Short-Time Alternating Current Electrical Stimulation and Cell Membrane-Related Components
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrical Stimulation via IonOptix System
2.2. Cell Culture
2.3. Aquaporin Expression in MG-63 Cells
2.4. AQP Expression and Granularity of MG-63 Cells after Electrical Stimulation
2.5. Hyaluronan Spherical Coat and Adhesion
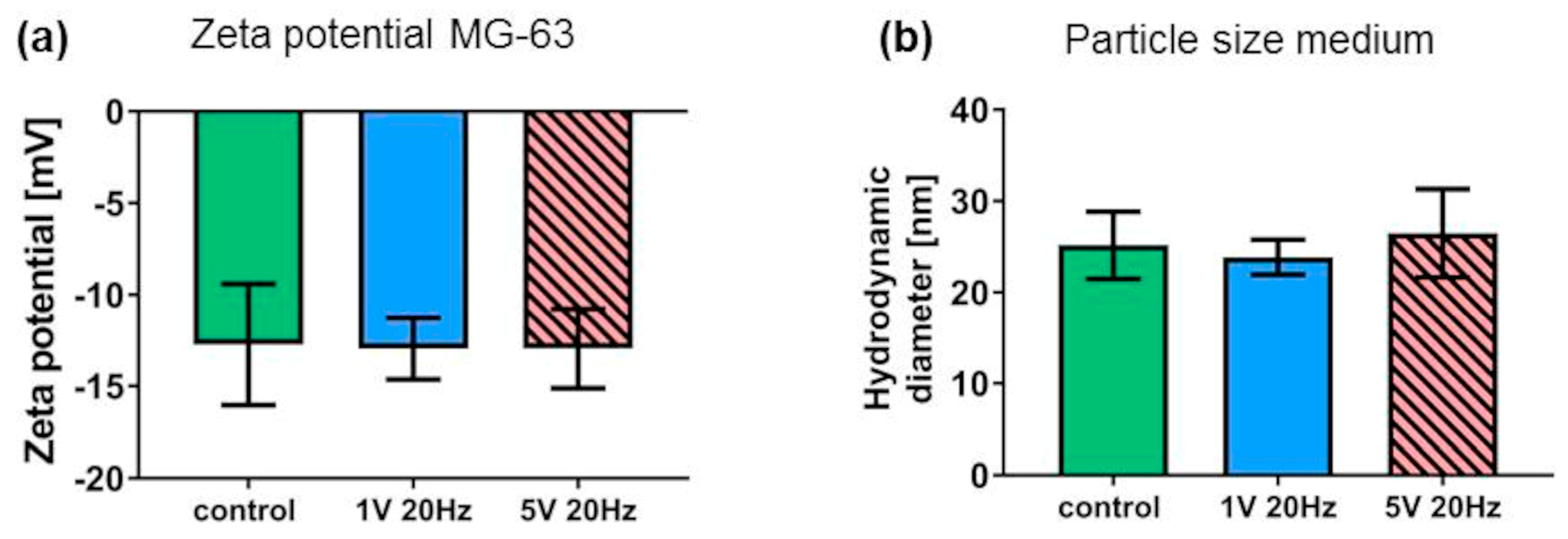
2.6. Cells‘ Surface Charge via Zeta Potential
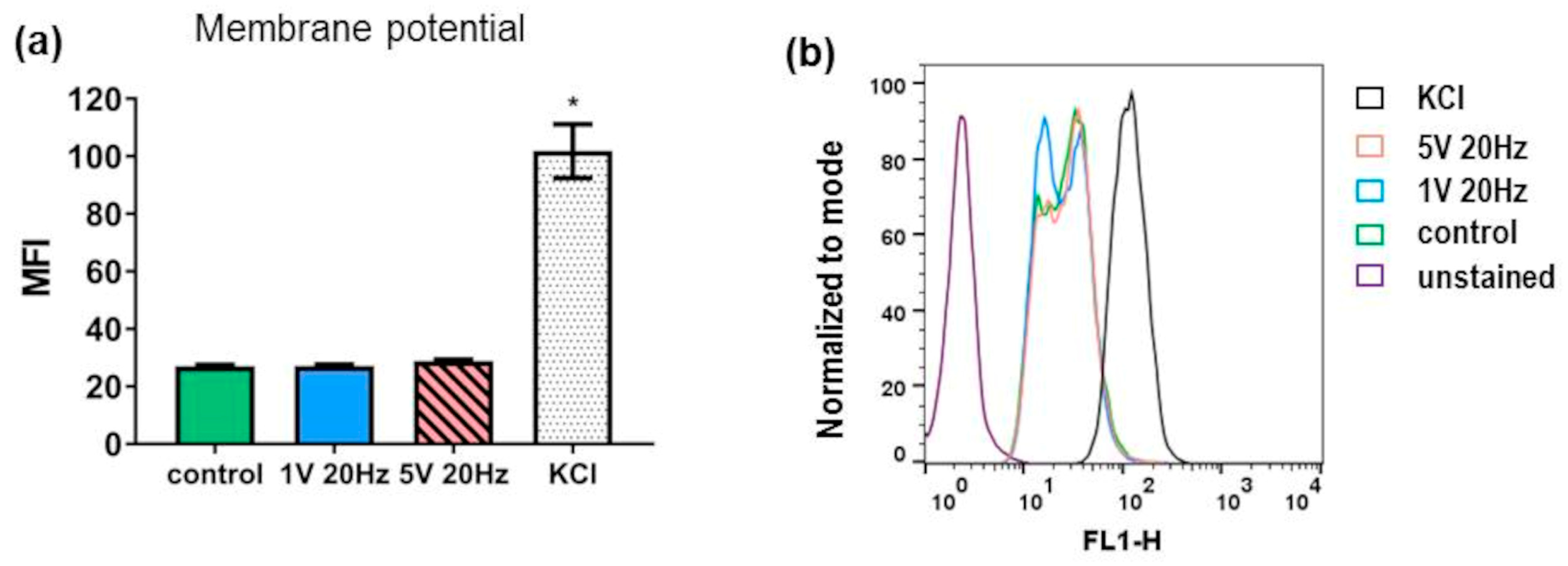
2.7. Membrane Potential
2.8. Particle Size in Medium
2.9. Quantification of Protein Adsorption
2.10. Statistics
3. Results
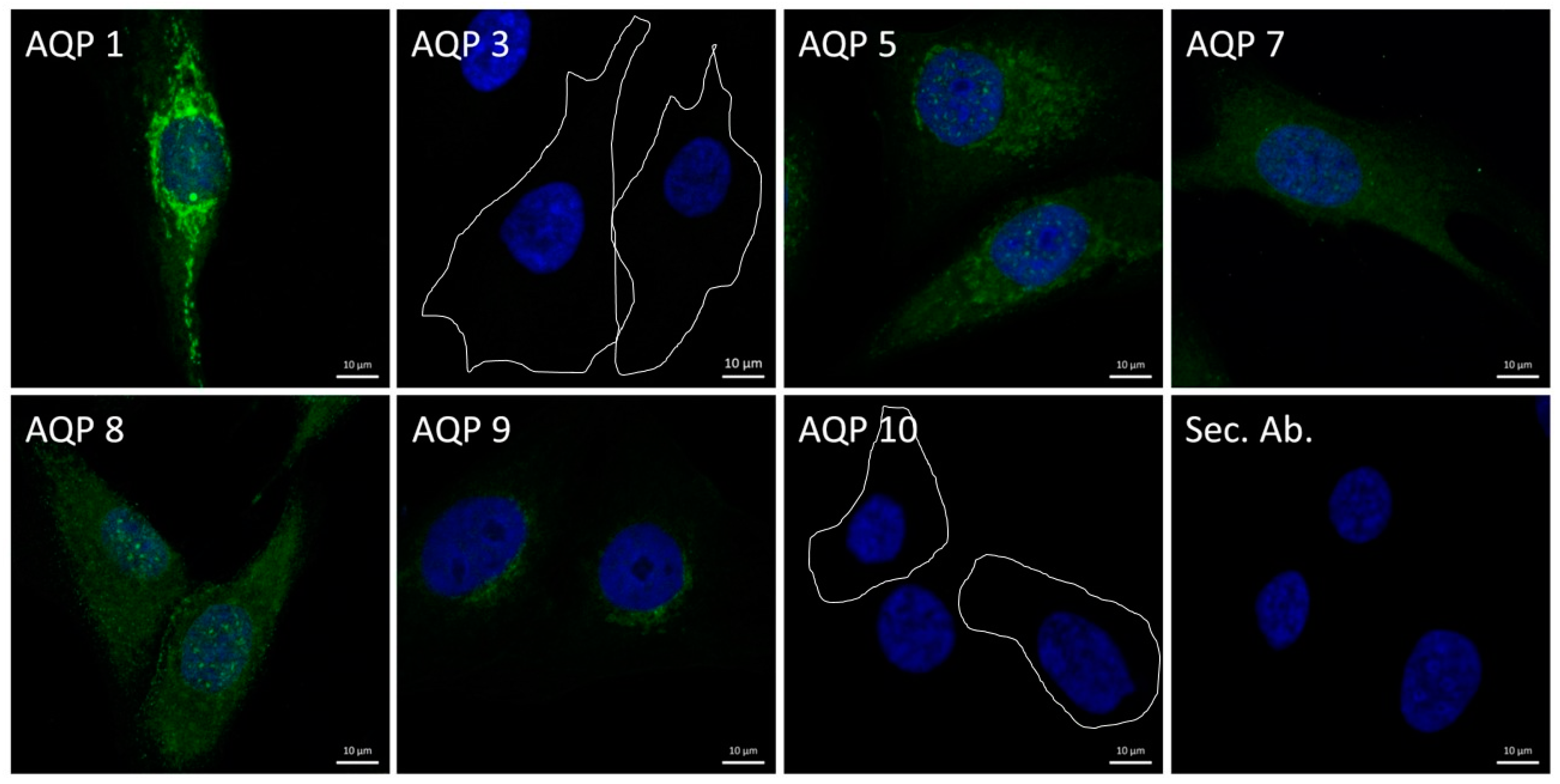
3.1. Expression of Aquaporins
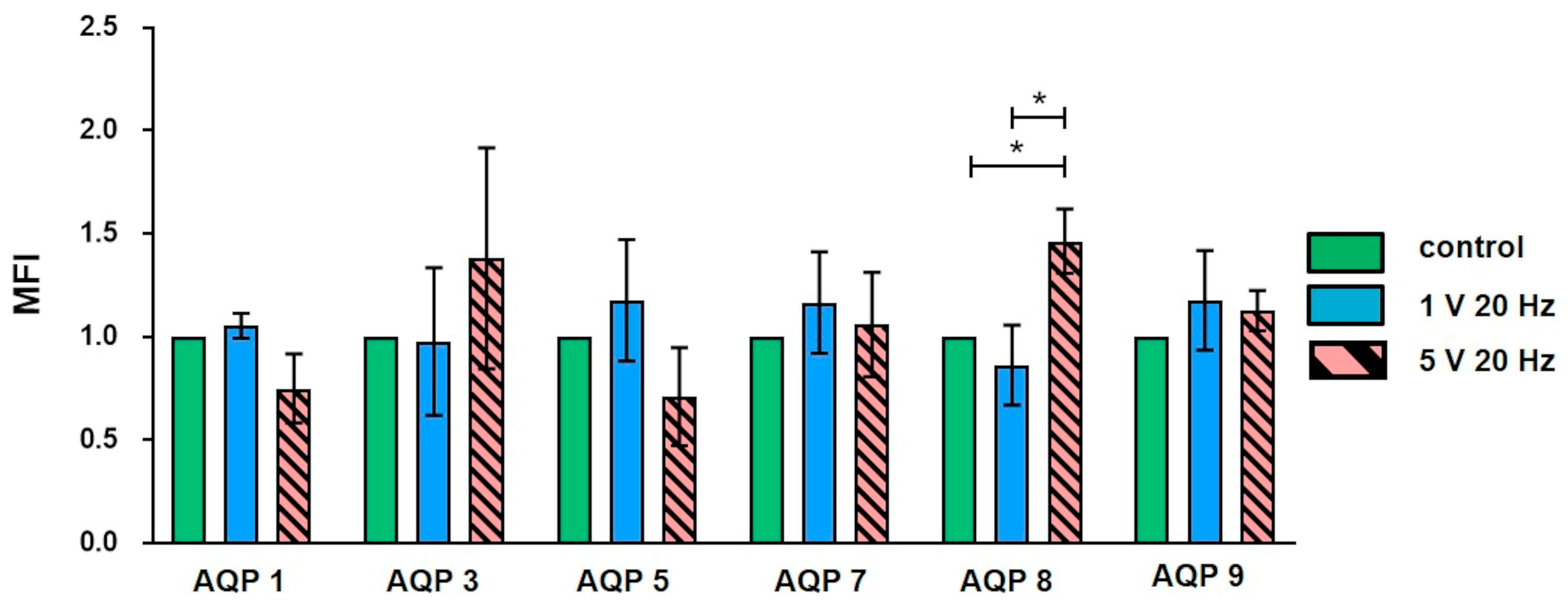
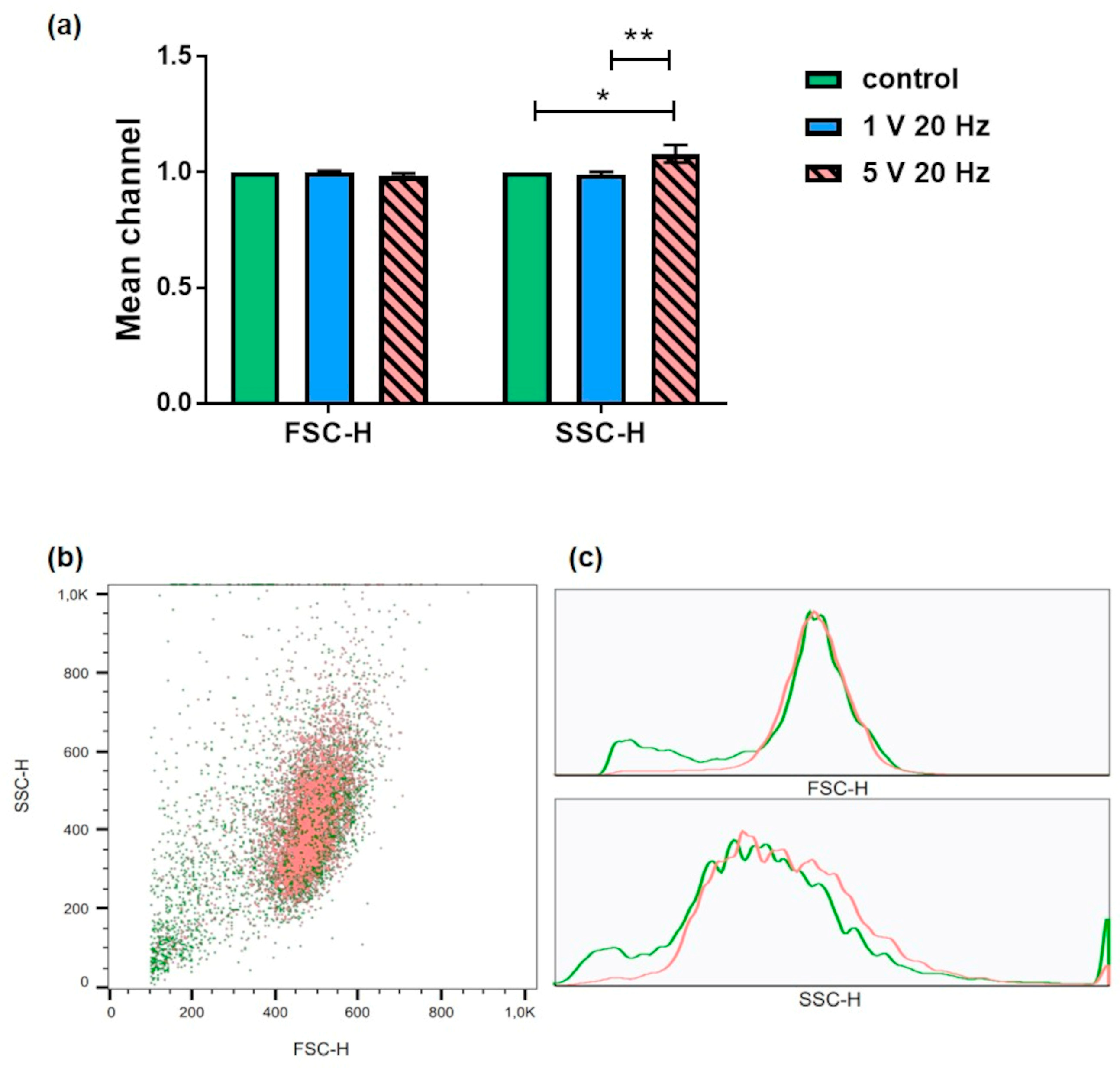
3.2. Flow Cytometry: Aquaporin Expression and Granularity of MG-63 Cells after AC Electrical Stimulation
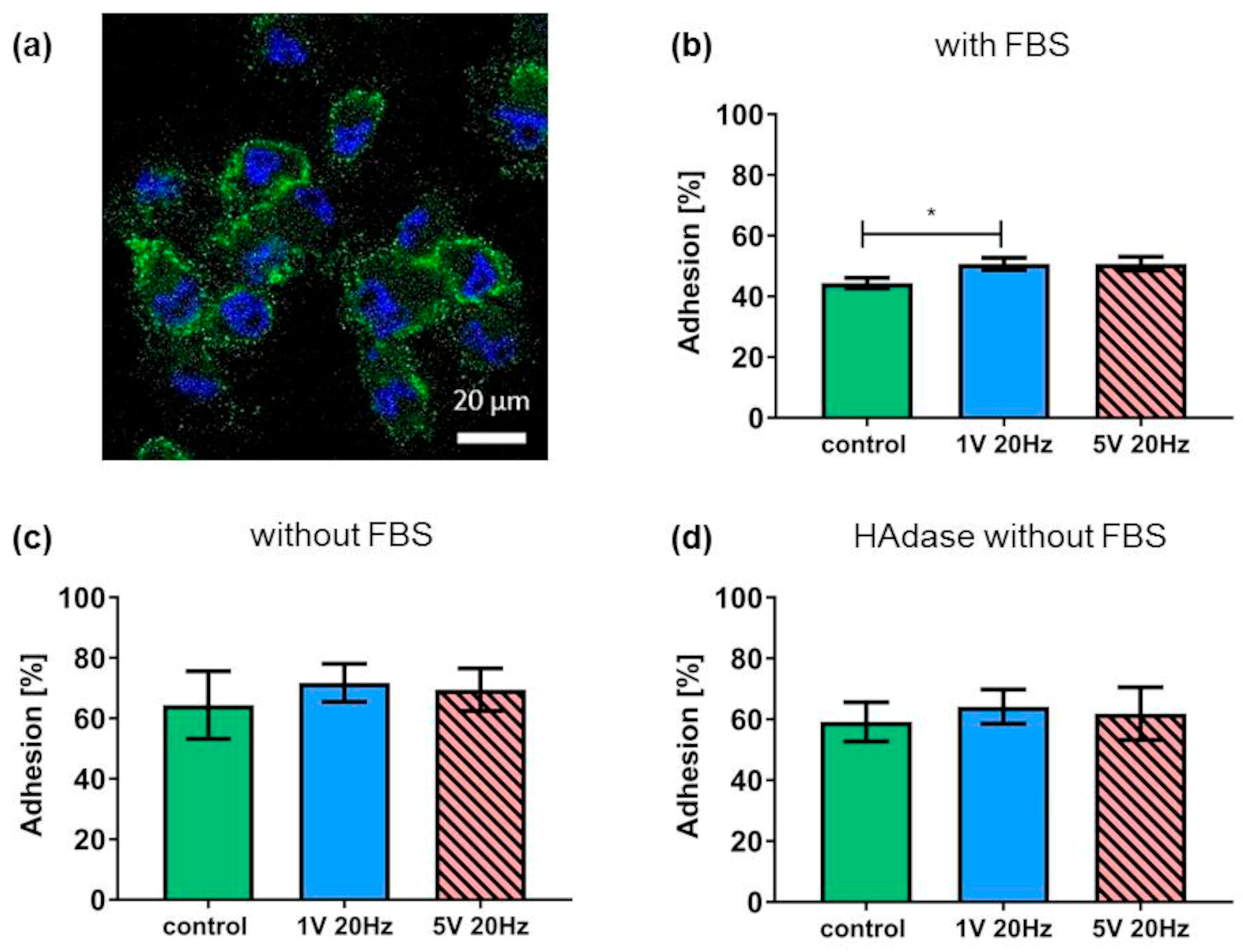
3.3. Hyaluronan Coat and Adhesion
3.4. Litesizer Measurements
3.5. Membrane Potential
3.6. Protein Adsorption
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vasquez-Sancho, F.; Abdollahi, A.; Damjanovic, D.; Catalan, G. Flexoelectricity in Bones. Adv. Mater. 2018, 30, 1705316. [Google Scholar] [CrossRef]
- Haglin, J.M.; Jain, S.; Eltorai, A.E.M.; Daniels, A.H. Bone Growth Stimulation: A Critical Analysis Review. JBJS Rev. 2017, 5, e8. [Google Scholar] [CrossRef] [PubMed]
- Busse, J.W.; Morton, E.; Lacchetti, C.; Guyatt, G.H.; Bhandari, M. Current management of tibial shaft fractures: A survey of 450 Canadian orthopedic trauma surgeons. Acta Orthop. 2008, 79, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Eischen-Loges, M.; Oliveira, K.M.C.; Bhavsar, M.B.; Barker, J.H.; Leppik, L. Pretreating mesenchymal stem cells with electrical stimulation causes sustained long-lasting pro-osteogenic effects. PeerJ 2018, 6, e4959. [Google Scholar] [CrossRef] [PubMed]
- Mobini, S.; Leppik, L.; Thottakkattumana Parameswaran, V.; Barker, J.H. In vitro effect of direct current electrical stimulation on rat mesenchymal stem cells. PeerJ 2017, 5, e2821. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.; Shah, F.A.; Ortiz-Catalan, M. Enhancing osteoblast survival through pulsed electrical stimulation and implications for osseointegration. Sci. Rep. 2021, 11, 22416. [Google Scholar] [CrossRef] [PubMed]
- Sahm, F.; Freiin Grote, V.; Zimmermann, J.; Haack, F.; Uhrmacher, A.M.; van Rienen, U.; Bader, R.; Detsch, R.; Jonitz-Heincke, A. Long-term stimulation with alternating electric fields modulates the differentiation and mineralization of human pre-osteoblasts. Front. Physiol. 2022, 13, 965181. [Google Scholar] [CrossRef]
- Dean, D.D.; Schwartz, Z.; Bonewald, L.; Muniz, O.E.; Morales, S.; Gomez, R.; Brooks, B.P.; Qiao, M.; Howell, D.S.; Boyan, B.D. Matrix vesicles produced by osteoblast-like cells in culture become significantly enriched in proteoglycan-degrading metalloproteinases after addition of beta-glycerophosphate and ascorbic acid. Calcif. Tissue Int. 1994, 54, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, M.; García, M.A.; Arrabal, P.M.; Martínez, F.; Yañez, M.J.; Jara, N.; Weil, B.; Domínguez, D.; Medina, R.A.; Nualart, F. Insulin regulates GLUT1-mediated glucose transport in MG-63 human osteosarcoma cells. J. Cell. Physiol. 2011, 226, 1425–1432. [Google Scholar] [CrossRef]
- Gruening, M.; Neuber, S.; Nestler, P.; Lehnfeld, J.; Dubs, M.; Fricke, K.; Schnabelrauch, M.; Helm, C.A.; Müller, R.; Staehlke, S.; et al. Enhancement of Intracellular Calcium Ion Mobilization by Moderately but Not Highly Positive Material Surface Charges. Front. Bioeng. Biotechnol. 2020, 8, 1016. [Google Scholar] [CrossRef]
- Staehlke, S.; Rebl, H.; Nebe, B. Phenotypic stability of the human MG-63 osteoblastic cell line at different passages. Cell Biol. Int. 2019, 43, 22–32. [Google Scholar] [CrossRef]
- Staehlke, S.; Bielfeldt, M.; Zimmermann, J.; Gruening, M.; Barke, I.; Freitag, T.; Sylvia, S.; Van Rienen, U.; Nebe, B. Pulsed Electrical Stimulation Affects Osteoblast Adhesion and Calcium Ion Signaling. Cells 2022, 11, 2650. [Google Scholar] [CrossRef] [PubMed]
- Ercan, B.; Webster, T.J. The effect of biphasic electrical stimulation on osteoblast function at anodized nanotubular titanium surfaces. Biomaterials 2010, 31, 3684–3693. [Google Scholar] [CrossRef] [PubMed]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L1005–L1028. [Google Scholar] [CrossRef] [PubMed]
- Leppik, L.; Oliveira, K.M.C.; Bhavsar, M.B.; Barker, J.H. Electrical stimulation in bone tissue engineering treatments. Eur. J. Trauma Emerg. Surg. 2020, 46, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Zannetti, A.; Benga, G.; Brunetti, A.; Napolitano, F.; Avallone, L.; Pelagalli, A. Role of Aquaporins in the Physiological Functions of Mesenchymal Stem Cells. Cells 2020, 9, 2678. [Google Scholar] [CrossRef]
- Mobasheri, A.; Wray, S.; Marples, D. Distribution of AQP2 and AQP3 water channels in human tissue microarrays. J. Mol. Histol. 2005, 36, 1–14. [Google Scholar] [CrossRef]
- Snuggs, J.W.; Day, R.E.; Bach, F.C.; Conner, M.T.; Bunning, R.A.D.; Tryfonidou, M.A.; Le Maitre, C.L. Aquaporin expression in the human and canine intervertebral disc during maturation and degeneration. JOR Spine 2019, 2, e1049. [Google Scholar] [CrossRef]
- Edamana, S.; Login, F.H.; Yamada, S.; Kwon, T.-H.; Nejsum, L.N. Aquaporin water channels as regulators of cell-cell adhesion proteins. In: American journal of physiology. Cell Physiol. 2021, 320, C771–C777. [Google Scholar] [CrossRef]
- Finke, B.; Luethen, F.; Schroeder, K.; Mueller, P.; Bergemann, C.; Frant, M.; Ohl, A.; Nebe, J.B. The effect of positively charged plasma polymerization on initial osteoblastic focal adhesion on titanium surfaces. Biomaterials 2007, 28, 4521–4534. [Google Scholar] [CrossRef]
- Cohen, M.; Kam, Z.; Addadi, L.; Geiger, B. Dynamic study of the transition from hyaluronan- to integrin-mediated adhesion in chondrocytes. EMBO J. 2006, 25, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Ercan, B.; Webster, T.J. Greater osteoblast proliferation on anodized nanotubular titanium upon electrical stimulation. Int. J. Nanomed. 2008, 3, 477–485. [Google Scholar] [CrossRef]
- Budde, K.; Zimmermann, J.; Neuhaus, E.; Schroder, M.; Uhrmacher, A.M.; van Rienen, U. Requirements for Documenting Electrical Cell Stimulation Experiments for Replicability and Numerical Modeling. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; Volume 41, pp. 1082–1088. [Google Scholar] [CrossRef]
- Saldaña, L.; Bensiamar, F.; Boré, A.; Vilaboa, N. In search of representative models of human bone-forming cells for cytocompatibility studies. Acta Biomater. 2011, 7, 4210–4221. [Google Scholar] [CrossRef]
- Leif, R.C. Practical flow cytometry, by Howard M. Shapiro. Alan R. Liss, New York, 1985, 295 pages. Cytometry 1986, 7, 111–112. [Google Scholar] [CrossRef]
- Hoentsch, M.; Bussiahn, R.; Rebl, H.; Bergemann, C.; Eggert, M.; Frank, M.; von Woedtke, T.; Nebe, B. Persistent Effectivity of Gas Plasma-Treated, Long Time-Stored Liquid on Epithelial Cell Adhesion Capacity and Membrane Morphology. PLoS ONE 2014, 9, e104559. [Google Scholar] [CrossRef] [PubMed]
- Fukada, E.; Yasuda, I. On the Piezoelectric Effect of Bone. J. Phys. Soc. Jpn. 1957, 12, 1158–1162. [Google Scholar] [CrossRef]
- Mobini, S.; Leppik, L.; Barker, J.H. Direct current electrical stimulation chamber for treating cells in vitro. Biotechniques 2016, 60, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Yang, G.-H.; Kim, G. Tissue engineering bioreactor systems for applying physical and electrical stimulations to cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 103, 935–948. [Google Scholar] [CrossRef]
- Griffin, M.; Bayat, A. Electrical Stimulation in Bone Healing: Critical Analysis by Evaluating Levels of Evidence. Eplasty 2011, 11, e34. [Google Scholar]
- Nicksic, P.J.; Donnelly, D.T.; Hesse, M.; Bedi, S.; Verma, N.; Seitz, A.J.; Shoffstall, A.J.; Ludwig, K.A.; Dingle, A.M.; Poore, S.O. Electronic Bone Growth Stimulators for Augmentation of Osteogenesis in In Vitro and In Vivo Models: A Narrative Review of Electrical Stimulation Mechanisms and Device Specifications. Front. Bioeng. Biotechnol. 2022, 10, 793945. [Google Scholar] [CrossRef]
- Pettersen, E.; Anderson, J.; Ortiz-Catalan, M. Electrical stimulation to promote osseointegration of bone anchoring implants: A topical review. J. NeuroEng. Rehabil. 2022, 19, 31. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Bai, X.; Ding, Y.; Lee, I.-S. Electrical stimulation as a novel tool for regulating cell behavior in tissue engineering. Biomater. Res. 2019, 23, 25. [Google Scholar] [CrossRef]
- Demais, V.; Audrain, C.; Mabilleau, G.; Chappard, D.; Baslé, M.F. Diversity of bone matrix adhesion proteins modulates osteoblast attachment and organization of actin cytoskeleton. Morphologie 2014, 98, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Vohra, S.; Hennessy, K.M.; Sawyer, A.A.; Zhuo, Y.; Bellis, S.L. Comparison of mesenchymal stem cell and osteosarcoma cell adhesion to hydroxyapatite. J. Mater. Sci. Mater. Med. 2008, 19, 3567–3574. [Google Scholar] [CrossRef] [PubMed]
- Freshney, R.I. Culture of Animal Cells. A Manual of Basic Technique and Specialized Applications, 6th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Grinnell, F.; Feld, M.K. Initial adhesion of human fibroblasts in serum-free medium: Possible role of secreted fibronectin. Cell 1979, 17, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Lüthen, F.; Lange, R.; Becker, P.; Rychly, J.; Beck, U.; Nebe, J.G. The influence of surface roughness of titanium on b1- and b3-integrin adhesion and the organization of fibronectin in human osteoblastic cells. Biomaterials 2005, 26, 2423–2440. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. The dynamic dialogue between cells and matrices: Implications of fibronectin’s elasticity. Proc. Natl. Acad. Sci. USA 1999, 96, 2588–2590. [Google Scholar] [CrossRef]
- Xian, M.; Fang, L.; Liu, Y.; Wei, Q.; Hao, L.; Yu, Z.; He, X.; Wu, G. Electrical field induce mBMSCs differentiation to osteoblast via protein adsorption enhancement. Colloids Surf. B Biointerfaces 2022, 209 Pt 2, 112158. [Google Scholar] [CrossRef]
- Benavidez, T.E.; Torrente, D.; Marucho, M.; Garcia, C.D. Adsorption of Soft and Hard Proteins onto OTCEs under the influence of an External Electric Field. Langmuir 2015, 31, 2455–2462. [Google Scholar] [CrossRef]
- Zimmermann, E.; Geiger, B.; Addadi, L. Initial stages of cell-matrix adhesion can be mediated and modulated by cell-surface hyaluronan. Biophys. J. 2002, 82, 1848–1857. [Google Scholar] [CrossRef]
- Nebe, B.; Finke, B.; Lüthen, F.; Bergemann, C.; Schröder, K.; Rychly, J.; Liefeith, K.; Ohl, A. Improved initial osteoblast functions on amino-functionalized titanium surfaces. Biomol. Eng. 2007, 24, 447–454. [Google Scholar] [CrossRef] [PubMed]
- O’Hearn, S.F.; Ackerman, B.J.; Mower, M.M. Paced monophasic and biphasic waveforms alter transmembrane potentials and metabolism of human fibroblasts. Biochem. Biophys. Rep. 2016, 8, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Khitrin, A.J.; Khitrin, K.A.; Model, M.A. A model for membrane potential and intracellular ion distribution. Chem. Phys. Lipids 2014, 184, 76–81. [Google Scholar] [CrossRef]
- Love, M.R.; Palee, S.; Chattipakorn, S.C.; Chattipakorn, N. Effects of electrical stimulation on cell proliferation and apoptosis. J. Cell. Physiol. 2018, 233, 1860–1876. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Liu, Y.; Lipsky, S.; Cho, M. Physical manipulation of calcium oscillations facilitates osteodifferentiation of human mesenchymal stem cells. FASEB J. 2007, 21, 1472–1480. [Google Scholar] [CrossRef]
- Snuggs, J.W.; Tessier, S.; Bunning, R.A.B.; Shapiro, I.M.; Risbud, M.V.; Le Maitre, C.L. TonEBP regulates the hyperosmotic expression of aquaporin 1 and 5 in the intervertebral disc. Sci. Rep. 2021, 11, 3164. [Google Scholar] [CrossRef] [PubMed]
- Chidawanyika, T.; Supattapone, S. Hydrogen Peroxide-induced Cell Death in Mammalian Cells. J. Cell. Signal. 2021, 2, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Pimpão, C.; Mósca, A.F.; Coxixo, A.S.; Lopes, D.; Da Silva, I.V.; Pedersen, P.A.; Antunes, F.; Soveral, G. Human aquaporin-5 facilitates hydrogen peroxide permeation affecting adaption to oxidative stress and cancer cell migration. Cancers 2019, 11, 29–34. [Google Scholar] [CrossRef]
- Adan, A.; Alizada, G.; Kiraz, Y.; Baran, Y.; Nalbant, A. Flow cytometry: Basic principles and applications. Crit. Rev. Biotechnol. 2017, 37, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Gera, N.; Yang, A.; Holtzman, T.S.; Lee, S.X.; Wong, E.T.; Swanson, K.D. Tumor treating fields perturb the localization of septins and cause aberrant mitotic exit. PLoS ONE 2015, 10, e0125269. [Google Scholar] [CrossRef]
- Giladi, M.; Schneiderman, R.S.; Porat, Y.; Munster, M.; Itzhaki, A.; Mordechovich, D.; Cahal, S.; Kirson, E.D.; Weinberg, U.; Palti, Y. Mitotic disruption and reduced clonogenicity of pancreatic cancer cells in vitro and in vivo by tumor treating fields. Pancreatology 2014, 14, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Giladi, M.; Schneiderman, R.S.; Voloshin, T.; Porat, Y.; Munster, M.; Blat, R.; Sherbo, S.; Bomzon, Z.; Urman, N.; Itzhaki, A.; et al. Mitotic Spindle Disruption by Alternating Electric Fields Leads to Improper Chromosome Segregation and Mitotic Catastrophe in Cancer Cells. Sci. Rep. 2015, 5, 18046. [Google Scholar] [CrossRef] [PubMed]
- Shteingauz, A.; Porat, Y.; Voloshin, T.; Schneiderman, R.S.; Munster, M.; Zeevi, E.; Kaynan, N.; Karnit, G.; Giladi, M.; Kirson, E.D.; et al. AMPK-dependent autophagy upregulation serves as a survival mechanism in response to Tumor Treating Fields (TTFields). Cell Death Dis. 2018, 9, 1074. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buenning, M.E.; Bielfeldt, M.; Nebe, B.; Staehlke, S. Short-Time Alternating Current Electrical Stimulation and Cell Membrane-Related Components. Appl. Sci. 2024, 14, 812. https://doi.org/10.3390/app14020812
Buenning ME, Bielfeldt M, Nebe B, Staehlke S. Short-Time Alternating Current Electrical Stimulation and Cell Membrane-Related Components. Applied Sciences. 2024; 14(2):812. https://doi.org/10.3390/app14020812
Chicago/Turabian StyleBuenning, Maren E., Meike Bielfeldt, Barbara Nebe, and Susanne Staehlke. 2024. "Short-Time Alternating Current Electrical Stimulation and Cell Membrane-Related Components" Applied Sciences 14, no. 2: 812. https://doi.org/10.3390/app14020812
APA StyleBuenning, M. E., Bielfeldt, M., Nebe, B., & Staehlke, S. (2024). Short-Time Alternating Current Electrical Stimulation and Cell Membrane-Related Components. Applied Sciences, 14(2), 812. https://doi.org/10.3390/app14020812







