Changes in Skeletal Muscle Atrophy over Time in a Rat Model of Adenine-Induced Chronic Kidney Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model and Experimental Design
2.2. Tissue Preparation
2.3. Histological Analysis of Muscle and Kidney
2.4. Mitochondrial Activity
2.5. Gene Expression Analysis of Skeletal Muscle
2.6. Statistical Analyses
3. Results
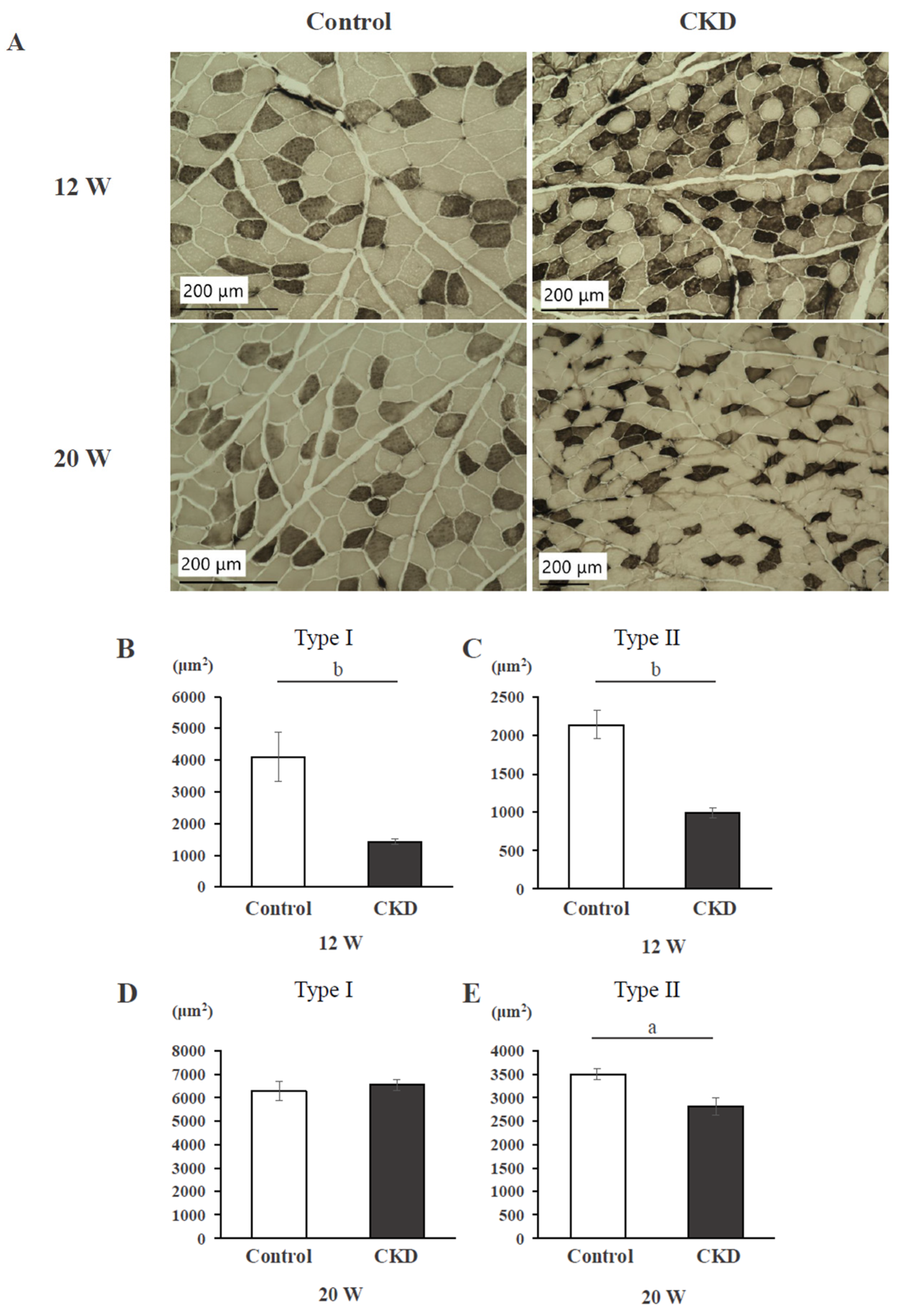
3.1. Histological Findings and Cross-Sectional Area of Back Muscle
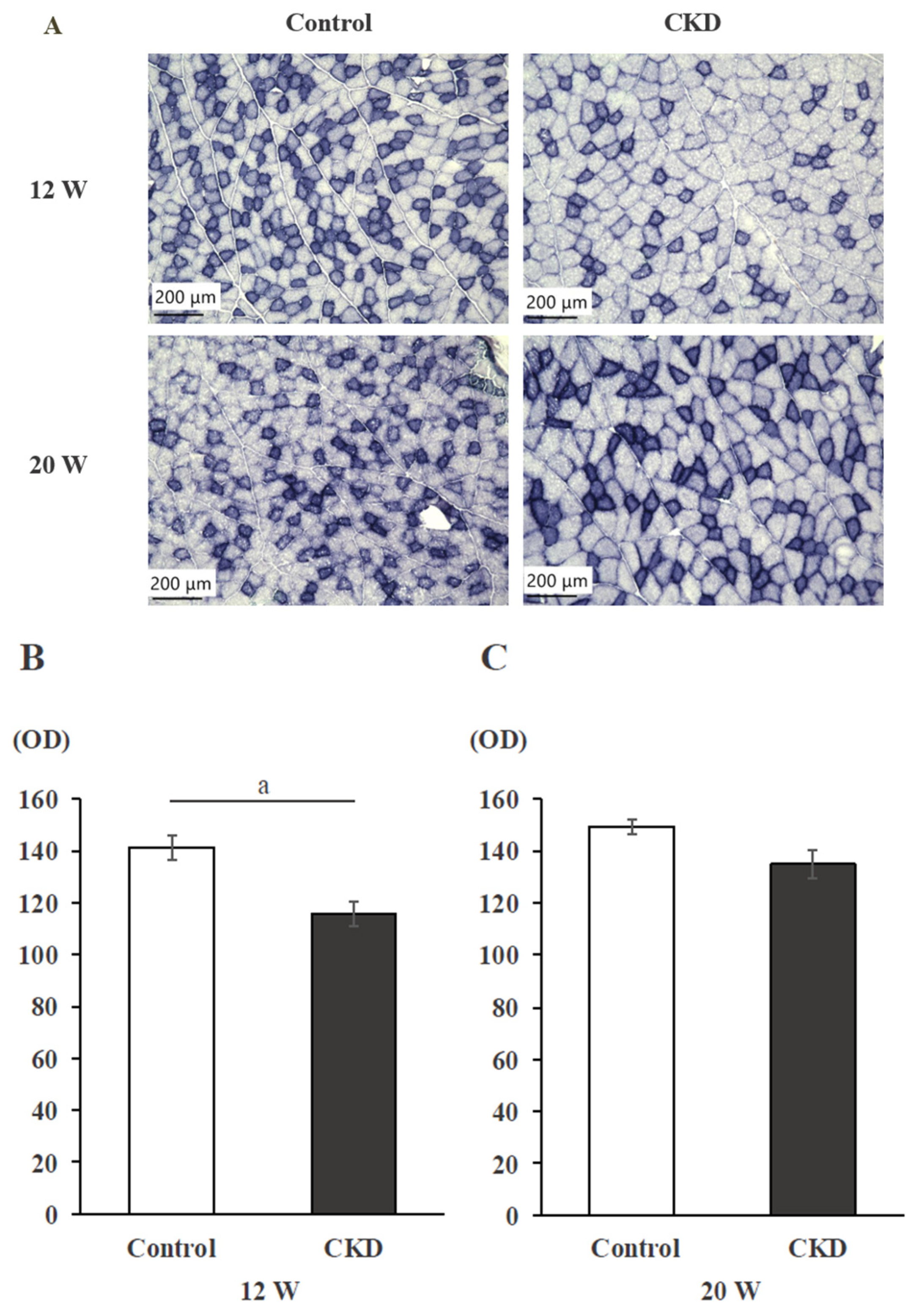
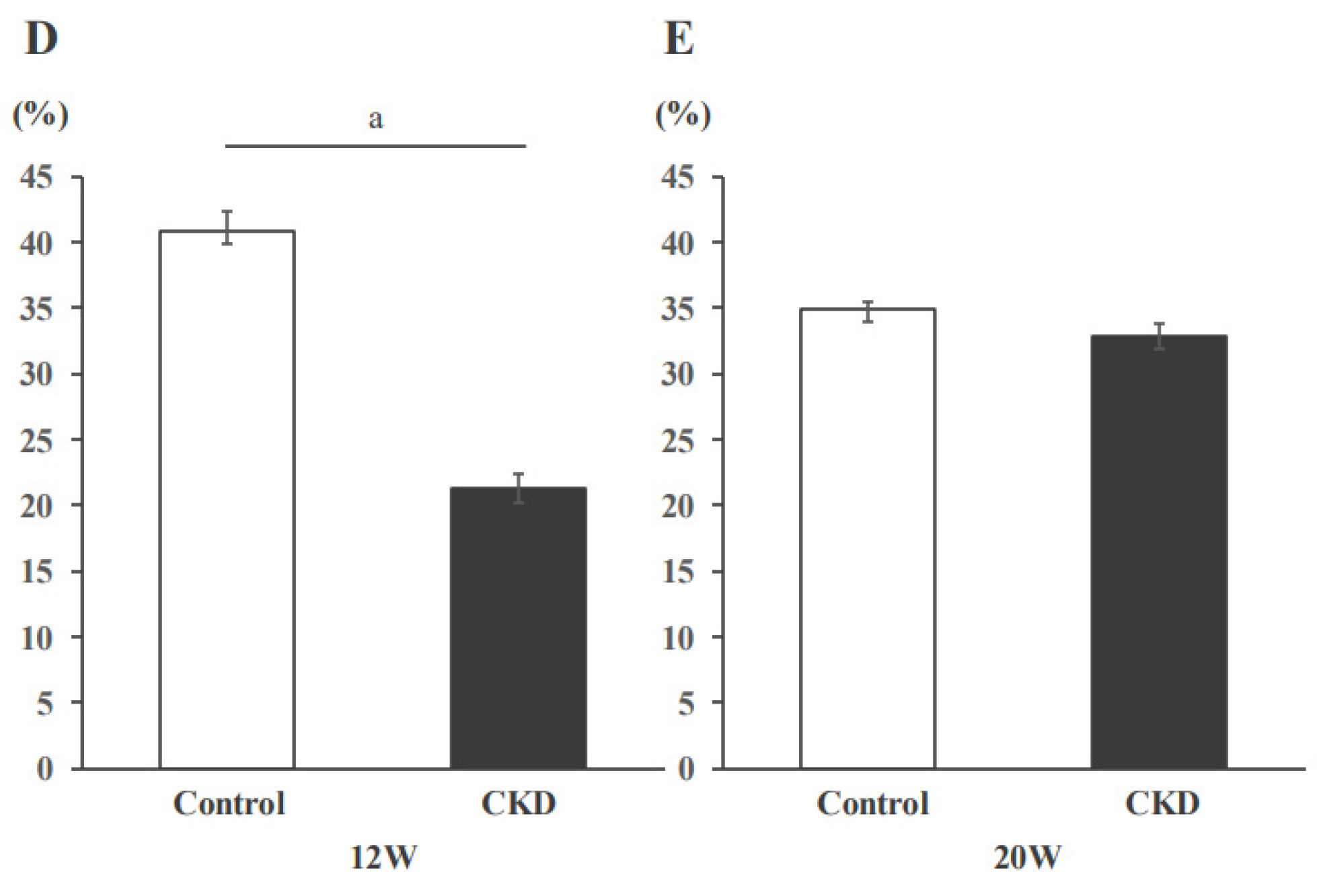
3.2. Mitochondrial Activity Evaluated with SDH Staining
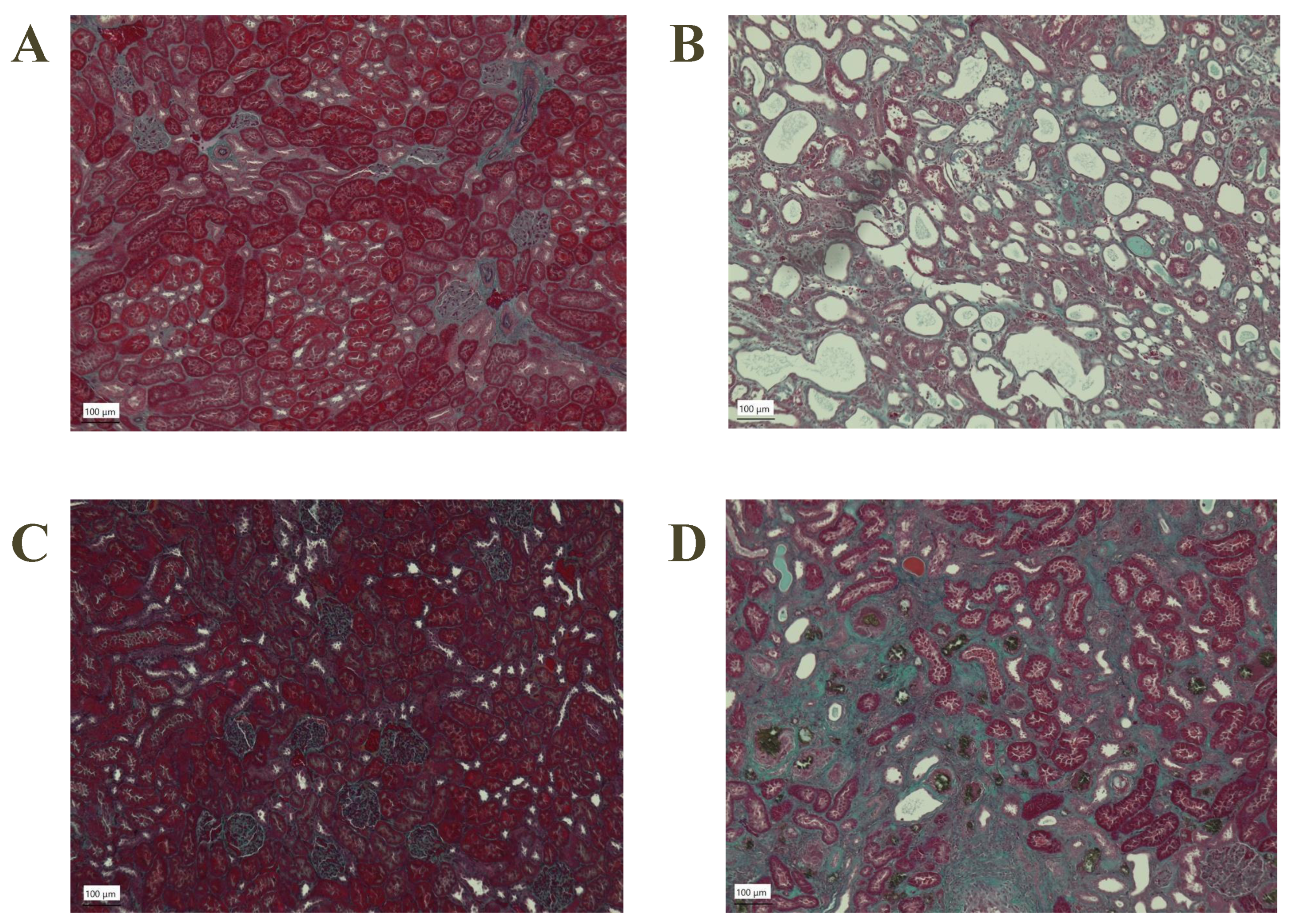
3.3. Histological Sections of the Kidney
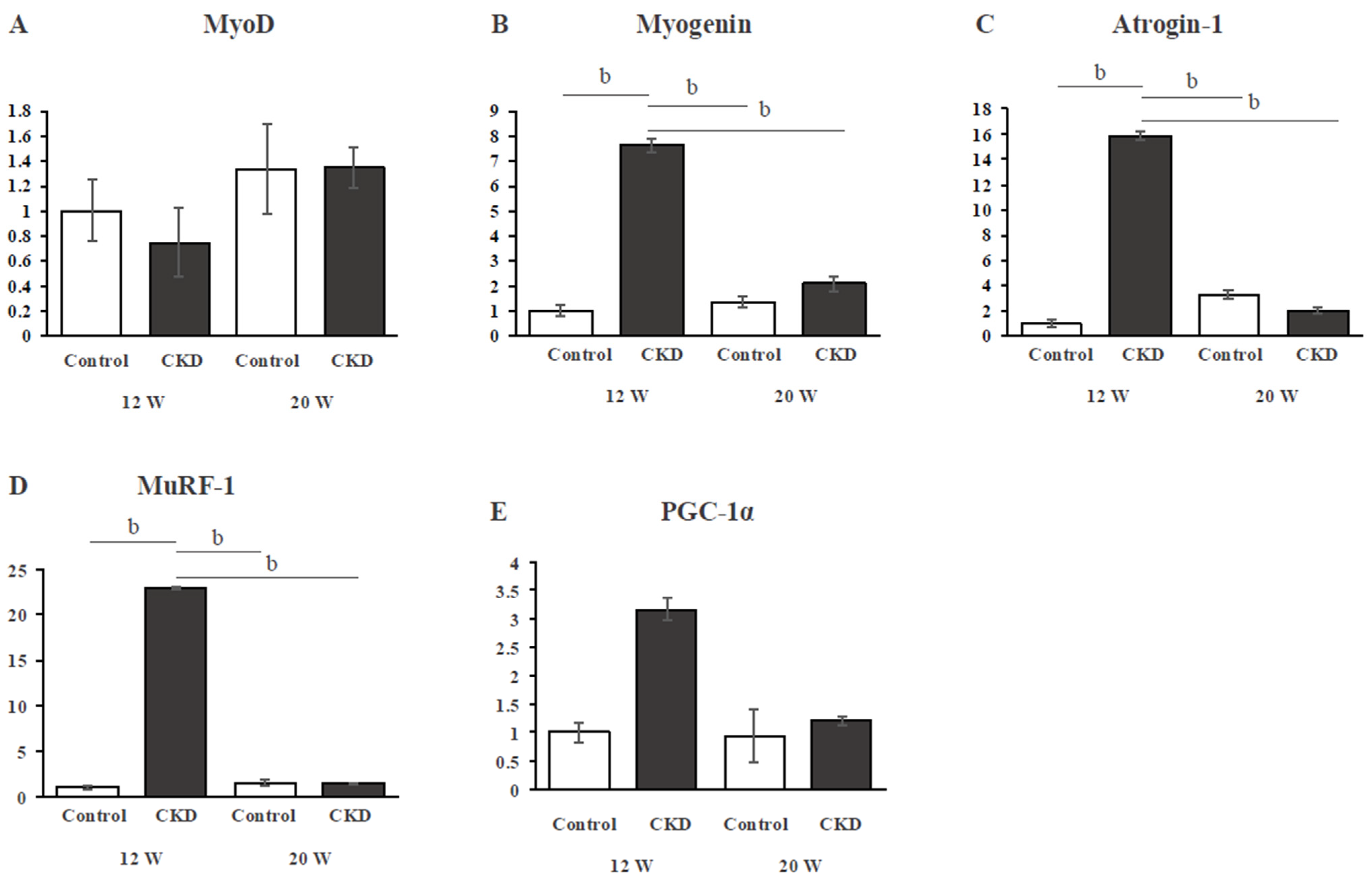
3.4. Gene Expression of Skeletal Muscle
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levey, A.S.; Atkins, R. Chronic kidney disease as a global public health problem: Approaches and initiatives—A position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007, 72, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.C.; Zhang, L.X. Prevalence and Disease Burden of Chronic Kidney Disease. Adv. Exp. Med. Biol. 2019, 1165, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Palit, S.; Kendrick, J. Vascular calcification in chronic kidney disease: Role of disordered mineral metabolism. Curr. Pharm. Des. 2014, 20, 5829–5833. [Google Scholar] [CrossRef]
- Cheng, T.C.; Huang, S.H. Muscle Wasting in Chronic Kidney Disease: Mechanism and Clinical Implications—A Narrative Review. Int. J. Mol. Sci. 2022, 23, 6047. [Google Scholar] [CrossRef] [PubMed]
- Beddhu, S.; Baird, B.C. Physical activity and mortality in chronic kidney disease (NHANES III). Clin. J. Am. Soc. Nephrol. 2009, 4, 1901–1906. [Google Scholar] [CrossRef]
- Watson, E.L.; Viana, J.L. The Effect of Resistance Exercise on Inflammatory and Myogenic Markers in Patients with Chronic Kidney Disease. Front. Physiol. 2017, 8, 541. [Google Scholar] [CrossRef]
- Avin, K.G.; Chen, N.X. Skeletal Muscle Regeneration and Oxidative Stress Are Altered in Chronic Kidney Disease. PLoS ONE 2016, 11, e0159411. [Google Scholar] [CrossRef]
- Serrano, E.; Whitaker-Menezes, D. Uremic Myopathy and Mitochondrial Dysfunction in Kidney Disease. Int. J. Mol. Sci. 2022, 23, 13515. [Google Scholar] [CrossRef] [PubMed]
- Flisiński, M.; Brymora, A. Influence of different stages of experimental chronic kidney disease on rats locomotor and postural skeletal muscles microcirculation. Ren. Fail. 2008, 30, 443–451. [Google Scholar] [CrossRef]
- Organ, J.M.; Srisuwananukorn, A. Reduced skeletal muscle function is associated with decreased fiber cross-sectional area in the Cy/+ rat model of progressive kidney disease. Nephrol. Dial. Transplant. 2016, 31, 223–230. [Google Scholar] [CrossRef]
- Zhang, A.; Li, M. miRNA-23a/27a attenuates muscle atrophy and renal fibrosis through muscle-kidney crosstalk. J. Cachexia Sarcopenia Muscle 2018, 9, 755–770. [Google Scholar] [CrossRef] [PubMed]
- Mogi, M.; Kohara, K. Diabetic mice exhibited a peculiar alteration in body composition with exaggerated ectopic fat deposition after muscle injury due to anomalous cell differentiation. J. Cachexia Sarcopenia Muscle 2016, 7, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.; Mori, T. Metabolic alterations by indoxyl sulfate in skeletal muscle induce uremic sarcopenia in chronic kidney disease. Sci. Rep. 2016, 6, 36618. [Google Scholar] [CrossRef]
- Andres-Hernando, A.; Lanaspa, M.A. Obesity causes renal mitochondrial dysfunction and energy imbalance and accelerates chronic kidney disease in mice. Am. J. Physiol. Renal Physiol. 2019, 317, F941–F948. [Google Scholar] [CrossRef]
- Kim, K.; Anderson, E.M. Skeletal myopathy in CKD: A comparison of adenine-induced nephropathy and 5/6 nephrectomy models in mice. Am. J. Physiol. Renal Physiol. 2021, 321, F106–F119. [Google Scholar] [CrossRef] [PubMed]
- Czaya, B.; Heitman, K. Hyperphosphatemia increases inflammation to exacerbate anemia and skeletal muscle wasting independently of FGF23-FGFR4 signaling. Elife 2022, 11, e74782. [Google Scholar] [CrossRef]
- Moecke, D.M.P.; Martins, G.H.C. Aerobic Exercise Attenuates Kidney Injury, Improves Physical Performance, and Increases Antioxidant Defenses in Lungs of Adenine-Induced Chronic Kidney Disease Mice. Inflammation 2022, 45, 1895–1910. [Google Scholar] [CrossRef]
- Kinoshita, H.; Miyakoshi, N. Effects of eldecalcitol on bone and skeletal muscles in glucocorticoid-treated rats. J. Bone Miner. Metab. 2016, 34, 171–178. [Google Scholar] [CrossRef]
- Yokozawa, T.; Zheng, P.D. Animal model of adenine-induced chronic renal failure in rats. Nephron 1986, 44, 230–234. [Google Scholar] [CrossRef]
- Fontecha-Barriuso, M.; Martin-Sanchez, D. The Role of PGC-1α and Mitochondrial Biogenesis in Kidney Diseases. Biomolecules 2020, 10, 347. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pessin, J.E. Mechanisms for fiber-type specificity of skeletal muscle atrophy. Curr. Opin. Clin. Nutr. Metab. Care. 2013, 16, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Ciciliot, S.; Rossi, A.C. Muscle type and fiber type specificity in muscle wasting. Int. J. Biochem. Cell Biol. 2013, 45, 2191–2199. [Google Scholar] [CrossRef]
- Sakkas, G.K.; Ball, D. Atrophy of non-locomotor muscle in patients with end-stage renal failure. Nephrol. Dial. Transplant. 2003, 18, 2074–2081. [Google Scholar] [CrossRef]
- Flisinski, M.; Brymora, A. Morphometric analysis of muscle fibre types in rat locomotor and postural skeletal muscles in different stages of chronic kidney disease. J. Physiol. Pharmacol. 2014, 65, 567–576. [Google Scholar] [PubMed]
- Hernández-Hernández, J.M.; García-González, E.G. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin. Cell Dev. Biol. 2017, 72, 10–18. [Google Scholar] [CrossRef]
- Sabourin, L.A.; Girgis-Gabardo, A. Reduced differentiation potential of primary MyoD-/- myogenic cells derived from adult skeletal muscle. J. Cell Biol. 1999, 144, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Zammit, P.S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 2017, 72, 19–32. [Google Scholar] [CrossRef]
- Ganassi, M.; Badodi, S. Myogenin promotes myocyte fusion to balance fibre number and size. Nat. Commun. 2018, 9, 4232. [Google Scholar] [CrossRef]
- Enoki, Y.; Watanabe, H. Indoxyl sulfate potentiates skeletal muscle atrophy by inducing the oxidative stress-mediated expression of myostatin and atrogin-1. Sci. Rep. 2016, 6, 32084. [Google Scholar] [CrossRef]
- Enoki, Y.; Watanabe, H. Potential therapeutic interventions for chronic kidney disease-associated sarcopenia via indoxyl sulfate-induced mitochondrial dysfunction. J. Cachexia Sarcopenia Muscle 2017, 8, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Alcalde-Estévez, E.; Sosa, P. Uraemic toxins impair skeletal muscle regeneration by inhibiting myoblast proliferation, reducing myogenic differentiation, and promoting muscular fibrosis. Sci. Rep. 2021, 11, 512. [Google Scholar] [CrossRef] [PubMed]
- Takemura, K.; Nishi, H. Mitochondrial Dysfunction in Kidney Disease and Uremic Sarcopenia. Front. Physiol. 2020, 11, 565023. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, M.; Miyashita, K. Chronic kidney disease reduces muscle mitochondria and exercise endurance and its exacerbation by dietary protein through inactivation of pyruvate dehydrogenase. Kidney Int. 2014, 85, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, B.; Li, J. Differentiation of the intracellular structure of slow- versus fast-twitch muscle fibers through evaluation of the dielectric properties of tissue. Phys. Med. Biol. 2014, 59, 2369–2380. [Google Scholar] [CrossRef]
- Hayeeawaema, F.; Muangnil, P. A novel model of adenine-induced chronic kidney disease-associated gastrointestinal dysfunction in mice: The gut-kidney axis. Saudi J. Biol. Sci. 2023, 30, 103660. [Google Scholar] [CrossRef]
- Saito, H.; Miyakoshi, N. Analysis of bone in adenine-induced chronic kidney disease model rats. Osteoporos. Sarcopenia 2021, 7, 121–126. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okamoto, K.; Kasukawa, Y.; Nozaka, K.; Tsuchie, H.; Kudo, D.; Kinoshita, H.; Ono, Y.; Igarashi, S.; Kasama, F.; Harata, S.; et al. Changes in Skeletal Muscle Atrophy over Time in a Rat Model of Adenine-Induced Chronic Kidney Disease. Appl. Sci. 2024, 14, 9106. https://doi.org/10.3390/app14199106
Okamoto K, Kasukawa Y, Nozaka K, Tsuchie H, Kudo D, Kinoshita H, Ono Y, Igarashi S, Kasama F, Harata S, et al. Changes in Skeletal Muscle Atrophy over Time in a Rat Model of Adenine-Induced Chronic Kidney Disease. Applied Sciences. 2024; 14(19):9106. https://doi.org/10.3390/app14199106
Chicago/Turabian StyleOkamoto, Kento, Yuji Kasukawa, Koji Nozaka, Hiroyuki Tsuchie, Daisuke Kudo, Hayato Kinoshita, Yuichi Ono, Shun Igarashi, Fumihito Kasama, Shuntaro Harata, and et al. 2024. "Changes in Skeletal Muscle Atrophy over Time in a Rat Model of Adenine-Induced Chronic Kidney Disease" Applied Sciences 14, no. 19: 9106. https://doi.org/10.3390/app14199106
APA StyleOkamoto, K., Kasukawa, Y., Nozaka, K., Tsuchie, H., Kudo, D., Kinoshita, H., Ono, Y., Igarashi, S., Kasama, F., Harata, S., Oya, K., Kawaragi, T., Tominaga, K., Watanabe, M., & Miyakoshi, N. (2024). Changes in Skeletal Muscle Atrophy over Time in a Rat Model of Adenine-Induced Chronic Kidney Disease. Applied Sciences, 14(19), 9106. https://doi.org/10.3390/app14199106





