meta-analysis. J. Dent. 2014, 42, 1509–1527. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Peri-odontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S68–S77. [Google Scholar] [CrossRef] [PubMed]
- Jazi, M.M.; Rodsari, H.R.S.P.; Mirmiran, F. Level of oxidative stress markers in peri-implant crevicular fluid and their correlation with clinical parameters. J. Dent. 2015, 12, 340. [Google Scholar]
- Sgolastra, F.; Petrucci, A.; Severino, M.; Gatto, R.; Monaco, A. Periodontitis, implant loss and peri-implantitis: A meta-analysis. Clin. Oral Implant. Res. 2015, 26, e8–e16. [Google Scholar] [CrossRef] [PubMed]
- Stacchi, C.; Berton, F.; Perinetti, G.; Frassetto, A.; Lombardi, T.; Khoury, A.; Andolsek, F.; Di Lenarda, R. Risk factors for peri-implantitis: Effect of history of periodontal dis-ease and smoking habits. A systematic review and meta-analysis. J. Oral Maxillofac. Res. 2016, 7, e3. [Google Scholar] [PubMed]
- Derks, J.; Tomasi, C. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015, 42 (Suppl. S16), S158–S171. [Google Scholar] [CrossRef] [PubMed]
- Zitzmann, N.U.; Berglundh, T. Definition and prevalence of peri-implant diseases. J. Clin. Periodontol. 2008, 35 (Suppl. S8), 286–291. [Google Scholar] [CrossRef]
- Gomes, A.M.; Douglas-de-Oliveira, D.W.; Oliveira Costa, F. Could the biomarker levels in saliva help distin-guish between healthy implants and implants with peri-implant disease? A systematic review. Arch. Oral Biol. 2018, 96, 216–222. [Google Scholar] [CrossRef]
- Roccuzzo, A.; Imber, J.C.; Marruganti, C.; Salvi, G.E.; Ramieri, G.; Roccuzzo, M. Clinical outcomes of dental implants in patients with and without history of periodontitis: A 20-year prospective study. J. Clin. Periodontol. 2022, 49, 1346–1356. [Google Scholar] [CrossRef]
- Liskmann, S.; Zilmer, M.; Vihalemm, T.; Salum, O.; Fischer, K. Correlation of peri-implant health and myeloperoxidase levels: A cross-sectional clinical study. Clin. Oral Implant. Res. 2004, 15, 546–552. [Google Scholar] [CrossRef]
- Yang, J.; Liu, C.; Sun, H.; Liu, Y.; Liu, Z.; Zhang, D.; Zhao, G.; Wang, Q.; Yang, D. The progress in titanium al-loys used as biomedical implants: From the view of reactive oxygen species. Front. Bioeng. Biotechnol. 2022, 10, 1092916. [Google Scholar] [CrossRef]
- Leone, F.D.; Blasi, G.; Amerio, E.; Valles, C.; Nart, J.; Monje, A. Influence of the Level of Compliance with Pre-ventive Maintenance Therapy upon the Prevalence of Peri-Implant Diseases: A cross-sectional study. J. Periodontol. 2023. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Navazesh, M.; Kumar, S.K.S. Measuring salivary flow: Challenges and opportunities. J. Am. Dent. Assoc. 2008, 139 (Suppl. S2), 35S–40S. [Google Scholar] [CrossRef] [PubMed]
- Barranco, T.; Rubio, C.P.; Tvarijonaviciute, A.; Rubio, M.; Damia, E.; Lamy, E.; Cugat, R.; Cerón, J.J.; Tecles, F.; Escribano, D. Changes of salivary biomarkers under different storage conditions: Effects of temperature and length of storage. Biochem. Med. 2019, 29, 010706. [Google Scholar] [CrossRef] [PubMed]
- Peres Rubio, C.; Hernández-Ruiz, J.; Martínez-Sabiela, S.; Tvarijonaviciute, A.; Ceron, J.J. Spectrophotometric as-says for total antioxidant capacity (TAC) in dog serum: An update. BMC Vet. Res. 2016, 12, 166. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability (FRAP) as a measure of ‘’antioxidant power’’: The FRAP as-say. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Campos, C.; Guzmán, R.; López-Fernández, E.; Casado, A. Evaluation of the copper(II) reduction assay using bathocuproinedisulfonic acid disodium salt for the total antioxidant capacity assessment: The CUPRAC-BCS assay. Anal. Biochem. 2009, 392, 37–44. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. Total antioxidant activity in plant material and its interest in food technology. Recent Res. Devel. Agric. Food Chem. 1998, 2, 893–905. [Google Scholar]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef]
- Weichselbaum, T.E. An accurate and rapid method for the determination of proteins in small amounts of blood se-rum and plasma. Am. J. Clin. Pathol. 1946, 10, 40–49. [Google Scholar] [CrossRef]
- de Sire, A.; Invernizzi, M.; Ferrillo, M.; Gimigliano, F.; Baricich, A.; Cisari, C.; De Marchi, F.; Foglio Bonda, P.L.; Mazzini, L.; Migliario, M. Functional status and oral health in patients with amyotrophic lateral sclerosis: A cross-sectional study. NeuroRehabilitation 2021, 48, 49–57. [Google Scholar] [CrossRef]
- de Sire, A.; Baricich, A.; Ferrillo, M.; Migliario, M.; Cisari, C.; Invernizzi, M. Buccal hemineglect: Is it useful to evalu-ate the differences between the two halves of the oral cavity for the multidisciplinary rehabilitative management of right brain stroke survivors? A cross-sectional study. Top. Stroke Rehabil. 2020, 27, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, R.; Nishiyama, A.; Matsuda, C.; Nakayama, Y.; Hakuta, C.; Shimada, M. Oral health status of hospitalized amyotrophic lateral sclerosis patients: A single-centre observational study. Acta Odontol. Scand. 2018, 76, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Gornitsky, M.; Velly, A.M.; Yu, H.; Benarroch, M.; Schipper, H.M. Salivary DNA, lipid, and protein oxidation in nonsmokers with periodontal disease. Free. Radic. Biol. Med. 2009, 46, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Zhang, X.L.; Wang, Y.Z.; Yang, C.X.; Chen, G. Lipid peroxidation levels, total oxidant status and superox-ide dismutase in serum, saliva and gingival crevicular fluid in chronic periodontitis patients before and after per-iodontal therapy. Aust. Dent. J. 2010, 55, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Karim, S.; Pratibha, P.K.; Kamath, S.; Bhat, G.S.; Kamath, U.; Dutta, B.; Sharma, N.; Archana, B.; Bhat, K.M.; Guddattu, V. Superoxide dismutase enzyme and thiol antioxidants in gingival crevicular fluid and saliva. Dent. Res. J. 2012, 9, 266–272. [Google Scholar]
- Dede, O.F.; Ozden, F.O.; Avci, B. 8-hydroxy-deoxyguanosine levels in gingival crevicular fluid and saliva in pa-tients with chronic periodontitis after initial periodontal treatment. J. Periodontol. 2013, 84, 821–828. [Google Scholar] [CrossRef]
- Guentsch, A.; Preshaw, P.M.; Bremer-Streck, S.; Klinger, G.; Glockmann, E.; Sigusch, B.W. Lipid peroxidation and antioxidant activity in saliva of periodontitis patients: Effect of smoking and periodontal treatment. Clin. Oral Investig. 2008, 12, 345–352. [Google Scholar] [CrossRef]

| No Implant Healthy | No Implant Periodontitis p Value | Healthy Implant | Periodontiti Implant | p-Value | ||
|---|---|---|---|---|---|---|
| Gender. n (%) | 0.822 | 0.630 | ||||
| Male | 17 (42.5) | 18 (45) | 12 (30) | 14 (35) | ||
| Female | 23 (57.5) | 22 (55) | 28 (70) | 26 (65) | ||
| Age. average | 36.9 (14.9) | 57.2 (15.3) | 55.43 (15.80) | 61.20 (11.77) | 0.068 | |
| Medical Treatment n (%) | 0.007 | 0.108 | ||||
| No | 36 (90) | 26 (65) | 28 (70) | 21 (52.5) | ||
| Yes | 4 (10) | 14 (35) | 12 (30) | 19 (47.5) | ||
| Tobacco consumption. n (%) | 0.045 | 0.160 | ||||
| No | 33 (82.5) | 25 (62.5) | 29 (72.5) | 23 (57.5) | ||
| Yes | 7 (17.5) | 15 (37.5) | 11 (27.5) | 17 (42.5) | ||
| Alcohol consumption. n (%) | 0.217 | 0.644 | ||||
| No | 31 (77.5) | 26 (65) | 26 (65) | 24 (60) | ||
| Yes | 9 (22.5) | 14 (35) | 14 (35) | 16 (40) | ||
| BMI. average | 23.59 (3.06) | 24.83 (4.29) | 0.14 | 24.92 (3.66) | 24.47 (2.65) | 0.528 |
| Clinical Variables | Healthy + No Implant | Periodontitis + No Implant | p-Value | Healthy + Implant | Periodontitis + Implant | p-Value |
|---|---|---|---|---|---|---|
| Amalgam Fillings. Median | 0 (0–0) | 0 (0–0) | 0.552 | 3 (0–7) | 2 (0–7) | 0.496 |
| Metal-Ceramic Crowns. n (%) | 0.003 | 0.883 | ||||
| - 0 | 38 (95%) | 28 (70%) | 28 (70%) | 27 (67.5%) | ||
| - 1 | 2 (5%) | 6 (15%) | 7 (17.5%) | 8 (20%) | ||
| - 2 | 3 (7.5%) | 3 (7.5%) | 5 (12.5%) | |||
| - 3 | 1 (2.5%) | 1 (2.5%) | ||||
| - 4 | 1 (2.5%) | 1 (2.5%) | ||||
| - 5 | 1 (2.5%) | 2 (5%) | ||||
| Metal-Ceramic Bridges. n (%) | 0.098 | 0.961 | ||||
| - 0 | 39 (97.5%) | 35 (87.5%) | 32 (80%) | 32 (80%) | ||
| - 1 | 3 (7.5%) | 5 (12.5%) | 4 (10%) | |||
| - 2 | 1 (2.5%) | 2 (5%) | 2 (5%) | 3 (7.5%) | ||
| - 3 | 1 (2.5%) | 1 (2.5%) | ||||
| % O’Leary’s Plaque Index Median | 8.3 (7.05–11.6) | 17.8 (11.3–24.55) | <0.001 | 12.5 (10.4–17.7) | 16.3 (11–21.7) | 0.037 |
| Last Dental Visit. n (%) | 0.001 | 0.062 | ||||
| - <1 year | 30 a (75%) | 13 b (32.5%) | 12 (30%) | 22 (55%) | ||
| - 1 year | 9 a (22.5%) | 21 b (52.5%) | 24 (60%) | 14 (35%) | ||
| - >1 year | 1 a (2.5%) | 6 b (15%) | 4 (10%) | 4 (10%) | ||
| Brushing Frequency. n (%) | 0.001 | 0.595 | ||||
| - 1/day | 1 a (2.5%) | 1 (2.5%) | ||||
| - 2/day | 18 a (45%) | 33 b (82.5%) | 27 (67.5%) | 27 (67.5%) | ||
| - 3 or more/day | 22 a (55%) | 6 b (15%) | 13 (32.5%) | 12 (30%) |
| Dental Implants | Test | p-Value | ||
|---|---|---|---|---|
| No | Yes | |||
| Periodontitis Stage | χ2(3) = 13.467 | 0.004 | ||
| None | 40 a (50) | 40 a (50) | ||
| I | 3 a (3.8) | 17 b (21.3) | ||
| II | 21 a (26.3) | 15 a (18.8) | ||
| III | 16 a (20) | 8 a (10) | ||
| Periodontitis Extention | χ2(2) = 0.672 | 0.715 | ||
| None | 40 (50) | 40 (50) | ||
| Localized | 7 (8.8) | 10 (12.5) | ||
| Generalized | 33 (41.3) | 30 (37.5) | ||
| Bleeding | χ2(1) = 7.53 | 0.006 | ||
| No | 60 (75) | 73 (91.3) | ||
| Yes | 20 (25) | 7 (8.8) | ||
| Pain | 1 * | |||
| No | 80 (100) | 79 (98.8) | ||
| Yes | 1 (1.3) | |||
| Mobility | 0.245 * | |||
| No | 80 (100) | 77 (96.3) | ||
| Yes | 3 (3.8) | |||
| Groups | Test | p-Value | ||
|---|---|---|---|---|
| Healthy + Implant | Periodontitis + Implant | |||
| Implant Width (mm) | 3.96 (0.28) | 3.85 (0.41) | 0.176 | |
| Implant Length (mm) | 10.49 (1.00) | 10.24 (1.42) | 0.366 | |
| Type of connection | 0.281 * | |||
| Internal | 119 (98.3) | 115 (95.8) | ||
| External | 2 (1.7) | 5 (4.2) | ||
| Abutment | χ2(5) = 29.943 | < 0.001 | ||
| 1 | 61 (50.4) | 70 (58.3) | ||
| 2 | 23 (19) | 31 (25.8) | ||
| 3 | 5 (4.1) | 9 (7.5) | ||
| 4 | 2 (1.7) | |||
| 5 | 6 (5) | 10 (8.3) | ||
| 6 | 24 (19.8) | |||
| Implant Material | χ2(1) = 1.411 | 0.235 | ||
| Titanium | 102 (84.3) | 94 (78.3) | ||
| Titanium + zirconium | 19 (15.7) | 26 (21.7) | ||
| Regeneration | χ2(2) = 7.846 | 0.02 | ||
| None | 69 a (57) | 89 b (74.2) | ||
| Guided Bone Regeneration | 45 a (37.2) | 27 b (22.5) | ||
| Sinus lift | 7 a (5.8) | 4 a (3.3) | ||
| Implant-Prosthetic Connection | 0.722 * | |||
| Screwed | 118 (97.5) | 116 (96.7) | ||
| Cemented | 3 (2.5) | 4 (3.3) | ||
| Implant bleeding on probing | χ2(1) = 0.334 | 0.564 | ||
| No | 114 (94.2) | 115 (95.8) | ||
| Yes | 7 (5.8) | 5 (4.2) | ||
| Peri-implant inflammation | 0.281 | |||
| No | 119 (98.3) | 115 (95.8) | ||
| Yes | 2 (1.7) | 5 (4.2) | ||
| Average probing epth (mm) | 3.94 (0.54) | 5.14 (0.76) | <0.001 | |
| Average insertion level (mm) | 4.27 (0.57) | 5.57 (0.96) | <0.001 | |
| Healthy No Implant | Periodontitis No Implant | p-Value | Healthy Implant | Periodontitis Implant p-Value | ||
|---|---|---|---|---|---|---|
| CUPRAC (mmol/L) | 0.29 (0.25) | 0.28 (0.17) | 0.903 | 0.27 (0.16) | 0.30 (0.14) | 0.352 |
| FRAP (mmol/L) | 0.60 (0.61) | 0.58 (0.39) | 0.861 | 0.57 (0.35) | 0.60 (0.32) | 0.695 |
| TEACH (mmol/L) | 0.32 (0.26) | 0.32 (0.18) | 0.939 | 0.31 (0.18) | 0.32 (0.17) | 0.653 |
| AOPP (µmol/L) | 318.68 (308.81) | 608.67 (1.088.45) | 0.11 | 331.85 (346.96) | 531.97 (1.008.24) | 0.239 |
| PT (mg/dL) | 65.74 (46.82) | 82.97 (54.15) | 0.141 | 66.33 (54.23) | 74.49 (60.00) | 0.528 |
| Univariant Logistic Regression | Multivariant Logistic Regression | |||
|---|---|---|---|---|
| OR (IC 95%) | p-Valor | OR (IC 95%) | p-Value | |
| Gender | ||||
| Male | 1 | |||
| Female | 0.85 (0.45–1.62) | 0.625 | ||
| Age | 2.05 (1.83–4.97) | <0.001 | 2.03 (1.72–4.86) | <0.001 |
| Medical Treatment | ||||
| No | 1 | 1 | ||
| Yes | 2.81 (1.39–5.69) | 0.004 | 1.24 (0.45–3.43) | 0.682 |
| Tabacco consumption | ||||
| No | 1 | |||
| Yes | 2.30 (1.15–4.58) | 0.018 | 2.87 (1.18–6.95) | 0.020 |
| Alcohol consumption | ||||
| No | 1 | |||
| Yes | 1.49 (0.77–2.89) | 0.241 | ||
| Body Mass Index | 1.03 (0.95–1.13) | 0.473 | ||
| % O’LEARY’s plaque index | 1.95 (1.18–3.22) | <0.001 | 1.07 (0.99–1.15) | 0.094 |
| Brushing Frequency | ||||
| 2/day | 1 | 1 | ||
| 3 or more/day | 0.39 (0.19–0.77) | 0.007 | 0.86 (0.35–2.10) | 0.732 |
| Bleeding | ||||
| No | ||||
| Yes | ||||
| CUPRAC (mmol/L) | 1.48 (0.27–8.25) | 0.655 | ||
| FRAP (mmol/L) | 1.03 (0.49–2.14) | 0.943 | ||
| TEACH (mmol/L) | 1.20 (0.24–5.89) | 0.827 | ||
| AOPP (µmol/L) | 1.00 (1.00–1.00) | 0.099 | ||
| PT (mg/dL) | 1.00 (1.00–1.01) | 0.151 | ||
| Risk of Implant Failure | Univariant Logistic Regression | |||
|---|---|---|---|---|
| No | Yes | OR (IC 95%) | p-Value | |
| Group. n (%) | ||||
| Healthy | 112 (92.6) | 9 (7.4) | ||
| Periodontitis | 112 (93.3) | 8 (6.7) | 0.89 (0.33–2.39) | 0.815 |
| Location. n (%) | ||||
| Mandibula | 103 (92) | 9 (8) | ||
| Maxilla | 121 (93.8) | 8 (6.2) | 0.76 (0.28–2.03) | 0.580 |
| Years implant placement. mean (St) | 2.8 (2.1) | 3.6 (3.5) | 1.14 (0.95–1.38) | 0.168 |
| Width (mm). mean (St) | 3.85 (0.38) | 3.8 (0.37) | 0.71 (0.19–2.62) | 0.603 |
| Length (mm). mean (St) | 10.2 (1.1) | 10 (1) | 0.84 (0.52–1.34) | 0.461 |
| Mesiovestibular probing. mean (St) | 2 (1) | 2 (1) | 1.35 (0.91–2.02) | 0.138 |
| Bleeding. n (%) | ||||
| No | 214 (93.4) | 15 (6.6) | ||
| Yes | 10 (83.3) | 2 (16.7) | 2.85 (0.57–14.22) | 0.201 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Jornet, P.; Hynninen, J.N.; Parra-Perez, F.; Peres-Rubio, C.; Pons-Fuster, E.; Tvarijonaviciute, A. The Role of Salivary Biomarkers in Monitoring Oral Health in Patients with Implants and Periodontitis. Appl. Sci. 2024, 14, 927. https://doi.org/10.3390/app14020927
López-Jornet P, Hynninen JN, Parra-Perez F, Peres-Rubio C, Pons-Fuster E, Tvarijonaviciute A. The Role of Salivary Biomarkers in Monitoring Oral Health in Patients with Implants and Periodontitis. Applied Sciences. 2024; 14(2):927. https://doi.org/10.3390/app14020927
Chicago/Turabian StyleLópez-Jornet, Pia, Joonas Nikolai Hynninen, Francisco Parra-Perez, Camila Peres-Rubio, Eduardo Pons-Fuster, and Asta Tvarijonaviciute. 2024. "The Role of Salivary Biomarkers in Monitoring Oral Health in Patients with Implants and Periodontitis" Applied Sciences 14, no. 2: 927. https://doi.org/10.3390/app14020927
APA StyleLópez-Jornet, P., Hynninen, J. N., Parra-Perez, F., Peres-Rubio, C., Pons-Fuster, E., & Tvarijonaviciute, A. (2024). The Role of Salivary Biomarkers in Monitoring Oral Health in Patients with Implants and Periodontitis. Applied Sciences, 14(2), 927. https://doi.org/10.3390/app14020927








