Abstract
This study aimed to determine the antibacterial activity of the essential oil of fingerroot (Boesenbergia pandurata) (EOF) as a natural preservative in ground meat and its effect on the formation of heterocyclic amines (HAs) in pan-fried meatballs. EOF was applied either by adding it to ground pork or marinating pork in it before grinding. In addition, the antibacterial activity of EOF was tested. Aerobic mesophilic total viable count (TVC), lactic acid bacteria (LAB), and Enterobacteriaceae bacteria were monitored. The results show that EOF exhibited strong antibacterial activity when added at concentrations of 1.0 and 2.5 wt%. Antimicrobial activity against TVC, LAB, and especially Enterobacteriaceae bacteria was observed at all EOF concentrations (0.25, 0.5, 1.0, and 2.5 wt%). A 2.5% concentration of EOF applied by marinating trimmings can extend the shelf-life of ground pork to 18 days, while 2.5% EOF applied via addition can extend the shelf-life to 15 days, compared with 3 days for the control sample. After frying the meatballs, the inhibitory effect on the formation of heterocyclic amines was only significant for MeIQx with the highest addition of EOF (2.5 wt%). Significant increases in the concentrations of all other HAs were determined by adding EOF (2.5 wt%).
1. Introduction
Meat and meat products are an important source of protein in the human diet. Ground meat is one of the most susceptible foods to bacterial spoilage under normal refrigerator storage conditions. The rich nutrient content and high moisture content of meat provide a suitable environment for the growth and multiplication of spoilage organisms and food-borne microorganisms. Microbial contamination of meat inevitably occurs during slaughtering and processing into fresh meat products. Using organic acid and hot water rinses in processed carcasses effectively reduces the microbial load in carcasses before chilling. During the processing of carcasses into primary cuts, sub-primary cuts, and trimmings, as well as during further processing, there is a potential for the recontamination of the meat surface by microorganisms [1,2]. The large surface of raw meat is the key to contamination in the minced meat process. The most important spoilage bacteria, such as Pseudomonas, Acinetobacter, Enterobacter, Lactobacillus, Leuconostoc, Proteus, etc., occur in aerobically stored meat [3,4,5]. The point of meat spoilage may be defined by a maximum acceptable bacterial level or an unacceptable off-odor and off-flavor or appearance. Therefore, adequate preservation technologies must be applied to maintain the safety and quality of meat and meat products [6,7].
Recently, there has been increased interest in a technology to reduce the microbial load on fresh meat before grinding. Much of the research has investigated the possible use of organic acid treatments to decontaminate trimmings [2,6,7,8]. In some countries, the meat industry uses chemical additives in several meat processes to extend shelf-life and prevent the growth of spoilage microorganisms and foodborne pathogenic bacteria [9]. However, growing awareness and concern about the quality and safety of meat has led to numerous developments in meat preservation. Meanwhile, consumers have been questioning the safety of synthetic chemical additives in food, and using natural additives as alternative preservatives has become popular, especially the potential application of plant extracts as safe additives for meat [10,11,12]. Herb and spice extracts are used as antioxidants, antimicrobials, anti-diabetics, anticarcinogens, and flavorings [13]. Therefore, the meat industry’s increasing interest in natural antimicrobials has led to extensive research on the utilization of spice and herb extracts as microorganism inhibitors. Plant essential oils are aromatic and volatile oily liquids obtained from plant material. In general, essential oils can be obtained using various production methods, e.g., steam distillation, solvent extraction, mostly with hexane, and CO2 extraction [14]. Many essential oils, such as cinnamon, ginger, garlic, oregano, lemon grass, lime, galangal, turmeric, and cloves, contain components that exhibit high antimicrobial efficiency against foodborne pathogens and spoilage bacteria [15,16,17]. In addition, using essential oils in nanoemulsions for meat and meat products has been addressed, including the increase in surface area and stability [18]. Fingerroot (Boesenbergia pandurata) is a widely distributed plant in Southeast Asia and Southern China. It is considered both a medicinal and a culinary herb. EOF presented the most broad-spectrum activity to inhibit foodborne pathogenic bacteria such as Listeria monocytogenes, Staphylococcus aureus, Escherichia coli, and Salmonella enterica [12,19,20]. However, to date, little research has been conducted to evaluate the functionality of essential oils for antimicrobials in food systems and, in particular, in meat products [21].
After the preparation and cooking of foods, some process contaminants could be formed at high temperatures [22,23]. One group of these process contaminants are heterocyclic amines (HAs), which are classified as hazardous substances [24]. In general, commercially produced or homemade cooked meat dishes contain HAs at low concentrations in the ppb range. These compounds are formed in vertebrate tissues after heating and can form DNA adducts in animal and human tissues [25]. Exposure levels vary according to the types of meat and fish eaten, cooking temperature and duration, and use of gravies, marinades, or sauces [22,23]. These parameters can cause dietary HA concentrations to vary more than 100-fold. An association has been reported in some of the detailed dietary studies on colorectal cancer or its precursor, adenoma [26,27]. HAs have been linked to an increased risk for the incidence of human cancer in epidemiologic studies, but the available data are not convincing. The International Agency for Research on Cancer has classified mutagenic HAs as probably carcinogenic (Group 2A) or possibly carcinogenic (Group 2B) to humans [28]. Several organizations have recommended reducing the daily intake of HAs by humans.
In addition to the antimicrobial effect of fingerroot, an antioxidant effect and an inhibitory effect on the formation of heterocyclic amines in cooked beef patties have also been reported [29]. The authors showed that pinocembrin, a flavonone, and pinostrobin, a flavone, were phenolic compounds found in fingerroot. Puangsombat et al. demonstrated the antioxidant activities of pinocembrin and pinostrobin by using the DPPH assay to measure the radical scavenging capacity of antioxidants. Phenolic compounds are not only antioxidants but are also effective in reducing the concentrations of HAs [22,30,31,32,33]. Free radicals are known to be involved in HA formation and the Maillard reaction. A trial using electron paramagnetic resonance demonstrated that antioxidants can scavenge free radicals, and the formation of HAs can be reduced [32,34].
This study’s objectives were to determine the antimicrobial activity of EOF in minced pork and to compare the antimicrobial activity of EOF with the methods of direct addition and marinating during storage at refrigeration temperatures. It was hypothesized that EOF has a strong antimicrobial effect in ground meat and can inhibit the formation of HAs in pan-fried meatballs.
2. Materials and Methods
2.1. Materials and Chemicals
EOF was purchased from Thai-China Flavours and Fragrances Industry Co., Ltd. (Ayutthaya, Thailand) (the GC/MS analysis of the steam-distilled fingerroot oil is contained in the Supplementary Data; Figure S1). Pork meat was obtained from a supplier (MEGA, Stuttgart, Germany).
For the analysis of HAs, the β-carbolines norharman and harman, as well as caffeine as an internal standard, were purchased from Sigma-Aldrich (Taufkirchen, Germany). IQ, MeIQ, MeIQx, IQx, 7.8-DiMeIQx, and 4.8-DiMeIQx, in addition to the fluorescence active HAs (PhIP, AαC, MeAαC, Glu-P-1, Glu-P-2, Trp-P-1, and Trp-P-2), were purchased from Toronto Research Chemicals (Toronto, ON, Canada). Methanol, acetonitrile toluene, and ethyl acetate (all gradient-grade) and aqueous ammonia (25%) were acquired from Carl Roth GmbH & Co. (Karlsruhe, Germany), and ammonium acetate, sodium or potassium hydroxide, hydrochloric, orthophosphoric, and perchloric acid, as well as triethylamine were purchased from VWR International (Darmstadt, Germany). All chemicals were analytical-grade. Blank cartridges (Isolute®) filled with diatomaceous earth (Isolute® HM-N) for the extraction were obtained from Separtis GmbH (Grenzach-Wyhlen, Germany). For the solid-phase extraction, Bond Elut® C18 (100 mg) and Bond Elut® PRS (500 mg) (Varian, Palo Alto, CA, USA) cartridges and a filter (type 0967, 11 mm ID) were obtained from Schleicher & Schuell GmbH (Dassel, Germany).
2.2. Preparation of Minced Pork
Fresh meat was vacuum-packed, refrigerated, and used immediately after purchase. The meat from pork shoulder was cut into 3 × 3 × 5 cm pieces and kept under refrigerated conditions before mincing.
For the addition method, pork was minced through 3 mm plates using a meat grinder (Stephan Machinery GmbH, Hamelin, Germany). After grinding, the minced meat was divided into 5 batches. One was the control batch (without EOF), and the remaining 4 batches were mixed with EOF in concentrations of 0.25, 0.50, 1.00, and 2.5 wt%, respectively.
For the marinating method, approximately 550 g of pork pieces was marinated with EOF in concentrations of 0.25, 0.50, 1.00, and 2.5 wt%, and without EOF (control). The EOF and pork pieces were mixed under aseptic conditions and stored at 6 ± 1 °C for 3 h before mincing.
After grinding, each treatment sample was formed into meatballs. Twenty-five grams of minced meat from each treatment sample was placed in a sterile plastic bag (stomacher bag). The bags were individually sealed and stored under aerobic conditions at 6 ± 1 °C for 21 days.
2.3. Microbiological Analysis
Microbiological analyses, including the determination of total viable counts (TVCs), lactic acid bacteria (LAB), and Enterobacteriaceae bacteria, were performed at 3-day intervals for up to 21 days of refrigerated storage. An individual package of a treated sample (25 g) was withdrawn to enumerate bacteria using the spread plate technique. At each sampling time, minced meat samples in a stomacher bag were aseptically filled with 225 mL of 0.1% sterile peptone water. The contents were homogenized in a stomacher for 2 min at room temperature. The resulting slurries were serially diluted (1:10) in 0.1% sterile peptone water. The TVCs of populations were determined on plate count agar at 37 °C for 24 h. Enterobacteriaceae bacteria were enumerated on Violet Red Bile Glucose Agar (VRBG) at 37 °C for 24 h. LAB was carried out on de Man–Rogosa–Sharpe agar incubated at 37 °C for 48 h.
2.4. Preparation and Heat Treatment of the Meatballs
An amount of 60 g ± 1 g of the minced pork without and with EOF concentrations of 0.25, 0.5, 1.0, and 2.5 wt% were formed into meatballs and pan-fried at a temperature of approximately 190 °C and a core temperature of 72 °C using a household pan with Teflon coating and a small amount of refined canola oil. The core temperature was measured with a temperature-measuring device (Almemo® 8990-8, Ahlborn, Holzkirchen, Germany) at the end of the frying process.
2.5. Color Measurement of the Meatballs
A color analysis of the meatballs (n = 8) was conducted after 1 h of the pan-frying process. The L*, a*, and b* values in the CIE tristimulus color space were measured in each case 3 times using a colorimeter (Konica Minolta, Langenhagen, Germany) with a standard illuminant D65.
2.6. Analysis of Heterocyclic Aromatic Amines
The application of the HA determination method [35,36] was based on the analytical method described by Gross and Grüter [37]. A solution of MeIQ, MeIQx, 4,8-DiMeIQx, 7,8-DiMeIQx, IQ, and IQx (0.13–0.25 ng/µL) and a solution of Glu-P-2, Glu-P-1, norharman, harman, PhIP, Trp-P-1, Trp-P-2, AαC, and MeAαC (0.05 ng/µL–0.1 ng/µL) were dissolved and mixed in methanol. This mixture (100 µL) was used for spiking, and a caffeine solution was applied as an internal standard (2.5 ng/µL; 1:1 methanol–ultrapure water, v/v). An amount of 30 g of each pork sample was mixed with 90 g of sodium hydroxide (1 mol/L) using an Ultra Turrax T-25 (IKA Labortechnik, Staufen, Germany) (at 24,000 rpm for 2 min). An amount of 5 g of each sample mixture was used, and approximately 3 g of diatomaceous earth was added and mixed into each portion. The method was described in detail [35].
The HPLC system was obtained from Gynkotek (Germering, Germany) equipped with an autosampler (Gina 50), degasser (DG 1310 S), M480 pump, diode array (UVD 320), and fluorescence detectors (RF 1002), as well as a chromatography data system (version 5.50). A SupelguardTM LC-18-DB guard column (Supelco, Bellefonte, PA, USA) connected to a TSK-gel® ODS-80TM column (reversed-phase C18, 4.6 mm Id, 250 mm, 5 µm; Tosoh Bioscience, Stuttgart, Germany) was used. Three elements were in the mobile phase: 10 mM triethylamine phosphate buffer (pH 3.2 (A)) or 10 mM triethylamine phosphate buffer (pH 3.6 (B)), in each case, with 8% acetonitrile and acetonitrile (C) (0–67% gradient program) using a flow rate of 1 mL/min at column temperature of 25 °C. The gradient program and UV and fluorescence detection are described in [35,36]. For injection, a volume of 50 µL for the non-polar and the polar fractions was applied. The peaks of HAs, as well as the β-carbolines norharman and harman, in the samples were identified by comparing the retention times and UV spectra with standards. The quantification of HAs was performed with the standard addition method and β-carbolines with an external calibration. The recoveries of HAs in the pan-fried meatballs are shown in the supplementary data (Table S1).
2.7. Statistical Analysis
All experiments were replicated twice on different occasions with different meat samples. Analyses were run in triplicate for each replicate (n = 2 × 3), and mean values are presented. The determination of HAs was repeated three times (n = 4). HAs and microbiological counts, which were converted to log cfu/g, were subjected to analysis of variance (ANOVA) using the statistical program SPSS version 12.0 or SigmaPlot version 14.0 with statistical significance determined at p ≤ 0.05. A correlation plot with the Pearson correlation coefficients was conducted using the program OriginLab© (OriginPro 2023).
3. Results and Discussion
3.1. Antibacterial Effect
The antibacterial effects of EOF in minced meat added via addition to the meat are illustrated in Figure 1. The minced meat samples were not sterilized in refrigerated storage at 6 ± 1 °C. The initial load of the TVC was 4.00 log cfu/g, indicative of acceptable-quality meat. Initial loads of TVC (24 h post-slaughter) of 2.60, 3.08, 4.60, and 5.39 log cfu/g were reported for lamb steaks [38], loins [39], lamb meat [40], and chicken meat [41] at a refrigerated temperature and in aerobic storage, respectively, reflecting differences in meat quality and microbial post-processing contamination. The initial load levels were very low in the cases of LAB (approximately 1.3 log cfu/g) and Enterobacteriaceae bacteria (approximately 0.87 log cfu/g).
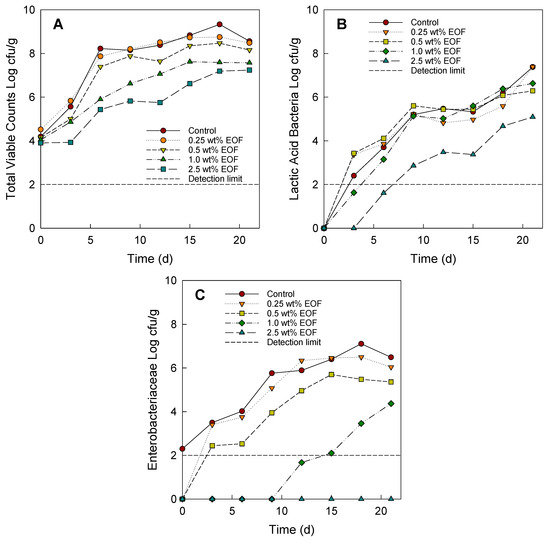
Figure 1.
Total bacteria count in ground meat (uncooked meatballs) (A), lactic acid bacteria (B), and Enterobacteriaceae bacteria (C) with control and added EOF at the concentrations of 0.25, 0.50, 1.00, and 2.50 wt% using the addition method (after grinding) during storage at 6 ± 1 °C for 21 days.
Concerning TVC, adding 2.50 wt% EOF to the minced meat samples showed a strong preservative effect (Figure 1A). The results present the microbial reduction between control samples and treated samples with 2.50 wt% EOF by marinating (3.83 log cfu/g) significantly higher (p < 0.05) than with the addition method (2.22 log cfu/g) after grinding. Samples treated with 1.00 wt% EOF using the marinating method showed a similar reductive effect on the TVC to samples treated with 2.50 wt% EOF using the addition method (p > 0.05). A comparison of the growth curves (TVC, LAB, and Enterobacteriaceae bacteria) using both methods of EOF addition before and after grinding is shown in the supplementary data (Figure S2).
The weakest effects of using EOF were observed in LAB, agreeing with the findings of Charnchai et al. [42] that Lactobacillus spp. and Pediococcus spp. were more resistant to EOF than other foodborne microorganisms such as S. aureus, B. cereus, and L. monocytogenes. Furthermore, based on the similar microbial growth patterns of TVC and LAB, it can be assumed that LAB are the major constituent of the microorganisms in TVC, which is consistent with the findings of Karabagias et al. [40] that LAB constitute a major part of the natural microflora of meat. The additive effects of 0.25 and 0.50 wt% EOF using the addition method were similar and very low compared with the control sample. Conversely, the addition of 0.25 and 0.50 wt% EOF by marinating inhibited the LAB population slightly less than the control sample, a reduction of slightly less than 1 log compared with the control sample (Figure 1B). The population of LAB in treated samples with 2.50 wt% EOF either via addition or marinating showed an initial decrease of 2 log cfu/g on day 3 and then increased, reaching ca. 4.46 log cfu/g by the end of storage. The LAB population in the control sample reached 6 log cfu/g on day 15. The discoloration and acidification observed in the meat were caused by LAB. Spoiled meat has a LAB load of approximately 6–7 log cfu/g [4]. In addition, the application of 2.50 wt% EOF by marinating and adding to the minced meat showed similar antimicrobial efficacy against LAB (p > 0.05). The positive effect of adding EOF was most evident for Enterobacteriaceae bacteria (Figure 1C).
The application of 1.00 and 2.50 wt% EOF by addition and all conditions treated with EOF by marinating showed a decrease in Enterobacteriaceae counts during refrigerated storage. The sample treated with 0.25 wt% EOF did not differ from the control (p > 0.05). The sample marinated with EOF showed a strong antibacterial effect (p < 0.05) against Enterobacteriaceae bacteria (a microbial reduction of 4.85 log cfu/g). With 0.50 wt% EOF, the Enterobacteriaceae counts were slightly lower than those of the control on days 6 to 12, and the population increased to 6.16 log cfu/g by the end of storage. There was no significant difference (p > 0.05) in the Enterobacteriaceae reduction compared with the control sample.
The application of 0.25 wt% EOF by marinating showed a similar (p > 0.05) antibacterial effect against Enterobacteriaceae (a microbial reduction of 4.85 log cfu/g) to 1.00 wt% EOF using the addition method (a microbial reduction of 3.76 log cfu/g). The additive effect of 0.50 and 1.00 wt% EOF by marinating showed a lower population of Enterobacteriaceae bacteria than the control sample on the first day and controlled coliform counts, which were not higher than 1 log cfu/g during storage. In addition, the bactericidal effect against the initial coliform load at 2.50 wt% EOF was evident using the methods of addition and marinating. It showed a bactericidal effect against Enterobacteriaceae until the end of storage.
The results show a microbial reduction of 6.65 log cfu/g for the addition method at 2.50 wt% after grinding and 6.89 log cfu/g at 2.50 wt% for the marinating method before grinding (Table 1). The Enterobacteriaceae microorganisms included coliform bacteria, which are hygiene indicators, as well as Escherichia coli and Salmonella spp. which are foodborne pathogenic bacteria.

Table 1.
Shelf-lives and microbial reductions in TVC, LAB, and Enterobacteriaceae in minced meat with the addition of EOF.
The results show EOF exhibited excellent antimicrobial activity against Gram-negative and food-borne spoilage bacteria in food. Govaris et al. [9] found that adding oregano essential oil to minced sheep meat was more effective against the growth of Salmonella enteritidis at a refrigerated temperature. This is in agreement with previous studies [20].
Per the EU regulation [43], Salmonella spp. were not present in minced meat, whereas E. coli were at 1.70–2.70 log cfu/g, and the aerobic colony count was 5.70–6.70 log cfu/g. The shelf-life of the minced meat was decided based on the TVC standard criterium (Table 1) due to this experiment using VRBG media for testing Enterobacteriaceae bacteria, which cannot identify E. coli and Salmonella spp. in minced meat.
Considering EU regulations for acceptable-quality meat products, control samples and treated samples with 0.25 and 0.50 wt% EOF using the mixed method demonstrated TVC levels under the standard criterium on day 3. When the sample was treated with 1.00 and 2.50 wt%, the shelf-life of the ground beef was extended to 9 and 15 days, respectively. Marinating with 0.25, 0.50, 1.00, and 2.50 wt% EOF extended the minced meat storage life to 9, 9, 12, and 18 days, respectively. When comparing the effectiveness of the same concentration of EOF applied with marinating versus the addition method, the former exhibited greater antibacterial properties in preserving the minced meat.
3.2. Effect of Added Fingerroot Essential Oil on Formation of HAs
The meatballs with added essential oil were fried at a temperature of approximately 190 °C. Figure 2A shows the results of the determination of the HAs. Only the concentration of MeIQx was significantly lower with the addition of 0.5 wt% EOF, with a reduction of 39%. The other compounds, PhIP, norharman, and harman, were significantly increased via the addition of EOF in increasing concentrations, with an increase of 59% for PhIP, 61% for norharman, and 118% for harman with the highest addition of EOF (2.5 wt%). The highest PhIP concentration of 0.64 ng/g was found with the addition of 0.5 wt% EOF. This PhIP concentration was significantly higher than all other PhIP concentrations when lower and higher amounts of EOF were added. An increase of 213% in the concentration of PhIP was observed compared with the control. However, the PhIP concentrations in all batches showed high standard deviations compared with the other HAs.
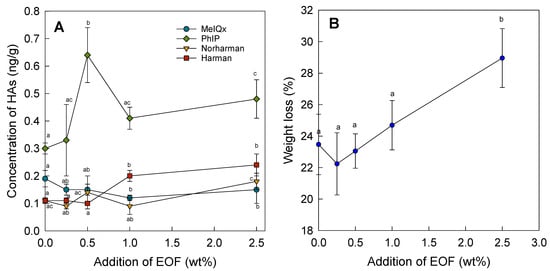
Figure 2.
(A) Concentrations of HAs (ng/g) in pan-fried meatballs after addition of different levels of EOF (wt%); (B) weight loss after pan-frying the meatballs without (control) and with addition of EOF (0.25–2.5 wt%) (a–c—different letters indicate significant differences; p < 0.05).
One reason for the increase in most of the HA concentrations in this study may be the use of the EOF compared with the study by Puangsombat et al. [29], where fresh or dried roots were used in the beef patties. The authors found that pinocembrin and pinostrobin, which are found in fingerroot, had high antioxidant activity, using the DPPH assay to determine free radical scavenging activity. Phenolic compounds are not only antioxidants but are also effective in reducing the concentrations of HAs, as reported in different studies [30,31,32,33,44,45]. Increases in the concentrations of β-carbolines were shown in earlier studies using different spices [22]. Increases in the levels of HAs, such as PhIP, in the presence of pro-oxidant substances have also been reported [46]. Increasing weight loss could also favor an increase in HA levels, as reported in [22,46].
The correlation plot is shown in Figure 3. Strong negative correlations were found between the addition of EOF and the TVC (r = −0.91; p < 0.05) as well as the Enterobactericeae count after storage for 18 days (−0.99; p < 0.001), which indicated the strong antimicrobial activity of EOF. Significant positive correlations were observed between the addition of EOF and weight loss (p < 0.05) as well as the harman concentration in pan-fried meatballs (p < 0.01). In addition, a positive correlation was found between weight loss and harman levels (p < 0.05). However, a significant correlation does not necessarily indicate a causal relationship.
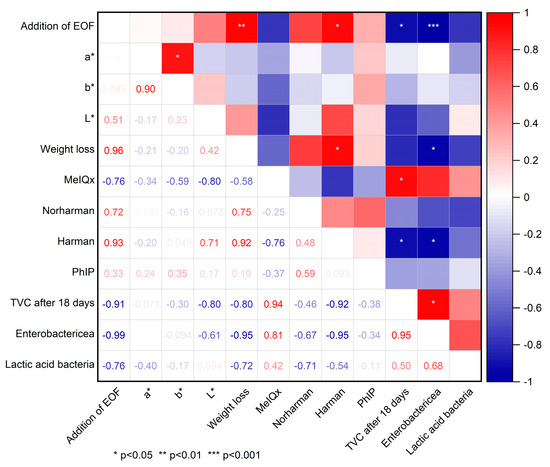
Figure 3.
Correlation plot (Pearson) of the different parameters (addition of EOF after grinding; L*, a*, and b* color values; weight loss; total viable counts; lactic acid; and Enterobacteriaceae bacteria in minced meat after storage for 18 days).
In addition, meatballs have a smaller surface area in contact with the hot iron plate compared with patties of the same volume. The area that comes into contact with the hotplate is approximately 20–25% less for a meatball than for a patty. This is supported by the low concentrations of HAs in the control. The weight losses were 23.4% approximately in all batches except for the highest addition of EOF, with 29.0%. This indicates that moisture as well as volatile essential oils are lost during frying. The L*, a*, and b* color values of the pan-fried meatballs are shown in Table 2. The lightness (L* value) was similar for all batches. However, the redness (a* value) of the control was significantly different from that of most of the EOF additions with increasing a* values, except for 1% EOF. In addition, the color difference ΔE had higher values than three, indicating differences in color perception among all batches with EOF compared with the control. A color difference can be perceived at a color distance above 2.5, and above 5, the color is perceived by the human eye as a different color [47]. Additionally, the sensory properties of the food were affected by EOF. Thus, sensory acceptance by consumers should be tested in a future study.

Table 2.
Color (L*, a*, and b*) and color difference ΔE values of pan-fried meatballs using different additions of EOF.
4. Conclusions
The addition of EOF demonstrated the potential for inhibiting microorganisms in minced meat. The different methods of applying EOF had different levels of antibacterial efficacy. Marinating meat in EOF before grinding resulted in a higher decrease in the initial load of microorganisms in the minced meat than adding and mixing the EOF after grinding. The reason for this is that microorganisms can easily contaminate the surface of raw meat, which leads to the distribution of microorganisms throughout the minced meat product when it is passed through a meat grinder. In most cases, however, HA levels (except for MeIQx) increased with the addition of EOF, most likely due to a pro-oxidant effect or weight loss during frying, which was visible in the EOF-treated samples. Before applying EOF as a natural antimicrobial agent in meat products, it is necessary to evaluate consumer sensory acceptability.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app14020712/s1, Analysis of fingerroot oil using GC/MS. Figure S1: Chromatogram of fingerroot oil with composition; Figure S2: Total viable count (TVC), lactic acid bacteria (LAB) and Enterobacteriaceae bacteria (ENB) in uncooked pork meatballs from the control (without EOF) and the group with added EOF in the concentrations of 0.25, 0.50, 1.00, and 2.50 wt% by marinating (before grinding) and adding after grinding during storage at 6 ± 1 °C for 21 days; Table S1: Recoveries of HAs in pan-fried meatballs (mean and SD standard deviation).
Author Contributions
Conceptualization, J.W. and M.G.; methodology and validation, S.S. and P.S.; formal analysis, P.S.; investigation, P.S. and M.G.; writing—original draft preparation, P.S. and M.G.; writing—review and editing, M.G. and S.S.; supervision, J.W., M.G., and C.R.; funding acquisition, C.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the International Affairs Division of Kasetsart University in 2554 B.E.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and supplementary materials.
Acknowledgments
The authors gratefully acknowledge the University of Hohenheim for providing the laboratory facility and the International Affairs Division of Kasetsart University for their financial support.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| DPPH | 1-diphenyl-2-picrylhydrazyl |
| Has | Heterocyclic Aromatic Amines |
| IQ | 2-Amino-3-methylimidazo[4,5-f]quinoline (CAS No. 76180-96-6) |
| IQx | 2-Amino-3-methylimidazo[4,5-f]quinoxaline (CAS No. 108354-47-8) |
| MeIQ | 2-Amino-3,4-dimethylimidazo[4,5-f]quinoline (CAS No. 77094-11-2) |
| MeIQx | 2-Amino-3,8-dimethylimidazo[4,5-f]quinoxaline (CAS No. 77500-04-0) |
| 4,8-DiMeIQx | 2-Amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline (CAS No. 95896-78-9) |
| 7,8-DiMeIQx | 2-Amino-3,7,8-trimethylimidazo[4,5-f]quinoxaline (CAS No. 92180-79-5) |
| PhIP | 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (CAS No. 105650-23-5) |
| Trp-P-1 | 3-Amino-1,4-dimethyl-5H-pyrido[4,3-b]indole (CAS No. 62450-06-0) |
| Trp-P-2 | 3-Amino-1-methyl-5H-pyrido[4,3-b]indole (CAS No. 62450-07-1) |
| Glu-P-1 | 2-Amino-6-methyldipyrido[1,2-a:3′,2′-d]imidazole (CAS No. 67730-11-4) |
| Glu-P-2 | 2-Aminodipyrido [1,2-a:3′,2′-d]imidazole (CAS No. 67730-10-3) |
| AαC | 2-Amino-9H-pyrido[2,3-b]indole (CAS No. 26148-68-5) |
| MeAαC | 2-Amino-3-methyl-9H-pyrido[2,3-b]indole (CAS No. 68006-83-7) |
| Harman | 1-Methyl-9H-pyrido[3,4-b]indole (CAS No. 486-84-0) |
| Norharman | 9H-pyrido[3,4-b]indole (CAS No. 244-63-3) |
References
- Ellebracht, J.W.; King, D.A.; Castillo, A.; Lucia, L.M.; Acuff, G.R.; Harris, K.B.; Savell, J.W. Evaluation of peroxyacetic acid as a potential pre-grinding treatment for control of Escherichia coli O157:H7 and Salmonella Typhimurium on beef trimmings. Meat Sci. 2005, 70, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Geornaras, I.; Yang, H.; Moschonas, G.; Nunnelly, M.C.; Belk, K.E.; Nightingale, K.K.; Woerner, D.R.; Smith, G.C.; Sofos, J.N. Efficacy of chemical interventions against Escherichia coli O157:H7 and multidrug-resistant and antibiotic-susceptible Salmonella on inoculated beef trimmings. J. Food Prot. 2012, 75, 1960–1967. [Google Scholar] [CrossRef] [PubMed]
- Borch, E.; Kant-Muermans, M.-L.; Blixt, Y. Bacterial spoilage of meat and cured meat products. Int. J. Food Microbiol. 1996, 33, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Gram, L.; Ravn, L.; Rasch, M.; Bruhn, J.B.; Christensen, A.B.; Givskov, M. Food spoilage—Interactions between food spoilage bacteria. Int. J. Food Microbiol. 2002, 78, 79–97. [Google Scholar] [CrossRef] [PubMed]
- Jayasena, D.D.; Jo, C. Essential oils as potential antimicrobial agents in meat and meat products: A review. Trends Food Sci. Technol. 2013, 34, 96–108. [Google Scholar] [CrossRef]
- Aymerich, T.; Picouet, P.A.; Monfort, J.M. Decontamination technologies for meat products. Meat Sci. 2008, 78, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.H.; Xu, X.L.; Liu, Y. Preservation technologies for fresh meat—A review. Meat Sci. 2010, 86, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Stivarius, M.R.; Pohlman, F.W.; McElyea, K.S.; Waldroup, A.L. Effects of hot water and lactic acid treatment of beef trimmings prior to grinding on microbial, instrumental color and sensory properties of ground beef during display. Meat Sci. 2002, 60, 327–334. [Google Scholar] [CrossRef]
- Govaris, A.; Solomakos, N.; Pexara, A.; Chatzopoulou, P.S. The antimicrobial effect of oregano essential oil, nisin and their combination against Salmonella Enteritidis in minced sheep meat during refrigerated storage. Int. J. Food Microbiol. 2010, 137, 175–180. [Google Scholar] [CrossRef]
- Holley, R.A.; Patel, D. Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials. Food Microbiol. 2005, 22, 273–292. [Google Scholar] [CrossRef]
- Hsouna, A.B.; Trigui, M.; Mansour, R.B.; Jarraya, R.M.; Damak, M.; Jaoua, S. Chemical composition, cytotoxicity effect and antimicrobial activity of Ceratonia siliqua essential oil with preservative effects against Listeria inoculated in minced beef meat. Int. J. Food Microbiol. 2011, 148, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Thongson, C.; Davidson, P.M.; Mahakarnchanakul, W.; Vibulsresth, P. Antimicrobial effect of Thai spices against Listeria monocytogenes and Salmonella Typhimurium DT104. J. Food Prot. 2005, 68, 2054–2058. [Google Scholar] [CrossRef] [PubMed]
- Hygreeva, D.; Pandey, M.C.; Radhakrishna, K. Potential applications of plant based derivatives as fat replacers, antioxidants and antimicrobials in fresh and processed meat products. Meat Sci. 2014, 98, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.M.B.; Khaneghah, A.M.; de Souza Sant’Ana, A. Essential Oils in Food Processing: Chemistry, Safety and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Bagamboula, C.F.; Uyttendaele, M.; Debevere, J. Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S. flexneri. Food Microbiol. 2004, 21, 33–42. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Oswell, N.J.; Thippareddi, H.; Pegg, R.B. Practical use of natural antioxidants in meat products in the U.S.: A review. Meat Sci. 2018, 145, 469–479. [Google Scholar] [CrossRef] [PubMed]
- da Silva, B.D.; do Rosário, D.K.A.; Weitz, D.A.; Conte-Junior, C.A. Essential oil nanoemulsions: Properties, development, and application in meat and meat products. Trends Food Sci. Technol. 2022, 121, 1–13. [Google Scholar] [CrossRef]
- Kingchaiyaphum, W.; Rachtanapun, C. Antimicrobial and antioxidative activities of essential oils in Chinese sausage (Kun-Chiang). Asian J. Food Agro-Ind. 2012, 5, 156–162. [Google Scholar]
- Pattaratanawadee, E.; Rachtanapun, C.; Wanchaitanawong, P.; Mahakarnchanakul, W. Antimicrobial activity of spice extracts against pathogenic and spoilage microorganisms. Agric. Nat. Resour. 2006, 40, 159–165. [Google Scholar]
- Pateiro, M.; Munekata, P.E.S.; Sant’Ana, A.S.; Domínguez, R.; Rodríguez-Lázaro, D.; Lorenzo, J.M. Application of essential oils as antimicrobial agents against spoilage and pathogenic microorganisms in meat products. Int. J. Food Microbiol. 2021, 337, 108966. [Google Scholar] [CrossRef]
- Gibis, M. Heterocyclic aromatic amines in cooked meat products: Causes, formation, occurrence, and risk assessment. Compr. Rev. Food Sci. Food Saf. 2016, 15, 269–303. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jia, W.; Zhu, L.; Mao, L.; Zhang, Y. Recent advances in heterocyclic aromatic amines: An update on food safety and hazardous control from food processing to dietary intake. Compr. Rev. Food Sci. Food Saf. 2020, 19, 124–148. [Google Scholar] [CrossRef]
- Mercogliano, R.; Murru, N.; De Felice, A. Food-borne heterocyclic amines and food safety. Ind. Aliment. 2014, 53, 17–28+34. [Google Scholar]
- Bellamri, M.; Le Hegarat, L.; Vernhet, L.; Baffet, G.; Turesky, R.J.; Langouët, S. Human T lymphocytes bioactivate heterocyclic aromatic amines by forming DNA adducts. Environ. Mol. Mutagen. 2016, 57, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Nowell, S.; Coles, B.; Sinha, R.; MacLeod, S.; Luke Ratnasinghe, D.; Stotts, C.; Kadlubar, F.F.; Ambrosone, C.B.; Lang, N.P. Analysis of total meat intake and exposure to individual heterocyclic amines in a case-control study of colorectal cancer: Contribution of metabolic variation to risk. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2002, 506–507, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Giovannucci, E.; Byrne, C.; Platz, E.A.; Fuchs, C.; Willett, W.C.; Sinha, R. Meat mutagens and risk of distal colon Adenoma in a cohort of U.S. men. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1120–1125. [Google Scholar] [CrossRef]
- WHO-IARC. IARC Monographs Evaluate Consumption of Red Meat and Processed Meat. Available online: http://www.iarc.fr/en/media-centre/pr/2015/pdfs/pr240_E.pdf (accessed on 9 November 2015).
- Puangsombat, K.; Jirapakkul, W.; Smith, J.S. Inhibitory activity of Asian spices on heterocyclic amines formation in cooked beef patties. J. Food Sci. 2011, 76, T174–T180. [Google Scholar] [CrossRef]
- Gibis, M.; Weiss, J. Antioxidant capacity and inhibitory effect of grape seed and rosemary extract in marinades on the formation of heterocyclic amines in fried beef patties. Food Chem. 2012, 134, 766–774. [Google Scholar] [CrossRef]
- Cao, H.; Chen, B.H.; Inbaraj, B.S.; Chen, L.; Alvarez-Rivera, G.; Cifuentes, A.; Zhang, N.; Yang, D.J.; Simal-Gandara, J.; Wang, M. Preventive potential and mechanism of dietary polyphenols on the formation of heterocyclic aromatic amines. Food Front. 2020, 1, 134–151. [Google Scholar] [CrossRef]
- Yang, X.; Blecker, C.; Liu, H.; Zhang, D.; Wang, Z. Effect of plant polyphenols with different m-hydroxy and o-hydroxy groups on the inhibition of heterocyclic amines formation in roasted meat. Food Control 2023, 153, 109960. [Google Scholar] [CrossRef]
- Oz, E. Inhibitory effects of black cumin on the formation of heterocyclic aromatic amines in meatball. PLoS ONE 2019, 14, e0221680. [Google Scholar] [CrossRef] [PubMed]
- Kikugawa, K. Involvement of free radicals in the formation of heterocyclic amines and prevention by antioxidants. Cancer Lett. 1999, 143, 123–126. [Google Scholar] [CrossRef]
- Gibis, M. Optimized HPLC method for analysis of polar and nonpolar heterocyclic amines in cooked meat products. J. AOAC Int. 2009, 92, 715–724. [Google Scholar] [CrossRef]
- Gibis, M. Effect of oil marinades with garlic, onion, and lemon juice on the formation of heterocyclic aromatic amines in fried beef patties. J. Agric. Food Chem. 2007, 55, 10240–10247. [Google Scholar] [CrossRef]
- Gross, G.A.; Grueter, A. Quantitation of mutagenic/carcinogenic heterocyclic aromatic amines in food products. J. Chromatogr. A 1992, 592, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, C.; Buckley, D.J.; Kerry, J.P. Display life of sheep meats retail packaged under atmospheres of various volumes and compositions. Meat Sci. 2004, 68, 649–658. [Google Scholar] [CrossRef]
- Kennedy, C.; Buckley, D.J.; Kerry, J.P. Influence of different gas compositions on the short-term storage stability of mother-packaged retail-ready lamb packs. Meat Sci. 2005, 69, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Karabagias, I.; Badeka, A.; Kontominas, M.G. Shelf life extension of lamb meat using thyme or oregano essential oils and modified atmosphere packaging. Meat Sci. 2011, 88, 109–116. [Google Scholar] [CrossRef]
- Radha Krishnan, K.; Babuskin, S.; Azhagu Saravana Babu, P.; Sasikala, M.; Sabina, K.; Archana, G.; Sivarajan, M.; Sukumar, M. Antimicrobial and antioxidant effects of spice extracts on the shelf life extension of raw chicken meat. Int. J. Food Microbiol. 2014, 171, 32–40. [Google Scholar] [CrossRef]
- Charnchai, P.; Gamjanagoonchorn, W.; Rachtanapun, C. Antimicrobial activity of sausage casing soaked in essential oil for inhibiting food microorganisms. In Proceedings of the 46th Kasetsart University Annual Conference, Bangkok, Thailand, 29 January–1 February 2008; Kasetsart University: Bangkok, Thailand, 2008; pp. 254–261. [Google Scholar]
- EC. Commission Regulation (EC) No 2073/2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2005, L338, 1–26. [Google Scholar]
- Puangsombat, K.; Smith, J.S. Inhibition of heterocyclic amine formation in beef patties by ethanolic extracts of rosemary. J. Food Sci. 2010, 75, T40–T47. [Google Scholar] [CrossRef] [PubMed]
- Zamora, R.; Aguilar, I.; Granvogl, M.; Hidalgo, F.J. Toxicologically relevant aldehydes produced during the frying process are trapped by food phenolics. J. Agric. Food Chem. 2016, 64, 5583–5589. [Google Scholar] [CrossRef] [PubMed]
- Elbir, Z.; Ekiz, E.; Aoudeh, E.; Oz, E.; Savaş, A.; Brennan, C.; Proestos, C.; Khan, M.R.; Elobeid, T.; Brennan, M.; et al. Enhancing effect of chia seeds on heterocyclic amine generation in meatball. Int. J. Food Sci. Technol. 2023, 58, 2560–2572. [Google Scholar] [CrossRef]
- Mokrzycki, W.S.; Tatol, M. Colour difference∆ E-A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).