Assessment of Remediation Efficiency for Soils Contaminated with Metallic Mercury in Hydrocarbon Extraction Zones
Abstract
1. Introduction
- Removing mercury (both elemental and bound) from the soil to an acceptable level;
- Reducing the mobility of mercury in the environment to prevent its emission into the atmosphere or migration into water;
- Landfilling at an appropriate site (hazardous waste landfill or non-hazardous waste landfill, depending on waste classification).
2. Materials and Methods
2.1. Chemicals and Analytical Methods
2.2. Description of the Sampling and Measurement Procedures
- Area near the gas drilling installation—samples were taken at 2 points (2 samples from 0 to 0.3 m below the surface and 2 samples from 0.3 to 0.6 m below the surface depth);
- Area at the in-line shut-off and relief valve systems—samples were taken at 8 points (8 samples from 0 to 0.3 m below the surface, 8 samples from 0.3 to 0.6 m below the surface, and 6 samples from 0.6 to 1.2 m below the surface);
- Area near the deposit water reservoir—samples were taken at 2 points (2 samples from 0 to 0.3 m below the surface and 2 samples from 0.3 to 0.6 m below the surface);
- Remaining area of the object (industrial facilities)—12 sampling points (12 samples in total from 0 to 0.6 m below the surface).
2.3. Selection and Developing a Method for Remediation of Soil Contaminated with Metallic Mercury in a Natural Gas Extraction Area
- Analysis of the total mercury content and mercury content capable of emissions in soil samples;
- Measurements of mercury emissions to air using a passive sensor meter and the diffusion chamber method.
3. Results
4. Discussion
4.1. Pilot Industrial Facilities—Characteristics of the Contaminated Area
4.2. Carrying Out a Mercury-Contaminated Soil Remediation Treatment on the Pilot Site
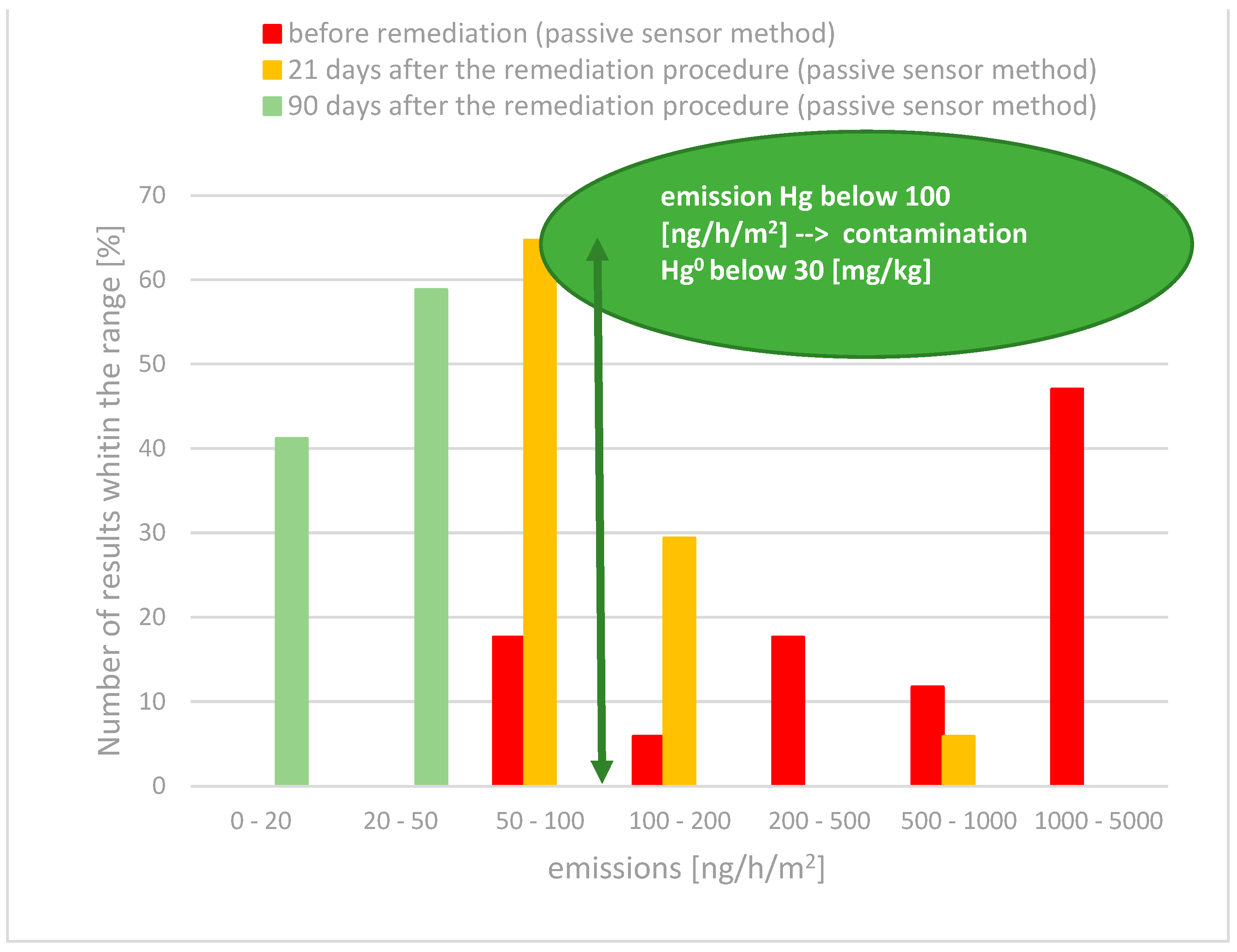
- At six out of seventeen measurement points (18% of the results), emissions were in the range of 50 to 100 ng/h/m2;
- For 35% of the measurements, emissions ranged from 100 to 1000 ng/h/m2;
- The remaining 47% of the results showed emissions ranging from 1000 to 5000 ng/h/m2.
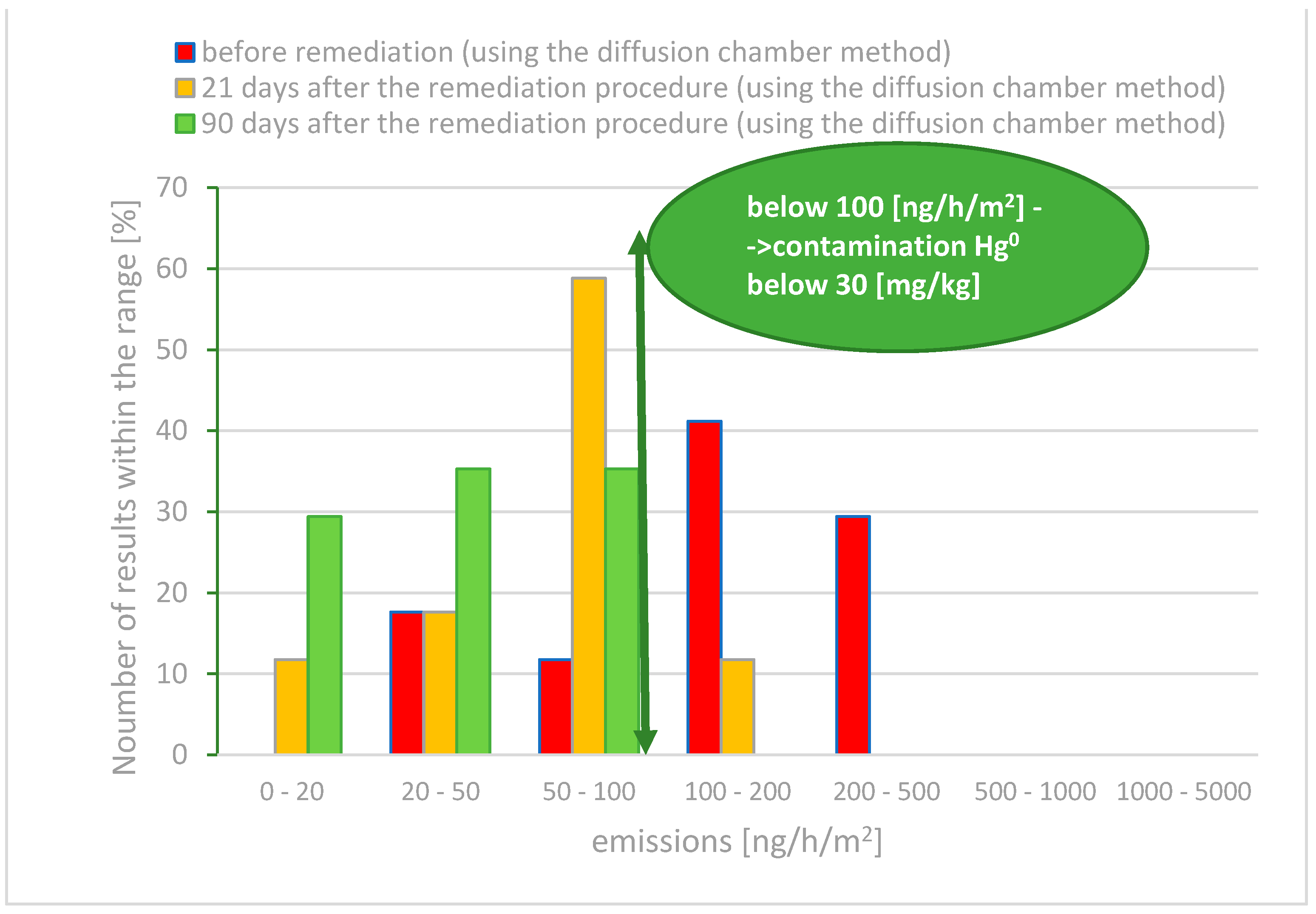
- For 30% of the measurements, the mercury emissions ranged from 0 to 50 ng/h/m2;
- In 59% of the measurements, the emissions were between 50 and 100 ng/h/m2;
- A total of 18% of the results indicated emissions in the range of 100 to 200 ng/h/m2.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gworek, B.; Dmuchowski, W.; Baczewska-Dąbrowska, A.H. Mercury in the terrestrial environment: A review. Environ. Sci. Eur. 2020, 32, 128. [Google Scholar] [CrossRef]
- Kung, H.-C.; Wu, C.-H.; Huang, B.-W.; Chang-Chien, G.-P.; Mutuku, J.K.; Lin, W.C. Mercury abatement in the environment: Insights from industrial emissions and fates in the environment. Heliyon 2024, 10, e28253. [Google Scholar] [CrossRef]
- Teng, D.; Mao, K.; Ali, W.; Xu, G.; Huang, G.; Niazi, N.K.; Feng, X.; Zhang, H. Describing the toxicity and sources and the remediation technologies for mercury contaminated soil. R. Soc. Chem. Adv. 2020, 10, 23221. [Google Scholar] [CrossRef] [PubMed]
- Gworek, B.; Bemowska, O.; Dmuchowski, W.; Szewczyk, A.; Wrzostek-Jakubowska, J. Sources of Mercury Release to the Environment—Legal Regulations, 1st ed.; Monografia IOŚ-PIB: Warsaw, Poland, 2013; ISBN 978-83-60312-42-1. [Google Scholar]
- Gworek, B.; Dmuchowski, W.; Bemowska, O.; Kucharczyk, K.; Wrzostek-Jakubowska, J.; Borzyszkowski, J. Mercury in the Environment, 1st ed.; Monografia IOŚ-PIB: Warsaw, Poland, 2013; ISBN 978-83-60312-47-6. [Google Scholar]
- UNEP. Global Mercury Assessment 2013: Sources, Emissions, Releases and Environmental Transport; UNEP Chemicals Branch: Geneva, Switzerland, 2013; Available online: https://www.unep.org/resources/report/global-mercury-assessment-2013-sources-emissions-releases-and-environmental (accessed on 5 August 2024).
- UNEP. Global Mercury Assessment. United Nations Environment Programme, Geneva. 2018. Available online: https://www.unep.org/resources/publication/global-mercury-assessment-2018 (accessed on 5 August 2024).
- Technical Background Report to the Global Mercury Assessment 2018. Available online: https://www.unep.org/resources/publication/global-mercury-assessment-2018 (accessed on 5 August 2024).
- Pacyna, E.G.; Pacyna, J.M. Global emission of Mercury from anthropogenic sources. Water Air Soil Pollut. 2002, 137, 149–165. [Google Scholar] [CrossRef]
- Pacyna, E.G.; Pacyna, J.M.; Sundseth, K.; Munthe, J.; Kindbom, K.; Wilson, S. Global emission of mercury to the atmosphere from anthropogenic sources in 2005 and projection to 2020. Atmos. Environ. 2010, 44, 2487–2499. [Google Scholar] [CrossRef]
- Kocman, D.; Horvat, M.; Pirrone, N.; Cinnirella, S. Contribution of contaminate stirs to the global mercury budget. Environ. Res. 2013, 125, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Frentiu, T.; Pintican, B.P.; Butaciu, S.; Mihaltan, A.I.; Ponta, M. Determination, speciation and distribution of mercury in soil in the surroundings of a former chlor-alkali plant: Assessment of sequential extraction procedure and analytical technique. Chem. Cent. J. 2013, 7, 178. [Google Scholar] [CrossRef] [PubMed]
- Bernaus, A.; Garona, X.; van Ree, D.; Valiente, M. Determination of mercury in polluted soil surrounding a chlor-alkali plant: Direct speciation by X ray absorption spectroscopy techniques and preliminary geochemical characterization of the area. Anal. Chim. Acta 2006, 565, 73–80. [Google Scholar] [CrossRef]
- Garcia-Sanchezded, A.; Murciego, A.; Alvarez-Ayuso, E.; Santa Regia, I.; Roriguez Gonzalez, M.A. Mercury in soils and plants in abandoned cinnabar mining area (SW Spain). J. Hazard. Mater. 2009, 168, 1319–1324. [Google Scholar] [CrossRef]
- Malferrari, D.; Brigatti, M.F.; Elmi, C.; Laurora, A. Determination of Hg binding forms in contaminated soils and sedimants: Stste of the art and a case study approaching abandonrd mercury mines from Mt. Amiata (Siena, Italy). Neues Jahrb. Mineral.-Abh. 2011, 188, 65–74. [Google Scholar] [CrossRef]
- Rimondi, V.; Bardelli, F.; Benvenuti, M.; Costagliola, P.; Gray, J.E.; Lattanzi, P. Mercury speciation in the Mt. Amiata mining district (Italy): Interplaybetween urban activities and mercury contamination. Chem. Geol. 2014, 380, 110–118. [Google Scholar] [CrossRef]
- Wang, J.; Feng, X.; Anderson, C.W.; Xing, Y.; Shang, L. Remediation of mercury contaminated sites—A review. J. Hazard. Mater. 2012, 221–222, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Santos-Francés, F.; García-Sánchez, A.; Alonso-Rojo, P.; Contreras, F.; Adams, M. Distribution and mobility of mercury in soils of a gold mining region, Cuyuni river basin, Venezuela. J. Environ. Manag. 2011, 92, 1268–1276. [Google Scholar] [CrossRef]
- Sierra, M.J.; Millán, R.; López, F.A.; Alguacil, F.J.; Cañadas, I. Sustainable remediation of mercury. Environ. Sci. Pollut. Res. 2016, 23, 4898–4907. [Google Scholar] [CrossRef]
- Lang, D.; Gardner, M.; Holmes, J. Mercury arising from oil and gas production in the United Kingdom and UK continental shelf. In IKIMP Mercury Knowledge Exchnge; Depth of Earth Science University of Oxford: Oxford, UK, 2012. [Google Scholar]
- Król, A.; Kukulska-Zając, E.; Macuda, J. Methods of monitoring and remediation of soil contaminated with mercury in industrial sites. Nafta Gaz 2016, 8, 626–632. [Google Scholar] [CrossRef]
- Krasińska, A.; Szlęk, M. Problems related to mercury pollution of the soil environment in the area of activity of the oil and gas industry. Nafta Gaz 2008, 5, 303–312. [Google Scholar]
- Steczko, K.; Rachwalski, J.; Krasińska, A. Contamination of soil with mercury—Assessment of the amount of mercury emission to the atmosphere and the effectiveness of its reduction as a result of sulphur stabilization. Nafta Gaz 2009, 10, 782–788. [Google Scholar]
- Lee, W.R.; Eorm, Y.; Lee, T.G. Mercury recovery from mercury-containing wastes using a vacuum thermal desorption system. Waste Manag. 2017, 60, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hou, D.; Cao, Y.; Ok, Y.S.; Tack, F.M.G.; Rinklebe, J.; O’Connor, D. Remediation of mercury contaminated soil, water, and air: A review of emerging materials and innovative technologies. Environ. Int. 2020, 134, 105281. [Google Scholar] [CrossRef]
- Piao, H.; Bishop, P.L. Stabilization of mercury-containing wastes using sulfide. Environ. Pollut. 2006, 139, 498–506. [Google Scholar] [CrossRef]
- Reddy, K.R.; Asce, M.; Chaparro, C.; Saichek, R.E. Iodide—Enhanced Electrokinetic Remediation of Mercury—Contaminated Soils. J. Environ. Eng. 2003, 129, 1137–1148. [Google Scholar] [CrossRef]
- Pusz, A. Assessment of effectiveness of remediation methods for soil contaminated with metals for reclamation needs of industrial degraded land. Prace Naukowe Politechniki Warszawskiej. Inżynieria Sr. 2013, 63, 3–160. [Google Scholar]
- O’Connor, D.; Hou, D.; Ok, Y.S.; Mulder, J.; Duan, L.; Wu, Q.; Wang, S.; Tack, F.M.G.; Rinklebe, J. Mercury speciation, transformation, and transportation in soils, atmospheric flux, and implications for risk management: A critical review. Environ. Int. 2019, 126, 747–761. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Castro, I.; Molina, L.; Prieto-Fernández, M.-A.; Segura, A. Past, present and future trends in the remediation of heavy-metal contaminated soil—Remediation techniques applied in real soil-contamination events. Heliyon 2023, 9, e16692. [Google Scholar] [CrossRef] [PubMed]
- Pei, P.; Mu, D.; Sun, T.; Wang, L.; Liang, X.; Xu, Y.; Sun, Y. Potential of mercaptomontmorillonites for immobilization remediation of Hg-contaminated paddy soil: Perspective from soil environmental quality. Appl. Clay Sci. 2022, 229, 106661. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.; Zhong, H. Remediation of mercury-contaminated soils and sediments using biochar: A critical review. Biochar 2021, 3, 23–35. [Google Scholar] [CrossRef]
- Chen, L.; Luo, X.; He, H.; Duan, T.; Zhou, Y.; Yang, L.; Zeng, Y.; Chen, H.; Fang, L. Hg-mining-induced soil pollution by potentially toxic metal(loid)s presents a potential environmental risk and threat to human health: A global meta-analysis. Soil Ecol. Lett. 2024, 6, 240233. [Google Scholar] [CrossRef]
- Gonçalves, G.; Domingues, E.M.; Ferreira, N.; Ranawadia, K.; Henriques, B.; Bessa, A.; Tavares, D.; Martins, N.; Pereira, E.; Marques, P.A.A.P. Enhanced Hg(II) removal using thiourea-functionalized graphene oxide: Lab to pilot scale evaluation. Sep. Purif. Technol. 2024, 351, 128053. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef]
- Renneberg, A.J.; Dudas, M.J. Transformations of elemental Mercury to inorganic and organic forms in mercury and hydrocarbon co-contaminated soils. Chemosphere 2001, 45, 1103–1109. [Google Scholar] [CrossRef]
- Innovative Approaches to Mercury Contamination in Soil, 2015. U.S. Department of Energy and Polish Institute for Ecology on Industrial Areas, Join Coordinating Committee for Environmental Systems, FY01 Annual Report. Available online: https://www.osti.gov/biblio/799446 (accessed on 5 August 2024).
- Richter, R.B.; Flachberger, H. Soil Washing and Thermal Desorption: Reliable Techniques for Remediating Materials Contaminated with Mercury. BHM 2010, 155, 1–7. [Google Scholar] [CrossRef]
- Bower, J.; Savage, K.S.; Weinman, B.; Barnett, M.O.; Hamilton, W.P.; Harper, W.F. Immobilization of mercury by pyrite (FeS2). Environ. Pollut. 2008, 156, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Król, A.; Kukulska-Zając, E.; Mostowska, J. Evaluation of the effectiveness of mercury immobilization procedures in soil using the index method. Gas Water Sanit. Eng. 2016, 12, 19–22. [Google Scholar] [CrossRef]
- Guidelines on Mercury Management in Oil and Gas Industry, 2011. Ministry of Human Resources, Malaysia. 2011. Available online: http://www.dosh.gov.my/index.php/competent-person-form/occupational-health/guidelines/chemical/615-01-guidelines-on-mercury-management-in-oil-and-gas-industry-2011?path=guidelines/chemical (accessed on 5 December 2019).
- Marrugo-Negrete, J.; Durango-Hernández, J.; Pinedo-Hernández, J.; Olivero-Verbel, J.; Díez, S. Phytoremediation of mercury-contaminated soils by Jatropha curcas. Chemosphere 2015, 127, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Norwegian Oil and Gas Recommended Guidelines for Handling Mercury 2012. Available online: https://www.norskoljeoggass.no/Global/Retningslinjer/HMS/Arbeidsmiljø/132 Kvikksølv/132Recommendedguidelinesforhandlingmercuryeng (accessed on 5 December 2019).
- Wang, F.Y.; Wang, S.X.; Zhang, L.; Yang, H.W.; Gao, Q.R.; Wu, Q.R.; Hao, J.M. Mercury mass flow in iron and steel production process and its implications for mercury emission control. J. Environ. Sci. 2016, 43, 293–301. [Google Scholar] [CrossRef]
- Wu, Q.R.; Wang, S.X.; Yang, M.; Su, H.T.; Li, G.L.; Tang, Y.; Hao, J.M. Mercury flows in large-scale gold production and implications for Hg pollution control. J. Environ. Sci. 2018, 68, 91–99. [Google Scholar] [CrossRef]
- Król, A.; Kukulska-Zając, E. Mercury in samples of gas mixtures—A review of mercury sampling and determination methods. Nafta-Gaz 2020, 76, 846–853. [Google Scholar] [CrossRef]
- Regulation the Minister of the Environment of 1 September 2016 on the Manner of Conducting the Assessment of Contamination of the Earth’s Surface (Dz.U. 2016 poz. 1395). Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=wdu20160001395 (accessed on 5 August 2024).
- Król, A.; Krasińska, A.; Kukulska-Zając, E. Rtęć w środowisku—Najnowsze wytyczne związane z gospodarowaniem zasobami rtęci. Chemik 2014, 68, 973–978. Available online: https://miesiecznikchemik.pl/2014/rtec-w-srodowisku-najnowsze-wytyczne-zwiazane-z-gospodarowaniem-zasobami-rteci/ (accessed on 5 August 2024).

| Type of Activity | Contaminated Area | Determined Soil Mercury Content [mg/kg] | Source of Data |
|---|---|---|---|
| Mercury mines | China, Spain, Slovenia, USA, Turkey | 0.05–9000 | [17] |
| Gold and silver mines | China, Venezuela, Mexico | 0.05–1100 | [1,4,5,6,7,17,19] |
| Cinnabar and sulfide mines | Spain, Italy, Portugal | 1–1709 | [4,14,15,16,17,19] |
| Chemical factory | Portugal, Romania, the Netherlands | 0.074–1150 | [4,12,17,18] |
| Discharge lamp production plant | Poland | 0.03–39.25 | [4] |
| Aerometer factory | Warsaw (Poland) | 62–393 | [2,4] |
| Coal power plant Siersza | Poland | 0.02–0.18 | [2,4] |
| Natural gas mines | Polish Lowland Region (Poland) | 0.05–495 | [21,22,23] |
| Chemical Methods | Biological Methods | Physical Methods | |
|---|---|---|---|
| The main technology | Mainly chemical methods: chemical stabilization, soil washing, oxidation, reduction, reduction dichlorination, solvent extraction, and others. | Mainly divided into plant remediation and microbial remediation. Phytoremediation includes phytostabilization, phytoextracion, phytotransphormation, phytovoltalization, and rhizofiltration methods. | Soil replacement, physical separation, soil vapor extraction, fixed/stabilized soil, vitrification, thermal desorption, electrokinetic remediation. |
| Main characteristic | This type of remediation involves methods in which chemical reagents, reactions, and principles are used to remove contaminants. These processes typically cause pollutants to degrade, which either removes or reduces the soil’s toxicity. | These methods reduce, remove, or immobilize harmful contaminants in the soil and purify it using biological agents. They involve the use of plants and their associated rhizospheric microorganisms to remove contaminants. Contaminants in soil are controlled by introducing specific microorganisms. Additionally, the activities of some lower animals can be utilized to enhance bioremediation. | This type of remediation requires the addition of chemical reagents to improve remediation efficiency. The main physical operations used are soil cleaning/washing, vapor pressure reduction, separation by heating, and establishing an electric field gradient. |
| Examples of used reagents or physical techniques | HCl, HNO3, H2SO4, H3PO4, NaCl, Na2S2O3, sulfide, phosphate | Hyperacumulators (plant), bacteria, earthworms. | High temperature, establishing an electric field gradient, reduction in the vapor pressure. |
| Advantages and disadvantages | Rapid and effective but depends on the type of soil, chemical, and metal. | Economical, eco-friendly, but time-consuming, limited to moderately contaminated sites. | Laborious and highly costly, but it can be applied to highly contaminated sites. |
| Matrix | Time of Drying [s] | Time of Mineralization [s] | Time of Waiting [s] |
|---|---|---|---|
| Soil | 0 | 150 | 45 |
| Gold element | 0 | 200 | 45 |
| Parameter | |
|---|---|
| Mass of sample (soil) | 2 g * ± 0.0001 g |
| Temperature | 13 °C ± 0.2 °C |
| Time of sorption | 3 min ± 10 s |
| Size of gold element (sampler) | 1 cm2 |
| Sampling Location | Results of Soil Sample Analysis from the Depth of 0.0–0.6 m bts | Field Measurements Emissions Levels | ||
|---|---|---|---|---|
| Total Mercury Content in Soil [mg/kg Dry Matter] | Emission-Capable Mercury Content [mg/kg Dry Matter] | |||
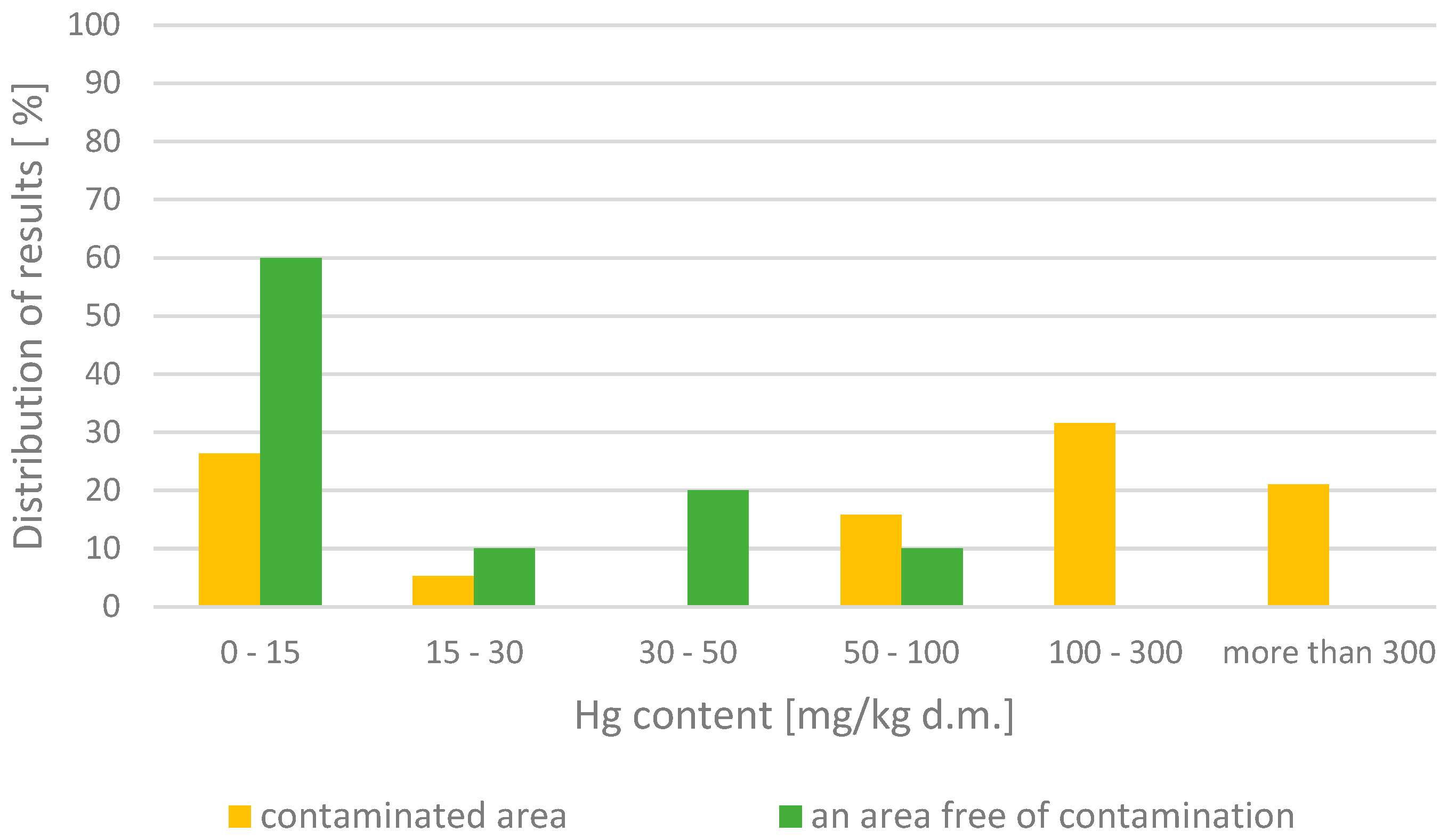
| Potential Contamination Area | Area near the blocking and bleed systems | 1.6–1200 | <15–>>300 | Emissions levels for the contaminated area |
| Area around the mercury removal facility | 0.6–3.61 | <15 | Emissions levels in mercury-free areas | |
| Area near the reservoir water separator | 26 | <15 | Emissions levels in mercury-free zones | |
| Pollution-Free Area | The remaining area of the industrial facility | 0.6 ÷ 27 | <15 | Emissions levels in mercury-free zones |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Król, A.; Kukulska-Zając, E.; Gajec, M. Assessment of Remediation Efficiency for Soils Contaminated with Metallic Mercury in Hydrocarbon Extraction Zones. Appl. Sci. 2024, 14, 8690. https://doi.org/10.3390/app14198690
Król A, Kukulska-Zając E, Gajec M. Assessment of Remediation Efficiency for Soils Contaminated with Metallic Mercury in Hydrocarbon Extraction Zones. Applied Sciences. 2024; 14(19):8690. https://doi.org/10.3390/app14198690
Chicago/Turabian StyleKról, Anna, Ewa Kukulska-Zając, and Monika Gajec. 2024. "Assessment of Remediation Efficiency for Soils Contaminated with Metallic Mercury in Hydrocarbon Extraction Zones" Applied Sciences 14, no. 19: 8690. https://doi.org/10.3390/app14198690
APA StyleKról, A., Kukulska-Zając, E., & Gajec, M. (2024). Assessment of Remediation Efficiency for Soils Contaminated with Metallic Mercury in Hydrocarbon Extraction Zones. Applied Sciences, 14(19), 8690. https://doi.org/10.3390/app14198690






