Exploring the Influence of Object, Subject, and Context on Aesthetic Evaluation through Computational Aesthetics and Neuroaesthetics
Abstract
1. Introduction
2. Materials and Methods
2.1. Features
2.1.1. Composition
2.1.2. Tone
2.1.3. Global Texture Features
2.1.4. Local Texture Features
- Gray Level Co-occurrence Matrix (GLCM);
- Local Binary Pattern (LBP);
2.2. Stimulus
2.3. EEG and ERP
2.4. Participants
2.5. Apparatus
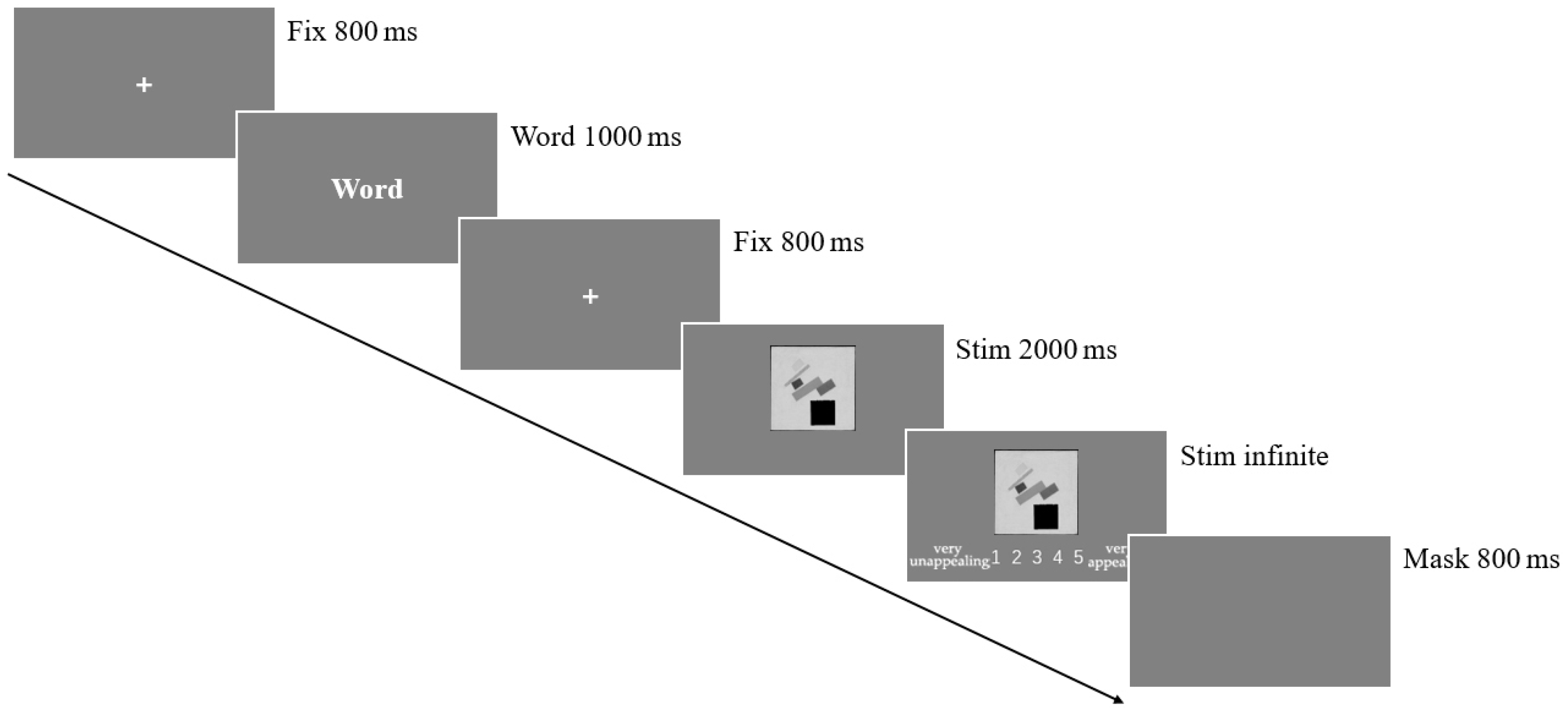
2.6. Procedure
2.7. EEG Recordings and Data Analysis
3. Results
3.1. Behavioral Data
3.2. EEG Data
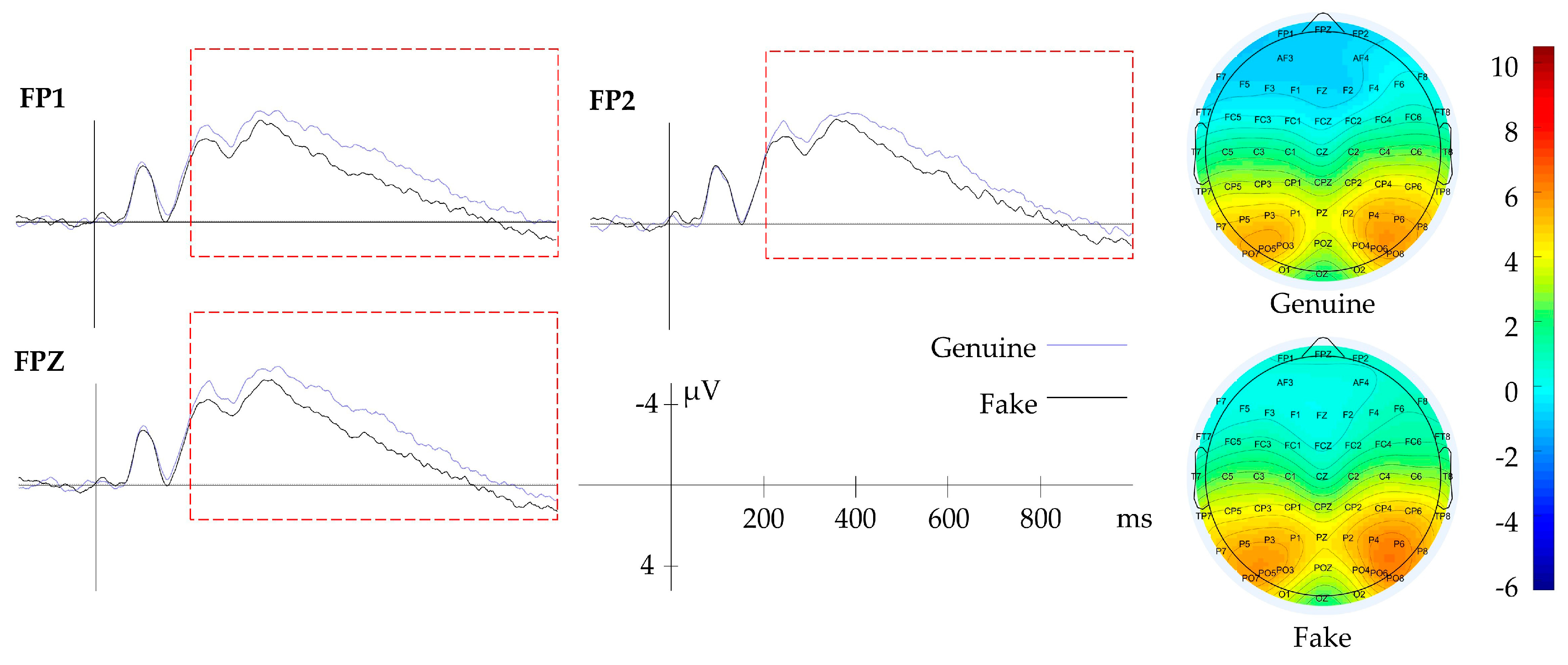
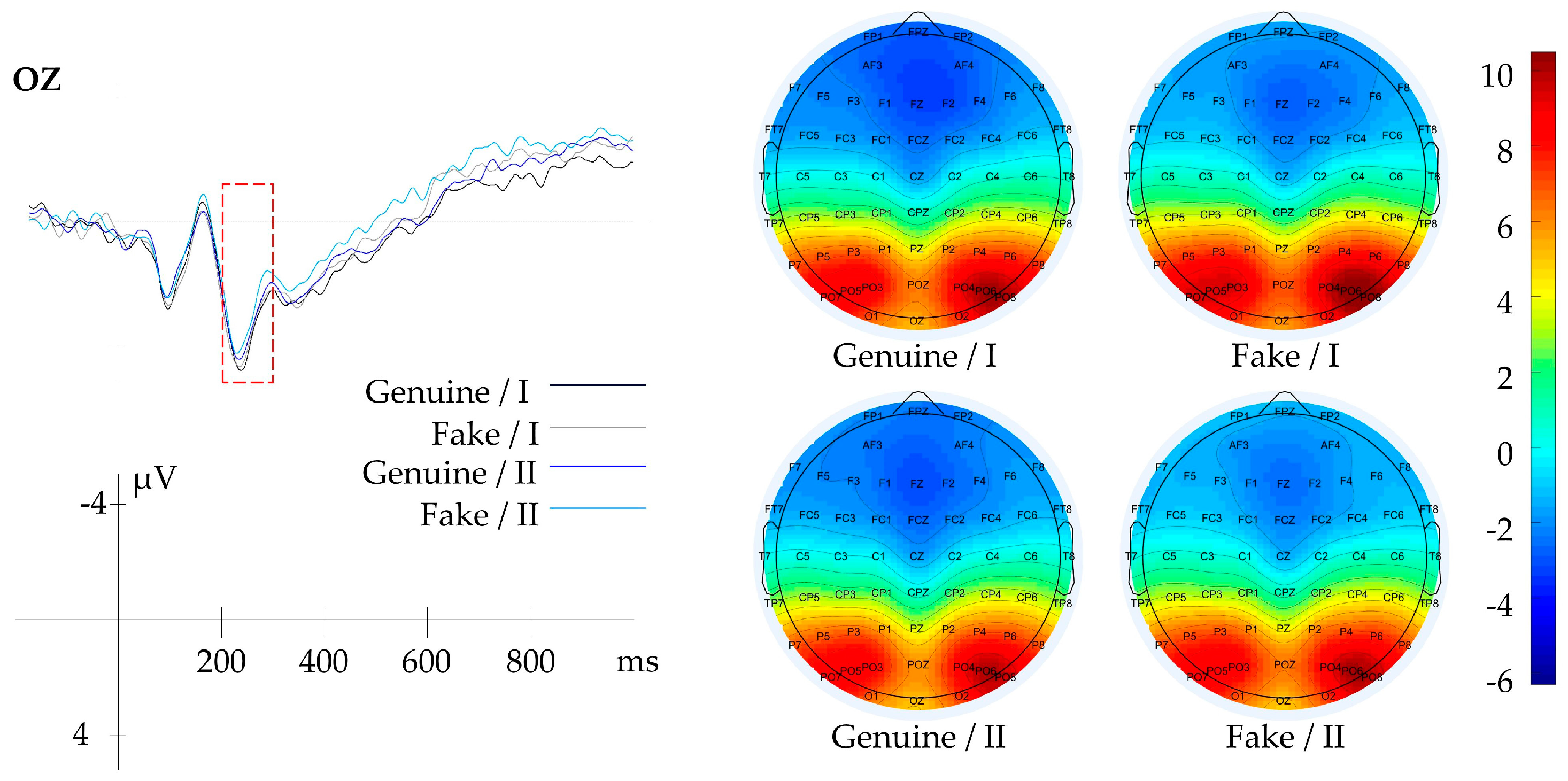
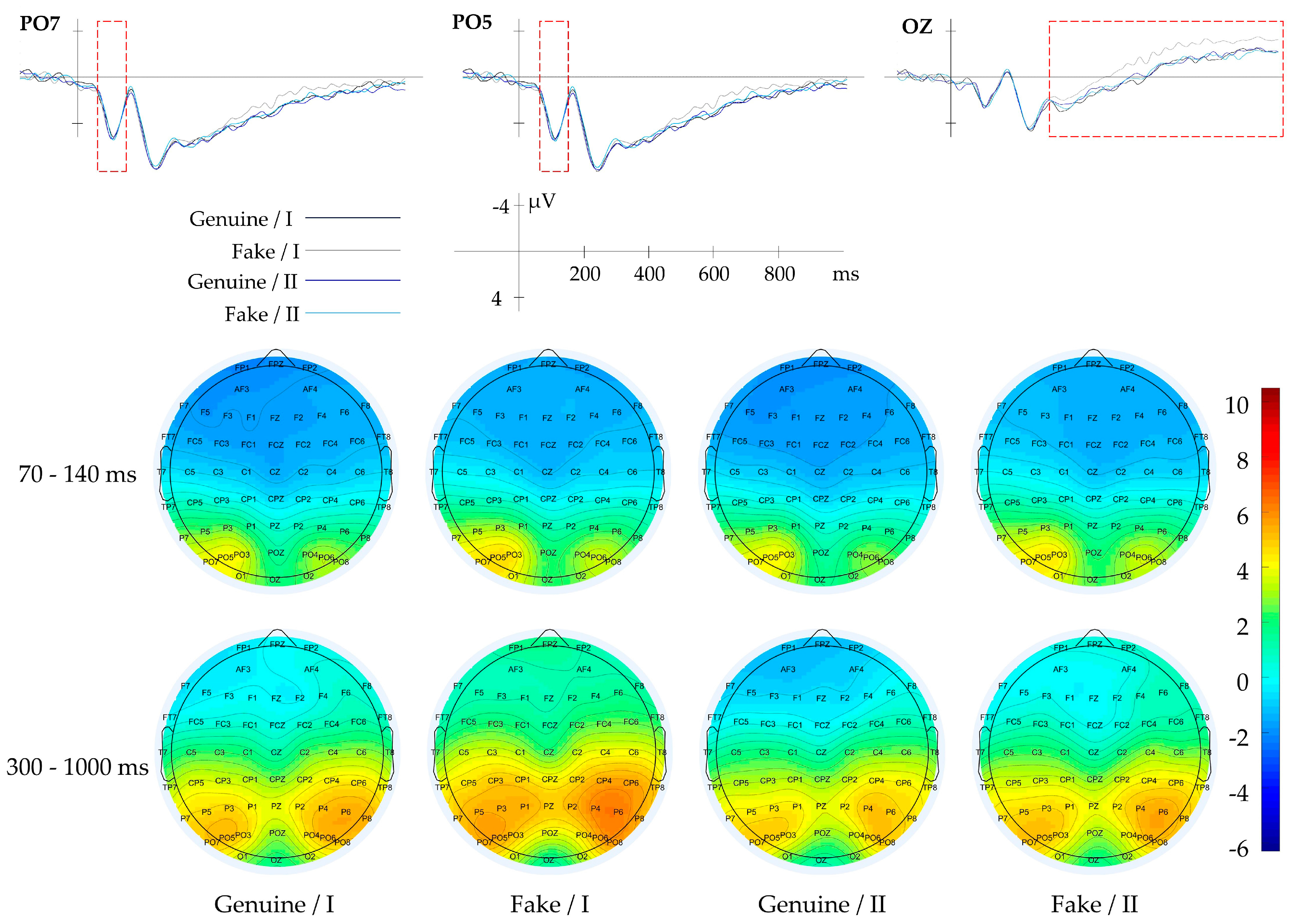
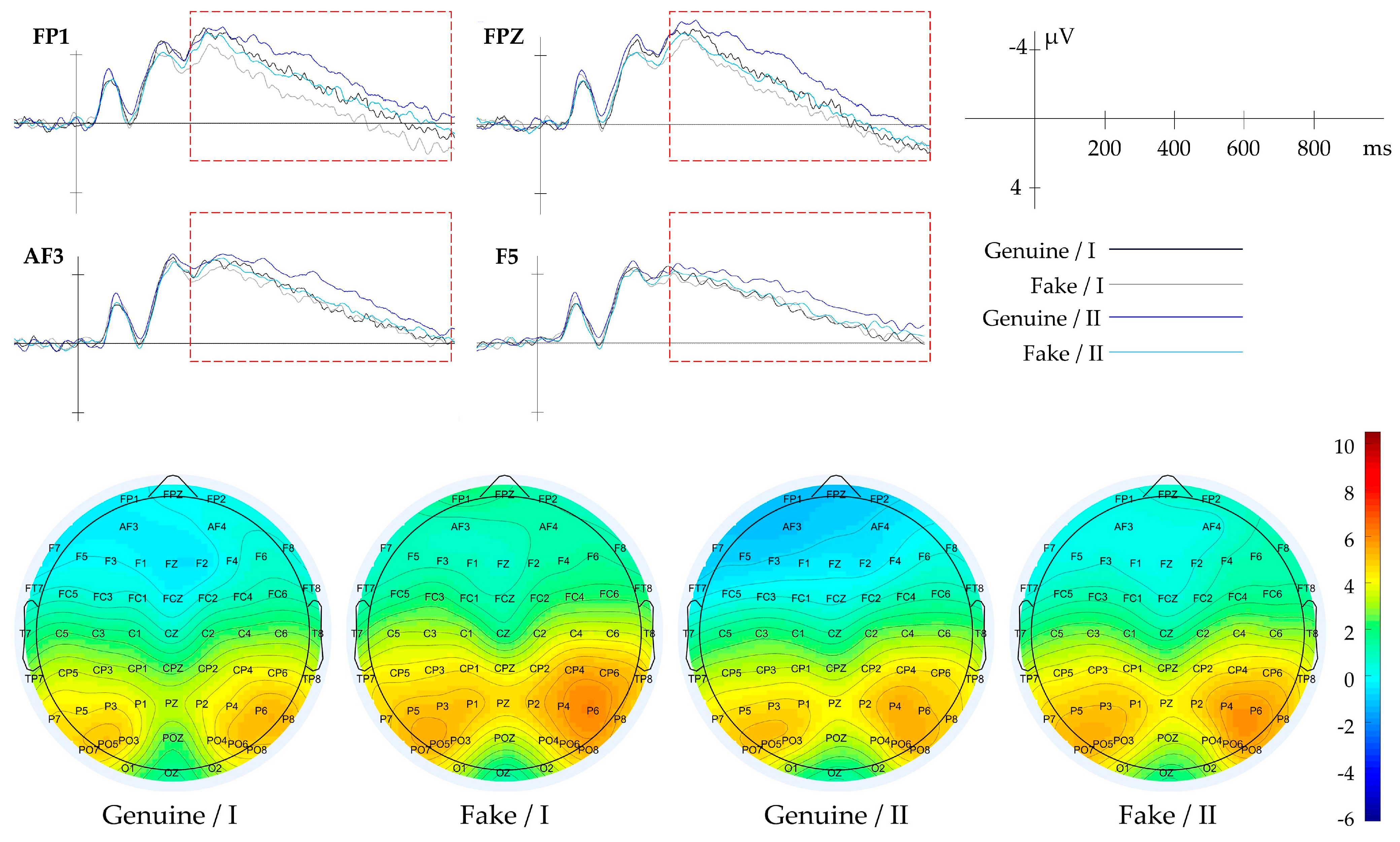
3.2.1. Result of Context
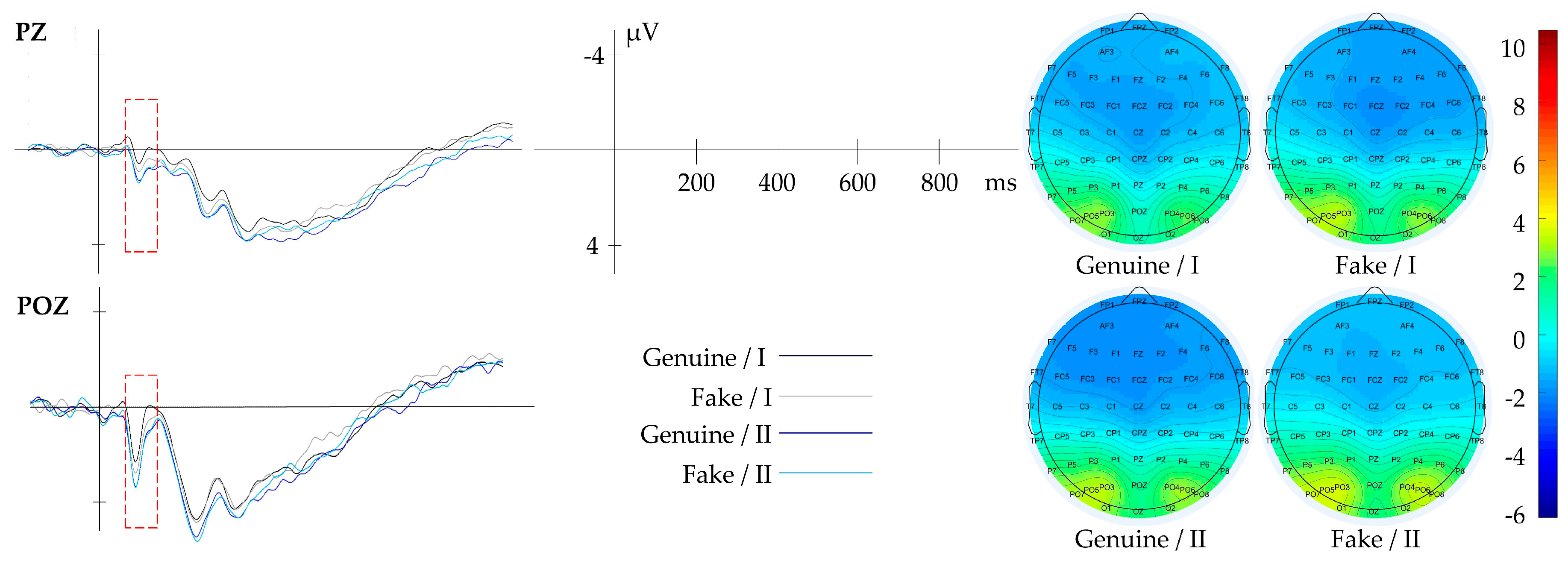
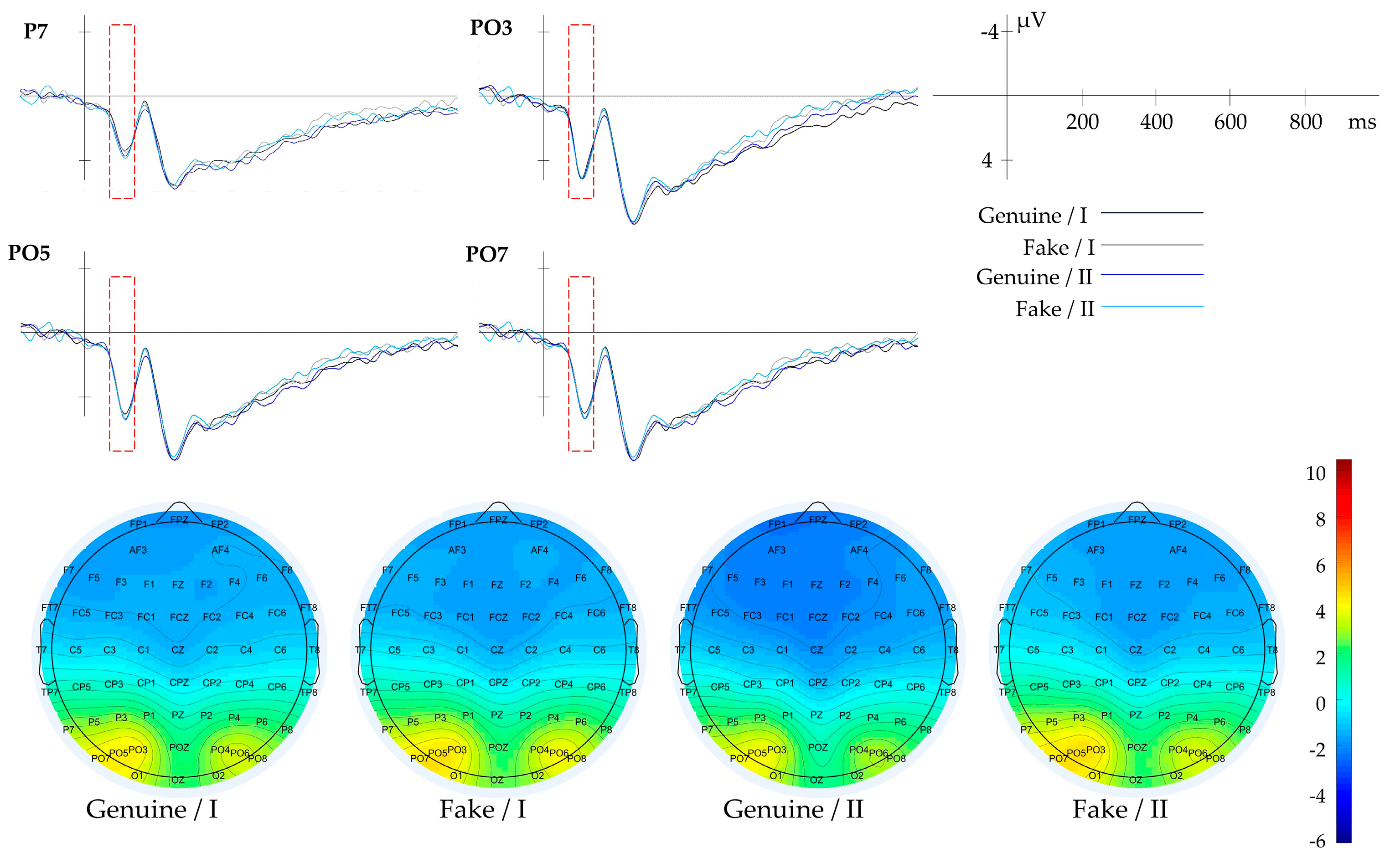
3.2.2. Result of Composition
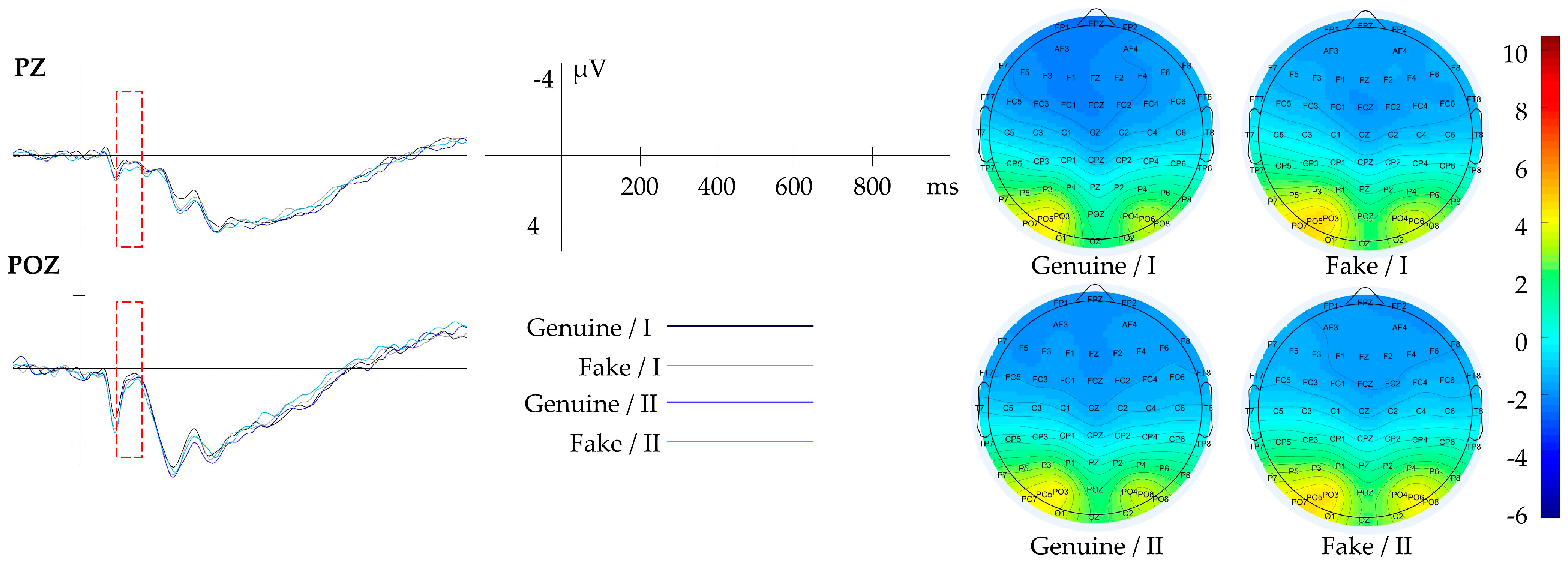
3.2.3. Result of Tone
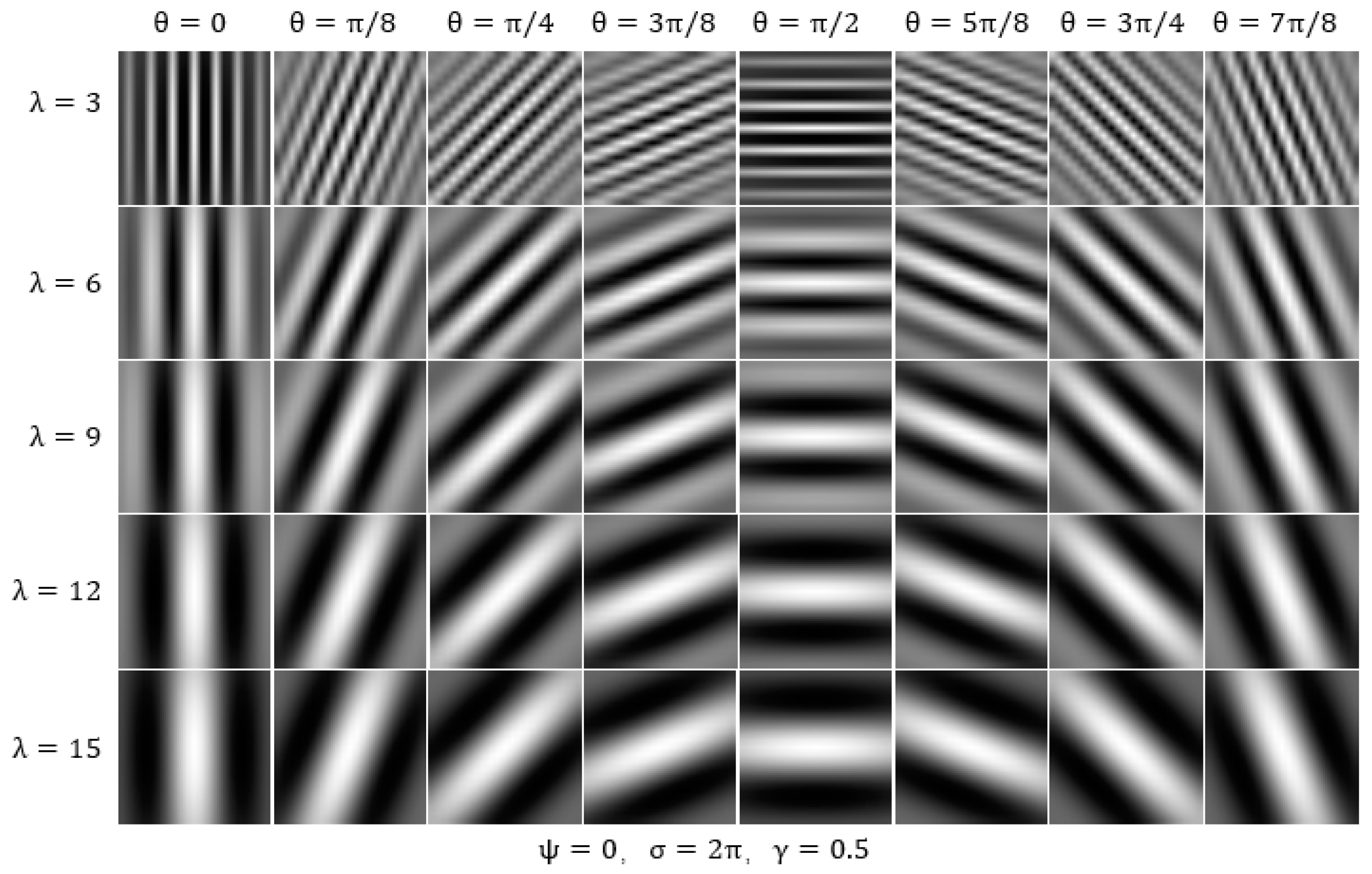
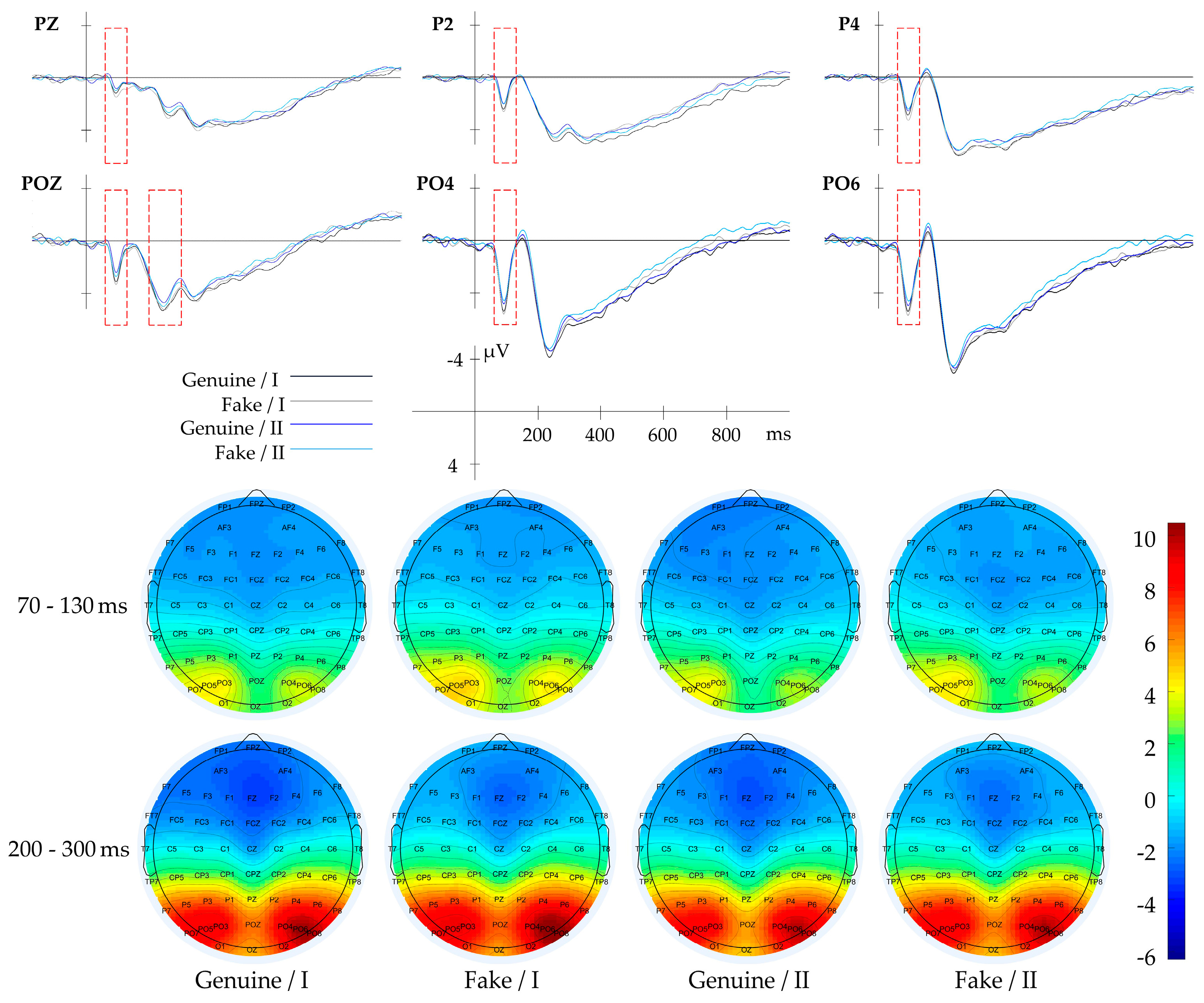
3.2.4. Result of Gabor-Mean
3.2.5. Result of Gabor-Variance
3.2.6. Result of Gabor-Energy
3.2.7. Result of Horizontal GLCM (θ = 0°)
3.2.8. Result of Diagonal GLCM (θ = 135°)
3.2.9. Result of LBP
4. Machine Learning Model
4.1. Dataset Construction
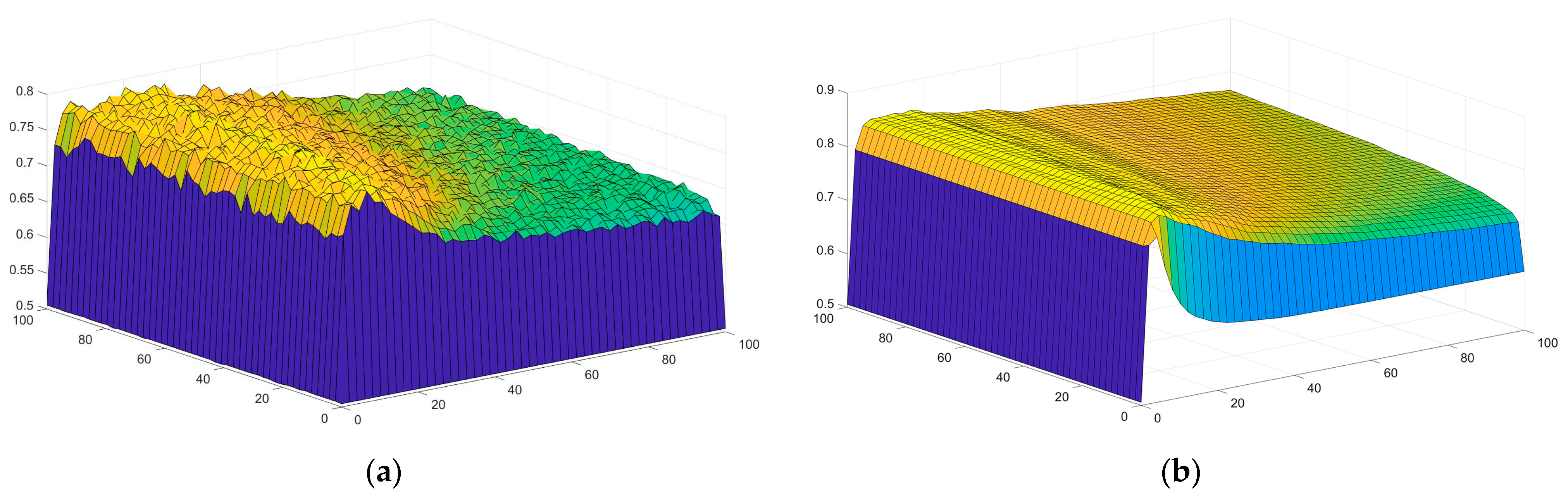
4.2. Parameter Determination
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Image Counts and Corresponding Parameter Values by Feature Classification (Composition, Tone, GLCM, LBP)
| Features | Class | Parameter | Mean | SD |
| Composition (Blank Space) | I | Mean | 0.769 | 0.066 |
| II | Mean | 0.573 | 0.059 | |
| Tone (Gray Histogram) | I | Mean | 108.774 | 31.488 |
| Variance | 5060.803 | 2820.122 | ||
| Skewness | 0.613 | 1.245 | ||
| Kurtosis | 0.774 | 5.617 | ||
| Energy | 4909.412 | 3298.131 | ||
| II | Mean | 179.912 | 25.290 | |
| Variance | 3306.247 | 1735.233 | ||
| Skewness | −1.676 | 1.167 | ||
| Kurtosis | 3.239 | 7.356 | ||
| Energy | 10,016.353 | 13,510.457 | ||
| Horizontal GLCM (θ = 0°) | I | Contrast | 60,369.94 | 10,198.14 |
| Energy | 72.746 | 7.304 | ||
| Entropy | 783.655 | 59.472 | ||
| II | Contrast | 3131.599 | 272.695 | |
| Energy | 6.365 | 0.431 | ||
| Entropy | 95.516 | 5.242 | ||
| Diagonal GLCM (θ = 135°) | I | Contrast | 115,494.1 | 15,748.78 |
| Energy | 88.365 | 8.009 | ||
| Entropy | 960.066 | 69.589 | ||
| II | Contrast | 7635.667 | 744.169 | |
| Energy | 7.038 | 0.468 | ||
| Entropy | 136.389 | 9.833 | ||
| LBP | I | Mean | 141.761 | 0.702 |
| Variance | 8982.159 | 23.793 | ||
| Skewness | −0.203 | 0.010 | ||
| Kurtosis | −1.530 | 0.005 | ||
| Energy | 29,133.405 | 200.916 | ||
| II | Mean | 170.961 | 1.740 | |
| Variance | 8333.661 | 85.958 | ||
| Skewness | −0.708 | 0.039 | ||
| Kurtosis | −0.895 | 0.095 | ||
| Energy | 16,563.702 | 439.355 |
Appendix B. Image Counts and Corresponding Parameter Values by Feature Classification (Gabor Filter)
| Features | Gabor-Mean | Gabor-Variance | Gabor-Energy | ||||||||||
| Class I | Class II | Class I | Class II | Class I | Class II | ||||||||
| λ | θ | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| 3 | 0 | 60.846 | 1.669 | 80.74 | 0.877 | 48.917 | 1.16 | 63.373 | 1.441 | 188.951 | 2.346 | 225.676 | 1.584 |
| 3 | π/8 | 71.264 | 2.009 | 104.904 | 1.247 | 44.264 | 1.193 | 57.174 | 1.165 | 209.145 | 2.047 | 239.915 | 1.021 |
| 3 | π/4 | 63.754 | 1.818 | 93.328 | 1.096 | 42.98 | 1.36 | 53.967 | 1.324 | 203.857 | 2.338 | 236.162 | 1.275 |
| 3 | 3π/8 | 71.366 | 1.996 | 104.918 | 1.270 | 44.871 | 1.208 | 57.028 | 1.101 | 209.131 | 2.063 | 239.566 | 0.999 |
| 3 | π/2 | 60.207 | 1.643 | 79.887 | 0.926 | 47.051 | 1.207 | 61.67 | 1.397 | 189.794 | 2.349 | 226.706 | 1.584 |
| 3 | 5π/8 | 71.118 | 1.984 | 104.798 | 1.266 | 44.118 | 1.227 | 56.482 | 1.106 | 209.672 | 2.102 | 240.196 | 0.987 |
| 3 | 3π/4 | 63.725 | 1.801 | 93.186 | 1.101 | 42.672 | 1.359 | 53.745 | 1.305 | 204.042 | 2.36 | 236.513 | 1.256 |
| 3 | 7π/8 | 71.324 | 1.979 | 104.947 | 1.26 | 44.414 | 1.2 | 57.431 | 1.094 | 209.228 | 2.049 | 239.901 | 0.997 |
| 6 | 0 | 7.041 | 0.527 | 2.328 | 0.199 | 19.378 | 0.975 | 36.913 | 1.481 | 8.703 | 0.602 | 2.509 | 0.203 |
| 6 | π/8 | 159.754 | 3.338 | 221.452 | 1.543 | 65.213 | 1.419 | 89.161 | 0.945 | 225.574 | 1.313 | 245.49 | 0.453 |
| 6 | π/4 | 175.236 | 3.273 | 230.479 | 1.223 | 57.481 | 1.541 | 83.868 | 1.03 | 233.441 | 1.208 | 248.162 | 0.41 |
| 6 | 3π/8 | 159.909 | 3.326 | 221.975 | 1.489 | 64.704 | 1.464 | 88.848 | 0.899 | 226.129 | 1.305 | 245.717 | 0.475 |
| 6 | π/2 | 6.448 | 0.483 | 1.678 | 0.143 | 16.578 | 0.886 | 35.335 | 1.331 | 7.877 | 0.552 | 1.903 | 0.16 |
| 6 | 5π/8 | 160.510 | 3.360 | 222.533 | 1.509 | 63.498 | 1.452 | 88.276 | 0.91 | 226.904 | 1.34 | 246.584 | 0.433 |
| 6 | 3π/4 | 175.775 | 3.306 | 230.954 | 1.230 | 56.498 | 1.482 | 83.439 | 1.048 | 233.823 | 1.22 | 248.884 | 0.353 |
| 6 | 7π/8 | 160.137 | 3.360 | 221.575 | 1.531 | 65.202 | 1.39 | 88.947 | 0.926 | 225.717 | 1.334 | 245.558 | 0.441 |
| 9 | 0 | 227.109 | 1.804 | 247.031 | 0.458 | 38.131 | 1.147 | 66.127 | 1.566 | 238.703 | 0.926 | 249.563 | 0.3 |
| 9 | π/8 | 13.813 | 0.708 | 6.5 | 0.347 | 32.714 | 0.922 | 55.135 | 1.089 | 19.708 | 0.807 | 7.066 | 0.34 |
| 9 | π/4 | 219.355 | 2.305 | 246.05 | 0.581 | 37.098 | 1.383 | 67.793 | 1.365 | 241.259 | 0.902 | 250.753 | 0.304 |
| 9 | 3π/8 | 13.679 | 0.722 | 6.055 | 0.343 | 31.914 | 1.038 | 54.132 | 1.163 | 18.918 | 0.809 | 6.808 | 0.372 |
| 9 | π/2 | 228.327 | 1.752 | 248.33 | 0.396 | 34.332 | 1.171 | 64.452 | 1.473 | 240.072 | 0.858 | 250.656 | 0.265 |
| 9 | 5π/8 | 12.660 | 0.712 | 5.391 | 0.314 | 29.388 | 0.889 | 52.421 | 1.118 | 18.194 | 0.813 | 5.901 | 0.303 |
| 9 | 3π/4 | 220.105 | 2.3465 | 246.49 | 0.566 | 35.687 | 1.277 | 66.999 | 1.372 | 241.749 | 0.917 | 251.293 | 0.259 |
| 9 | 7π/8 | 13.887 | 0.739 | 6.581 | 0.342 | 33.252 | 0.921 | 55.093 | 1.127 | 19.683 | 0.858 | 7.124 | 0.326 |
| 12 | 0 | 10.766 | 0.633 | 4.053 | 0.236 | 27.941 | 0.925 | 48.489 | 1.302 | 12.15 | 0.673 | 4.069 | 0.229 |
| 12 | π/8 | 16.008 | 0.787 | 7.124 | 0.342 | 36.547 | 0.946 | 59.815 | 1.162 | 19.566 | 0.826 | 7.116 | 0.32 |
| 12 | π/4 | 241.567 | 1.385 | 250.487 | 0.346 | 26.42 | 1.118 | 48.929 | 1.526 | 246.509 | 0.611 | 251.899 | 0.236 |
| 12 | 3π/8 | 14.901 | 0.774 | 6.42 | 0.332 | 34.564 | 0.971 | 57.421 | 1.213 | 18.097 | 0.822 | 6.668 | 0.334 |
| 12 | π/2 | 9.820 | 0.589 | 3.202 | 0.200 | 24.567 | 0.931 | 46.261 | 1.208 | 10.97 | 0.62 | 3.291 | 0.211 |
| 12 | 5π/8 | 14.085 | 0.770 | 5.674 | 0.308 | 32.073 | 0.86 | 56.061 | 1.181 | 17.412 | 0.819 | 5.726 | 0.275 |
| 12 | 3π/4 | 242.164 | 1.386 | 250.926 | 0.325 | 24.369 | 0.967 | 47.861 | 1.561 | 246.948 | 0.615 | 252.455 | 0.189 |
| 12 | 7π/8 | 15.648 | 0.808 | 7.137 | 0.328 | 36.724 | 0.912 | 59.076 | 1.216 | 19.129 | 0.858 | 7.114 | 0.296 |
| 15 | 0 | 39.947 | 1.420 | 26.953 | 0.998 | 69.6 | 1.166 | 89.118 | 1.119 | 54.754 | 1.404 | 30.268 | 1.034 |
| 15 | π/8 | 226.967 | 1.642 | 244.075 | 0.502 | 45.802 | 1.016 | 74.387 | 1.257 | 232.332 | 1.016 | 246.376 | 0.373 |
| 15 | π/4 | 248.298 | 0.978 | 252.998 | 0.208 | 16.308 | 1.008 | 33.632 | 1.602 | 250.527 | 0.443 | 253.465 | 0.156 |
| 15 | 3π/8 | 228.557 | 1.579 | 245.44 | 0.487 | 42.801 | 1.111 | 71.819 | 1.3 | 234.201 | 0.951 | 247.353 | 0.38 |
| 15 | π/2 | 37.557 | 1.419 | 24.238 | 0.957 | 65.499 | 1.305 | 86.464 | 1.166 | 52.398 | 1.363 | 27.638 | 1.024 |
| 15 | 5π/8 | 229.281 | 1.585 | 245.979 | 0.474 | 41.009 | 1.037 | 71.066 | 1.304 | 234.8 | 0.958 | 248.074 | 0.333 |
| 15 | 3π/4 | 248.6452 | 0.968 | 253.244 | 0.192 | 14.363 | 0.868 | 32.833 | 1.622 | 250.759 | 0.437 | 253.803 | 0.131 |
| 15 | 7π/8 | 227.472 | 1.653 | 244.578 | 0.5 | 44.604 | 0.995 | 73.665 | 1.309 | 232.715 | 1.043 | 246.833 | 0.348 |
Appendix C. Table of Names and Sources of Experimental Stimuli
References
- Baumgarten, A.G. Aesthetica; Impens Ioannis Christiani Kleyb: 1763. Available online: https://books.google.com.sg/books?id=wMfu3eOBiVEC&pg (accessed on 4 March 2024).
- Zeki, S. Inner Vision: An Exploration of Art and the Brain; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Leder, H.; Belke, B.; Oeberst, A.; Augustin, D. A model of aesthetic appreciation and aesthetic judgments. Br. J. Psychol. 2004, 95, 489–508. [Google Scholar] [CrossRef] [PubMed]
- Leder, H.; Nadal, M. Ten years of a model of aesthetic appreciation and aesthetic judgments: The aesthetic episode—Developments and challenges in empirical aesthetics. Br. J. Psychol. 2014, 105, 443–464. [Google Scholar] [CrossRef] [PubMed]
- Guy, M.W.; Reynolds, G.D.; Mosteller, S.M.; Dixon, K.C. The effects of stimulus symmetry on hierarchical processing in infancy. Dev. Psychobiol. 2017, 59, 279–290. [Google Scholar] [CrossRef]
- Jacobsen, T.; Höfel, L. Aesthetics Electrified: An Analysis of Descriptive Symmetry and Evaluative Aesthetic Judgment Processes Using Event-Related Brain Potentials. Empir. Stud. Arts 2001, 19, 177–190. [Google Scholar] [CrossRef]
- Leder, H.; Tinio, P.P.L.; Brieber, D.; Kröner, T.; Jacobsen, T.; Rosenberg, R. Symmetry Is Not a Universal Law of Beauty. Empir. Stud. Arts 2019, 37, 104–114. [Google Scholar] [CrossRef]
- van Paasschen, J.; Bacci, F.; Melcher, D.P.; Brattico, E. The Influence of Art Expertise and Training on Emotion and Preference Ratings for Representational and Abstract Artworks. PLoS ONE 2015, 10, e0134241. [Google Scholar] [CrossRef]
- Zeki, S.; Chén, O.Y.; Romaya, J.P. The Biological Basis of Mathematical Beauty. Front. Hum. Neurosci. 2018, 12, 467. [Google Scholar] [CrossRef]
- Kirk, U.; Skov, M.; Hulme, O.; Christensen, M.S.; Zeki, S. Modulation of aesthetic value by semantic context: An fMRI study. Neuroimage 2009, 44, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Gonzalez, V.; East, S.; Garbutt, M.; Spehar, B. Viewing Art in Different Contexts. Front. Psychol. 2020, 11, 569. [Google Scholar] [CrossRef] [PubMed]
- Birkhoff, G.D. Aesthetic Measure; Harvard University Press: Cambridge, MA, USA, 1933. [Google Scholar]
- Liu, Y. Engineering aesthetics and aesthetic ergonomics: Theoretical foundations and a dual-process research methodology. Ergonomics 2003, 46, 1273–1292. [Google Scholar] [CrossRef]
- Perc, M. Beauty in artistic expressions through the eyes of networks and physics. J. R. Soc. Interface 2020, 17, 20190686. [Google Scholar] [CrossRef] [PubMed]
- Hoenig, F. Defining Computational Aesthetics, Computational Aesthetics in Graphics. Vis. Imaging 2005, 2005, 13–18. [Google Scholar]
- Haralick, R.M. Statistical and Structural Approaches to Texture. Proc. IEEE 1979, 67, 786–804. [Google Scholar] [CrossRef]
- Falomir, Z.; Museros, L.; Sanz, I.; Gonzalez-Abril, L. Categorizing paintings in art styles based on qualitative color descriptors, quantitative global features and machine learning (QArt-Learn). Expert Syst. Appl. 2018, 97, 83–94. [Google Scholar] [CrossRef]
- Sandoval, C.; Pirogova, E.; Lech, M. Two-stage deep learning approach to the classification of fine-art paintings. IEEE Access 2019, 7, 41770–41781. [Google Scholar] [CrossRef]
- Zhong, S.; Huang, X.; Xiao, Z. Fine-art painting classification via two-channel dual path networks. Int. J. Mach. Learn. Cybern. 2020, 11, 137–152. [Google Scholar] [CrossRef]
- Baraldi, L.; Cornia, M.; Grana, C.; Cucchiara, R. Aligning Text and Document Illustrations: Towards Visually Explainable Digital Humanities, In Proceedings of the 2018 24th International Conference on Pattern Recognition (ICPR), Beijing, China, 20–24 August 2018.
- Tan, W.; Wang, J.; Wang, Y.; Lewis, M.; Jarrold, W. CNN Models for Classifying Emotions Evoked by Paintings; Technical Report; SVL Lab, Stanford University: Stanford, CA, USA, 2018. [Google Scholar]
- Yanulevskaya, V.; Uijlings, J.; Bruni, E.; Sartori, A.; Zamboni, E.; Bacci, F.; Melcher, D.; Sebe, N. In the eye of the beholder: Employing statistical analysis and eye tracking for analyzing abstract paintings, In Proceedings of the 20th ACM International Conference on Multimedia, Nara, Japen, 29 October–2 November 2012.
- Markovic, S. Perceptual, Semantic and Affective Dimensions of Experience of Abstract and Representational Paintings. Psihologija 2011, 44, 191–210. [Google Scholar] [CrossRef]
- Navon, D. The forest revisited: More on global precedence. Psychol. Res. 1981, 43, 1–32. [Google Scholar] [CrossRef]
- Oliva, A.; Torralba, A. Building the gist of a scene: The role of global image features in recognition. Prog. Brain Res. 2006, 155, 23. [Google Scholar] [PubMed]
- Navon, D. Forest before trees: The precedence of global features in visual perception. Cogn. Psychol. 1977, 9, 353–383. [Google Scholar] [CrossRef]
- Schütz, A.C.; Braun, D.I.; Gegenfurtner, K.R. Eye movements and perception: A selective review. J. Vis. 2011, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Love, B.C.; Rouder, J.N.; Wisniewski, E.J. A structural account of global and local processing. Cogn. Psychol. 1999, 38, 291–316. [Google Scholar] [CrossRef] [PubMed]
- Sharvashidze, N.; Schutz, A.C. Task-Dependent Eye-Movement Patterns in Viewing Art. J. Eye Mov. Res. 2020, 13, 1–17. [Google Scholar] [CrossRef]
- Park, S.; Wiliams, L.; Chamberlain, R. Global Saccadic Eye Movements Characterise Artists’ Visual Attention While Drawing. Empir. Stud. Arts 2022, 40, 228–244. [Google Scholar] [CrossRef]
- Koide, N.; Kubo, T.; Nishida, S.; Shibata, T.; Ikeda, K. Art expertise reduces influence of visual salience on fixation in viewing abstract-paintings. PLoS ONE 2015, 10, e0117696. [Google Scholar] [CrossRef]
- Soxibov, R. Composition and Its Application in Painting. Sci. Innov. 2023, 2, 108–113. [Google Scholar]
- Lelievre, P.; Neri, P. A deep-learning framework for human perception of abstract art composition. J. Vis. 2021, 21, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Shen, J.; Yue, M.; Ma, Y.; Wu, S. A Computational Study of Empty Space Ratios in Chinese Landscape Painting, 618–2011. Leonardo 2022, 55, 43–47. [Google Scholar] [CrossRef]
- Fan, Z.; Zhang, K.; Zheng, X.S. Evaluation and Analysis of White Space in Wu Guanzhong’s Chinese Paintings. Leonardo 2019, 52, 111–116. [Google Scholar] [CrossRef]
- Mollica, P. Color Theory: An Essential Guide to Color-From Basic Principles to Practical Applications; Walter Foster: Irvine, CA, USA, 2013; p. 17. [Google Scholar]
- Zhai, Q.; Luo, M.R.; Liu, X.Y. The impact of illuminance and colour temperature on viewing fine art paintings under LED lighting. Lighting Res. Technol. 2015, 47, 795–809. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Z.; Zhang, M.; Qin, X.; Liu, T. Exploring the Influence of the Illumination and Painting Tone of Art Galleries on Visual Comfort. Photonics 2022, 9, 981. [Google Scholar] [CrossRef]
- Haralick, R.M.; Shanmugam, K.; Dinstein, I. Textural Features for Image Classification. IEEE Trans. Syst. Man Cybern. 1973, SMC-3, 610–621. [Google Scholar] [CrossRef]
- Ojala, T.; Pietikainen, M.; Maenpaa, T. Multiresolution gray-scale and rotation invariant texture classification with local binary patterns. IEEE Trans. Pattern Anal. Mach. Intell. 2002, 24, 971–987. [Google Scholar] [CrossRef]
- Hammouda, K.; Jernigan, E. Texture segmentation using gabor filters. Cent. Intell. Mach 2000, 2, 64–71. [Google Scholar]
- Fan, Z.; Li, Y.; Zhang, K.; Yu, J.; Huang, M.L. Measuring and Evaluating the Visual Complexity Of Chinese Ink Paintings. Comput. J. 2022, 65, 1964–1976. [Google Scholar] [CrossRef]
- Finkel, R.A.; Bentley, J.L. Quad trees a data structure for retrieval on composite keys. Acta Inform. 1974, 4, 1–9. [Google Scholar] [CrossRef]
- Berger, H. Über das elektroenkephalogramm des menschen. Arch. Psychiatr. Nervenkrankh. 1929, 87, 527–570. [Google Scholar] [CrossRef]
- Walter, W.G.; Cooper, R.; Aldridge, V.J.; Mccallum, W.C.; Winter, A.L. Contingent Negative Variation: An Electric Sign of Sensori-Motor Association and Expectancy in the Human Brain. Nature 1964, 203, 380–384. [Google Scholar] [CrossRef]
- Boser, B.; Guyon, I.; Vapnik, V. A training algorithm for optimal margin classifiers. In Proceedings of the Fifth Annual Workshop on Computational Learning Theory, Pittsburgh, PA, USA, 27–29 July 1992. [Google Scholar]
- Wong, K.K. Cybernetical intelligence: Engineering Cybernetics with Machine Intelligence; John Wiley & Sons: Hoboken, NJ, USA, 2023. [Google Scholar]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Chang, C.; Lin, C. LIBSVM: A library for support vector machines. ACM Trans. Intell. Syst. Technol. 2011, 2, 1–27. [Google Scholar] [CrossRef]
- Shawe-Taylor, J.; Cristianini, N. Kernel Methods for Pattern Analysis; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Bishop, C.M.; Nasrabadi, N.M. Pattern Recognition and Machine Learning; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Noguchi, Y.; Murota, M. Temporal dynamics of neural activity in an integration of visual and contextual information in an esthetic preference task. Neuropsychologia 2013, 51, 1077–1084. [Google Scholar] [CrossRef]
- Grüner, S.; Specker, E.; Leder, H. Effects of Context and Genuineness in the Experience of Art. Empir. Stud. Arts 2019, 37, 138–152. [Google Scholar] [CrossRef]
- Petcu, E.B. The Rationale for a Redefinition of Visual Art Based on Neuroaesthetic Principles. Leonardo 2018, 51, 59–60. [Google Scholar] [CrossRef]
- Sbriscia-Fioretti, B.; Berchio, C.; Freedberg, D.; Gallese, V.; Umiltà, M.A.; Di Russo, F. ERP modulation during observation of abstract paintings by Franz Kline. PLoS ONE 2013, 8, e75241. [Google Scholar] [CrossRef]
- O’Doherty, J.; Critchley, H.; Deichmann, R.; Dolan, R.J. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. J. Neurosci. 2003, 23, 7931–7939. [Google Scholar] [CrossRef] [PubMed]
- Schultz, W.; Tremblay, L. Relative reward preference in primate orbitofrontal cortex. Nature 1999, 398, 704–708. [Google Scholar]
- Thai, C.H. Electrophysiological Measures of Aesthetic Processing. Doctoral Dissertation, Swinburne University of Technology, Melbourne, Australia, 2019. [Google Scholar]
- Munar, E.; Nadal, M.; Rosselló, J.; Flexas, A.; Moratti, S.; Maestú, F.; Marty, G.; Cela-Conde, C.J.; Martinez, L.M. Lateral orbitofrontal cortex involvement in initial negative aesthetic impression formation. PLoS ONE 2012, 7, e38152. [Google Scholar] [CrossRef] [PubMed]
- Medathati, N.V.K.; Neumann, H.; Masson, G.S.; Kornprobst, P. Bio-inspired computer vision: Towards a synergistic approach of artificial and biological vision. Comput. Vis. Image Underst. 2016, 150, 1–30. [Google Scholar] [CrossRef]
- Milner, A.D.; Goodale, M.A. Two visual systems re-viewed. Neuropsychologia 2008, 46, 774–785. [Google Scholar] [CrossRef]
- Goodale, M.A.; Milner, A.D. Separate visual pathways for perception and action. Trends Neurosci. 1992, 15, 20–25. [Google Scholar] [CrossRef]
- Zhong, H.; Wang, R. Neural mechanism of visual information degradation from retina to V1 area. Cogn. Neurodyn. 2021, 15, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Bayram, A.; Karahan, E.; Bilgiç, B.; Ademoglu, A.; Demiralp, T. Achromatic temporal-frequency responses of human lateral geniculate nucleus and primary visual cortex. Vis. Res. 2016, 127, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Willmore, B.D.; Prenger, R.J.; Gallant, J.L. Neural representation of natural images in visual area V2. J. Neurosci. 2010, 30, 2102–2114. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T.; Deco, G.; Huang, C.; Feng, J. Multiple cortical visual streams in humans. Cereb. Cortex 2023, 33, 3319–3349. [Google Scholar] [CrossRef] [PubMed]
- Hegde, J.; Van Essen, D.C. Selectivity for Complex Shapes in Primate Visual Area V2. J. Neurosci. 2000, 20, 61. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.E.; Thompson, B.; Liu, Z. Motion opponency examined throughout visual cortex with multivariate pattern analysis of fMRI data. Hum. Brain Mapp. 2021, 42, 5–13. [Google Scholar] [CrossRef]
- Bannert, M.M.; Bartels, A. Human V4 Activity Patterns Predict Behavioral Performance in Imagery of Object Color. J. Neurosci. 2018, 38, 3657–3668. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Bair, W.; Pasupathy, A. Neural Coding for Shape and Texture in Macaque Area V4. J. Neurosci. 2019, 39, 4760–4774. [Google Scholar] [CrossRef]
- Vetter, P.; Grosbras, M.; Muckli, L. TMS over V5 disrupts motion prediction. Cereb. Cortex 2015, 25, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.M.; Graulty, C.J.; Hillyard, S.A.; Pitts, M.A. Does spatial attention modulate the earliest component of the visual evoked potential? In The Cognitive Neuroscience of Attention; Routledge: London, UK, 2020; pp. 9–24. [Google Scholar]
- Conte, S.; Richards, J.E.; Guy, M.W.; Xie, W.; Roberts, J.E. Face-sensitive brain responses in the first year of life. Neuroimage 2020, 211, 116602. [Google Scholar] [CrossRef]
- Schindler, S.; Bruchmann, M.; Gathmann, B.; Moeck, R.; Straube, T. Effects of low-level visual information and perceptual load on P1 and N170 responses to emotional expressions. Cortex 2021, 136, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X. Evaluation of Aesthetic Response to Clothing Color Combination: A Behavioral and Electrophysiological Study. J. Fiber Bioeng. Inform. 2018, 6, 405–414. [Google Scholar] [CrossRef]
- Liang, T.; Lau, B.T.; White, D.; Barron, D.; Zhang, W.; Yue, Y.; Ogiela, M. Artificial Aesthetics: Bridging Neuroaesthetics and Machine Learning. In Proceedings of the 2024 8th International Conference on Control Engineering and Artificial Intelligence, Shanghai, China, 26–28 January 2024; ACM: New York, NY, USA, 2024. [Google Scholar]
- Li, R.; Zhang, J. Review of computational neuroaesthetics: Bridging the gap between neuroaesthetics and computer science. Brain Inform. 2020, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Botros, C.; Mansour, Y.; Eleraky, A. Architecture Aesthetics Evaluation Methodologies of Humans and Artificial Intelligence. MSA Eng. J. 2023, 2, 450–462. [Google Scholar] [CrossRef]
- Skov, M.; Nadal, M. A Farewell to Art: Aesthetics as a Topic in Psychology and Neuroscience. Perspect. Psychol. Sci. 2020, 15, 630–642. [Google Scholar] [CrossRef]
- Zeki, S.; Bao, Y.; Poppel, E. Neuroaesthetics: The art, science, and brain triptych. Psychol. J. 2020, 9, 427–428. [Google Scholar] [CrossRef]



| Features | Class | N | Description |
|---|---|---|---|
| Composition | I | 123 | Class I has a larger blank area compared to Class II. |
| II | 126 | ||
| Tone | I | 105 | The average brightness of Class I is lower than that of Class II, and the gray level distribution is more discrete. |
| II | 144 | ||
| Gabor-Mean | I | 117 | combinations—(6, 0), (6, π/2), (9, π/8), (9, 3π/8), (9, 5π/8), (9,7π/8), (12,0), (12, π/8), (12, 3π/8), (12, π/2), (12, 5π/8), (12, 7π/8), (15,0), (15, π/2), the grayscale mean of the filtered images in Class I is higher than that of Class II. This indicates that Class I has a higher overall brightness in these combinations. Conversely, Class II exhibits more high-brightness features in other combinations. |
| II | 132 | ||
| Gabor-Variance | I | 130 | combinations, Class II has more high-frequency components and greater texture changes after Gabor filter processing, which suggests that it contains more diverse and complex textures. |
| II | 119 | ||
| Gabor-Energy | I | 122 | combinations—(6, 0), (6, π/2), (9, π/8), (9, 3π/8), (9, 5π/8), (9,7π/8), (12,0), (12, π/8), (12, 3π/8), (12, π/2), (12, 5π/8), (12, 7π/8), (15,0), (15, π/2)—the energy gray level of Class I after Gabor filter processing is greater. The features in Class I are more pronounced and intense, likely containing more edges, lines, or texture features in these combinations. Conversely, Class II contains more prominent edges or complex textures in other combinations. |
| II | 127 | ||
| Horizontal GLCM (θ = 0°) | I | 123 | In the horizontal direction, compared to Class II, the texture features of Class I are more obvious, with high-contrast edges and lines, higher regularity, and more texture patterns. |
| II | 126 | ||
| Diagonal GLCM (θ = 135°) | I | 118 | In the diagonal direction, compared to Class II, the texture features of Class I are more obvious, with high-contrast edges and lines, higher regularity, and more texture patterns. |
| II | 131 | ||
| LBP | I | 113 | Compared to Class II, Class I has weaker texture contrast but richer details, uniform texture distribution, and contains more small and intense details. |
| II | 136 |
| Input Layer | Output Layer | |||
|---|---|---|---|---|
| Context and Features | Average Amplitude of ERP/μV | |||
| Channel | Time Window/ms | |||
| Context | Genuine/Fake | FP1, FPZ, FP2 | 200–1000 | Aesthetic Evaluation |
| Composition | Blank Space | PZ, POZ | 50–120 | |
| Tone | Gray Histogram | OZ | 200–300 | |
| Global Texture | Gabor-Mean | P7, PO3, PO5, PO7 | 70–130 | |
| Gabor-Variance | PZ, POZ | 70–130 | ||
| Gabor-Energy | P2, P4, PZ, PO4, PO6, POZ | 70–130 | ||
| Local Texture | Horizontal GLCM | OZ | 500–1000 | |
| Diagonal GLCM | PO5, PO7 | 70–140 | ||
| OZ | 300–1000 | |||
| LBP | AF3, P5, FP1, FPZ | 300–1000 | ||
| Metrics | Values | |
|---|---|---|
| Best C | 28.5786 | |
| Best γ | 14.2943 | |
| Train ACC | 0.79801 | |
| Test ACC | 0.76866 | |
| AUC | 0.74155 | |
| Precision | 0.78241 | 0.75269 |
| Recall | 0.78605 | 0.74866 |
| F1 | 0.78422 | 0.75067 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, F.; Xu, W.; Li, Y.; Song, W. Exploring the Influence of Object, Subject, and Context on Aesthetic Evaluation through Computational Aesthetics and Neuroaesthetics. Appl. Sci. 2024, 14, 7384. https://doi.org/10.3390/app14167384
Lin F, Xu W, Li Y, Song W. Exploring the Influence of Object, Subject, and Context on Aesthetic Evaluation through Computational Aesthetics and Neuroaesthetics. Applied Sciences. 2024; 14(16):7384. https://doi.org/10.3390/app14167384
Chicago/Turabian StyleLin, Fangfu, Wanni Xu, Yan Li, and Wu Song. 2024. "Exploring the Influence of Object, Subject, and Context on Aesthetic Evaluation through Computational Aesthetics and Neuroaesthetics" Applied Sciences 14, no. 16: 7384. https://doi.org/10.3390/app14167384
APA StyleLin, F., Xu, W., Li, Y., & Song, W. (2024). Exploring the Influence of Object, Subject, and Context on Aesthetic Evaluation through Computational Aesthetics and Neuroaesthetics. Applied Sciences, 14(16), 7384. https://doi.org/10.3390/app14167384






