Isolation and Characterization of Starch from Different Potato Cultivars Grown in Croatia
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method of Isolation of Starch from Potatoes
2.3. Determination of Basic Chemical Composition and Amylose Content
2.4. Determination of the Color of Starch
2.5. Determination of Paste Properties by Brabender’s Micro Visco-Amylograph
- Heating from 30 to 92 °C, heating rate 7.5 °C/min.
- Isothermal at 92 °C, 15 min.
- Cooling from 92 to 50 °C, cooling rate 7.5 °C/min.
- Isothermal at 50 °C, 15 min.
2.6. Determination of Swelling Power and Solubility Index of Starches
2.7. Determination of Clarity of Starch Pastes
2.8. Determination of the Texture of Starch Gels
- Speed before measuring—3 mm/s;
- Speed of measuring (during penetration)—2 mm/s;
- Speed after measuring—10 mm/s.
2.9. Determination of Thermophysical Properties
2.10. Statistical Analysis
3. Results and Discussion
3.1. Basic Chemical Composition of Tested Potato Cultivars
3.2. Basic Chemical Composition of Isolated Potato Starches
3.3. The Color of Potato Starches
3.4. Paste Properties by Brabender’s Micro Visco-Amylograph
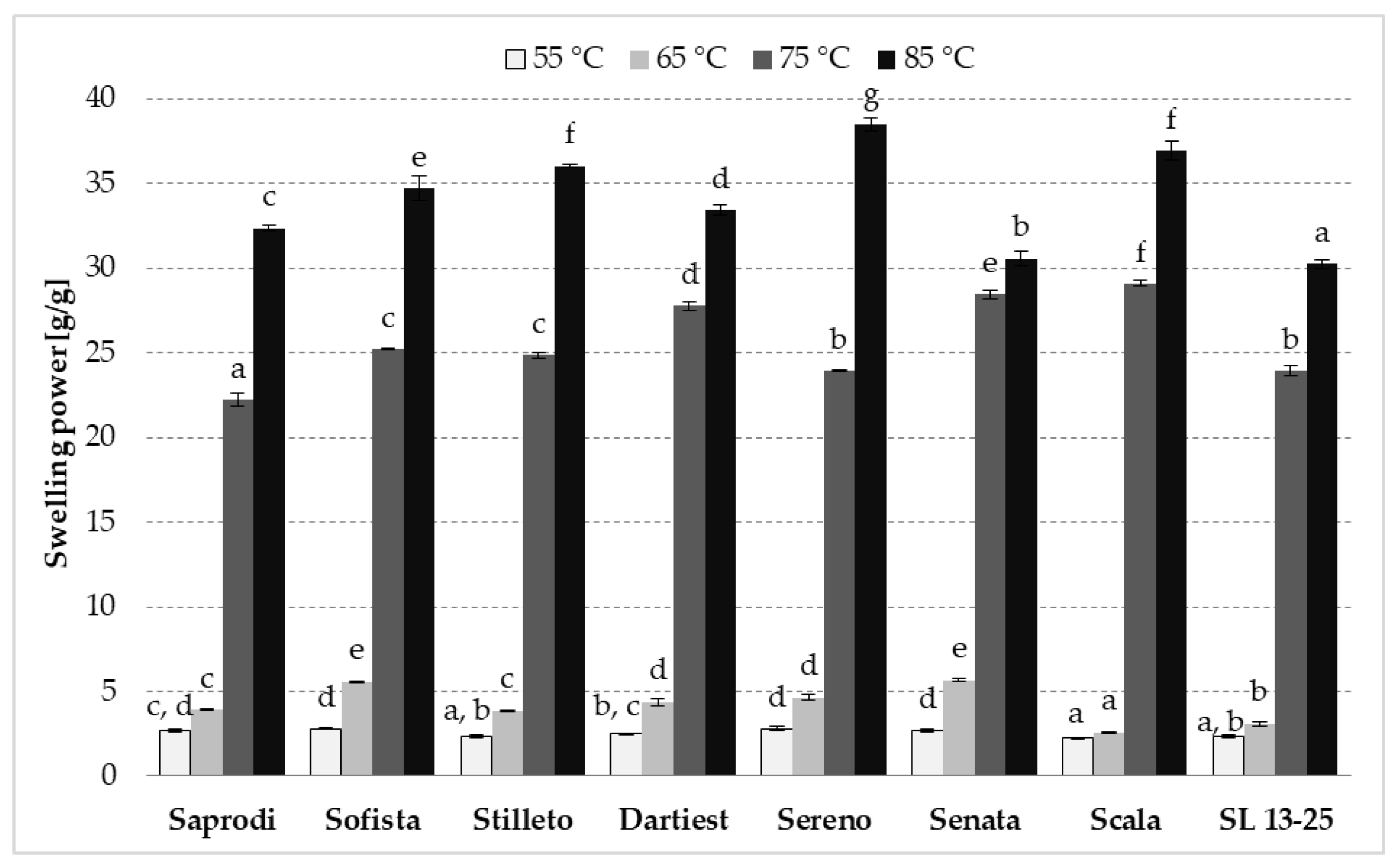
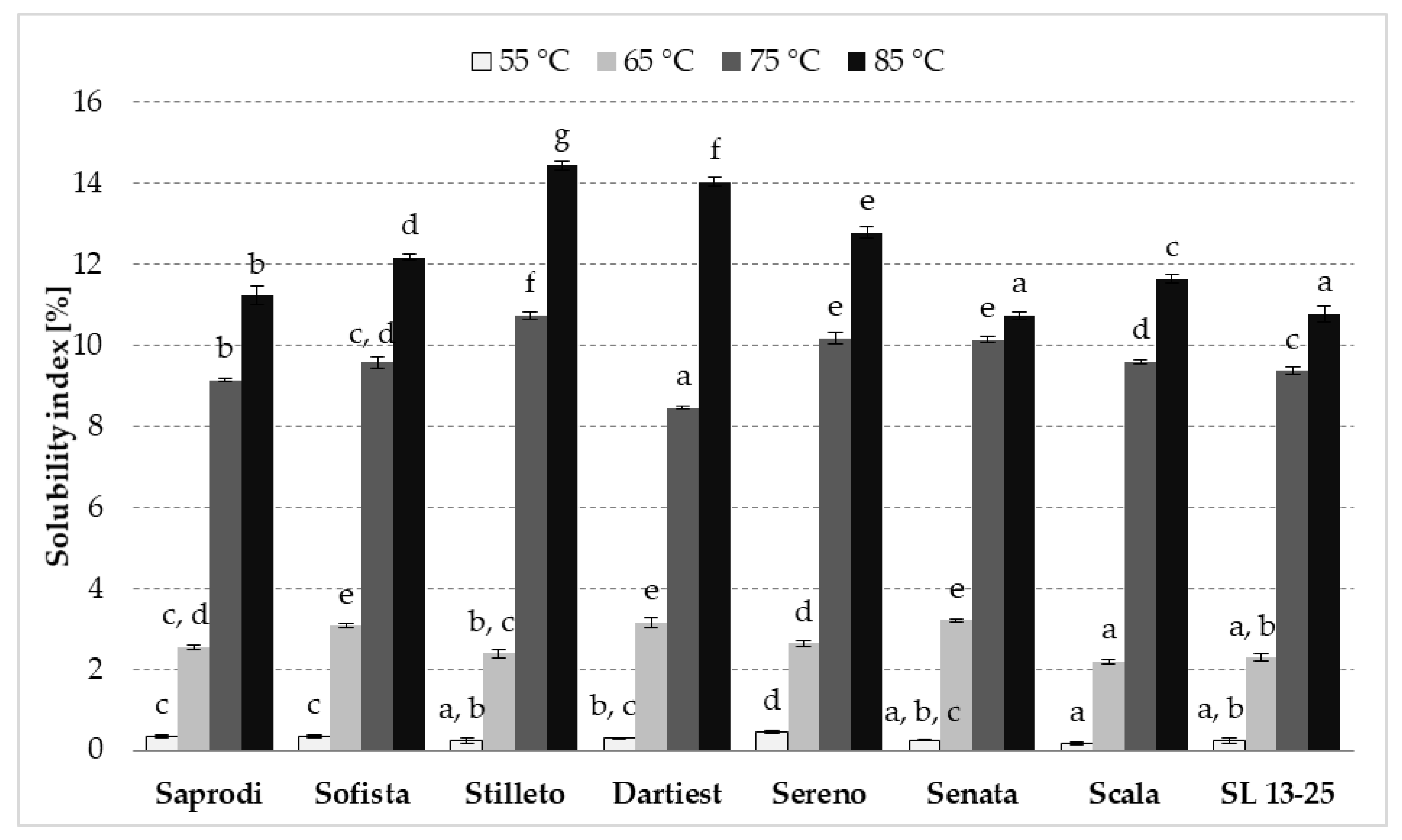
3.5. Swelling Power and Solubility Index
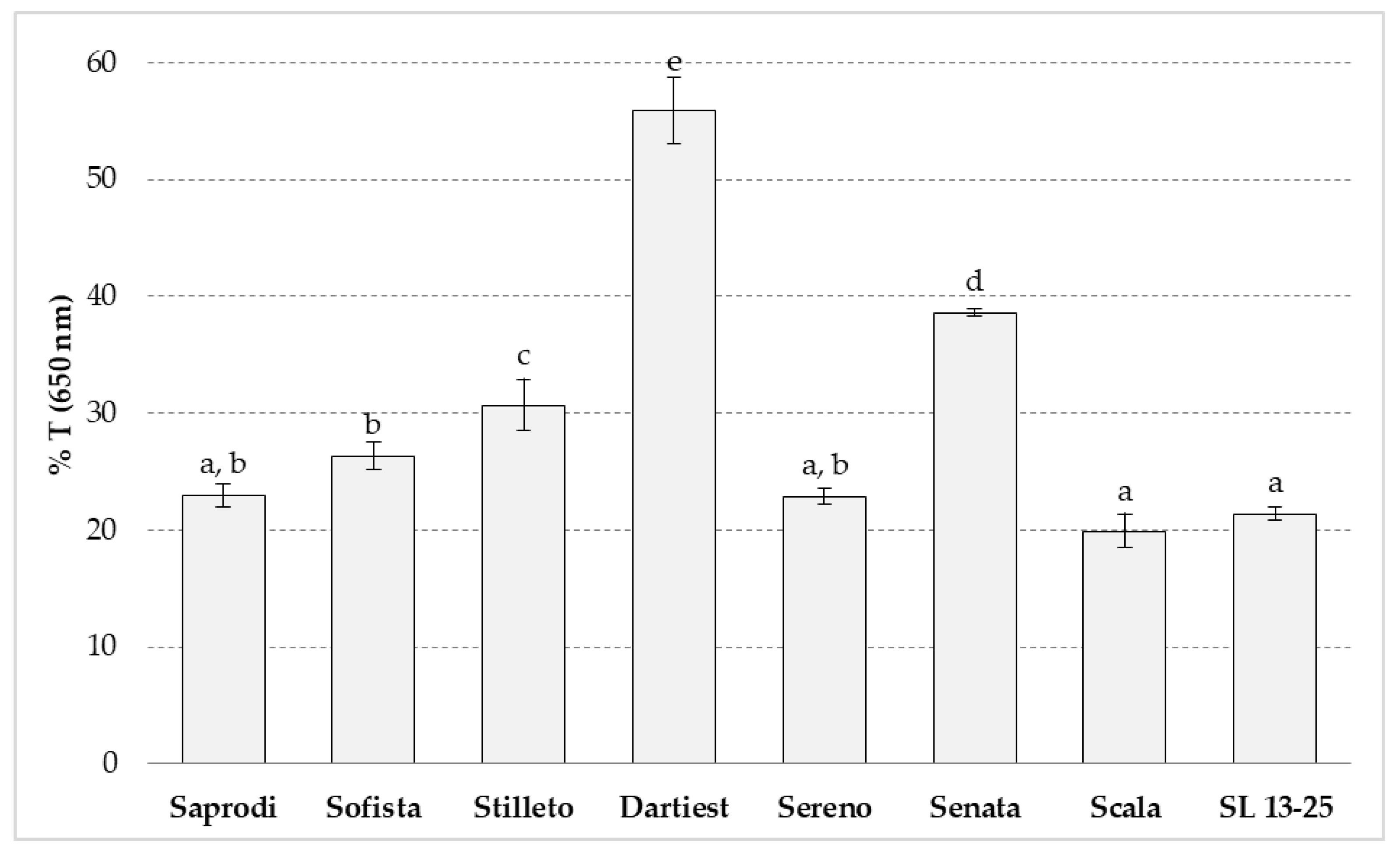
3.6. Clarity of Starch Paste
3.7. Texture of Starch Gels
3.8. Determination of Thermophysical Properties
3.8.1. Properties of Gelatinization
3.8.2. Properties of Retrogradation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goffart, J.P.; Haverkort, A.; Storey, M.; Haase, N.; Martin, M.; Lebrun, P.; Demeulemeester, K. Potato Production in Northwestern Europe (Germany, France, the Netherlands, United Kingdom, Belgium): Characteristics, Issues, Challenges and Opportunities. Potato Res. 2022, 65, 503–547. [Google Scholar] [CrossRef] [PubMed]
- Djaman, K.; Koudahe, K.; Koubodana, H.D.; Saibou, A.; Essah, S. Tillage practices in potato (Solanum tuberosum L.) production: A review. Am. J. Potato Res. 2022, 99, 1–12. [Google Scholar] [CrossRef]
- Gulati, A.; Wardhan, H.; Sharma, P. Tomato, Onion and Potato (TOP) value chains. In Agricultural Value Chains in India: Ensuring Competitiveness, Inclusiveness, Sustainability, Scalability, and Improved Finance; Springer Nature: Berlin/Heidelberg, Germany, 2022; pp. 33–97. [Google Scholar]
- Singh, P.; Arif, Y.; Siddiqui, H.; Upadhyaya, C.P.; Pichtel, J.; Hayat, S. Critical Factors Responsible for Potato Tuberization. Bot. Rev. 2023, 89, 421–437. [Google Scholar] [CrossRef]
- Adewale, P.; Yancheshmeh, M.S.; Lam, E. Starch modification for non-food, industrial applications: Market intelligence and critical review. Carbohydr. Polym. 2022, 291, 119590. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Z.; Fan, L.; Ma, S. A review of wheat starch analyses: Methods, techniques, structure and function. Int. J. Biol. Macromol. 2022, 203, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Grommers, H.E.; van der Krogt, D.A. Potato starch: Production, modifications and uses. In Starch; Academic Press: Cambridge, MA, USA, 2009; pp. 511–539. [Google Scholar]
- Van Es, A.; Hartmans, K.J. Structure and Chemical Composition of the Potato; Pudoc Publishers: Wageningen, The Netherlands, 1981. [Google Scholar]
- Ashwar, B.A.; Gani, A.; Shah, A.; Wani, I.A.; Masoodi, F.A. Preparation, health benefits and applications of resistant starch—A review. Starch-Stärke 2016, 68, 287–301. [Google Scholar] [CrossRef]
- Morrison, I.M.; Cochrane, M.P.; Cooper, A.M.; Dale, M.F.B.; Duffus, C.M.; Ellis, R.P.; Lynn, A.; Mackay, G.R.; Paterson, L.J.; Prentice, R.D.M.; et al. Potato starches: Variation in composition and properties between three genotypes grown at two different sites and in two different years. J. Sci. Food Agric. 2000, 81, 319–328. [Google Scholar] [CrossRef]
- Martínez, P.; Peña, F.; Bello-Pérez, L.A.; Núñez-Santiago, C.; Yee-Madeira, H.; Velezmoro, C. Physicochemical, functional and morphological characterization of starches isolated from three native potatoes of the Andean region. Food Chem. 2019, X, 100030. [Google Scholar] [CrossRef]
- Alvani, K.; Qi, X.; Tester, R.F.; Snape, C.E. Physico-chemical properties of potato starches. Food Chem. 2011, 125, 958–965. [Google Scholar] [CrossRef]
- Jiménez-Hernández, J.; Salazar-Montoya, J.A.; Ramos-Ramírez, E.G. Physical, chemical and microscopic characterization of a new starch from chayote (Sechium edule) tuber and its comparison with potato and maize starches. Carbohydr. Polym. 2007, 68, 679–686. [Google Scholar] [CrossRef]
- Lovera, M.; Pérez, E.; Laurentin, A. Digestibility of starches isolated from stem and root tubers of arracacha, cassava, cush–cush yam, potato and taro. Carbohydr. Polym. 2017, 176, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Sit, N.; Misra, S.; Deka, S.C. Characterization of physicochemical, functional, textural and color properties of starches from two different cultivars of taro and their comparison to potato and rice starches. Food Sci. Technol. Res. 2014, 20, 357–365. [Google Scholar] [CrossRef]
- Pérez Sira, E.E.; Amaiz, M.L. A laboratory scale method for isolation of starch from pigmented sorghum. J. Food Eng. 2004, 64, 515–519. [Google Scholar] [CrossRef]
- Mitch, E.L. Potato starch: Production and uses. In Starch: Chemistry and Technology; Whistler, R.L., BeMiller, J.N., Paschall, E.F., Eds.; Academic Press: New York, NY, USA, 1984; pp. 479–490. [Google Scholar]
- Matveev, Y.I.; Van Soest, J.J.G.; Nieman, C.; Wasserman, L.A.; Protserov, V.A.; Ezernitskaja, M.; Yuryev, V.P. The relationship between thermodynamic and structural properties of low and high amylose maize starches. Carbohydr. Polym. 2001, 44, 151–160. [Google Scholar] [CrossRef]
- Chung, H.Y.; Lim, H.S.; Lim, S.T. Effect of partial gelatinization and retrogradation on the enzymatic digestion of waxy rice starch. J. Cereal Sci. 2006, 43, 353–359. [Google Scholar] [CrossRef]
- Grgić, I.; Ačkar, Đ.; Barišić, V.; Vlainić, M.; Knežević, N.; Medverec Knežević, Z. Nonthermal methods for starch modification—A review. J. Food Process. Preserv. 2019, 43, e14242. [Google Scholar] [CrossRef]
- Šubarić, D.; Babić, J.; Ačkar, Đ. Tehnologija Škroba; Prehrambeno-tehnološki fakultet: Osijek, Croatia, 2016. [Google Scholar]
- Kaur, L.; Singh, N.; Sodhi, N.S. Some properties of potatoes and their starches II. Morphological, thermal and rheological properties of starches. Food Chem. 2002, 79, 183–192. [Google Scholar] [CrossRef]
- Bhandari, P.N.; Singhal, R.S.; Kale, D.D. Effect of succinylation on the rheological profile of starch pastes. Carbohydr. Polym. 2002, 47, 365–371. [Google Scholar] [CrossRef]
- Craig, S.A.S.; Maningat, C.C.; Seib, P.A.; Hoseney, R.C. Starch Paste Clarity. Am. Assoc. Cereal Chem. 1989, 66, 173–182. [Google Scholar]
- Karim, A.A.; Toon, L.C.; Lee, V.P.L.; Ong, W.Y.; Fazilah, A.; Noda, T. Effects of phosphorus contents on the gelatinization and retrogradation of potato starch. J. Food Sci. 2007, 72, 132–138. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; Hoover, R.; Donner, E.; Liu, Q.; Jaiswal, S.; Chibbar, R.; Seetharaman, K. Structure of faba bean, black bean and pinto bean starches at different levels of granule organization and their physicochemical properties. Food Res. Int. 2011, 44, 2962–2974. [Google Scholar] [CrossRef]
- Saartrat, S.; Puttanlek, C.; Rungsardthong, V.; Uttapap, D. Paste and gel properties of low-substituted acetylated canna starches. Carbohydr. Polym. 2005, 61, 211–221. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Kaur, M.M. Studies on noodle quality of potato and rice starches and their blends in relation to their physicochemical, pasting and gel textural properties. LWT Food Sci. Technol. 2010, 43, 1289–1293. [Google Scholar] [CrossRef]
- Galkowska, D.; Pycia, K.; Juszczak, L.; Pajak, P. Influence of cassia gum on rheological and textural properties of native potato and corn starch. Starch-Stärke 2014, 66, 1060–1070. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, L.; Singh, N. Effect of Acetylation on Some Properties of Corn and Potato Starches. Starch-Stärke 2004, 56, 586–601. [Google Scholar] [CrossRef]
- Singh, N.; Singh, J.; Kaur, L.; Sing, S.N.; Singh, G.B. Morphological, thermal and rheological properties of starches from different botanical sources. Food Chem. 2003, 81, 219–231. [Google Scholar] [CrossRef]
| Sample | Dry Matter [%] | Protein [%] | Fat [%] | Ash [%] | Starch [%] | Crude Fiber [%] |
|---|---|---|---|---|---|---|
| Saprodi | 25.05 ± 0.29 c | 2.53 ± 0.11 c | 0.09 ± 0.01 a,b | 1.24 ± 0.01 d,e | 19.78 ± 0.17 b | 1.08 ± 0.01 d |
| Sofista | 24.43 ± 1.99 b,c | 2.15 ± 0.13 b | 0.09 ± 0.00 a,b | 1.24 ± 0.01 d,e | 19.91 ± 0.12 b | 0.88 ± 0.04 a |
| Stilleto | 28.54 ± 0.69 d | 3.47 ± 0.16 d | 0.10 ± 0.00 b | 1.02 ± 0.01 b | 22.49 ± 0.71 c | 0.96 ± 0.00 b,c |
| Dartiest | 21.95 ± 1.14 a,b | 2.37 ± 0.06 b,c | 0.10 ± 0.00 b | 0.96 ± 0.01 a | 17.42 ± 0.00 a | 1.08 ± 0.01 d |
| Sereno | 24.48 ± 0.76 b,c | 3.37 ± 0.03 d | 0.09 ± 0.00 a | 1.20 ± 0.01 d | 17.63 ± 0.43 a | 1.18 ± 0.04 e |
| Senata | 21.66 ± 0.97 a | 1.79 ± 0.09 a | 0.08 ± 0.00 a | 1.11 ± 0.01 c | 17.36 ± 0.32 a | 1.07 ± 0.05 d |
| Scala | 23.57 ± 0.84 a,b,c | 2.15 ± 0.08 b | 0.12 ± 0.00 c | 1.14 ± 0.02 c | 19.06 ± 0.70 b | 0.90 ± 0.03 a,b |
| SL 13–25 | 23.48 ± 0.72 a,b,c | 2.33 ± 0.09 b,c | 0.16 ± 0.00 d | 1.25 ± 0.03 e | 17.26 ± 0.37 a | 1.03 ± 0.04 c,d |
| Sample | Dry Matter [%] | Protein [%] | Fat [%] | Ash [%] | Starch [%] | Crude Fiber [%] | Amylose [%] |
|---|---|---|---|---|---|---|---|
| Saprodi | 84.66 ± 0.02 c | 0.09 ± 0.00 a | 0.01 ± 0.00 a | 0.22 ± 0.00 a,b | 82.67 ± 0.27 b | 1.01 ± 0.03 d | 22.42 ± 0.67 f |
| Sofista | 85.33 ± 0.02 e | 0.10 ± 0.01 a | 0.01 ± 0.00 a | 0.26 ± 0.00 c | 83.25 ± 0.41 b,c | 0.77 ± 0.01 b | 20.67 ± 0.61 c,d |
| Stilleto | 84.67 ± 0.01 c | 0.09 ± 0.01 a | 0.01 ± 0.00 a | 0.22 ± 0.01 a,b | 82.96 ± 0.06 b,c | 0.95 ± 0.04 c,d | 21.16 ± 0.29 d,e |
| Dartiest | 85.69 ± 0.02 f | 0.10 ± 0.00 a | 0.01 ± 0.00 a | 0.21 ± 0.00 a,b | 83.75 ± 0.13 c | 0.70 ± 0.01 a | 20.28 ± 0.37 b,c |
| Sereno | 84.33 ± 0.08 b | 0.15 ± 0.03 b | 0.01 ± 0.00 a | 0.23 ± 0.01 b | 82.51 ± 0.40 b | 0.70 ± 0.02 a | 21.40 ± 0.48 e |
| Senata | 84.92 ± 0.03 d | 0.10 ± 0.01 a | 0.01 ± 0.00 a | 0.26 ± 0.00 c | 83.32 ± 0.58 b,c | 0.90 ± 0.01 c | 19.93 ± 0.34 b |
| Scala | 84.95 ± 0.01 d | 0.09 ± 0.00 a | 0.01 ± 0.00 a | 0.25 ± 0.00 c | 82.95 ± 0.42 b,c | 0.90 ± 0.01 c | 16.97 ± 0.37 a |
| SL 13–25 | 83.51 ± 0.13 a | 0.09 ± 0.00 a | 0.01 ± 0.00 a | 0.21 ± 0.01 a | 81.23 ± 0.10 a | 1.02 ± 0.05 d | 16.82 ± 0.34 a |
| Sample | L* | a* | b* | C | h° |
|---|---|---|---|---|---|
| Saprodi | 94.85 ± 0.31 c | −1.45 ± 0.19 a | 2.29 ± 0.12 a | 2.72 ± 0.05 b,c,d | 122.36 ± 4.59 e |
| Sofista | 94.08 ± 0.45 a,b | −1.32 ± 0.02 a,b | 2.33 ± 0.07 a | 2.67 ± 0.06 b,c | 119.46 ± 0.88 d,e |
| Stilleto | 93.71 ± 0.17 a | −1.21 ± 0.02 b | 2.49 ± 0.04 b | 2.77 ± 0.04 c,d | 115.94 ± 0.59 b,c,d |
| Dartiest | 94.49 ± 0.30 b,c | −1.15 ± 0.29 b | 2.52 ± 0.14 b | 2.79 ± 0.14 d | 114.40 ± 6.06 b,c |
| Sereno | 93.81 ± 0.56 a,b | −1.31 ± 0.04 a,b | 2.65 ± 0.01 c | 2.96 ± 0.02 e | 116.35 ± 0.75 c,d |
| Senata | 93.67 ± 1.19 a | −1.18 ± 0.26 b | 2.93 ± 0.05 d | 3.17 ± 0.11 f | 111.86 ± 4.25 a,b |
| Scala | 94.18 ± 0.25 a,b,c | −1.32 ± 0.02 a,b | 2.31 ± 0.07 a | 2.65 ± 0.05 b | 119.77 ± 0.96 d,e |
| SL 13–25 | 94.85 ± 0.04 c | −0.79 ± 0.03 c | 2.27 ± 0.04 a | 2.41 ± 0.04 a | 109.16 ± 0.51 a |
| Sample | Peak Viscosity [BU] | Viscosity at 92 °C [BU] | Viscosity after Shearing at 92 °C [BU] | Viscosity at 50 °C [BU] | Viscosity after Shearing at 50 °C [BU] | Breakdown [BU] | Setback [BU] |
|---|---|---|---|---|---|---|---|
| Saprodi | 1505.5 ± 33.2 b | 1130.5 ± 6.4 f | 600.0 ± 2.8 f | 1046.0 ± 18.4 e | 854.5 ± 13.4 e | 905.5 ± 30.4 a | 446.0 ± 15.6 d |
| Sofista | 1499.5 ± 19.1 b | 1016.0 ± 7.1 d | 539.0 ± 8.5 d | 924.5 ± 3.5 c | 758.5 ± 0.7 c | 960.5 ± 27.6 b,c | 385.5 ± 4.9 b,c |
| Stilleto | 1410.0 ± 2.8 a | 1006.0 ± 18.4 d | 514.5 ± 12.0 c | 898.0 ± 18.4 c | 745.5 ± 33.2 c | 895.5 ± 9.2 a | 383.5 ± 6.4 b,c |
| Dartiest | 1446.5 ± 9.2 a | 904.5 ± 16.3 b | 468.0 ± 7.1 a | 834.5 ± 19.1 b | 693.0 ± 11.3 b | 978.5 ± 2.1 b,c | 366.5 ± 12.0 b |
| Sereno | 1518.5 ± 21.9 b | 1048.0 ± 11.3 e | 562.5 ± 6.4 e | 962.5 ± 19.1 d | 799.0 ± 15.6 d | 956.0 ± 28.3 b | 400.0 ± 12.7 c |
| Senata | 1490.0 ± 1.4 b | 888.5 ± 9.2 a,b | 487.0 ± 4.2 b | 806.5 ± 6.4 a,b | 641.5 ± 10.6 a | 1003.0 ± 5.7 c,d | 319.5 ± 2.1 a |
| Scala | 1673.5 ± 20.5 c | 974.0 ± 9.9 c | 521.0 ± 4.2 c | 915.0 ± 4.2 c | 738.0 ± 4.2 c | 1152.5 ± 16.3 e | 394.0 ± 0.0 c |
| SL 13–25 | 1519.0 ± 9.9 b | 870.0 ± 9.9 a | 474.0 ± 5.7 a,b | 796.0 ± 0.0 a | 663.0 ± 7.1 a,b | 1045.0 ± 4.2 d | 322.0 ± 5.7 a |
| Sample | Gel Strength [g] | Rupture Strength [g] | Adhesiveness [g sec] |
|---|---|---|---|
| Saprodi | 3.14 ± 0.08 b,c | 928.89 ± 18.84 d | −219.78 ± 6.00 b,c |
| Sofista | 2.98 ± 0.01 b | 840.18 ± 17.90 b | −202.52 ± 4.58 d |
| Stilleto | 3.25 ± 0.08 b,c | 959.33 ± 6.77 e | −224.69 ± 4.04 b |
| Dartiest | 3.30 ± 0.16 c | 776.63 ± 9.16 a | −73.02 ± 9.50 e |
| Sereno | 2.48 ± 0.23 a | 998.99 ± 10.61 f | −348.49 ± 2.23 a |
| Senata | 3.08 ± 0.00 b,c | 758.10 ± 4.46 a | −219.27 ± 8.41 b,c |
| Scala | 3.03 ± 0.08 b,c | 912.11 ± 17.51 d | −192.56 ± 5.68 d |
| SL 13–25 | 3.08 ± 0.00 b,c | 873.06 ± 1.98 c | −205.13 ± 8.11 c,d |
| Sample | To (°C) | Tp (°C) | Te (°C) | ∆H (J/g) |
|---|---|---|---|---|
| Saprodi | 64.80 ± 0.11 b | 70.32 ± 0.36 d | 74.24 ± 0.02 c,d | 2.16 ± 0.04 c |
| Sofista | 63.80 ± 0.18 a | 67.56 ± 0.28 b | 73.80 ± 0.13 b | 1.22 ± 0.06 a |
| Stilleto | 64.22 ± 0.42 a,b | 68.27 ± 0.33 c | 76.73 ± 0.21 f | 1.15 ± 0.04 a |
| Dartiest | 63.73 ± 0.16 a | 66.21 ± 0.13 a | 70.24 ± 0.08 a | 2.27 ± 0.08 c,d |
| Sereno | 67.08 ± 0.06 c | 70.11 ± 0.06 d | 74.62 ± 0.25 d | 2.32 ± 0.05 d |
| Senata | 68.45 ± 0.28 d | 71.89 ± 0.12 e | 75.94 ± 0.08 e | 2.01 ± 0.08 b |
| Scala | 67.52 ± 0.59 c | 71.41 ± 0.06 e | 77.05 ± 0.17 f | 2.30 ± 0.04 c,d |
| SL 13–25 | 69.18 ± 0.15 e | 71.84 ± 0.17 e | 75.81 ± 0.49 e | 2.21 ± 0.02 c,d |
| Sample | To (°C) | Tp (°C) | Te (°C) | ∆H (J/g) |
|---|---|---|---|---|
| Saprodi | 44.57 ± 0.43 b | 53.11 ± 0.49 d | 58.89 ± 0.12 a | 0.38 ± 0.00 c,d |
| Sofista | 44.80 ± 0.27 b | 51.23 ± 0.24 a,b | 62.06 ± 0.07 b,c | 0.35 ± 0.02 c |
| Stilleto | 44.76 ± 0.26 b | 55.56 ± 0.59 e | 63.19 ± 0.17 d | 0.28 ± 0.01 b |
| Dartiest | 44.39 ± 0.70 b | 55.11 ± 0.04 e | 63.27 ± 0.29 d | 0.29 ± 0.01 b |
| Sereno | 44.70 ± 0.13 b | 53.39 ± 0.24 d | 62.23 ± 0.28 c | 0.11 ± 0.04 a |
| Senata | 42.40 ± 0.39 a | 50.72 ± 0.35 a | 61.53 ± 0.56 b | 0.36 ± 0.01 c |
| Scala | 45.27 ± 0.42 b | 51.99 ± 0.13 b,c | 63.09 ± 0.21 d | 0.15 ± 0.01 a |
| SL 13–25 | 49.19 ± 0.08 c | 52.64 ± 0.47 c,d | 62.25 ± 0.08 c | 0.42 ± 0.03 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovač, M.; Ravnjak, B.; Šubarić, D.; Vinković, T.; Babić, J.; Ačkar, Đ.; Lončarić, A.; Šarić, A.; Bulatović, V.O.; Jozinović, A. Isolation and Characterization of Starch from Different Potato Cultivars Grown in Croatia. Appl. Sci. 2024, 14, 909. https://doi.org/10.3390/app14020909
Kovač M, Ravnjak B, Šubarić D, Vinković T, Babić J, Ačkar Đ, Lončarić A, Šarić A, Bulatović VO, Jozinović A. Isolation and Characterization of Starch from Different Potato Cultivars Grown in Croatia. Applied Sciences. 2024; 14(2):909. https://doi.org/10.3390/app14020909
Chicago/Turabian StyleKovač, Mario, Boris Ravnjak, Drago Šubarić, Tomislav Vinković, Jurislav Babić, Đurđica Ačkar, Ante Lončarić, Antonija Šarić, Vesna Ocelić Bulatović, and Antun Jozinović. 2024. "Isolation and Characterization of Starch from Different Potato Cultivars Grown in Croatia" Applied Sciences 14, no. 2: 909. https://doi.org/10.3390/app14020909
APA StyleKovač, M., Ravnjak, B., Šubarić, D., Vinković, T., Babić, J., Ačkar, Đ., Lončarić, A., Šarić, A., Bulatović, V. O., & Jozinović, A. (2024). Isolation and Characterization of Starch from Different Potato Cultivars Grown in Croatia. Applied Sciences, 14(2), 909. https://doi.org/10.3390/app14020909












