Abstract
In the present study, an immersion experiment was carried out to examine how N80 steel corrodes when exposed to formation water containing dissolved CO2 and supercritical CO2 (Sc-CO2) along with water vapor. We employed electrochemical and surface analysis methods to examine the influence of various factors, including the temperature and duration of immersion, on the extent of corrosion. The results show that the corrosion patterns of N80 steel in a supercritical CO2 environment and CO2-saturated formation water differed significantly. The presence of similar corrosion features was suggested by the constant structure of the corrosion products identified in the formation water. However, the morphology of the corrosion product was complex in the supercritical CO2 environment, exhibiting features of pitting and localized corrosion. Furthermore, a non-linear trend in the corrosion rate was observed between 40 °C and 120 °C. Specifically, the rate of corrosion declined from 40 °C to 80 °C, but it then resumed its growth from 80 °C to 120 °C. These findings suggest that very high temperatures could lead to the destruction of corrosion products and subsequently enhance the corrosion process.
1. Introduction
In light of the continuous development of global industry, substantial quantities of greenhouse gases, primarily CO2, are being released into the atmosphere, leading to a profound climate warming phenomenon on Earth. According to the annual report of the United Nations Intergovernmental Panel on Climate Change (IPCC) in 2021 [1], the annual average CO2 concentration surged to 410 ppm in 2019. This concentration level of atmospheric CO2 represents the highest point observed in the past 2 million years [1], thereby posing a significant threat to both the natural ecosystem and human habitation. At the moment, storing CO2 underground in geological formations is one of the most promising methods for lowering CO2 emissions and slowing down global warming [2]. This approach, known as CO2 capture, utilization, and storage (CCUS) technology, has garnered widespread acknowledgment and support from numerous national and regional entities as a response to global climate change [3]. Consequently, CCUS stands indisputably as a pivotal technological direction for curbing the repercussions of global warming [4,5].
The CCUS process encompasses several key stages, namely CO2 capture from power plants or industrial operations, the utilization of CO2 for energy production or resource extraction enhancement, CO2 transportation, and storage in geological reservoirs. Among the various options for CO2 transportation, the use of metal pipes emerges as the most economically viable choice. The CO2 that is carried via the pipeline is compressed to a supercritical state (with a pressure exceeding 7.38 MPa and a temperature above 31.1 °C) in order to maximize the CO2 density and reduce the likelihood of two-phase flow. From a corrosion perspective, pure and dry supercritical CO2 (Sc-CO2)does not pose a corrosion threat to metal pipes [6,7]. Unfortunately, Sc-CO2 fluids naturally contain impurities like O2, H2O, H2S, SO2 and NOx [8,9,10,11]. In the event that the metal pipes include free water, Sc-CO2 and other contaminants can dissolve into the water phase, changing its chemical composition and potentially escalating the environment’s corrosiveness [12]. CO2 acts as an acid gas and dissolves in water as H2CO3, undergoing ionization into H+. This process can be represented by the following reactions [13,14,15,16]:
Subsequently, a cathodic corrosion process and an anodic corrosion process take place on the metal surface,
and overall,
The presence of H can cause cracking in the metal matrix, and the formation of FeCO3 can result in scale formation on metal surfaces. This leads to a complex corrosion process when CO2 is present. Crucially, at the same temperatures, the rate of metal corrosion in the Sc-CO2 environment may be significantly greater than in a low-pressure gaseous environment. This can be attributed to two main factors. The first factor results in enhanced amounts of H2CO3, HCO3−, and CO32− in the aqueous solution due to the high solubility of CO2 in water at supercritical conditions. Consequently, the corresponding cathodic reaction rate increases, ultimately elevating the overall corrosion rate and substantially increasing the risk of damage to metal pipelines and casings [17,18,19]. The second factor stems from the ability of water to evaporate into Sc-CO2, leading the Sc-CO2 phase to contain trace amounts of water, making it highly corrosive to metals. Interestingly, metals in the low-water Sc-CO2 phase may experience more corrosion than in the water phase when CO2 has been dissolved. In recent times, there has been considerable attention in the literature on understanding the corrosion behavior of metals in Sc-CO2 environments [19,20,21,22,23,24]. Certain researchers have explicitly investigated the properties of metal corrosion in Sc-CO2 environments with low water percentages [25,26,27].
This paper investigates the corrosion characteristics of N80 steel under distinct reaction environments, specifically comparing formation water with Sc-CO2 conditions. Immersion tests are used to assess the corrosion, and energy dispersive spectroscopy (EDS) and scanning electron microscopy (SEM) are used to analyze the corrosion products of N80 steel. Additionally, electrochemical testing is utilized for further examination of the corrosion behavior. This study is interesting because it examines the corrosion behavior of N80 steel in a two-phase environment with formation water and Sc-CO2. Furthermore, this study provides a detailed analysis of how immersion time and temperature influence the N80 steel corrosion process and the rate of corrosion.
2. Experiments
2.1. Materials, Solution and Preprocessing
N80 steel was chosen for the immersion experiments and electrochemical tests in this study. The composition of N80 steel (wt.%) is shown in Table 1. The dimensions of the steel samples used in the immersion experiments were 50 × 10 × 3 mm. The samples were prepared for the studies by grinding them with 800# silicon carbide sandpaper, washing them with deionized water, cleaning them with ethanol, and then drying them in a desiccator. Similarly, the electrochemical test samples had the same dimensions of 50 × 10 × 3 mm and were polished using silicon carbide sandpaper ranging from 1000# to 2000#. The samples were then cleaned using ethanol and deionized water and were allowed to air dry. Once dry, they were then embedded in 704 silicone rubber, leaving an area of 0.35 cm2 which was exposed to the test solution. The test solution used was a laboratory-simulated formation water, which was formulated to mimic the composition of the formation water found in the Sudong Block of the Sulige Gas Field. It had the following composition (g/L): 5.99 Na+, 5.22 K+, 7.14 Ca2+, 0.29 Mg2+, 26.96 Cl−, 0.56 SO42−, and 0.14 HCO3−.

Table 1.
The chemical composition of N80 steel (wt.%).
2.2. Immersion Experiments
To examine the corrosion properties of N80 steel in a two-phase situation with formation water and Sc-CO2, an immersion test was conducted in an autoclave. Every experiment was carried out in a stationary state, and Table 2 contains the specific test conditions. The prepared N80 steel sample was affixed to the top terminal post of the autoclave using a fishing line, enabling half of the sample to be submerged in the solution while the other half was exposed to a wet Sc-CO2 environment (PCO2 = 10 MPa). This arrangement allowed for the observation of N80 steel’s corrosion behavior at the junction of the two phases (Figure 1). After every immersion test, the samples were removed from the autoclave, with one specimen set aside for examining the surface features of the corrosion products. The remaining samples were placed in vacuum-sealed bags. Prior to the corrosion test, formation water was introduced into the autoclave. To deoxidize the solution, purified N2 was injected into the autoclave for 3 h. This was followed by pumping high-purity CO2 into the solution for 2 h until it became a saturated CO2 solution. After that, the solution was heated to the appropriate temperature, and CO2 was pressurized to 10 MPa using an ISCO pump and then injected into the autoclave, creating a Sc-CO2 phase above the solution. As the Sc-CO2 was in direct contact with the solution, water vapor was able to transfer into the Sc-CO2 phase.

Table 2.
Immersion experiment conditions (PCO2 = 10 MPa).
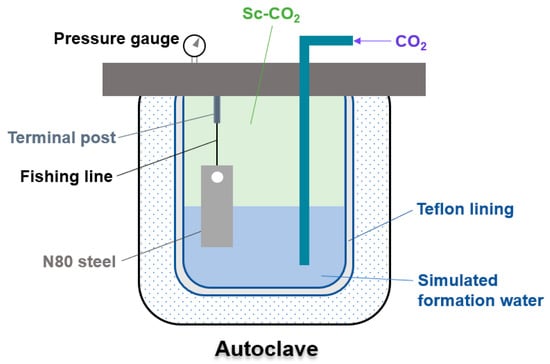
Figure 1.
Diagrammatic representation of the autoclave immersion experiment.
2.3. Electrochemical Tests
A CHI600E electrochemical workstation (Chenhua Instrument Co., Ltd., Shanghai, China) was utilized to perform the electrochemical tests, employing a standard three-electrode system. The electrochemical tests in this paper did not use in situ test methods. Following the immersion, the N80 steel sample was taken out of the autoclave and subjected to electrochemical testing under ambient temperature and pressure. The solution used in the electrochemical tests was the same as the solution used in the immersion tests. The working electrode in this configuration was N80 steel, the reference electrode was a saturated potassium chloride calomel electrode, and the auxiliary electrode was a platinum sheet. The electrochemical test parameters in this experiment primarily encompassed the open-circuit potential (OCP) evaluation, the potentiodynamic polarization assessment (Tafel curve), and the electrochemical impedance spectroscopy (EIS) analysis. The corrosion characteristics of N80 steel were investigated using these three electrochemical test methods. Prior to conducting EIS and potentiodynamic polarization analyses, the OCP was monitored for 1 h. A sinusoidal potential perturbation with a 5 mV amplitude was applied over a frequency range of 10−2 Hz to 105 Hz at the OCP for the EIS measurements. Meanwhile, the potential was swept from −500 mV against OCP to +500 mV against OCP using a sweep rate of 10 mV/s for the potentiodynamic polarization test.
2.4. Surface Analysis
SEM was used to study the surface corrosion morphology of N80 steel after the corrosion process was finished. Moreover, EDS was used to ascertain the elemental makeup of the corrosion products. The Zeiss Sigma 300 System (Carl Zeiss Microscopy, Oberkochen, Germany) was utilized for SEM testing, while the Oxford X-MAX System (Oxford Instruments, Abingdon, UK) was utilized for EDS scanning in both the SEM and EDS analyses, which were carried out using field emission high-resolution scanning electron microscopy (FESEM).
3. Results and Discussion
3.1. Corrosion Morphology
The sample’s exterior (Figure 2) exhibited distinct signs of corrosion following the immersion experiments, particularly noticeable at the interface between the solution and Sc-CO2. The corrosion morphology showed significant alterations to the steel, with some corrosion products formed due to being in contact with the solution appearing dark and uniform, while those exposed to Sc-CO2 exhibited various shades of color and were uneven. Based on the EDS image analysis (Figure 3), the distribution of elements in the corrosion products on the surface of N80 steel samples in the solution environment was relatively uniform, whereas the distribution of elements in the corrosion products in the Sc-CO2 environment was non-uniform, particularly with a significant presence of Ca elements in localized areas. Since the N80 steel used in the experiment did not contain Ca elements, the Ca in the corrosion products originated from the Ca2+ in the solution. The occurrence of this phenomenon might be attributed to the migration of formation water from the solution phase to the Sc-CO2 phase through the pores within the corrosion products. Due to pore structure variation at different locations on the corrosion product layer, some locations had more water accumulation than others, causing pitting corrosion in water-rich regions. As a result, some regions are heavily corroded and abundant Ca is left in those regions in the Sc-CO2 phase. Regrettably, we did not obtain solid evidence to validate this hypothesis, and future investigation was needed. Because the specimen was not immediately stored under vacuum conditions upon removal from the reactor, some corrosion products might have interacted with H2O and O2 in the air, leading to a darkening of the specimen’s surface color. However, this did not mean that these darker samples were more severely corroded in the autoclave, which was an error in the experiment.
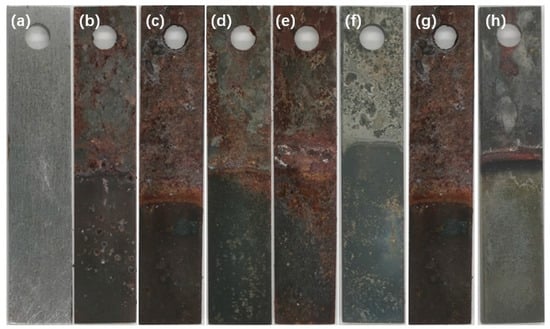
Figure 2.
Pictures of the N80 steel surface after immersion experiments. (a) Before corrosion; (b–e) 3.5, 7, 14, and 28 d after corrosion, T = 80 °C; (f–h) 40 °C, 80 °C, and 120 °C, time = 7 d.
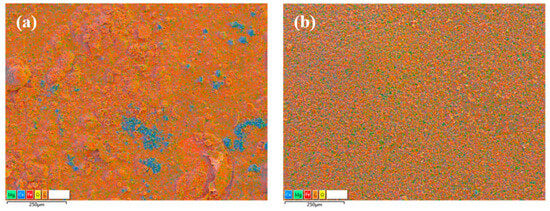
Figure 3.
EDS images of the N80 steel samples after 7 days of corrosion, T = 80 °C. (a) Exposed to Sc-CO2; (b) exposed to the solution.
Throughout the 28-day corrosion period, the color of the resulting corrosion constituents on the exterior of the N80 sample gradually deepened, with significant corrosion detected at the interface between the formation water and Sc-CO2. The corrosion severity at the interface was significantly higher compared to that observed in the Sc-CO2 and formation water environments. Identical corrosion patterns were noted at various temperatures.
3.2. SEM and EDS Observation
SEM images of N80 steel at the interface between the CO2-saturated solution and Sc-CO2, under a CO2 partial pressure of 10 MPa, are depicted in Figure 4. The images clearly exhibited distinct differences in the corrosion morphology of N80 steel between the two environments. Upon closer examination through local magnification views, it became apparent that the corrosion product structure in the formation water environment exhibited regularity, indicative of predominantly uniform corrosion. In contrast, the corrosion product morphology within the Sc-CO2 environment was more intricate, displaying various structures such as spherical, needle-like, and sheet-like shapes, with an uneven corrosion surface. Moreover, convexities and pits were observed, primarily manifesting as instances of local corrosion and pitting corrosion. These observed features might be linked to the random distribution of water vapor in the Sc-CO2 environment.
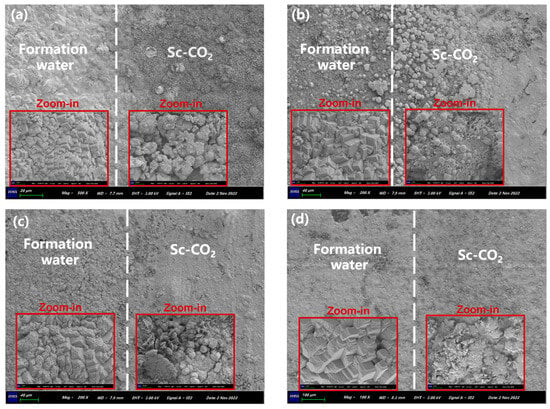
Figure 4.
SEM images at the interface between the formation water and Sc-CO2 after 3.5 (a), 7 (b), 14 (c) and 28 (d) days of corrosion.
In Figure 5, a comparative analysis is conducted using EDS linear scanning to examine the surface of N80 steel samples exposed to different temperatures. This analysis focuses on the horizontal distribution of elements Mg, Ca, Fe, C, and O along the scan line on the surface. At 40 °C, the corrosion products of the sample in the solution environment primarily comprise Fe, O, Ca, and C, with the Fe and Ca contents being closely matched. With the increase in temperature, the Fe content in the corrosion products gradually increases, indicating that higher temperatures facilitate the formation of FeCO3. In contrast, the Ca content decreases gradually, which may be attributed to the possibility of Ca2+ ions replacing some Fe2+ ions at lower temperatures, resulting in the formation of FexCa1−xCO3 compounds [28].
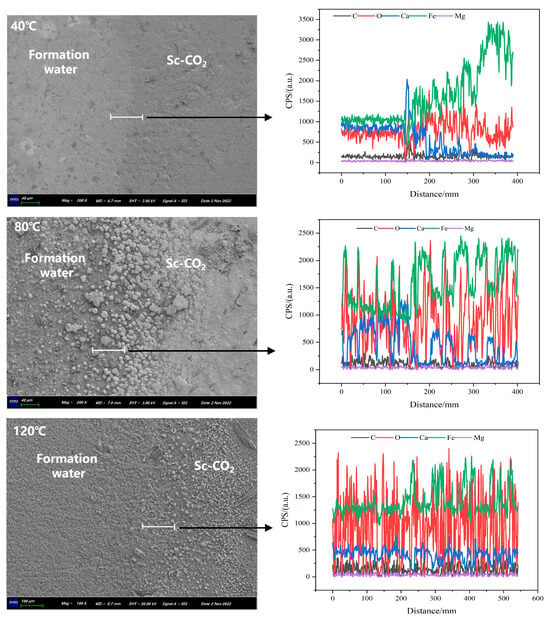
Figure 5.
Distribution of the key elements at the surfaces of N80 steel samples determined by means of EDS linear scanning (along the white lines) after immersion experiments at 40 °C, 80 °C and 120 °C for 7 days.
Figure 6 depicts the distribution of Ca and Mg at the interface of the solution and Sc-CO2 in samples immersed in solutions for 7 days, as determined by means of EDS surface scanning. Analysis of the graph indicated that in the constituents formed due to corrosion on the exterior of N80 steel, Ca was primarily located in the liquid phase, with only a small amount present in the Sc-CO2. On the other hand, Mg had a relatively uniform distribution in both the liquid phase and the Sc-CO2. The existence of Mg and Ca in the Sc-CO2 may be due to the splashing of some solution onto the upper portion of the steel sample during the CO2 gas-injection process, resulting in a residue of the solution on the specimen’s surface after exposure to the Sc-CO2 environment. This phenomenon indicated that in the Sc-CO2 environment, Mg from the solution was more prone to enter the corrosion products compared to Ca.
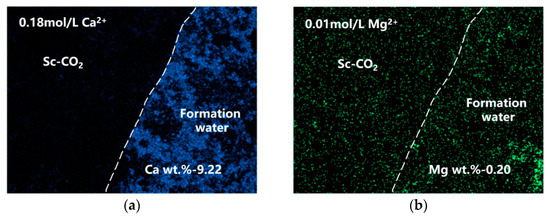
Figure 6.
Ca (a) and Mg (b) distribution at the steel surface determined by means of EDS surface scanning of N80 steel after immersion tests for 7d (Ca wt.% and Mg wt.% represent the content of Ca and Mg elements in the corrosion products, respectively).
3.3. Electrochemical Analysis
3.3.1. Open Circuit Potential
OCP refers to the potential difference between a working electrode and a reference electrode when no load is applied. It can be used to assess the corrosion of metals and provide fundamental data for other electrochemical tests. Figure 7 shows how the OCP of N80 steel changed in various environments with a 10 MPa CO2 partial pressure. The results demonstrate the distinct behavior of the OCP curve at different immersion times. During the initial stage of corrosion (0 to 3.5 days), ions such as Cl− and SO42− in the solution penetrated the double layer at the electrode interface due to their strong penetration ability. These ions adsorbed onto the electrode, leading to a decrease in the OCP of the N80 steel sample. As the corrosion time continued (3.5 to 7 days), the surface Fe of the electrode transformed into Fe2+, causing changes in the charge distribution of the double layer and a positive shift in the double layer potential, resulting in an increase in the OCP. Additionally, the formation of corrosion products on the electrode surface led to resistance polarization, effectively preventing the adsorption of active ions and further contributing to the increase in the OCP. After 7 days, the continued generation and stacking of corrosion products were accompanied by detachment and shedding, influencing the compactness of the surface corrosion products and indirectly affecting the contact between active ions and the N80 steel sample, ultimately causing fluctuations in the OCP. Within the temperature range of 40 °C to 120 °C, as the temperature increased, the movement frequency of active ions accelerated, leading to an increased frequency of contact with the electrode surface. This resulted in a gradual decrease in the OCP of the N80 steel sample.
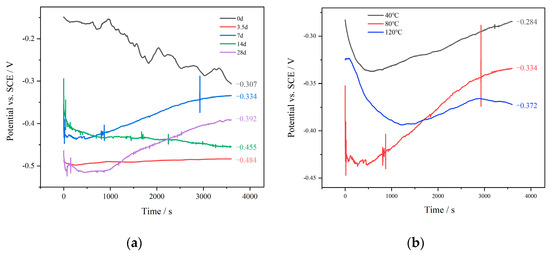
Figure 7.
OCP variation for N80 steel in CO2-saturated formation water at various immersion times (a) and temperatures (b).
3.3.2. Polarization Curves
Figure 8 displays the N80 steel polarization curves that were obtained at different immersion periods. The rate of corrosion for the N80 steel sample was determined using the Tafel extrapolation method. Specifically, a linear fit was applied within a linear interval where either the anode or cathode curve exceeded or fell below the corrosion potential by 50 mV. The rate of corrosion was calculated by converting the corrosion current, which was determined at the point of intersection between the straight line and the potential for corrosion. The results of these calculations are provided in Table 3 and Table 4. The calculated values revealed that the corrosion rate gradually decreased within the immersion period of 3.5 days to 14 days, and then accelerated at 28 days (Figure 9). Specifically, the corrosion rates after 3.5 days, 7 days, and 14 days of immersion were 4.31 × 10−3 mm/y, 8.78 × 10−5 mm/y, and 7.54 × 10−5 mm/y, respectively. As a result, the rate of corrosion for the sample declined as the immersion duration increased. One possible explanation for this decrease might be the formation of corrosion products on the material’s surface, which provide protection. Electrochemically, this phenomenon could be explained through resistance polarization. However, by the 28th day, the corrosion rate had slightly increased. There were several factors that could have contributed to this, such as secondary dissolution of the coating of corrosion products and the replacement of Ca and Mg ions, leading to structural damage of the corrosion product layer.
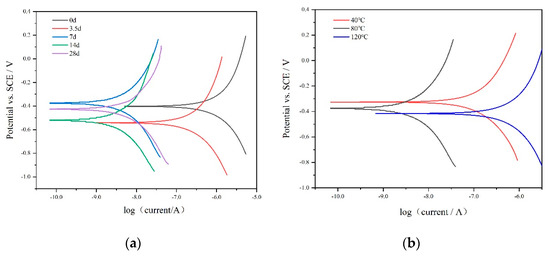
Figure 8.
Polarization curves for N80 steel in CO2-saturated formation water at various immersion times (a) and temperatures (b).

Table 3.
Corrosion rate of N80 steel measured by means of LPR at various immersion times, given a temperature of 80 °C.

Table 4.
Corrosion rate of N80 steel measured by means of LPR at various temperatures, given a corrosion duration of 7 days.
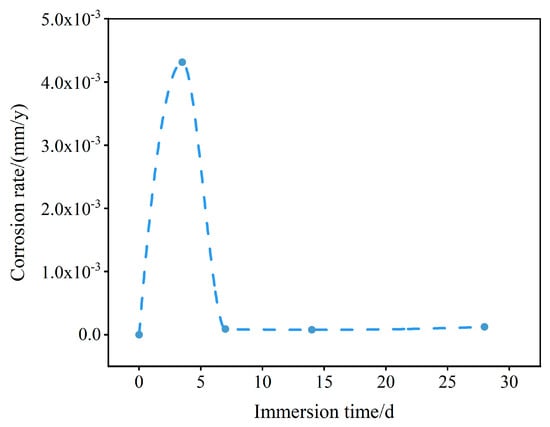
Figure 9.
N80 steel corrosion rate as measured by means of LPR across a range of immersion times. Blue spots represent the durations of the immersion experiments (i.e., 0, 3.5, 7, 14, and 28 days).
As the immersion time extended, the cathodic polarization curve exhibited a general leftward and downward shift, indicating changes in the cathodic reaction behavior. On the other hand, the changes observed in the anodic polarization curve were more intricate. When the electrostatic current density of the electrochemical polarization reached zero, the corresponding polarization potential corresponded to the self-corrosion potential (Ecorr), which progressively shifted in the forward direction with increasing mineralization. Notably, this trend was largely consistent with the observed changes in the OCP with respect to the immersion time.
The examination of the Tafel curve indicated that with an increase in temperature, the rate of corrosion for N80 steel followed a pattern of initial reduction and subsequent elevation. This pattern suggested that the protective layer of corrosion products established at higher temperatures did not have an effective safeguarding impact on the structure. Within the temperature range of 40 °C to 80 °C, the corrosion products formed on the electrode surface exhibited increased density, thereby enhancing their ability to protect the matrix. However, as the temperature continued to rise, the diffusion of ions in the solution and their penetration through the corrosion product film became more prominent. This effect surpassed the film’s compactness, resulting in a weakening of its protective effect on the matrix between 80 °C and 120 °C.
The polarization curves depicted in Figure 8 were processed and analyzed using the CHI660E Electrochemical Workstation software to perform fitting. This fitting process facilitated the determination of corrosion-related parameters for N80 steel samples exposed to different immersion time and temperatures. This analysis allowed us to obtain specific parameters, including the corrosion current density (Icorr), the corrosion potential (Ecorr), and the Tafel constants βa (anode slope) and βc (cathode slope). Furthermore, we calculated the polarization resistance (Rp) using the Stein–Geary equation [29,30]. Detailed values for Icorr, Ecorr, and Rp are presented in Table 5.

Table 5.
Polarization parameters of N80 steel specimens at different immersion times and temperatures.
When considering the immersion time, the N80 steel sample displayed the highest corrosion current density (Icorr) of 3.70 × 10−7 A·cm−2, the most negative corrosion potential (Ecorr) of −0.541 V, and the lowest polarization resistance (Rp) of 9.15 × 105 Ω·cm2 at an immersion time of 3.5 days. These results indicate the poor corrosion resistance of N80 steel under this condition. Between the time periods of 3.5 days and 14 days, there was a gradual increase in the polarization resistance (Rp) of the N80 steel samples, suggesting an improvement in the safeguarding impact of corrosion products on the steel substrate and an enhancement in the samples’ ability to withstand corrosion. However, at 28 days, the Rp started to decline, indicating a reduction in the protective effect of corrosion products and an aggravation of the corrosion severity. As the temperature increased, the Rp of the sample initially increased, followed by a subsequent decrease, with the change exceeding one order of magnitude. Consequently, the corrosion resistance demonstrated an initial improvement and then a weakening trend. This corresponded exactly to the results obtained through calculation of the corrosion rate.
3.3.3. Electrochemical Impedance Spectroscopy
To further explore the corrosion behavior of N80 steel in different conditions, we conducted EIS tests at various stages. During the immersion period of 0 to 14 days, the EIS results showed the presence of high-frequency capacitive reactance arcs and low-frequency capacitive reactance arcs, as illustrated in Figure 10. Typically, larger diameters of the capacitive arcs indicated a more difficult charge transfer on the metal surface and a lower degree of corrosion [31]. As the immersion time extended, the complete capacitive arc shifted gradually towards the low-frequency region, and its diameter progressively increased, indicating a gradual reduction in the level of corrosion. As a result, the Bode figure showed a progressive increase in the impedance modulus at lower frequencies, accompanied by an increase in the maximum phase angle. This observation suggested an enhancement in the sample’s ability to resist corrosion over time [32]. Concurrently, the buildup of products from corrosion on the metal exterior led to a gradual strengthening and densification of the film resulting from corrosion, which continued until the 14th day of immersion. However, by the 28th day, the intact corrosion product film either peeled off or suffered destruction, leading to a continuation of the corrosion intensification. This assessment was based on the comparison of the Bode plot data, where both the impedance modulus and the maximum phase angle at 28 days exhibited a decrease in comparison to the 14-day immersion period.
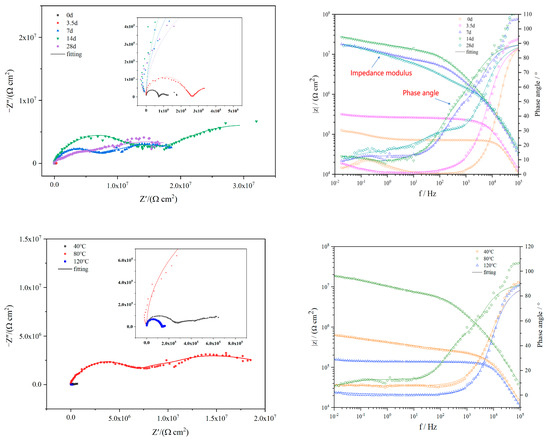
Figure 10.
EIS spectra for N80 steel immersed in CO2-saturated formation water at varying temperatures and immersion times.
The Nyquist diagram and the corresponding Bode diagram at different temperatures displayed distinctive trends. With the rise in temperature, there was an initial enlargement followed by a subsequent reduction in the radius of the capacitive arc. Notably, the capacitive arc at 80 °C exhibited a significantly larger radius compared to the other two temperatures. Moreover, the impedance modulus and the corrosion resistance also followed a similar pattern of first increasing and then decreasing with increasing temperature.
4. Conclusions
The corrosion characteristics of N80 steel exhibits notable differences when it is exposed to CO2-saturated formation water and when it is exposed to Sc-CO2. The N80 steel immersed in the solution undergoes uniform corrosion, and the corrosion products exhibit a regular structure. Meanwhile, the samples exposed to Sc-CO2 present corrosion products with spherical, needle-shaped, and lamellar structures. As time progresses, the corrosion rate of N80 steel exhibits fluctuating changes. These fluctuations can be attributed to variations in the compactness of the film resulting from corrosion and the penetration of ions, which have a notable influence on the process of corrosion.
Within the range of temperatures from 40 °C to 120 °C, there is a decrease in the corrosion rate from 40 °C to 80 °C, subsequently experiencing a rise from 80 °C to 120 °C. This suggests that the film of corrosion products formed at elevated temperatures (i.e., 120 °C and above) does not offer sufficient protection for the steel in an environment rich in CO2.
Author Contributions
Conceptualization, L.Z. and H.W.; methodology, K.M. and H.W.; software, Q.X. and M.G.; validation, M.G., Y.W. and X.F.; formal analysis, X.S.; writing—original draft preparation, H.W.; writing—review and editing, L.Z. and H.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant Number 42172315 and U1967208) and the Science and Technology Plan Project of Sichuan Province (grant number 2022YFSY0018).
Institutional Review Board Statement
This article does not contain any studies with human or animal subjects.
Informed Consent Statement
This study did not involve humans.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
Author Xuebin Su was employed by the company China National Uranium Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; p. 2391. [Google Scholar]
- Rani, S.; Padmanabhan, E.; Prusty, B.K. Review of gas adsorption in shales for enhanced methane recovery and CO2 storage. J. Petrol. Sci. Eng. 2019, 175, 634–643. [Google Scholar] [CrossRef]
- Czernichowski-Lauriol, I.; Berenblyum, R.; Bigi, S.; Car, M.; Gastine, M.; Persoglia, S.; Poulsen, N.; Schmidt-Hattenberger, C.; Stead, R.; Vincent, C.; et al. CO2 GeoNet actions in Europe for advancing CCUS through global cooperation. Energy Procedia 2018, 154, 73–79. [Google Scholar] [CrossRef]
- Bhave, A.; Taylor, R.H.S.; Fennell, P.; Livingston, W.; Shah, N.; Dowell, N.M.; Dennis, J.; Kraft, M.; Pourkashanian, M.; Insa, M.; et al. Screening and techno-economic assessment of biomass-based power generation with CCS technologies to meet 2050 CO2 targets. Appl. Energ. 2017, 190, 481–489. [Google Scholar] [CrossRef]
- Reiner, D.M. Learning through a portfolio of carbon capture and storage demonstration projects. Nat. Energy 2016, 1, 15011. [Google Scholar] [CrossRef]
- Zeng, Y.; Pang, X.; Shi, C.; Arafin, M.; Zavadil, R.; Collier, J. Influence of impurities on corrosion performance of pipeline steels in supercritical carbon dioxide. In Proceedings of the Corrosion 2015, Dallas, Texas, USA, 15–19 March 2015. [Google Scholar]
- Zhang, Y.; Gao, K.; Schmitt, G. Effect of water on steel corrosion under supercritical CO2 conditions. Mater. Perform. 2011, 50, 62–68. [Google Scholar]
- Cui, L.; Kang, W.; You, H.; Cheng, J.; Li, Z. Experimental study on corrosion of J55 casing steel and N80 tubing steel in high pressure and high temperature solution containing CO2 and NaCl. J. Bio-Tribo-Corros. 2020, 7, 13. [Google Scholar] [CrossRef]
- Wang, Y. Effect of temperature on CO2/H2S corrosion of oil tube steels. Adv. Mat. Res. 2014, 908, 111–114. [Google Scholar]
- Jingen, D.; Wei, Y.; Xiaorong, L.; Xiaoqin, D. Influence of H2S content on CO2 corrosion behaviors of N80 tubing steel. Pet. Sci. Technol. 2011, 29, 1387–1396. [Google Scholar] [CrossRef]
- Huang, X.B.; Yin, Z.F.; Li, H.L.; Bai, Z.Q.; Zhao, W.Z. Corrosion of N80 tubing steel in brine at 1.2 MPa CO2 containing trace amounts of H2S. Corros. Eng. Sci. Technol. 2013, 47, 78–83. [Google Scholar] [CrossRef]
- Cole, I.S.; Paterson, D.A.; Corrigan, P.; Sim, S.; Birbilis, N. State of the aqueous phase in liquid and supercritical CO2 as relevant to CCS pipelines. Int. J. Greenh. Gas Con. 2012, 7, 82–88. [Google Scholar] [CrossRef]
- Basilico, E.; Marcelin, S.; Mingant, R.; Kittel, J.; Fregonese, M.; Ropital, F. The effect of chemical species on the electrochemical reactions and corrosion product layer of carbon steel in CO2 aqueous environment: A review. Corros. Mater. 2021, 72, 1152–1167. [Google Scholar] [CrossRef]
- Pentland, N.; Bockris, J.M.; Sheldon, E. Hydrogen evolution reaction on copper, gold, molybdenum, palladium, rhodium, and iron: Mechanism and measurement technique under high purity conditions. J. Electrochem. Soc. 1957, 104, 182. [Google Scholar] [CrossRef]
- Bockris, J.M.; Potter, E. The mechanism of the cathodic hydrogen evolution reaction. J. Electrochem. Soc. 1952, 99, 169. [Google Scholar] [CrossRef]
- Devanathan, M.; Stachurski, Z. The mechanism of hydrogen evolution on iron in acid solutions by determination of permeation rates. J. Electrochem. Soc. 1964, 111, 619. [Google Scholar] [CrossRef]
- Zhang, G.A.; Cheng, Y.F. Electrochemical characterization and computational fluid dynamics simulation of flow-accelerated corrosion of X65 steel in a CO2-saturated oilfield formation water. Corros. Sci. 2010, 52, 2716–2724. [Google Scholar] [CrossRef]
- Duan, Z.; Sun, R. An improved model calculating CO2 solubility in pure water and aqueous NaCl solutions from 273 to 533 K and from 0 to 2000 bar. Chem. Geol. 2003, 193, 257–271. [Google Scholar] [CrossRef]
- Zhang, Y.; Pang, X.; Qu, S.; Li, X.; Gao, K. Discussion of the CO2 corrosion mechanism between low partial pressure and supercritical condition. Corros. Sci. 2012, 59, 186–197. [Google Scholar] [CrossRef]
- Wei, L.; Pang, X.; Liu, C.; Gao, K. Formation mechanism and protective property of corrosion product scale on X70 steel under supercritical CO2 environment. Corros. Sci. 2015, 100, 404–420. [Google Scholar] [CrossRef]
- Farelas, F.; Choi, Y.S.; Nešić, S.; Magalhães, A.A.O.; de Azevedo Andrade, C. Corrosion behavior of deep water oil production tubing material under supercritical CO2 environment: Part 2—Effect of crude oil and flow. Nace Corros. 2014, 70, 137–145. [Google Scholar] [CrossRef]
- Choi, Y.S.; Farelas, F.; Nešić, S.; Magalhães, A.A.O.; de Azevedo Andrade, C. Corrosion behavior of deep water oil production tubing material under supercritical CO2 environment: Part 1—Effect of pressure and temperature. Corrosion 2014, 70, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Pang, X.L.; Gao, K.W. Corrosion mechanism discussion of X65 steel in NaCl solution saturated with supercritical CO2. Acta Met. Sin 2015, 51, 701–712. [Google Scholar]
- Zhang, Y.; Gao, K.; Schmitt, G.; Hausler, R.H. Modeling steel corrosion under supercritical CO2 conditions. Corros. Mater. 2013, 64, 478–485. [Google Scholar] [CrossRef]
- Jiang, X.; Qu, D.R.; Song, X.L.; Liu, X.H.; Zhang, Y.L. Critical water content for corrosion of X65 mild steel in gaseous, liquid and supercritical CO2 stream. Int. J. Greenh. Gas Control 2019, 85, 11–22. [Google Scholar] [CrossRef]
- De Visser, E.; Hendriks, C.; Barrio, M.; Mølnvik, M.J.; De Koeijer, G.; Liljemark, S.; Le Gallo, Y. Dynamis CO2 quality recommendations. Int. J. Greenh. Gas Control 2008, 2, 478–484. [Google Scholar] [CrossRef]
- McGrail, B.P.; Schaef, H.T.; Glezakou, V.A.; Dang, L.X.; Owen, A.T. Water reactivity in the liquid and supercritical CO2 phase: Has half the story been neglected? Energy Procedia 2009, 1, 3415–3419. [Google Scholar] [CrossRef]
- Tavares, L.M.; da Costa, E.M.; de Oliveira Andrade, J.J.; Hubler, R.; Huet, B. Effect of calcium carbonate on low carbon steel corrosion behavior in saline CO2 high pressure environments. Appl. Surf. Sci. 2015, 359, 143–152. [Google Scholar] [CrossRef]
- Duan, H.; Du, K.; Yan, C.; Wang, F. Electrochemical corrosion behavior of composite coatings of sealed MAO film on magnesium alloy AZ91D. Electrochim. Acta 2006, 51, 2898–2908. [Google Scholar] [CrossRef]
- Cui, L.Y.; Zeng, R.C.; Guan, S.K.; Qi, W.C.; Zhang, F.; Li, S.Q.; Han, E.H. Degradation mechanism of micro-arc oxidation coatings on biodegradable Mg-Ca alloys: The influence of porosity. J. Alloys Compd. 2017, 695, 2464–2476. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, L.; Tang, A.; Ma, Y.; Song, G.L.; Zheng, D.; Jiang, B.; Atrens, A.; Pan, F. Active corrosion protection by a smart coating based on a MgAl-layered double hydroxide on a cerium-modified plasma electrolytic oxidation coating on Mg alloy AZ31. Corros. Sci. 2018, 139, 370–382. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Yarmand, B.; Mozafari, M. Enhanced corrosion resistance and in-vitro biodegradation of plasma electrolytic oxidation coatings prepared on AZ91 Mg alloy using ZnO nanoparticles-incorporated electrolyte. Surf. Coat. Technol. 2019, 360, 153–171. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).