THC Modelling of Bentonite Barrier of Geological Repository in Granite and Its Impact on Long-Term Safety
Abstract
1. Introduction
2. Materials and Methods
2.1. Conceptual Model
2.2. Mathematical Model
2.2.1. Transport Processes
2.2.2. Two-Phase Flow of Miscible Fluids
2.3. Numerical Model
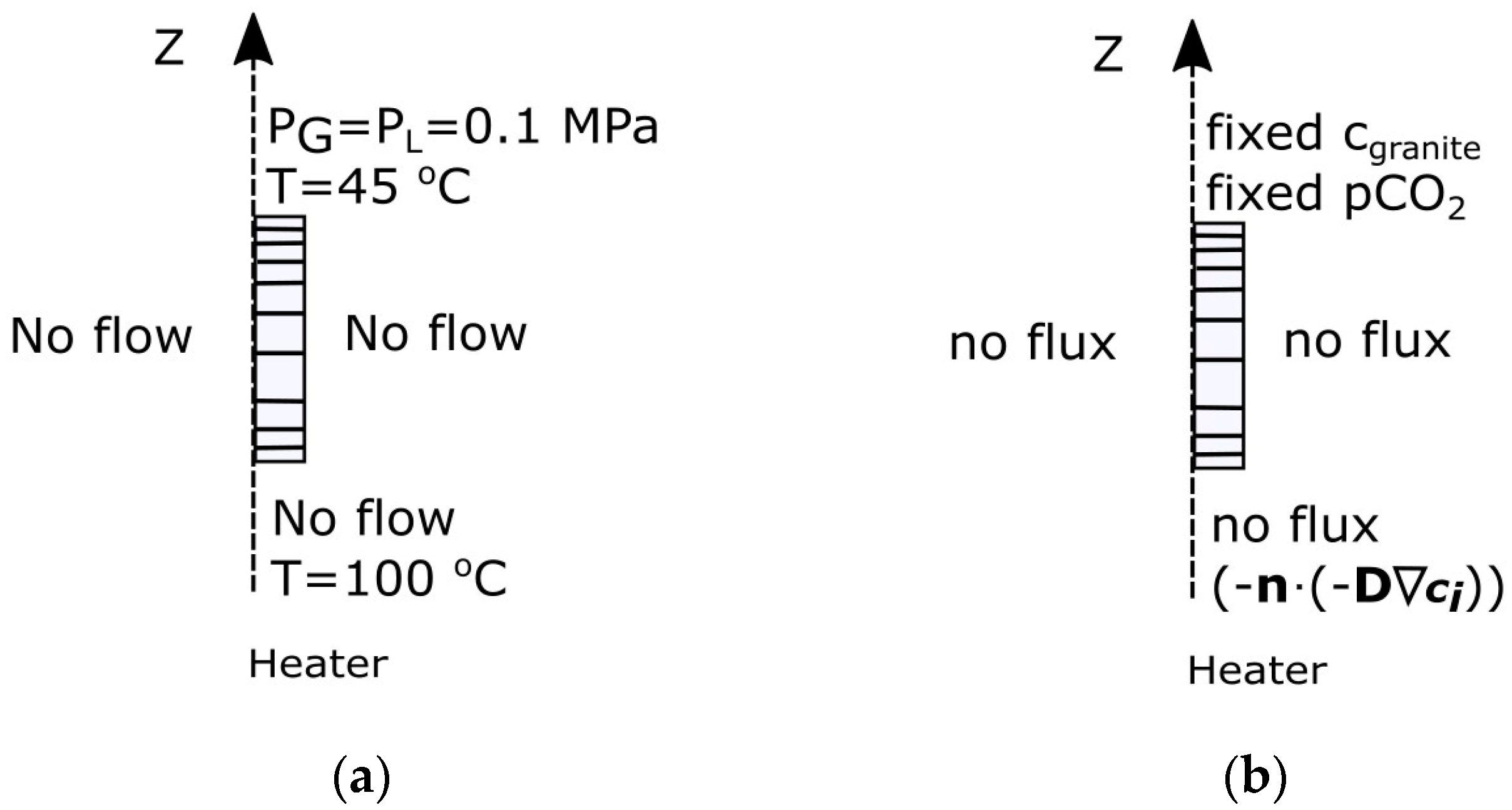
- With the heater temperature of 100 °C and the initial temperature in granite of 12 °C, the temperature gradient establishes quickly across the bentonite layer [12], with ~45 °C on the bentonite–granite interface. Thus, fixed-temperature boundary conditions were applied on the bottom (z = 0.45 m) boundary (T = 100 °C), representing the heater–bentonite interface, and on the upper (z = 1.14 m) boundary (T = 45 °C), representing the bentonite–granite interface.
- Before transport simulation, the equilibrated porewater compositions with mineral phases, cation exchange sites, and surface protonation at 45 °C were derived with Phreeqc.
- Initial suction (PG − PL) of 117.8 MPa was prescribed in the bentonite. With the defined water retention curve, this suction corresponded to 60% saturation.
- No flow boundary conditions were applied to the heater–bentonite interface for water and air, and Dirichlet-type boundary conditions were applied for the bentonite–granite interface (PG = PL = 0.1 MPa).
- No flux boundary condition was applied on the bottom model boundary representing the heater-bentonite interface for the transport of dissolved species and CO2.
- A fixed concentration boundary condition was applied on the upper boundary, representing the bentonite–granite interface for the transport of dissolved species.
- Initially, after the equilibration of predefined porewater composition, the initial ion exchange concentration and minerals resulted in log(pCO2) = –2 with the ThermoChimie database.
- A fixed partial CO2 pressure (log(pCO2) = −4) boundary condition was applied on the top boundary, representing the bentonite–granite interface for CO2 transport following CO2 concentration in granite porewater (Case C1).
- A fixed partial CO2 pressure (log(pCO2) = −1) boundary condition was applied on the top boundary, representing the bentonite–granite interface for CO2 transport (Case C2).
3. Results
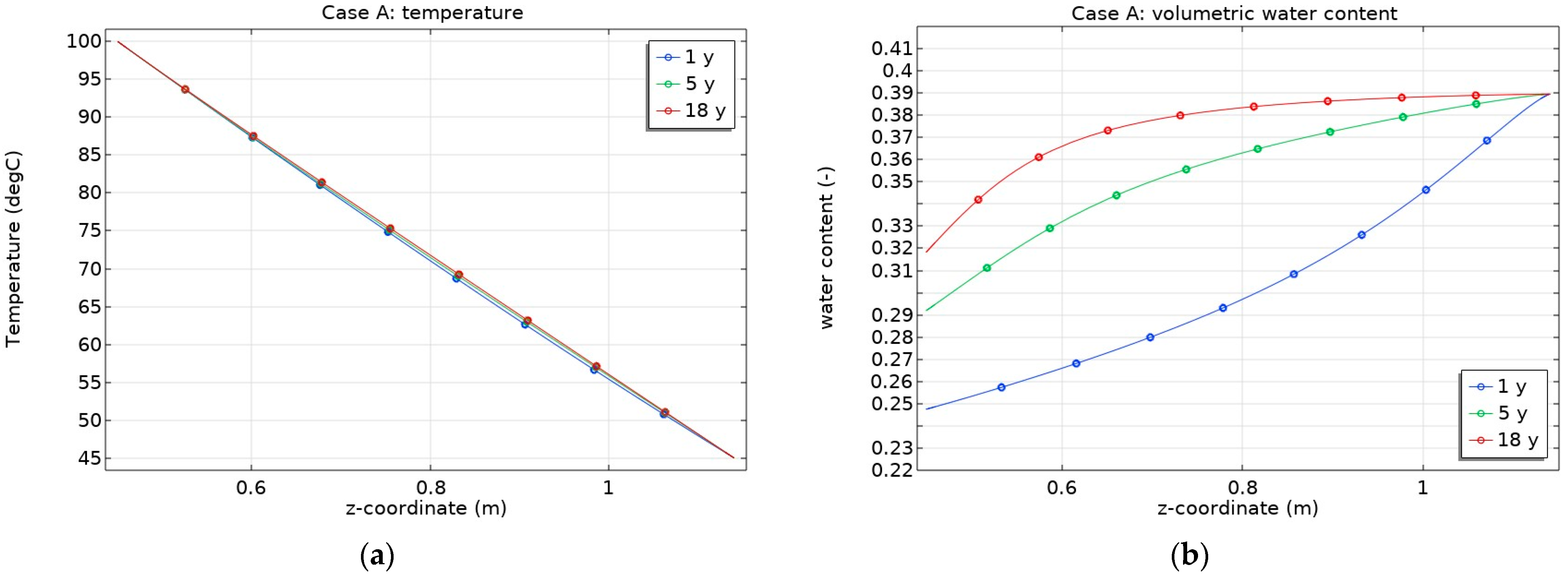
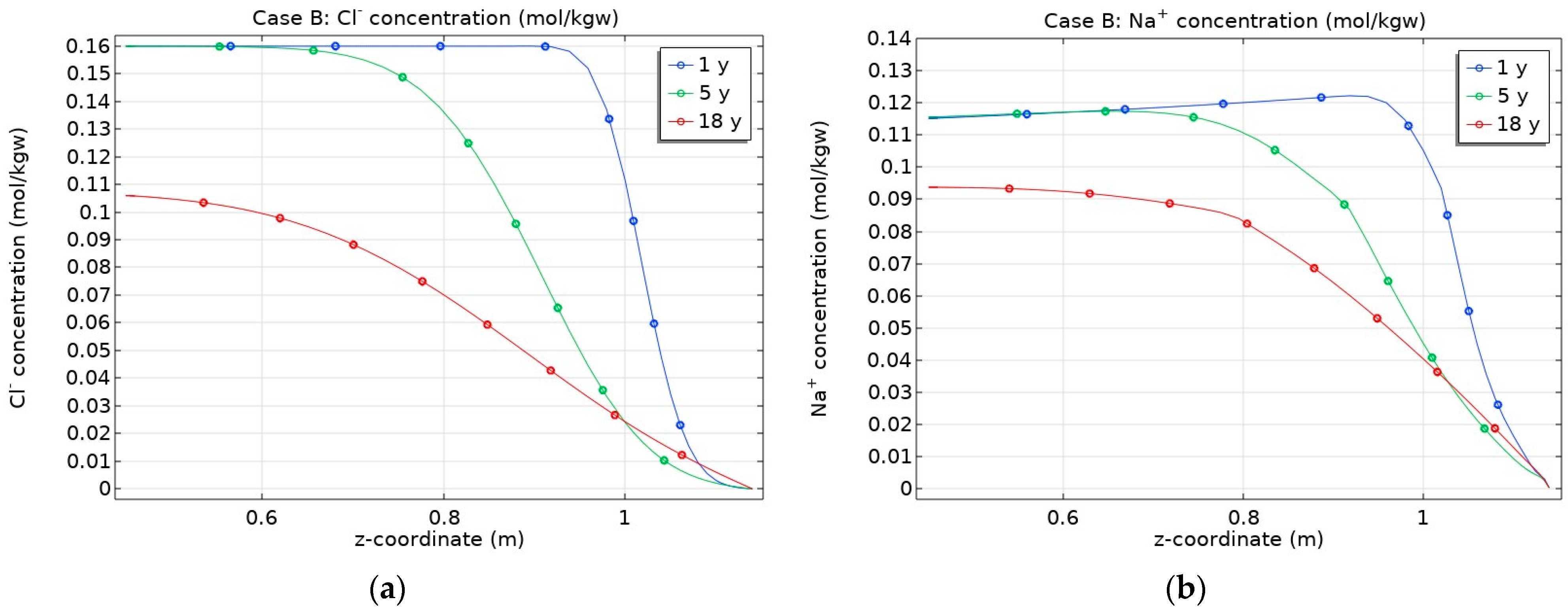
3.1. Mineral Dissolution/Precipitation, Cation Exchange and Surface Complexation Effect on THC State of Bentonite
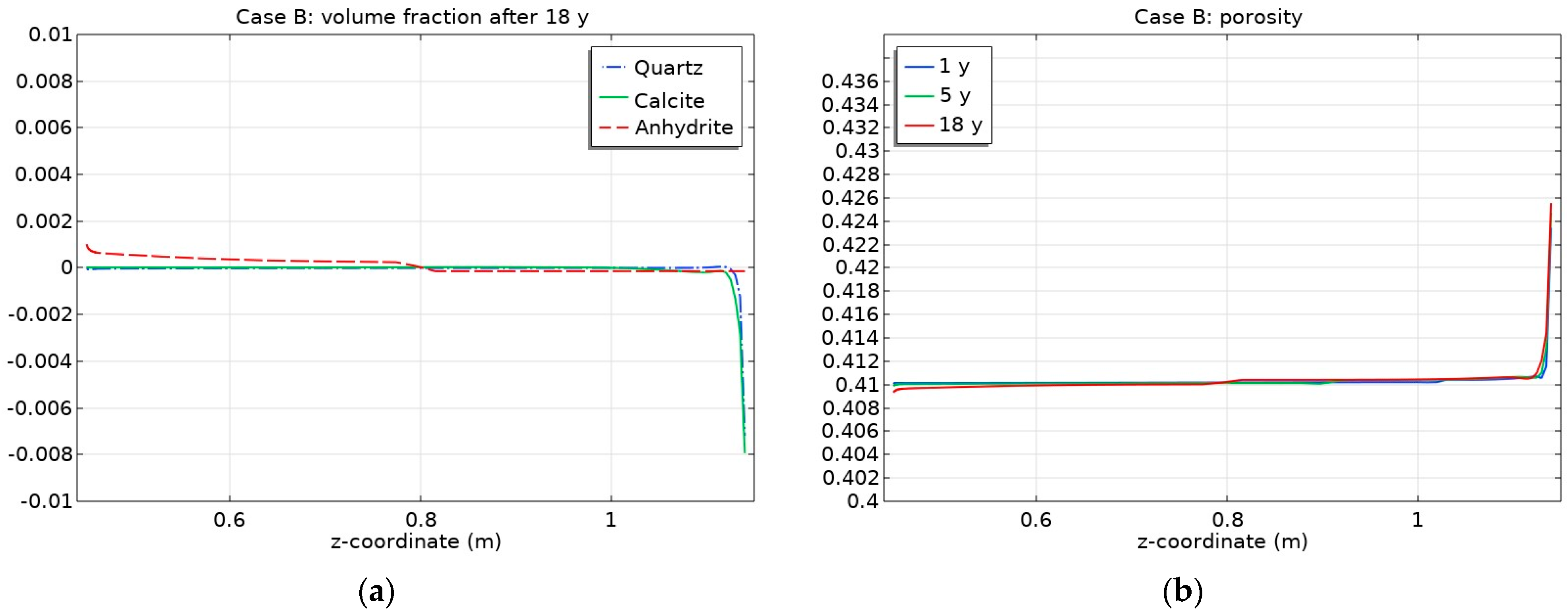
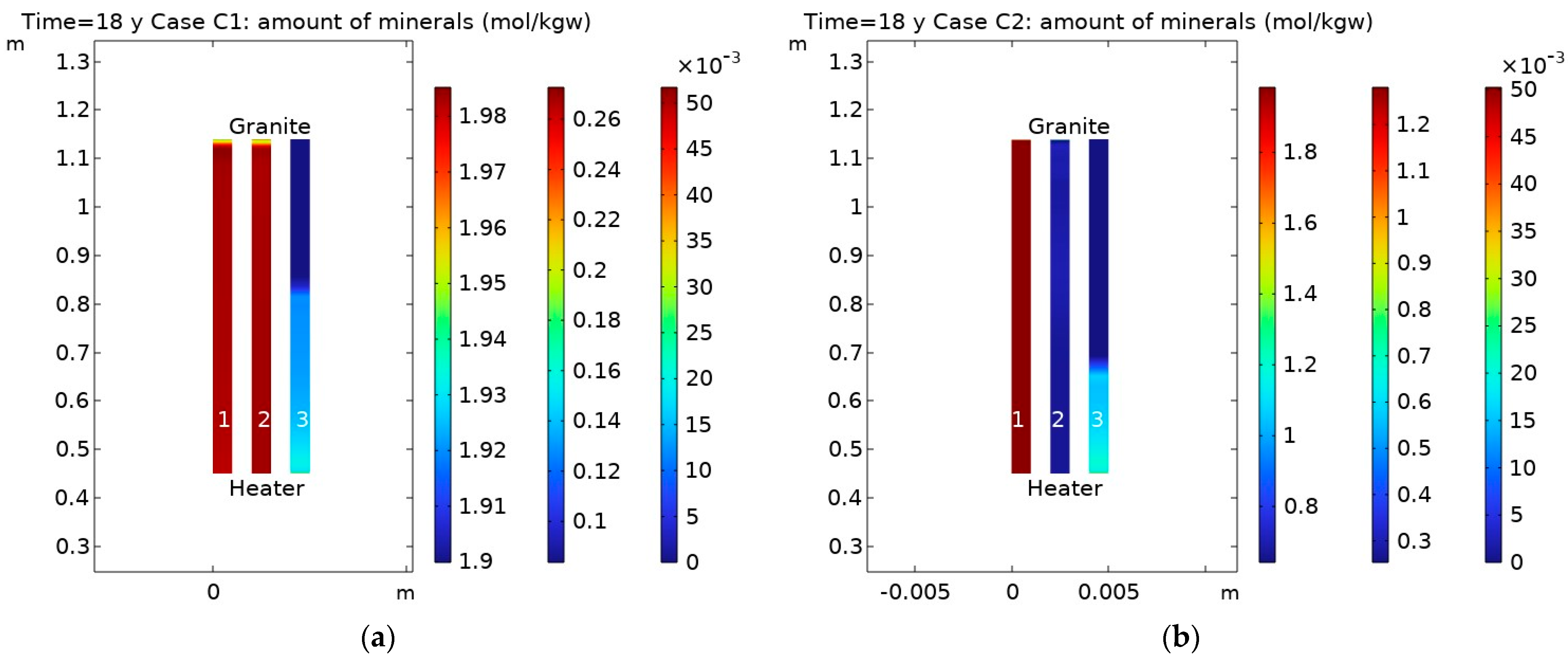
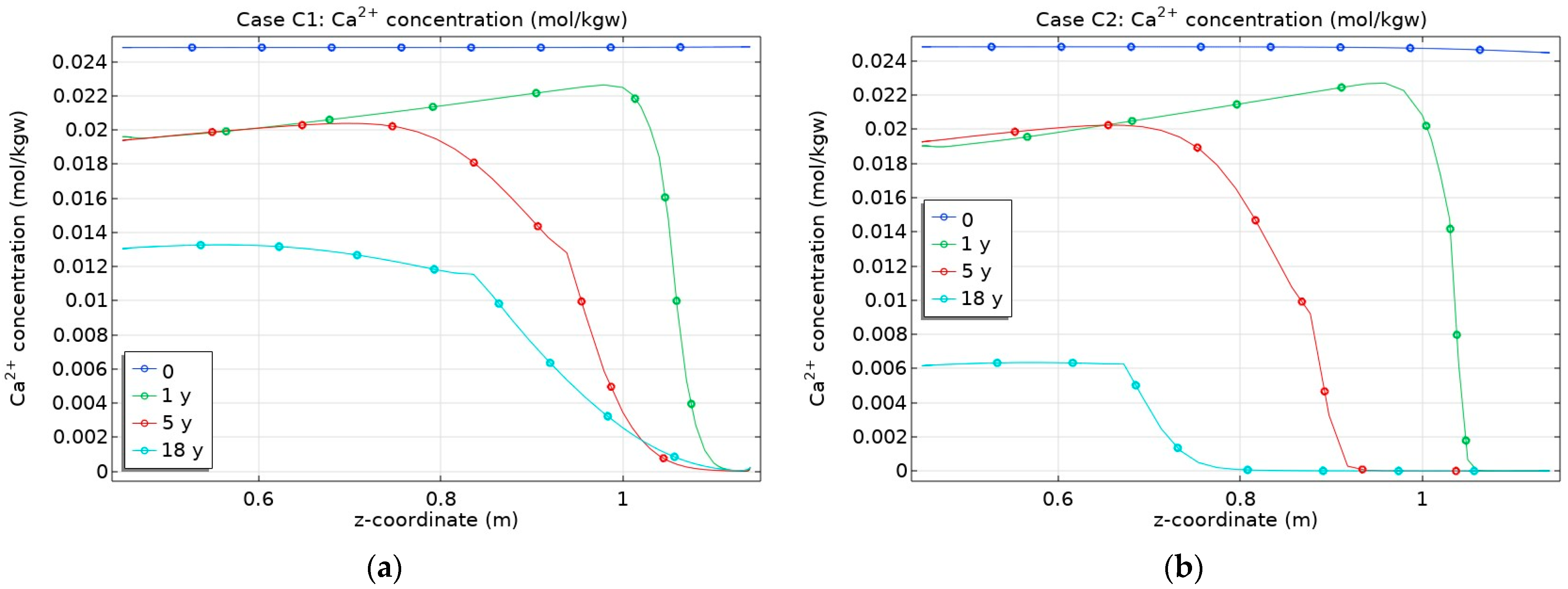
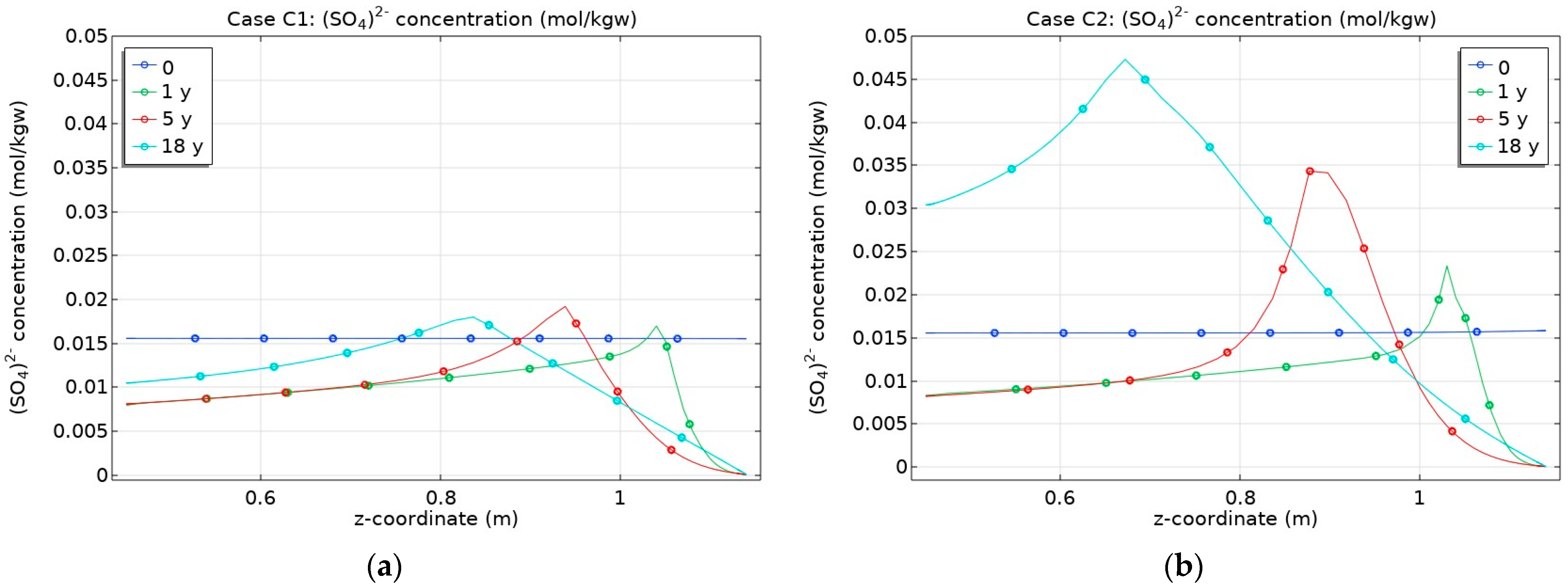
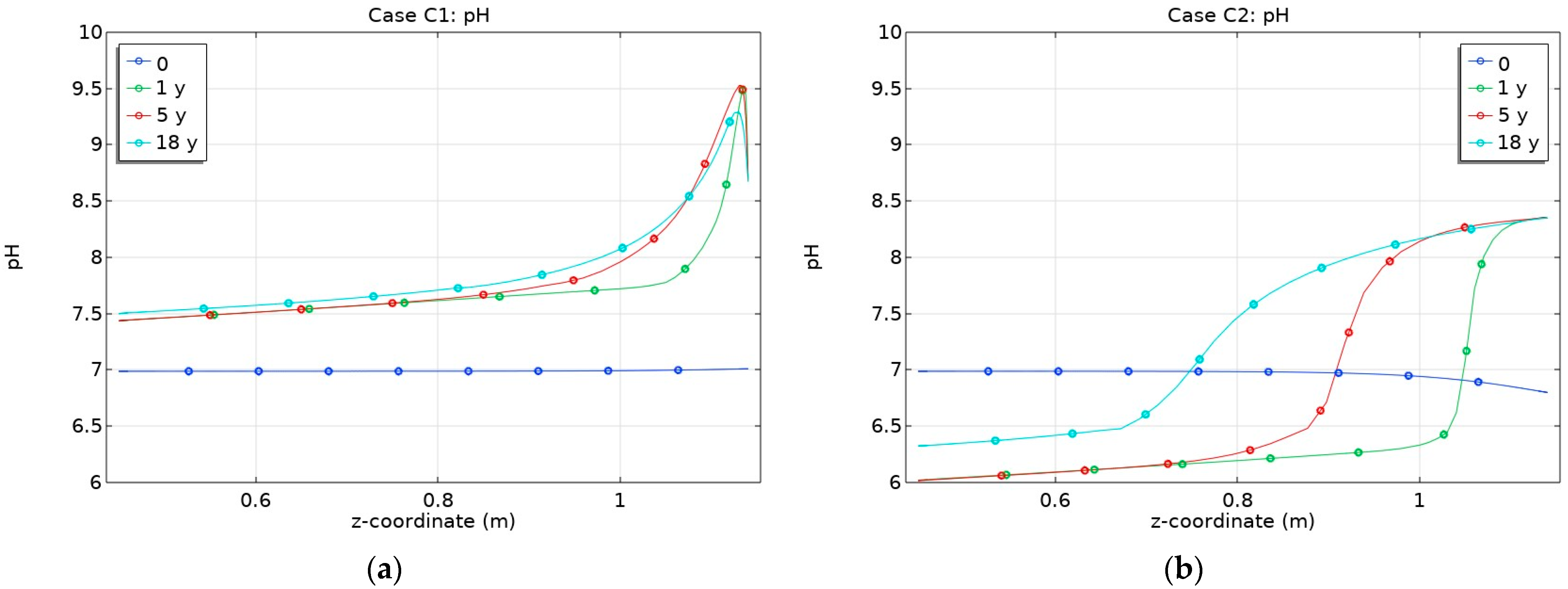
3.2. Influence of CO2 Transport
4. Conclusions
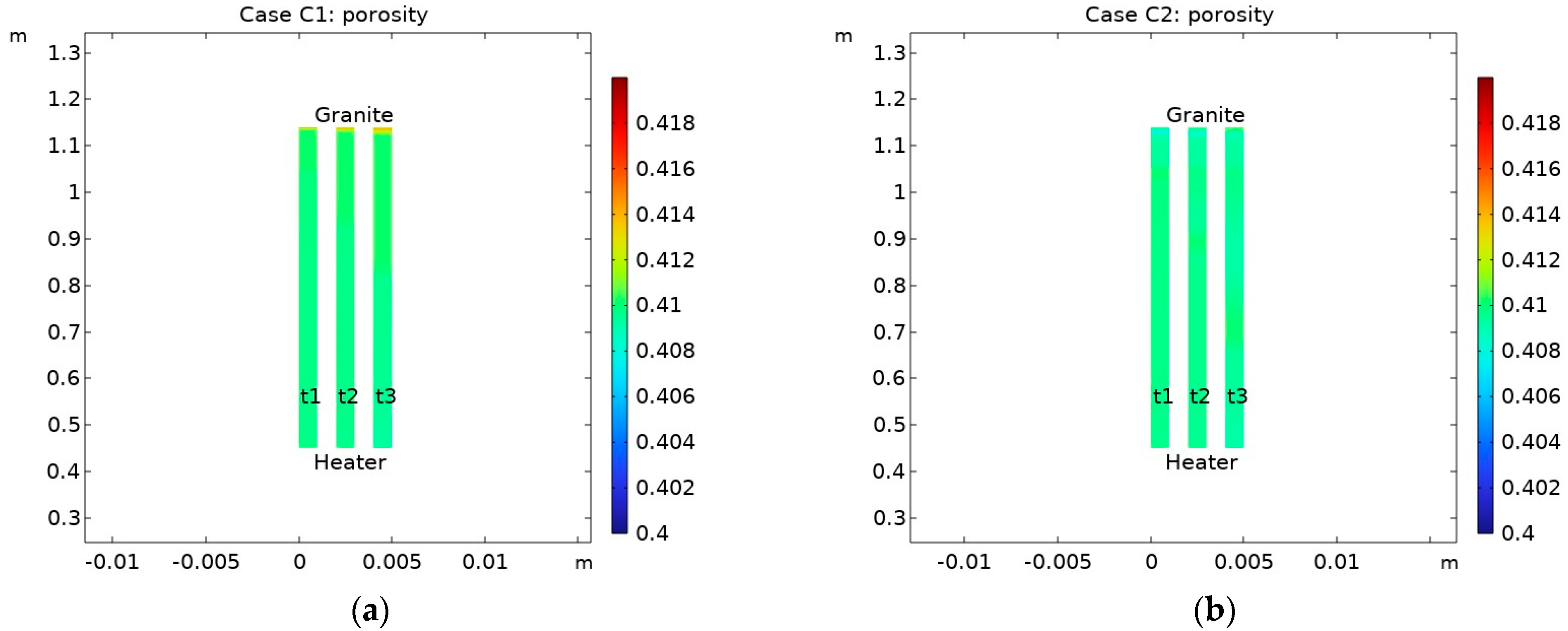
- Mineral dissolution/precipitation induced slight changes in the bentonite porosity. The most significant porosity increase was observed near the bentonite–granite interface (by ~3%) in Case B (THC without consideration of CO2 gas transport);
- Changes in porosity depend on the partial CO2 pressure at the bentonite–granite boundary. In the case of a low pCO2 at the bentonite–granite interface (Case C1), the calcite dissolution led to a slight porosity increase, while a higher pCO2 (Case C2) led to decreased porosity. In the latter case, the changes were observed at the larger distance from the bentonite–granite interface;
- Consideration of chemical processes taking place in the bentonite had no significant influence on non-reactive Cl transport for the considered period of time, but it would be important for other radionuclides whose sorption in porous media depends on porewater pH;
- CO2 de-gassing due to temperature-dependent solubility did not have a significant impact on porosity change;
- Different boundary conditions in terms of pCO2 at the bentonite–granite interface had a larger impact on the sulphate concentration compared to cation distribution in the bentonite barrier;
- Modelling results presented in this study could be useful for further interpretation of the FEBEX test measurement results before model application for large scale predictions;
- In the future, the modelling of the FEBEX in situ test considering more detailed experiment geometry and heating history is foreseen.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bennett, D.G.; Gens, R. Overview of European concepts for high-level waste and spent fuel disposal with special reference waste container corrosion. J. Nucl. Mater. 2008, 379, 1–8. [Google Scholar] [CrossRef]
- Zhao, X.; Qiang, S.; Wu, H.; Yang, Y.; Shao, D.; Fang, L.; Liang, J.; Li, P.; Fan, Q. Exploring the Sorption Mechanism of Ni(II) on Illite: Batch Sorption, Modelling, EXAFS and Extraction Investigations. Sci. Rep. 2017, 7, 8495. [Google Scholar] [CrossRef] [PubMed]
- Belousov, P.; Semenkova, A.; Egorova, T.; Romanchiuk, A.; Zakusin, S.; Dorzhieva, O.; Tyupina, E.; Izosimova, Y.; Tolpeshta, I.; Chernov, M.; et al. Cesium Sorption and Desorption on Glauconite, Bentonite, Zeolite, and Diatomite. Minerals 2019, 9, 625. [Google Scholar] [CrossRef]
- Thatcher, K.E.; Bond, A.E.; Norris, S. Pore pressure response to disposal of heat generating radioactive waste in a low permeability host rock. Int. J. Rock. Mech. Min. Sci. 2020, 135, 104456. [Google Scholar] [CrossRef]
- Bumbieler, F.; Plua, C.; Tourchi, S.; Vu, M.-N.; Vaunat, J.; Gens, A.; Armand, G. Feasibility of constructing a full-scale radioactive high-level waste disposal cell and characterization of its thermo-hydro-mechanical behavior. Int. J. Rock. Mech. Min. Sci. 2021, 137, 104555. [Google Scholar] [CrossRef]
- Müller, H.R.; Garitte, B.; Vogt, T.; Kohler, S.; Sakaki, T.; Weber, H.; Spillmann, T.; Herthrich, M.; Becker, J.K.; Giroud, N.; et al. Implementation of the full-scale emplacement (FE) experiment at the Mont Terri rock laboratory. Swiss J. Geosci. 2017, 110, 287–306. [Google Scholar] [CrossRef]
- Li, X.L.; Bastiaens, W.; Van Marcke, P.; Verstricht, J.; Chen, G.J.; Weetjens, W.; Sillen, X. Design and development of large-scale insitu PRACLAY heater test and horizontal high-level radioactive waste disposal gallery seal test in Belgian HADES. J. Rock. Mech. Geotech. Eng. 2010, 2, 103–110. [Google Scholar] [CrossRef]
- Grimsel Test Site. High Temperature Effects on Bentonite Buffers (HotBENT)—Aims & Objectives. Available online: https://www.grimsel.com/gts-projects/hotbent-high-temperature-effects-on-bentonite-buffers/hotbent-introduction (accessed on 8 March 2024).
- Sánchez, M.; Pomaro, B.; Gens, A. Coupled THM analysis of a full-scale test for high-level nuclear waste and spent fuel disposal under actual repository conditions during 18 years of operation. Géotechnique 2023, 73, 418–438. [Google Scholar] [CrossRef]
- Bosch, J.A.; Qiao, Y.; Ferrari, A.; Laloui, L. Thermo-hydro-mechanical analysis of the complete lifetime of the bentonite barrier in the FEBEX in-situ test. Geomech. Energy Environ. 2023, 34, 100472. [Google Scholar] [CrossRef]
- Zhang, L.; Samper, J.; Montenegro, L. A coupled THC model of the FEBEX in situ test with bentonite swelling and chemical and thermal osmosis. J. Contam. Hydrol. 2011, 126, 45–60. [Google Scholar] [CrossRef]
- Zheng, L.; Fernández, A.M. Prediction of Long-Term Geochemical Change in Bentonite Based on the Interpretative THMC Model of the FEBEX In Situ Test. Minerals 2023, 13, 1522. [Google Scholar] [CrossRef]
- Samper, J.; Mon, A.; Montenegro, L. A revisited thermal, hydrodynamic, chemical and mechanical model of compacted bentonite for the entire duration of the FEBEX in situ test. Appl. Clay Sci. 2018, 160, 58–70. [Google Scholar] [CrossRef]
- Zheng, L.; Xu, H.; Rutquist, J.; Reagan, M.; Birkholzer, J.; Villar, V.; Fernandez, A.M. The hydration of bentonite buffer material revealed by modeling analysis of a long-term in situ test. Appl. Clay Sci. 2020, 185, 105360. [Google Scholar] [CrossRef]
- Sedighi, M.; Thomas, H.R.; Vardon, P.J. Reactive transport of chemicals in compacted bentonite under non-isothermal water infiltration. J. Geotech. Geoenviron. Eng. 2018, 144, 04018075. [Google Scholar] [CrossRef]
- Samper, J.; Zheng, L.; Montenegro, L.; Fernandez, A.M.; Rivas, P. Coupled thermo-hydro-chemical models of compacted bentonite after FEBEX in situ test. Appl. Geochem. 2008, 23, 1186–1201. [Google Scholar] [CrossRef]
- Samper, J.; Mon, A.; Ahusborde, E.; Yu, H.; Narkuniene, A.; Hokr, M.; Montenegro, L.; Amaziane, B.; Ossmani, M.E.; Xu, T.; et al. Multiphase flow and reactive transport benchmark for radioactive waste disposal. Environ. Earth Sci. (under review).
- Nardi, A.; Idiart, A.; Trinchero, P.; de Vries, L.M.; Molinero, J. Interface COMSOL-PHREEQC (iCP), an efficient numerical framework for the solution of coupled Multiphysics and geochemistry. Comput. Geosci. 2014, 69, 10–21. [Google Scholar] [CrossRef]
- Huertas, F.; Farina, P.; Farias, J.; Garcia-Sineriz, J.L.; Villar, M.V.; Fernandez, A.M.; Martin, P.L.; Elorza, F.J.; Gens, A.; Sanchez, M.; et al. Full-Scale Engineered Barriers Experiment Updated Final Report 1994–2004; Tech. Publ 05-0/2006; Enresa: Madrid, Spain, 2006; p. 590. [Google Scholar]
- Villar, M.V.; Arnaud, D.; Besuelle, P.; Cernochova, K.; Collin, F.; Cuevas, J.; Cuss, R.; de Lesquen, C.; El Tabbal, G.; Graham, C.; et al. D7.2 HITEC. Updated State-of-the-Art on THM Behaviour of i) Buffer Clay Materials and of ii) Host Clay Materials; Deliverable D7.2 HITEC (2023); EURAD Project, Horizon: Bruxelles, Belgium, 2020; No 847593; p. 121. [Google Scholar]
- Bradbury, M.H.; Baeyens, B. Porewater chemistry in compacted re-saturated MX-80 bentonite. J. Contam. Hydrol. 2003, 61, 329–338. [Google Scholar] [CrossRef]
- Birgersson, M. A general framework for ion equilibrium calculations in compacted bentonite. Geochim. Cosmochim. Acta 2017, 200, 186–200. [Google Scholar] [CrossRef]
- Bradbury, M.H.; Baeyens, B. A mechanistic description of Ni and Zn sorption on Na–montmorillonite: Part II. Modelling. J. Contam. Hydrol. 1997, 27, 223–248. [Google Scholar] [CrossRef]
- Subsurface Flow Module. User’s Guide. Comsol Multiphysics. 2021. Available online: https://doc.comsol.com/6.1/doc/com.comsol.help.ssf/SubsurfaceFlowModuleUsersGuide.pdf (accessed on 20 March 2024).
- Parkhurst, D.L.; Appelo, C.A.J. User’s Guide to PHREEQC (Version 2): A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; USSG: Denver, CO, USA, 1999; 312p. [Google Scholar]
- Millington, R.J.; Quirk, J.M. Transport in Porous Media. Transp. Porous Media 1960, 49, 377–378. [Google Scholar] [CrossRef]
- Lasaga, A.C. Kinetic Theory in the Earth Sciences; Princeton Series in Geochemistry; Princeton University Press: Princeton, NJ, USA, 1998; 811p, ISBN 0-691-03748-5. [Google Scholar]
- NIST Chemistry WebBook, SRD 69. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C124389&Mask=10 (accessed on 21 March 2024).
- Navarro, V.; Asensio, L.; Gharbieh, H.; De la Morena, G.; Pulkkanen, V.M. Development of a multiphysics numerical solver for modeling the behavior of clay-based engineered barriers. Nucl. Eng. Technol. 2019, 51, 1047–1059. [Google Scholar] [CrossRef]
- Pollock, D.W. Simulation of fluid flow and energy transport processes associated with high-level radioactive waste disposal in unsaturated alluvium. Water Resour. Res. 1986, 22, 765–775. [Google Scholar] [CrossRef]
- Thermodynamic Database Andra—RWM—Ondraf Thermo_Chimie. Available online: www.thermochimie-tdb.com (accessed on 22 February 2023).
- Rodríguez, J.; Colàs, E.; Duro, L.; Fuller, A.J.; Harvey, L.; Thermo Chimie. Track-Changes and Track-Error Document: From Version 10d to Version 11. Technical Report TCIII-2022-07E_vs1. April 2022.. Available online: https://www.thermochimie-tdb.com/pages/version.php (accessed on 22 February 2023).






| Range | Mesh Size, m |
|---|---|
| 0.45 m–0.46 m | 0.000625 |
| 0.46 m–0.49 m | 0.0025 |
| 0.49 m–0.59 m | 0.005 |
| 0.59 m–1.00 m | 0.01 |
| 1.00 m–1.10 m | 0.005 |
| 1.10 m–1.13 m | 0.0025 |
| 1.13 m–1.14 m | 0.00625 |
| Cases | Transported Species | Geochemical Model |
|---|---|---|
| Case A | Na, Cl | Aqueous complexation, acid–base reactions |
| Case B | Na, Cl, C, Ca, S, Si, Mg, K | Aqueous complexation, acid–base reactions, mineral dissolution/precipitation, ion exchange, surface complexation |
| Case C1 | Na, Cl, C, Ca, S, Si, Mg, K, CO2 gas diffusion | Aqueous complexation, acid–base reactions, mineral dissolution/precipitation, ion exchange, surface complexation, fixed pCO2 at bentonite–granite boundary (log (pCO2) = −4) |
| Case C2 | Na, Cl, C, Ca, S, Si, Mg, K, CO2 gas diffusion | Consideration of mineral dissolution/precipitation, ion exchange, fixed pCO2 at bentonite–granite boundary (log (pCO2) = −1) |
| Parameter [13,18,29] | Value or Dependence |
|---|---|
| Initial porosity of bentonite | 0.41 |
| Solid density (kg/m3) (T in Celsius) | |
| Water density (kg/m3) (T in Celsius) | |
| Vapour and air density (kg/m3) (T in Celsius) | |
| Thermal properties [13,18,29] | |
| Thermal conductivity of the liquid (W/m °C) | 1.5 |
| Thermal conductivity of the air (W/m °C) | 2.6·10−2 |
| Thermal conductivity of the vapour (W/m °C) | 4.2·10−2 |
| Thermal conductivity of the solid (W/m °C) | 1.23 |
| Specific heat of the water (J/kg °C) | 4202 |
| Specific heat of the air (J/kg °C) | 1000 |
| Specific heat of the vapour (J/kg °C) | 1620 |
| Specific heat of the solid (J/kg °C) | 835.5 |
| Vaporisation enthalpy (J/kg) | 2.45·106 |
| Thermal compressibility of the water (°C−1) | 2.1·10−4 |
| Thermal compressibility of the solid (°C−1) | 2·10−5 |
| Hydraulic properties [13,18] | |
| Intrinsic permeability for liquid flow (m2) | = 3.75 10−21 m2 |
| Relative permeability to liquid | |
| Intrinsic permeability for gas flow (m2) | 5·10−10 |
| Relative permeability to gas (m2) | |
| Van Genuchten retention curve | |
| Liquid viscosity (kg/m∙s) (T in Celsius) | |
| Gas viscosity (kg/m∙s) | 1.76·10−5 |
| Vapour tortuosity factor | 0.10 |
| Henry’s constant for air (T in Celsius) | |
| Henry’s constant for CO2 at 25 °C (mol/(m3∙Pa)) | 3.4·10−4 |
| Transport-related properties [18,29] | |
| Molecular diffusion in water (m2/s) | = 25 °C |
| Longitudinal dispersivity (m) | 0.01 |
| Molecular diameter of the gases species (m) | 10−10 |
| Vapour diffusivity (m2/s) |
| Bentonite | Granite | |
|---|---|---|
| Initial porewater composition | ||
| pH | 7.72 | 8.35 |
| Na+ (mol/L) | 1.3 × 10−1 | 3.83 × 10−4 |
| K+ (mol/L) | 1.73 × 10−3 | 7.83 × 10−6 |
| Ca2+ (mol/L) | 2.23 × 10−2 | 1.83 × 10−4 |
| Mg2+ (mol/L) | 2.33 × 10−2 | 1.33 × 10−6 |
| HCO3− (mol/L) | 4.13 × 10−4 | 3.93 × 10−6 |
| SO42− (mol/L) | 3.23 × 10−2 | 7.93 × 10−5 |
| Cl− (mol/L) | 1.63 × 10−1 | 1.33 × 10−5 |
| SIO2(aq) (mol/L) | 1.13 × 10−6 | 1.43 × 10−4 |
| Initial volume fraction of the minerals (%) | ||
| Calcite | 1 | 5 |
| Quartz | 4.5 | 20 |
| Anhydrite | 0 | 0 |
| Gypsum | 0.016 | 0 |
| Halite | 0 | 0 |
| Initial ion exchange concentration meq/100 g bentonite | ||
| Na+ | 21.10 | - |
| K+ | 1.94 | - |
| Ca2+ | 31.31 | - |
| Mg2+ | 41.41 | - |
| Total concentration of surface complexation sites (mol/kg) | ||
| 2 × 10−3 | - | |
| 4 × 10−3 | - | |
| 4 × 10−3 | - |
| Selectivity Constant KNa-cation [13] | Log K | |
|---|---|---|
| Cation exchange reactions on montmorillonite | ||
| Na+ + X-K ⇔ K+ + X-Na | 0.138 | |
| Na+ + 0.5 X2-Ca ⇔ 0.5 Ca2+ + X-Na | 0.294 | |
| Na+ + 0.5 X2-Mg ⇔ 0.5 Mg2+ + X-Na | 0.288 | |
| Or expressed with half reactions: | ||
| X− = X− | 0.0 | |
| K+ + X− = KX | 0.86 | |
| Ca2+ + 2X− = CaX2 | 1.064 | |
| Mg2+ + 2X− = MgX2 | 1.082 | |
| Surface complexation | [23] | |
| −4.5 | ||
| 7.9 | ||
| −4.5 | ||
| 7.9 | ||
| −6.0 | ||
| −10.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narkuniene, A.; Grigaliuniene, D.; Poskas, G. THC Modelling of Bentonite Barrier of Geological Repository in Granite and Its Impact on Long-Term Safety. Appl. Sci. 2024, 14, 7851. https://doi.org/10.3390/app14177851
Narkuniene A, Grigaliuniene D, Poskas G. THC Modelling of Bentonite Barrier of Geological Repository in Granite and Its Impact on Long-Term Safety. Applied Sciences. 2024; 14(17):7851. https://doi.org/10.3390/app14177851
Chicago/Turabian StyleNarkuniene, Asta, Dalia Grigaliuniene, and Gintautas Poskas. 2024. "THC Modelling of Bentonite Barrier of Geological Repository in Granite and Its Impact on Long-Term Safety" Applied Sciences 14, no. 17: 7851. https://doi.org/10.3390/app14177851
APA StyleNarkuniene, A., Grigaliuniene, D., & Poskas, G. (2024). THC Modelling of Bentonite Barrier of Geological Repository in Granite and Its Impact on Long-Term Safety. Applied Sciences, 14(17), 7851. https://doi.org/10.3390/app14177851






