Abstract
The research on entomopathogenic viruses is of major significance as they could serve as alternatives to chemical pesticides. There are various types of entomopathogenic viruses; among them, Baculoviruses (BVs) are a potential option because they are eco-friendly and target specific. The experiment in question aimed to evaluate the effect of three insect-specific commercial viruses, Cydia pomonella Granulovirus (CpGV), Helicoverpa armigera Nucleopoyhedrovirus (HearNPV), and Phthorimaea operculella Granulovirus (PoG), on the third-instar larvae of Helicoverpa armigera Hübner (Lepidoptera: Noctuidae) and Thaumetopoea pityocampa Schiff (Lepidoptera: Notodontidae). The viruses’ concentrations when tested were 500 ppm, 1000 ppm, 1500 ppm, 3000 ppm, and 6000 ppm, and were applied on the eating medium. Both mortality and larval weight were monitored for 6 days. All three viruses had significant mortality rates on both moths (23.3–83.3% in the highest dose) and larval weights had considerable decreases (70–80% in the highest dose). Generally, noteworthy insecticidal action was recorded after 4 days and in doses higher than 1500 ppm. These results highlight that entomopathogenic viruses may infect species other than their natural host and can be implemented in terms of Integrated Pest Management.
1. Introduction
For many years, chemical pesticides were the only option to employ in agriculture. However, as more and more of these substances are becoming obsolete or even withdrawn from the market due to the risks linked to them, finding eco-friendly and healthier alternatives has become a vital need. Such alternative tools are biopesticides that are derived from naturally occurring matter and are defined by their biodegradability and their low impact on other organisms and the environment. They can include fungi, bacteria, viruses, nematodes, protozoa, or metabolites of the forementioned, which are known for their insecticidal effect.
Among various groups of insect pathogens that have been used for pest control, entomopathogenic viruses are distinct as they are known for their safety and target specificity. Apart from this, they are very widespread, well s
.tudied, and can be easily replicated, characteristics that reinforce their selection for further research and commercial development [1]. The pathogenicity caused by these microorganisms is not the same in all insects and even differs between the insect’s developmental stages. Despite the fact that entomopathogenic viruses infect a plethora of species, they exhibit very high selectivity, as many of them infect only one host. Although they do not cause acute and immediate mortality, dramatic reductions in their host populations have often been observed because of their actions [1].
A special group of entomopathogenic viruses, Nucleopolyhedroviruses (NPVs), belonging to the family Baculoviridae, have been suggested as potential bioinsecticides, and already some of them have successfully been implemented as pest agents [2,3]. This family consists of 600 viruses, including two genera, NPV and Granuloviruses (GVs) [4]. NPVs have been found to be effective against many lepidopterous insects, while different factors may influence outcomes, such as dose, temperature, nutrition, physical character, and the larval stage [5,6,7]. However, there are certain disadvantages, such as their narrow host range that limits their success against the diverse insect species in the field, and the slow speed of action that allows the pests to infest crops and forests for considerable periods of time [3,4,8,9].
The pine processionary moth T. pityocampa is a significant pest of pines in Mediterranean countries, Central Europe, the Middle East, and North Africa [10,11]. It is distributed virtually everywhere in Greece, with the exception of certain regions in Central Greece and the Aegean Sea islands because of unfavorable climate conditions and isolation [12]. It is regarded as one of the most dangerous forest pests in Greece because it can cause severe defoliation [13]. The adult moth lays eggs on pine trees and some other conifer tree species. The hatched larvae that feed on pine needles form visible white winter nests, which provide unambiguous evidence of their presence [14]. Such infections can defoliate young trees severely; older ones may become weakened and more susceptible to other pathogenic organisms, or to environmental stress induced by drought or excessive moisture [15]. Although old trees rarely die, notable increment losses in diameter and volume can be seen [16]. Aside from damaging forest trees, larvae can also cause dermatitis and ocular lesions in humans and animals, as well as respiratory symptoms and anaphylactic reactions in rare cases [17].
The cotton bollworm H. armigera is one of the most dangerous agricultural pests [18,19]. Currently, it is estimated that this species is responsible for about 3 billion USD in annual global losses [20]. In addition to being a cosmopolitan species, it is also a polyphagous moth as its diet consists of a variety of crops, such as cotton, tomato, sorghum, and chickpea, among others [21]. Eggs are deposited on fruits and flowers and hatched larvae feed on plant tissue, causing significant damage [22,23]. Aside from the destruction its larvae can cause on many economically important crops, its resistance to chemical insecticides ranks H. armigera among the most serious of crop pests [24,25,26,27,28].
Control of these pests is of vital need as they can cause great damage to agriculture and forestry, imposing great impact on the economy and human health. A promising tool either as an alternative or as an assistant to chemicals would be entomopathogenic viruses which, among other things, can effectively help to prevent moth pests from developing resistance to conventional insecticides. This research aimed to evaluate larval mortality with the application of commercial biopesticides based on entomopathogenic viruses, which were used against T. pityocampa and H. armigera in laboratory conditions.
2. Materials and Methods
2.1. Biological Material
Larvae of H. armigera were originally picked from biological tomato fields in Kourtesi, Ilia, Greece (37°58′44″ N 21°19′4″ E), and their identification was established stereoscopically. An artificial diet made in the laboratory was provided as cited in Matzoukas et al., 2022 [19]. The ingredients were separated into three mixtures and treated as follows. The first step included a mixture of vitamins [Micotineacitamide (9.30 g), riboflavin (4.64 g), pyridoxine hydrochloride (2.32 g), biotin (0.18 g), vitamin B12 (0.01 g), folic acid (4.64 g), and thiamine hydrochloride (2.32 g)] and agar (45 g) that was boiled in distilled water (1000 mL). After that, the second mixture [Biological yeast powder (60 g), sucrose (60 g), formaldehyde 10% (15 mL), choline chloride 20% (30 mL), and distilled water (1200 mL)] and the third mixture [Ascorbic acid (12 g), methyl 4 hydroxy benzoate (7.5 g), sorbic acid (4.5 g), streptomycine sulphate (0.1 g), cholesterol (0.6 g), and wheat germ oil (0.6 mL)] were prepared separately by grinding. Finally, the second and third mixtures were combined, and 45 gr of agar and another 1000 mL of distilled water were added. The final mixture was brought to a boil and after it cooled (around 70 °C) the first vitamin mixture was added. It was kept in the refrigerator at 6–8 °C.
For larval rearing, plastic trays (26 cm wide, 4 cm deep, 5.5 cm3 in volume) were carefully covered with fine muslin cloth for aeration. The pupae were removed daily and placed in empty glass vials sealed with cotton wool. They were placed in an incubator and maintained at 24 ± 3 °C, 70 ± 5% RH, and L14:D10 until adult emergence [18]. The newly emerged adult moths were sexed and transferred to boxes to acquire eggs for future progeny development.
Lab culture of T. pityocampa was set up by collecting 1500 larvae from five habitats in stands of Pinus halepensis Mill. in Patras (Dassylio), Achaia, from February to May 2022. Several infested pine samples (50–60) were placed in sterile, wet sand in plastic boxes with vented openings and transferred to the laboratory. The larvae were fed on pine needles (P. halepensis) at room temperature [29]. Every 1 or 2 days, fresh twigs were provided. All larvae were maintained in constant conditions of temperature, 25 ± 1 °C, relative humidity 60–70%, and photoperiod L16:D8 (PHC Europe/Sanyo/Panasonic Biomedical MLR-352-PE, Nijverheidsweg 120, 4879 AZ Etten-Leur, The Netherlands).
2.2. Insect Toxicity Assays
The following insect pathogens were obtained for this experiment: Cydia pomonella Granulovirus (CpGV) (Madex 6 × 1012 OB/mL from Hellafarm, Athens, Greece), Helicoverpa armigera Nucleopoyhedrovirus (HearNPV) (Helicovex SC 7.5 × 1012 OB/mL from Hellafarm, Athens, Greece), and Phthorimaea operculella Granulovirus (PoG) (Tutavir 2 × 1013 OB/mL produced in Greece by Athesis Hellas). Each viral solution was prepared inside a laminar flow chamber (Equip Vertical Air Laminar Flow Cabinet Clean Bench, Mechanical Application Ltd., Athens, Greece).
Virus pathogenicity against 3rd-instar larvae of H. armigera and T. pityocampa was tested at five different doses using a Potter spray tower on the larval diet (Burkard Manufacturing Co., Ltd., Rickmansworth, Hertfordshire, UK) at 1 kgf cm−2. The concentrations applied were 500 ppm (3 × 109 Obs/mL CpGV, 3.75 × 109 Obs/mL HearNPV, 10 × 109 Obs/mL PoG), 1000 ppm (6 × 109 Obs/mL CpGV, 7.5 × 109 Obs/mL HearNPV, 20 × 109 Obs/mL PoG), 1500 ppm (9 × 109 Obs/mL CpGV, 11.25 × 109 Obs/mL HearNPV, 30 × 109 Obs/mL PoG), 3000 ppm (18 × 109 Obs/mL CpGV, 22.5 × 109 Obs/mL HearNPV, 60 × 109 Obs/mL PoG), and 6000 ppm (36 × 109 Obs/mL CpGV, 45 × 109 Obs/mL HearNPV, 120 × 109 Obs/mL PoG).
Experimental larvae were placed on plastic sterilized six-well plates (Labbox Labware, Barcelona, Spain) with a 2 gr diet each where they were monitored for 6 days. For T. pityocampa, fresh pine leaves (70–90 cm2) were sprayed with the viral solution on both surfaces and were air-dried. The artificial feed (100 gr) of H. armigera was sprayed and left for 20 minutes to dry naturally before placing it on the experimental plates. Six 3rd-instar larvae were used per dose. Each dose was replicated 10 times. The same procedure was performed for the control larvae (sprayed with double distilled water only). Larval mortality and weight were measured every 2 days. Weight was determined through the Gravimetric method.
2.3. Statistical Analysis
Mean values of larval mortality were compared using analysis of variance, with the main factors being treatment, concentration, insect species, and day of the experiment. The Kolmogorov–Smirnov test was used for testing normality. Where necessary, experimental data were arcsine-transformed to meet the requirements of parametric analysis for equal variation among treatments. To find statistically significant differences between factors, the Tukey’s test was used with a significance level of 0.05. All statistical tests were performed using SPSS (SPSS, Inc., Chicago, IL, USA, version 24). Moreover, the Kaplan–Meier method was applied to determine the mean survival time of the larvae. Parameters for data files analyzed by SPSS 24 were as follows: probit model, natural response, and concentrations converted to logarithms. The LC50 was then obtained together with 95% upper and lower confidence limits.
3. Results
3.1. Larval Mortality
Significant differences appeared among treatments; days of the experiment and used concentrations were proven to have a significant effect on larval mortality. The factors’ interactions showed a considerable effect; this suggests that experimental factors affected the insects’ survival time in various ways (Table 1).

Table 1.
An analysis of variance (3-way ANOVA) for the main effects and interactions of the mortality levels of experimental larvae.
The mortality percentage is contingent on the concentration of the used treatment. The final mortality percentages of T. pityocampa larvae after 6 days were 26.7 to 76.6% with HearNPV, 16.7 to 70% with CpGV, and 30 to 73.3% with POG. Control larvae, which were treated only with ddH2O, recorded minor mortality (0.6%) until the end of the experiment (Figure 1). Similarly, the final mortality of H. armigera larvae was 36.7 to 83.3% with HearNPV, 40 to 76.6% with CpGV, and 46.7 to 70% with POG, while the control mortality was also very low (1.7%) (Figure 1).
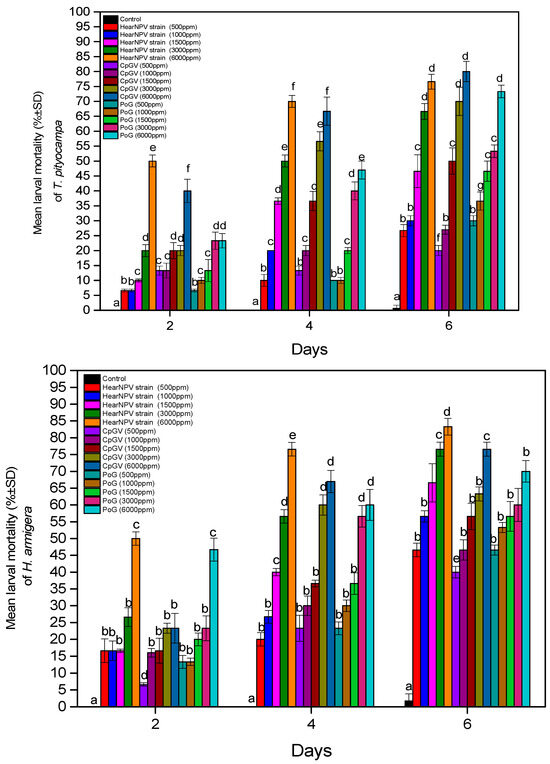
Figure 1.
Mean larval mortality of T. pityocampa (up) and H. armigera (down) treated with an entomopathogenic virus for a period of 6 days. Bars represent the standard error. Columns with the same letter did not differ significantly.
The survival time of T. pityocampa larvae treated with the viruses was significantly reduced in comparison to that of the control larvae. More specifically, after exposure to HearNPV the lethal time of the larvae ranged from 5.5 to 2.1 days, after exposure to CpGV this was 5.6–2.2 days, and, finally, after exposure to PoG this was 5.5–2.7 days. For the control larvae, the survival time was 5.9 days (Table 2). As far as H. armigera is concerned, the survival duration of treated larvae was likewise markedly shortened. More precisely, the survival time varied from 5.0 to 2.0 days following exposure to HearNPV, 5.1 to 2.3 days following exposure to CpGV, and 4.4 to 2.8 days following exposure to PoG. The respective period for untreated larvae was 5.9 days.

Table 2.
Median survival time of T. pityocampa and H. armigera larvae (Kaplan–Meier method, F: 26.096; df: 29; p = 0.000). Columns with the same letter did not differ significantly.
Five different concentrations of the tested virus on the larvae of T. pityocampa and H. armigera yielded an LC50 of 1.374–0.981 ppm for HearNPV, 1.691–0.664 ppm for CpGV, and 1.033–0.524 ppm for PoG (Table 3).

Table 3.
Lethal concentration (LC50) of the three viruses against T. pityocampa and H. armigera larvae after 6 days.
3.2. Larval Weight
Larval weight was significantly affected by the treatments, days of the experiment, and concentrations used. This indicates that experimental factors affected larval weight in a variety of ways (Table 4).

Table 4.
An analysis of variance (3-way ANOVA) for the main effects and interactions of the larval weight levels.
The mean weight of the tested larvae depended on the concentration of the used treatment. The final weights of the T. pityocampa larvae 6 days after exposure were 9.0 to 2.3 mg with HearNPV, 8.7 to 2.5 mg with CpGV, and 9.1 to 2.2 mg with PoG (Table 5). Similarly, the ultimate larval weights of H. armigera were 9.0 to 1.5 mg with HearNPV, 9.1 to 2.2 mg with CpGV, and 9.1 to 2.8 mg with PoG. The weight of the control larvae was found to be 10.5 mg for T. pityocampa and 10.8 mg for H. armigera (Table 5).

Table 5.
Mean weight (mg ± SD) of T. pityocampa and H. armigera larvae. Means of the same column followed by the same letter are not significantly different (Tukey’s test, a = 0.05).
4. Discussion
Baculoviruses are insect pathogens that are occasionally developed as biopesticides to control plant pests, especially moth species [2]. There are now about 16 baculovirus-based biopesticides available for use or in development, most of which are applied against certain moth pests, like C. pomonella [30]. Notwithstanding the advantages, viral biopesticides account only for a small portion of the pesticide market, mostly because of the previously noted drawbacks (limited host range, slow killing). Remarkably, just four insect viruses have been approved by EU countries for commercial use as biopesticides [31]. Therefore, the ability to successfully overcome these obstacles through scientific research will determine whether or not insect viruses will be used continuously in the future.
In this frame, commercial viral biopesticides were evaluated for the management of two common lepidopteran pests in the present study. This is the first time that HearNPV, CpGV, and PoG have been tested as potential biological control agents against T. pityocampa. Also, for the first time, CpGV and PoG were tested against H. armigera.
During the last decade, many lab bioassays and field tests have been carried out, evaluating NPVs as biocontrol agents of lepidopteran pests with very promising results. NPVs have demonstrated effective insecticidal action against many notorious moth species, like H. armigera [32], Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae) [33], Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) [34,35,36,37], Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae) [38,39], Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae) [40,41], and Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) [42,43].
Specifically, S. frugiperda that infested maize plants suffered more than 80% mortality when a formulated SfNPV strain from Colombia was applied [34], while an even higher control was achieved by mixing this NPV with Granulovirus (GPV) [35]. Moreover, three Mexican NPV isolates caused almost complete mortality (˃98%) after 7 days on the same moth [2]. Another NPV caused 77.5% larval mortality within 5 days on S. exigua and suppressed its feeding capacity [38]. In a recent similar study, an NPV was effective at killing young (1st–3rd instars) larvae of H. armigera, recording 99% mortality after 4–6 days, but the grown larvae (4th–5th instar) survived (35% mortality) [33].
Usually, infected larvae cease to gain weight after 24 h of viral infection. Healthy larvae will easily maximize their weight and size in a period of 3–4 days, while the infected larvae stop growing and start losing weight [7]. This happens because viruses are released into the host’s alkaline midgut when the occlusion bodies dissolve in the stomach of lepidopterous larvae. The same pattern was found in the current study at the end of the experiment, given that the final larval weight was gradually decreased in both species due to viral treatments, reaching a reduction of 80% compared to the control in the highest dose and exposure time. This antifeedant effect of viral infection in moth larvae has been well documented for SpliMNPV on S. littoralis [44], for LoGV on the tomato moth Lacanobia oleracea (Lepidoptera: Noctuidae) [45], and for other moth pests [46,47,48,49]. Similarly to our results, a 45% weight reduction was recorded on S. litura larvae treated with a commercial virus suspension (Spodavax, SpltNPV) [46].
Apart from their single action, it has been well documented that NPVs can be perfectly combined with various chemical insecticides, like azadirachtin [50,51,52,53], emamectin [52,54], chlorantraniliprole [50,55], spinetoram [54], thiamethoxam, diflubenzuron [56], endosulfan [57,58], and metaflumizone [52]. Most of the time, this synergy presented an additive effect in contrast with their separate application, providing successful control.
Although many cases of moth pests that have been successfully controlled by their own NPVs have been reported, others isolated from different species have failed to cause high mortality. HaNPV treatment failed to control Plutella xylostella (L.) (Lepidoptera: Plutellidae) [59]. On the contrary, we showed that viruses originally isolated from other hosts significantly decreased the numbers of T. pityocampa and H. armigera larvae under laboratory conditions. Similarly, viruses from the alfalfa looper Autographa californica (Speyer) (AcMNPV) and the celery looper Anagrapha falcifera (Kirby) (AfMNPV) have been very potent against codling moth Cydia pomonella (L.) (Lepidoptera: Tortricidae) [60], Mamestra brassicae NPV (MbNPV) demonstrated high virulence against P. xylostella [61], S. litura NPV (SliMNPV) also killed Arna pseudoconspersa (Strand) (Lepidoptera: Erebidae) [44], and Mythimna separata NPV (Ms-NPV) caused higher mortality in S. exigua than its own virus (Se-NPV) [38]. Moreover, an NPV isolate from the greater wax moth Galleria mellonella L. (Lepidoptera: Pyralidae) caused severe infection in several other moth pests, like P. xylostella, Crocidolomia binotalis Zeller (Lepidoptera: Crambidae), the tobacco budworm Heliothis virescens (Fabricius), and the cabbage moth Mamestra brassicae (L.) (Lepidoptera: Noctuidae) [62]. All these examples highlight the existence of certain viral entomopathogens with relatively broad host ranges, a theory that has been verified by this study as well.
5. Conclusions
It has been well documented that serious moth pests can be controlled effectively with viral biopesticides. Based on our results, the three tested viruses proved to be valuable bioinsecticides and have the potential to be implemented in Integrated Pest Management strategies. Apart from causing significant mortality, they also demonstrated a noteworthy antifeedant effect on both moth pests. Such findings could also be of service in selecting natural virus strains for use against certain insect species. In the case of a viral insecticide, if one species dominates the lepidopterous pest population of a particular crop, it might be advisable to choose a virus that is most effective against that species. Moreover, the potency of any virus is affected by several factors not examined in this study, including the viral strain, age of the host, weather, and adjuvants. Further studies and experimental data on these and other factors are needed if viruses are to become commonly adopted pest management tools.
Author Contributions
Conceptualization, S.M., G.P. and P.A.E.; methodology, S.M.; software, S.M.; validation S.M., G.P. and P.A.E.; formal analysis, S.M.; investigation, S.M., C.Z., F.K. and I.L.; resources, S.M.; data curation, S.M., C.Z. and F.K.; writing—original draft preparation, S.M., F.K., I.L., G.P. and P.A.E.; writing—review and editing, S.M., F.K., I.L., G.P. and P.A.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to 2025. Because it is a new topic for research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Singh, A.; Bhardwaj, R.; Singh, I.K. Biocontrol Agents: Potential of Biopesticides for Integrated Pest Management. In Biofertilizers for Sustainable Agriculture and Environment; Giri, B., Prasad, R., Wu, Q.S., Varma, A., Eds.; Springer: Cham, Switzerland, 2019; pp. 413–433. [Google Scholar] [CrossRef]
- Black, B.C.; Brennan, L.A.; Dierks, P.M.; Gard, I.E. Commercialization of Baculoviral Insecticides. In The Baculoviruses; Miller, L.K., Ed.; Springer: Boston, MA, USA, 1997; pp. 341–387. [Google Scholar] [CrossRef]
- Moscardi, F. Assessment of the Application of Baculoviruses for Control of Lepidoptera. Annu. Rev. Entomol. 1999, 44, 257–289. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chen, X.; Sun, X. Molecular Biology of Insect Viruses. In Advances in Microbial Control of Insect Pests; Springer: Boston, MA, USA, 2003; pp. 83–107. [Google Scholar] [CrossRef]
- Tang, X.X.; Sun, X.L.; Pu, G.Q.; Wang, W.B.; Zhang, C.X.; Zhu, J. Expression of a Neurotoxin Gene Improves the Insecticidal Activity of Spodoptera litura Nucleopolyhedrovirus (SpltNPV). Virus Res. 2011, 159, 51–56. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, F.; Li, Z.; Lu, Z.; Zhang, X.; Zhang, Q.; Liu, X. Effects of Nucleopolyhedrovirus Infection on the Development of Helicoverpa armigera (Lepidoptera: Noctuidae) and Expression of Its 20-Hydroxyecdysone—And Juvenile Hormone—Related Genes. Fla. Entomol. 2015, 98, 682–689. [Google Scholar] [CrossRef]
- Federici, B.A. Baculovirus Pathogenesis. In The Baculoviruses; Miller, L.K., Ed.; Springer: Boston, MA, USA, 1997; pp. 33–59. [Google Scholar] [CrossRef]
- Federici, B.A.; Maddox, J.V. Host specificity in microbe-insect interactions. BioScience 1996, 46, 410–421. [Google Scholar] [CrossRef]
- Lacey, L.A.; Frutos, R.; Kaya, H.K.; Vail, P. Insect Pathogens as Biological Control Agents: Do They Have a Future? Biol. Control 2001, 21, 230–248. [Google Scholar] [CrossRef]
- Battisti, A.; Avcı, M.; Avtzis, D.N.; Jamaa, M.L.B.; Berardi, L.; Berretima, W.; Branco, M.; Chakali, G.; El Alaoui El Fels, M.A.; Frérot, B.; et al. Natural history of the processionary moths (Thaumetopoea spp.): New insights in relation to climate change. In Processionary Moths and Climate Change: An Update; Roques, A., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 15–79. [Google Scholar] [CrossRef]
- Jakubowska, A.K.; Nalcacioglu, R.; Millán-Leiva, A.; Sanz-Carbonell, A.; Muratoglu, H.; Herrero, S.; Demirbag, Z. In Search of Pathogens: Transcriptome-Based Identification of Viral Sequences from the Pine Processionary Moth (Thaumetopoea pityocampa). Viruses 2015, 7, 456–479. [Google Scholar] [CrossRef]
- Roques, A. Processionary Moths and Climate Change: An Update; Springer: Dordrecht, The Netherlands, 2015; Volume 427, 440p. [Google Scholar]
- Battisti, A. Host-plant relationships and population dynamics of the Pine Processionary Caterpillar Thaumetopoea pityocampa (Denis & Schiffermuller). J. Appl. Entomol. 1996, 105, 393–402. [Google Scholar] [CrossRef]
- Jacquet, J.-S.; Orazio, C.; Jactel, H. Defoliation by processionary moth significantly reduces tree growth, a quantitative review. Ann. For. Sci. 2012, 69, 857–866. [Google Scholar] [CrossRef]
- Rodriguez-Mahillo, A.I.; Gonzalez-Muñoz, M.; Vega, J.M.; López, J.A.; Yart, A.; Kerdelhué, C.; Camafeita, E.; Ortiz, J.C.G.; Vogel, H.; Toffolo, E.P.; et al. Setae from the pine processionary moth (Thaumetopoea pityocampa) contain several relevant allergens. Contact Derm. 2012, 67, 367–374. [Google Scholar] [CrossRef]
- Kanat, M.; Alma, M.H.; Sivrikaya, F. Effect of defoliation by Thaumetopoea pityocampa (Den. & Schiff.)(Lepidoptera: Thaumetopoeidae) on annual diameter increment of Pinus brutia Ten. in Turkey. Ann. For. Sci. 2005, 62, 91–94. [Google Scholar] [CrossRef]
- Bonamonte, D.; Foti, C.; Vestita, M.; Angelini, G. Skin reactions to pine processionary caterpillar Thaumetopoea pityocampa Schiff. Sci. World J. 2013, 2013, 867431. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hao, H. Effects of Climate Change and Crop Planting Structure on the Abundance of Cotton Bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Ecol. Evol. 2020, 10, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Mantzoukas, S.; Kitsiou, F.; Lagogiannis, I.; Eliopoulos, P.A. Potential Use of Fusarium Isolates as Biological Control Agents: Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) Case Study. Appl. Sci. 2022, 12, 8918. [Google Scholar] [CrossRef]
- Haile, F.; Nowatzki, T.; Storer, N. Overview of pest status, potential risk, and management considerations of Helicoverpa armigera (Lepidoptera: Noctuidae) for U.S. Soybean Production. J. Integr. Pest Manag. 2021, 12, 3. [Google Scholar] [CrossRef]
- Talekar, N.S.; Opena, R.T.; Hanson, P. Helicoverpa armigera Management: A Review of AVRDC’s Research on Host Plant Resistance in Tomato. Crop Prot. 2006, 25, 461–467. [Google Scholar] [CrossRef]
- Gu, M.; Xue, Z.; Lv, S.; Cai, Y.; Zhang, L.; Gao, X. Corynebacterium sp. 2-TD Mediated Toxicity of 2-Tridecanone to Helicoverpa armigera. Toxins 2022, 14, 698. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, D.J.; Mares, C.L.; Fitt, G.P. Field Performance and Seasonal Changes in the Efficacy against Helicoverpa armigera (Hübner) of Transgenic Cotton Expressing the Insecticidal Protein Vip3A. Agric. For. Entomol. 2007, 9, 93–101. [Google Scholar] [CrossRef]
- Karim, S. Management of Helicoverpa armigera: A Review and Prospectus for Pakistan. Pak. J. Biol. Sci. 2000, 3, 1213–1222. [Google Scholar] [CrossRef]
- Alvi, A.H.; Sayyed, A.H.; Naeem, M.; Ali, M. Field Evolved Resistance in Helicoverpa armigera (Lepidoptera: Noctuidae) to Bacillus Thuringiensis Toxin Cry1Ac in Pakistan. PLoS ONE 2012, 7, e47309. [Google Scholar] [CrossRef]
- Faheem, U.; Nazir, T.; Saleem, M.; Yasin, M.; Bakhsh, M. Status of Insecticide Resistance in Helicoverpa armigera (Hübner) in Southern Punjab, Pakistan. Sarhad J. Agric. 2013, 29, 563–572. [Google Scholar] [CrossRef]
- Qayyum, M.A.; Wakil, W.; Arif, M.J.; Sahi, S.T.; Saeed, N.A.; Russell, D.A. Multiple Resistances against Formulated Organophosphates, Pyrethroids, and Newer-Chemistry Insecticides in Populations of Helicoverpa armigera (Lepidoptera: Noctuidae) from Pakistan. J. Econ. Entomol. 2015, 108, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Blanco, C.A.; Chiaravalle, W.; Dalla-Rizza, M.; Farias, J.R.; García-Degano, M.F.; Gastaminza, G.; Willink, E. Current Situation of Pests Targeted by Bt Crops in Latin America. Curr. Opin. Insect Sci. 2016, 15, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Lagogiannis, I.; Mantzoukas, S.; Eliopoulos, P.A.; Poulas, K. First Record of Beauveria varroae, Cordyceps blackwelliae, and Purpureocillium lavendulum from Greece and Their Pathogenicity against Thaumetopoea pityocampa. Diversity 2023, 15, 312. [Google Scholar] [CrossRef]
- Abd-Alla, A.M.; Meki, I.K.; Demirbas-Uzel, G. Insect viruses as biocontrol agents: Challenges and opportunities. In Cottage Industry of Biocontrol Agents and Their Applications; El-Wakeil, N., Saleh, M., Abu-hashim, M., Eds.; Springer Nature: Dordrecht, The Netherlands, 2020; pp. 277–295. [Google Scholar]
- Karamaouna, F.; Economou, L.P.; Lykogianni, M.; Mantzoukas, S.; Eliopoulos, P.A. Βiopesticides in the EU: State of play and perspectives after the Green Deal for agriculture. In Development and Commercialization of Biopesticides; Koul, O., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 213–239. [Google Scholar]
- Arrizubieta, M.; Williams, T.; Caballero, P.; Simon, O. Selection of a nucleopolyhedrovirus isolate from Helicoverpa armigera as the basis for a biological insecticide. Pest Manag. Sci. 2014, 70, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Black, J.L.; Lorenz, G.M.; Cato, A.J.; Bateman, N.R.; Seiter, N.J. Efficacy of Helicoverpa armigera nucleopolyhedrovirus on soybean for control of Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae) in Arkansas Agriculture. Insects 2022, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Barrera-Cubillos, G.P.; Gómez-Valderrama, J.A.; Rivero, L.F.V. Efficacy of microencapsulated nucleopolyhedroviruses from Colombia as biological insecticides against Spodoptera frugiperda (Lepidoptera: Noctuidae). Acta Agronómica 2017, 66, 267–274. [Google Scholar] [CrossRef]
- Cuartas-Otálora, P.E.; Gómez-Valderrama, J.A.; Ramos, A.E.; Barrera-Cubillos, G.P.; Villamizar-Rivero, L.F. Bio-insecticidal potential of nucleopolyhedrovirus and granulovirus mixtures to control the fall armyworm Spodoptera frugiperda (JE Smith, 1797) (Lepidoptera: Noctuidae). Viruses 2019, 11, 684. [Google Scholar] [CrossRef]
- Ordóñez-García, M.; Rios-Velasco, C.; Ornelas-Paz, J.D.J.; Bustillos-Rodríguez, J.C.; Acosta-Muñiz, C.H.; Berlanga-Reyes, D.I.; Salas-Marina, M.Á.; Cambero-Campos, O.J.; Gallegos-Morales, G. Molecular and morphological characterization of multiple nucleopolyhedrovirus from Mexico and their insecticidal activity against Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Appl. Entomol. 2020, 144, 123–132. [Google Scholar] [CrossRef]
- Popham, H.J.; Rowley, D.L.; Harrison, R.L. Differential insecticidal properties of Spodoptera frugiperda multiple nucleopolyhedrovirus isolates against corn-strain and rice-strain fall armyworm, and genomic analysis of three isolates. J. Invertebr. Pathol. 2021, 183, 107561. [Google Scholar] [CrossRef]
- Supyani, S.S.; Noviayanti, P.N.; Wijayanti, R.W. Insecticidal properties of Spodoptera exigua nuclear polihedrosis virus local isolate against Spodoptera exigua on shallot. Int. J. Entomol. Res. 2014, 2, 175–180. [Google Scholar]
- Elvira, S.; Ibargutxi, M.A.; Gorria, N.; Muñoz, D.; Caballero, P.; Williams, T. Insecticidal characteristics of two commercial Spodoptera exigua nucleopolyhedrovirus strains produced on different host colonies. J. Econ. Entomol. 2013, 106, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Elmenofy, W.; Salem, R.; Osman, E.; Yasser, N.; Abdelmawgod, A.; Saleh, M.; Zaki, A.; Hanafy, E.; Tamim, S.; Amin, S.; et al. Evaluation of two viral isolates as a potential biocontrol agent against the Egyptian cotton leafworm, Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae). Egypt. J. Biol. Pest Control 2020, 30, 75. [Google Scholar] [CrossRef]
- El Sayed, Y.A.; Sayed, S.; Magdy, A.; Elmenofy, W. Detection, characterization and virulence analysis of nucleopolyhedrovirus isolated from the cotton leafworm, Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae). Egypt. J. Biol. Pest Control 2022, 32, 74. [Google Scholar] [CrossRef]
- Ayyub, M.B.; Nawaz, A.; Arif, M.J.; Amrao, L. Individual and combined impact of nuclear polyhedrosis virus and spinosad to control the tropical armyworm, Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae), in cotton in Pakistan. Egypt. J. Biol. Pest Control 2019, 29, 67. [Google Scholar] [CrossRef]
- Kaur, M.; Joshi, N.; Sharma, S.; Kalia, A. Pathogenicity of Nucleopolyhedrovirus (NPV) against Spodoptera litura (Fabricius). J. Biol. Control 2021, 35, 218–226. [Google Scholar] [CrossRef]
- Takatsuka, J.; Okuno, S.; Ishii, T.; Nakai, M.; Kunimi, Y. Host Range of Two Multiple Nucleopolyhedroviruses Isolated from Spodoptera litura. Biol. Control 2007, 41, 264–271. [Google Scholar] [CrossRef]
- Matthews, H.J.; Smith, I.; Edwards, J.P. Lethal and sublethal effects of a granulovirus on the tomato moth Lacanobia oleracea. J. Invertebr. Pathol. 2002, 80, 73–80. [Google Scholar] [CrossRef]
- Nathan, S.S.; Kalaivani, K. Efficacy of nucleopolyhedrovirus and azadirachtin on Spodoptera litura Fabricius (Lepidoptera: Noctuidae). Biol. Control 2005, 34, 93–98. [Google Scholar] [CrossRef]
- Ali, G.; van der Werf, W.; Vlak, J.M. Infection with Spodoptera litura NPV Reduces Food Consumption and Weight Gain of Spodoptera litura Larvae. Pak. J. Zool. 2019, 51, 495–501. [Google Scholar] [CrossRef]
- Beach, M.R.; Todd, J.W. Discrete and interactive effects of plant resistance and nuclear polyhedrosis viruses for suppression of soybean looper and velvet bean caterpillar (Lepidoptera: Noctuidae) on soybean. J. Econ. Entomol. 1988, 81, 684–691. [Google Scholar] [CrossRef]
- Subrahmanyam, B.; Ramakrishnan, N. Influence of a baculovirus infection on molting and food consumption by Spodoptera litura. J. Invertebr. Pathol. 1981, 38, 161–168. [Google Scholar] [CrossRef]
- Wakil, W.; Ghazanfar, M.U.; Nasir, F.; Qayyum, M.A.; Tahir, M. Insecticidal efficacy of Azadirachta indica, nucleopolyhedrovirus and chlorantraniliprole singly or combined against field populations of Helicoverpa armigera Hübner (Lepidoptera: Noctuidae). Chil. J. Agric. Res. 2012, 72, 53–61. [Google Scholar] [CrossRef]
- Pineda, S.; Pérez-Robledo, C.A.; Hernández, R.E.; Figueroa De La Rosa, J.I.; Chavarrieta, J.M.; Martínez, A.M. Combined and individual effects of a nucleopolyhedrovirus and azadirachtin on the mortality and maize-leaf consumption of Spodoptera frugiperda. Phytoparasitica 2014, 42, 571–578. [Google Scholar] [CrossRef]
- Dáder, B.; Aguirre, E.; Caballero, P.; Medina, P. Synergy of lepidopteran nucleopolyhedroviruses AcMNPV and SpliNPV with insecticides. Insects 2020, 11, 316. [Google Scholar] [CrossRef] [PubMed]
- Senthil Kumar, N.; Murugan, K.; Zhang, W. Additive interaction of Helicoverpa armigera nucleopolyhedrovirus and azadirachtin. BioControl 2008, 53, 869–880. [Google Scholar] [CrossRef]
- Abid, A.D.; Zaka, S.M.; Saeed, S.; Iqbal, N.; Naqqash, M.N.; Shahzad, M.S. Sub-lethal doses of Nucleopolyhedrosis Virus and synthetic ınsecticides alter the biological parameters of Helicoverpa armigera Hübner (Lepidoptera: Noctuidae). PLoS ONE 2021, 16, e0259867. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, G.; Maan, N.A.; Ayub, M.A.; Shahid, M.R.; Malik, M.A.; Farooq, M. Evaluation of indigenous the nucleopolyhedrovirus (NPV) of Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) in combination with chlorantraniliprole against Spodoptera species. Egypt. J. Biol. Pest Control 2021, 31, 58. [Google Scholar] [CrossRef]
- Trang, T.; Chaudhari, S. Bioassay of nuclear polyhedrosis virus (NPV) and in combination with insecticide on Spodoptera litura (Fab). Omonrice 2002, 10, 45–53. [Google Scholar]
- Mir, M.U.D.; Gaurav, S.S.; Prasad, C.S.; Tyagi, A. Field efficacy of HaNPY against Helicoverpa armigera on Tomato. Ann. Plant Prot. Sci. 2010, 18, 301–303. [Google Scholar] [CrossRef]
- Siddique, S.S.; Ram, B.; Mohd, A. Efficacy of Trichogramma brasiliense, nuclear polyhedrosis virus and endosulfan for the management of Helicoverpa armigera on tomato. J. Exp. Zool. India 2010, 13, 177–180. [Google Scholar]
- Magholi, Z.; Abbasipour, H.; Marzban, R. Effects of Helicoverpa armigera nucleopolyhedrosis virus (HaNPV) on the larvae of the diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae). Plant Prot. Sci. 2014, 4, 184–189. [Google Scholar] [CrossRef]
- Lacey, L.A.; Vail, P.V.; Hoffmann, D.F. Comparative activity of baculoviruses against the codling moth Cydia pomonella and three other tortricid pests of tree fruit. J. Invertebr. Pathol. 2002, 80, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Fahimi, A.; Kharazi-Pakdel, A.; Talaei-Hassanloui, R.; Rezapanah, M.R.; Maleki, F. Evaluation of the effect of MbNPV on cabbage moth, Plutella xylostella (Lepidoptera: Plutellidae), in laboratory conditions. J. Entomol. Soc. Iran 2008, 28, 63–74. [Google Scholar] [CrossRef]
- Bin Abdul Kadir, H.; Payne, C.C.; Crook, N.E.; Fenlon, J.S.; Winstanley, D. The comparative susceptibility of the diamondback moth Plutella xylostella and some other major lepidopteran pests of brassica crops to a range of baculoviruses. Biocontrol Sci. Technol. 1999, 9, 421–433. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).