Study on the Anti-Interference Performance of Substrate-Free PEDOT:PSS ECG Electrodes
Abstract
1. Introduction
2. Materials and Methods
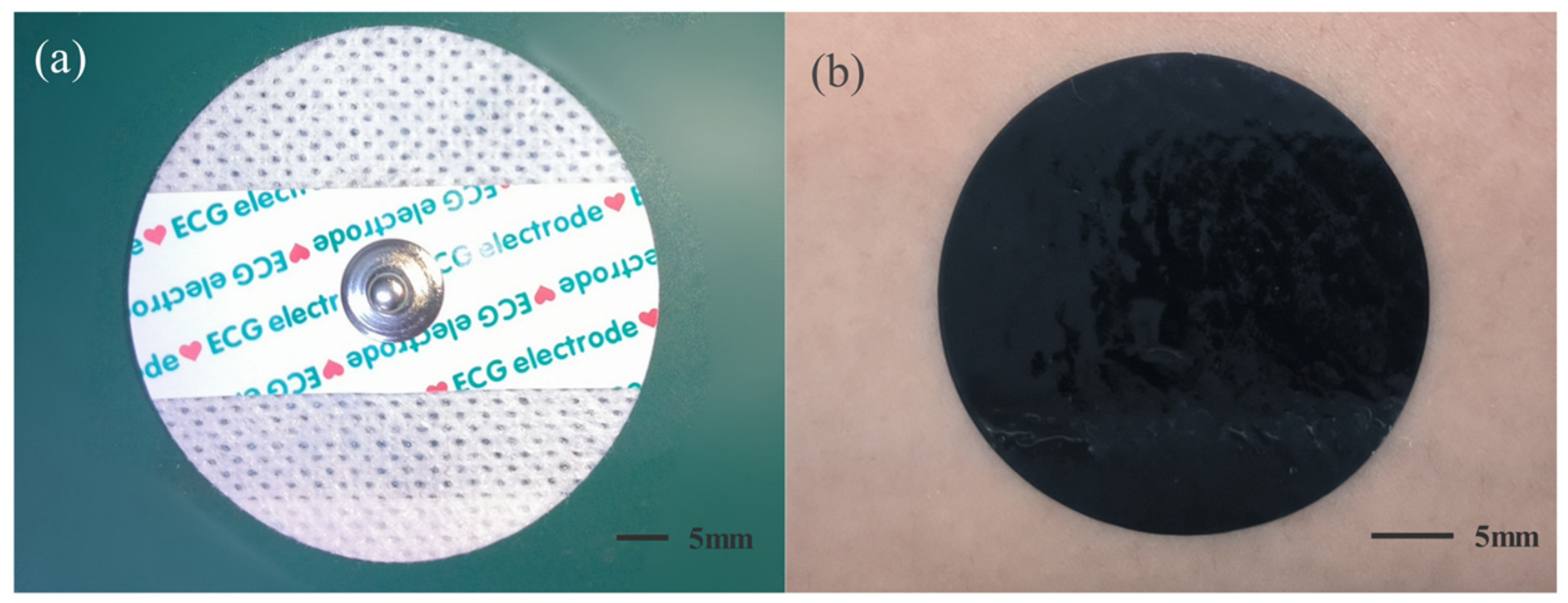
2.1. Experimental Electrodes
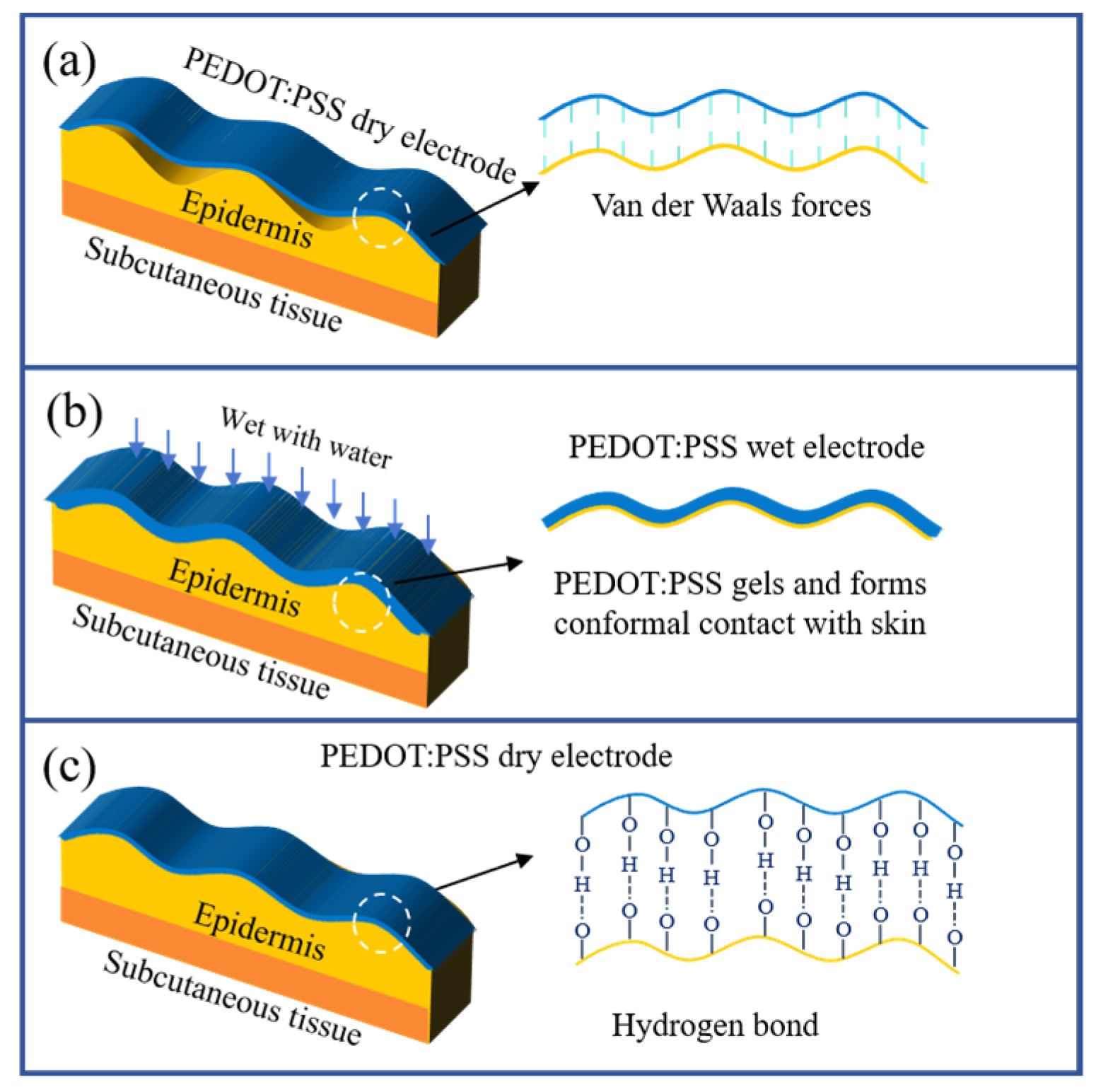
2.2. Electrode Adhesion Mechanism
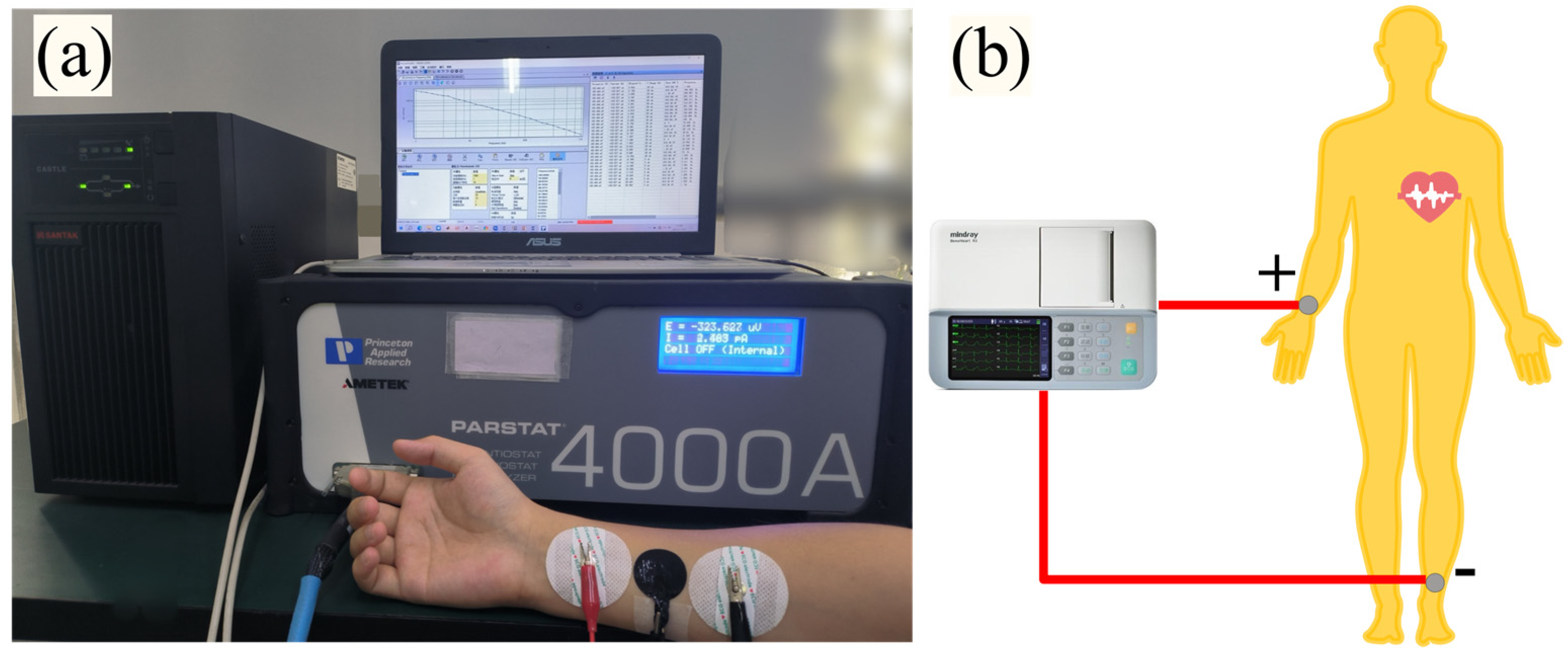
2.3. Impedance Mesurement and ECG Monitoring
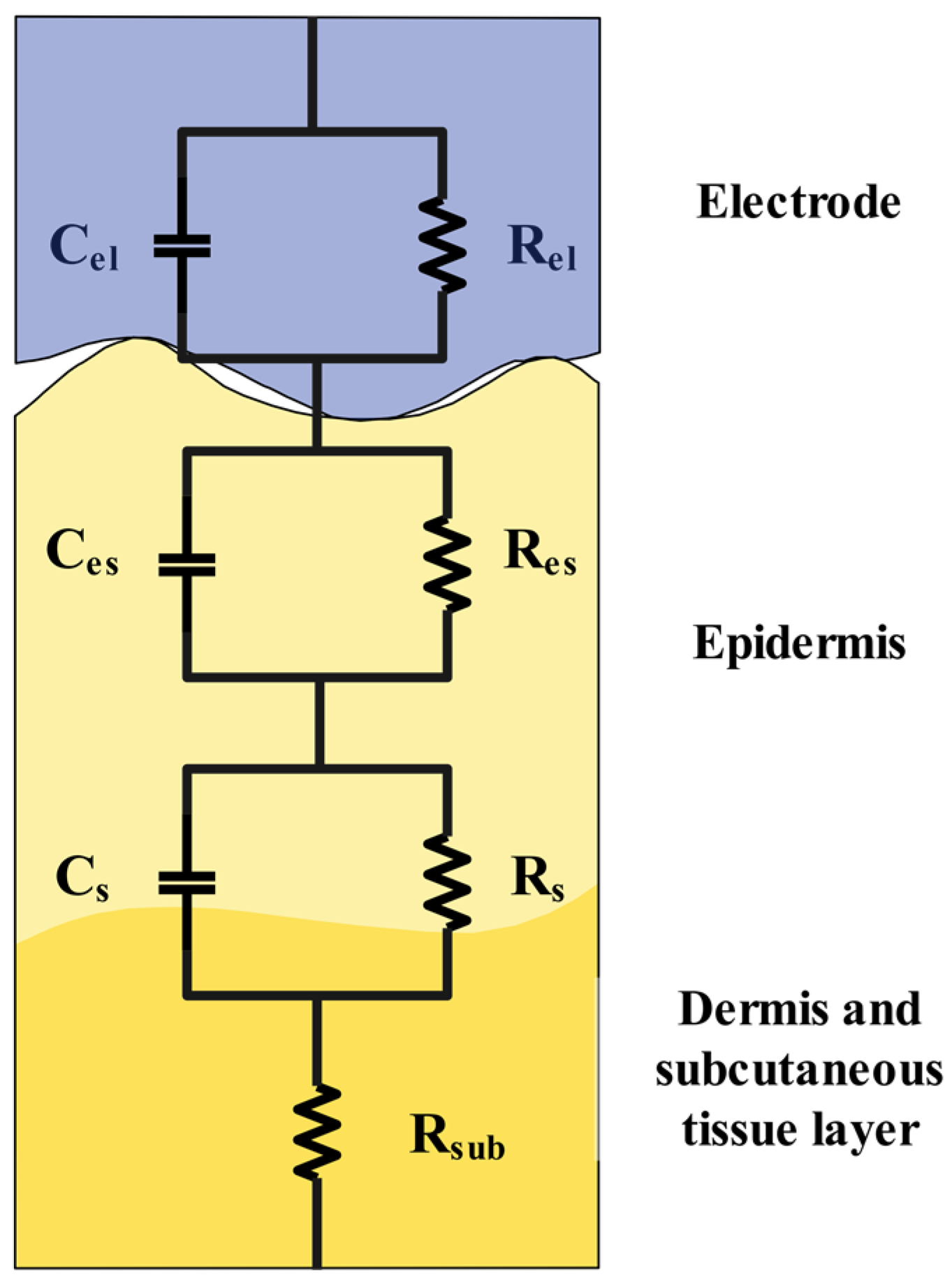
2.4. Skin–Electrode Impedance Equivalent Circuit Model
2.5. Experimental Design
2.6. Signal-to-Noise Ratio
3. Results and Discussion
3.1. Effect of Static Factors on Impedance and ECG
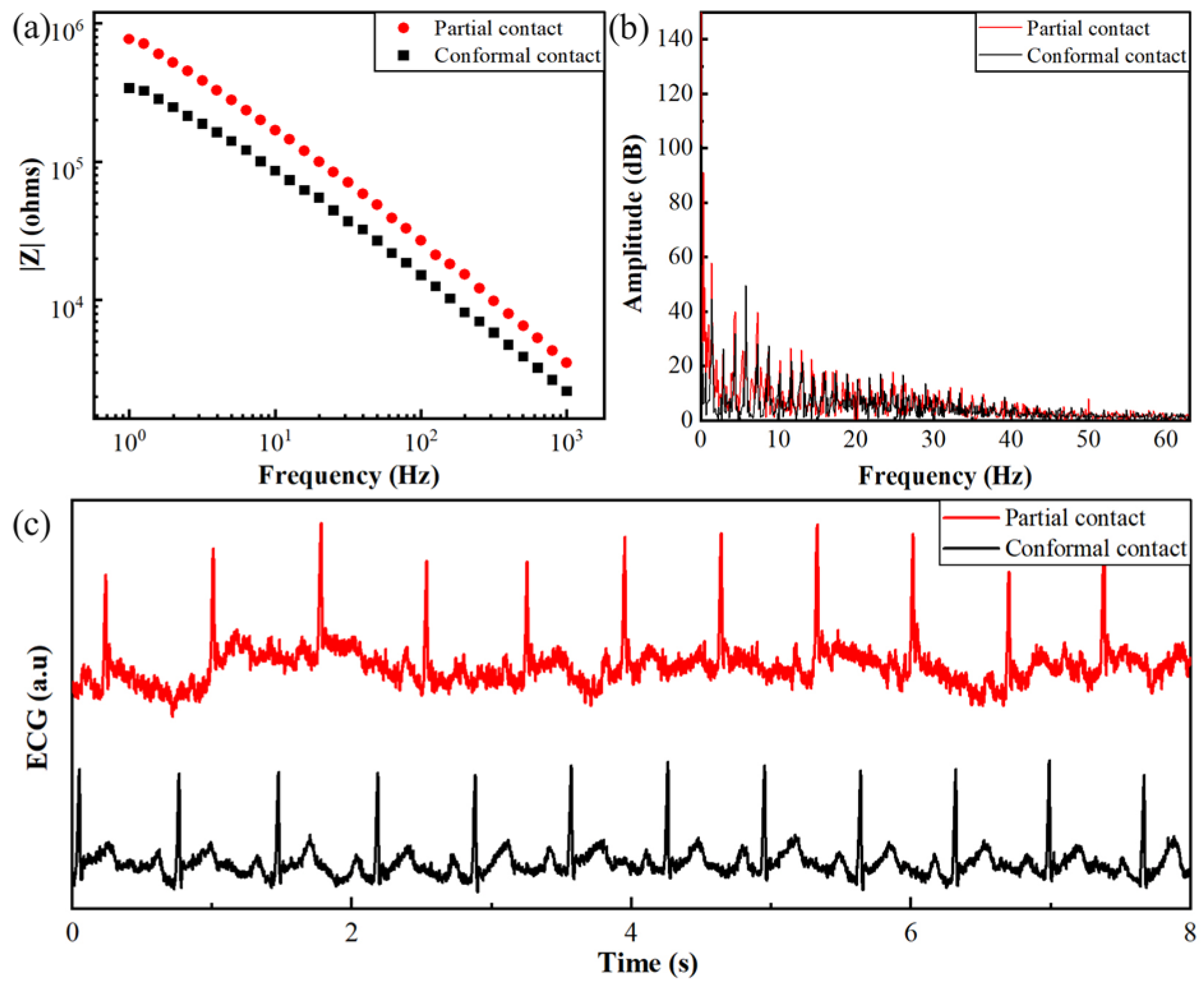
3.1.1. Influence of the Contact State
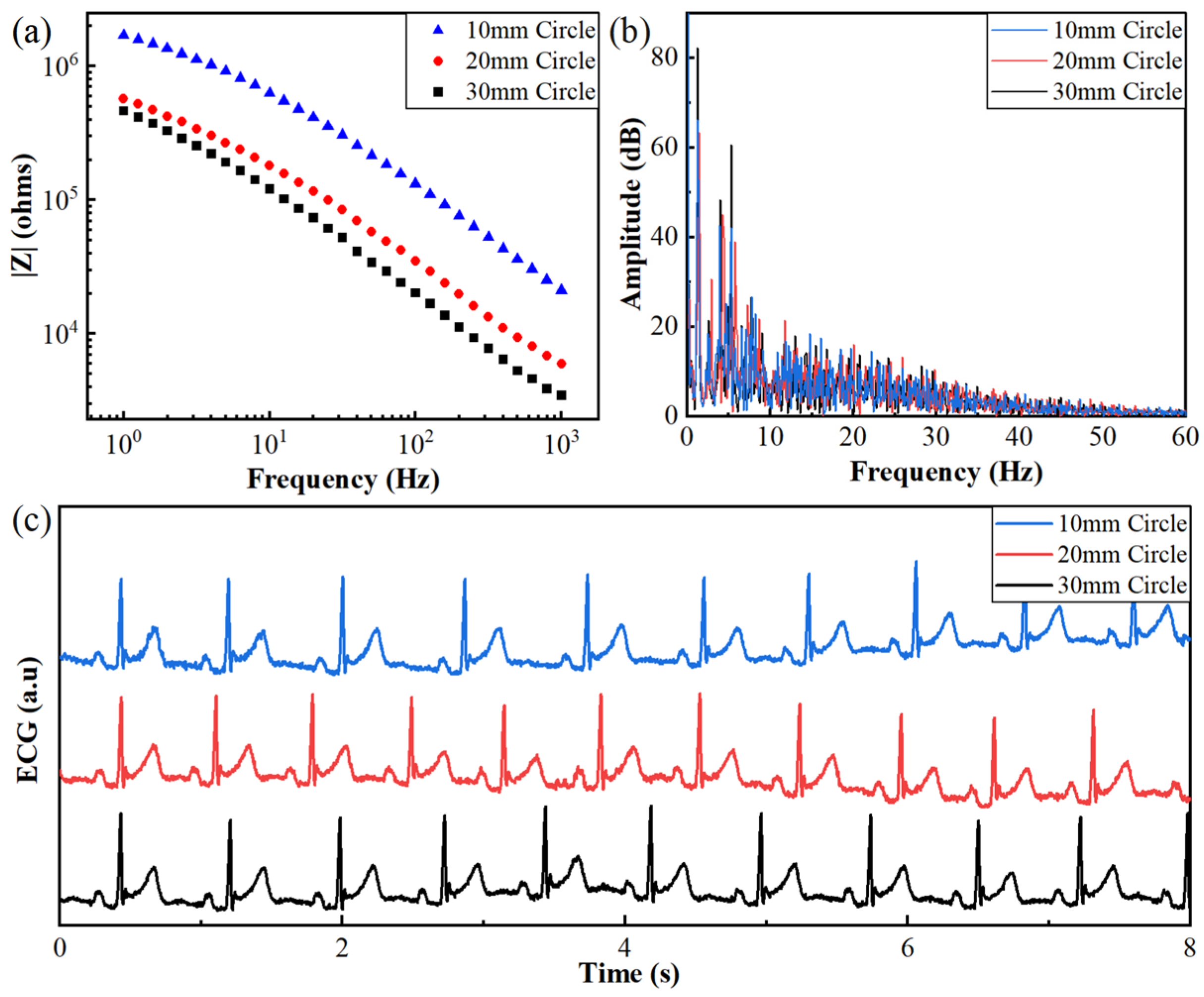
3.1.2. Effect of Electrode Area
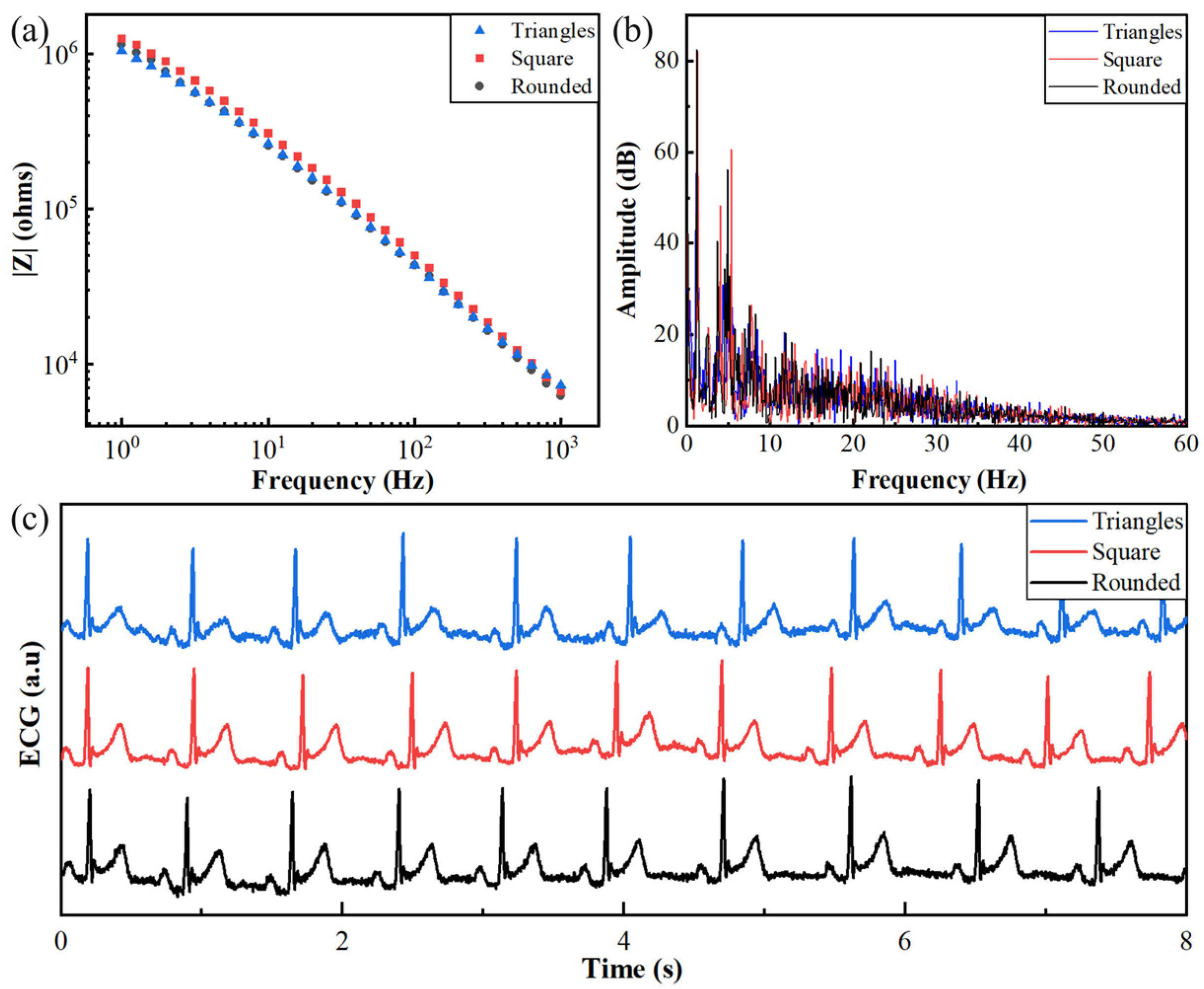
3.1.3. Effect of Electrode Shape
3.2. Effect of Dynamic Factors on Impedance and ECG
3.2.1. Effect of Friction between the Fabric and the Electrode
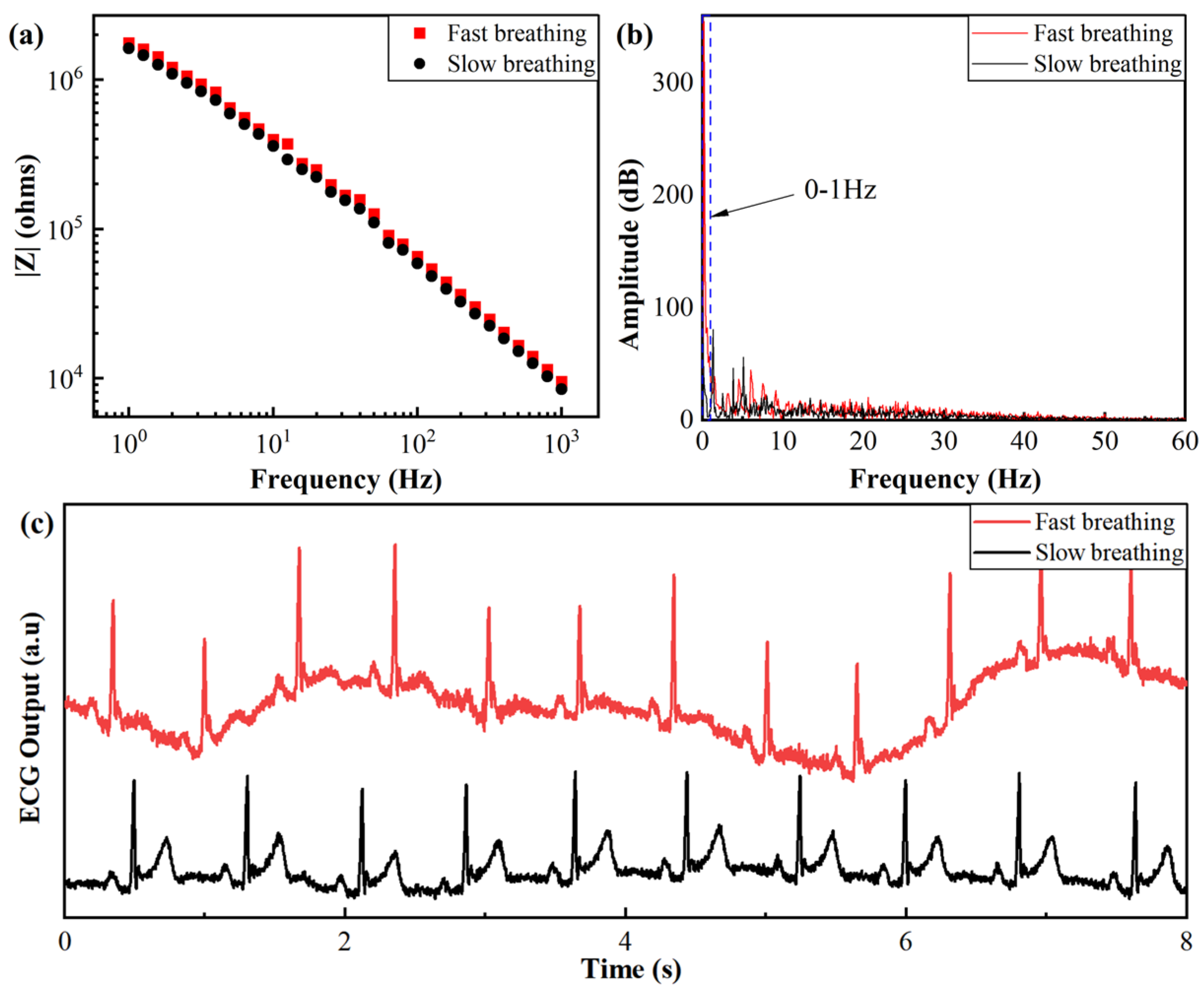
3.2.2. Effect of Breathing Frequency
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laghlimi, C.; Moutcine, A.; Chtaini, A.; Isaad, J.; Soufi, A.; Ziat, Y.; Amhamdi, H.; Belkhanchi, H. Recent advances in electrochemical sensors and biosensors for monitoring drugs and metabolites in pharmaceutical and biological samples. Admet Dmpk 2023, 11, 151–173. [Google Scholar] [CrossRef] [PubMed]
- Bihar, E.; Roberts, T.; Ismailova, E.; Saadaoui, M.; Isik, M.; Sanchez-Sanchez, A.; Mecerreyes, D.; Hervé, T.; De Graaf, J.B.; Malliaras, G.G. Fully Printed Electrodes on Stretchable Textiles for Long-Term Electrophysiology. Adv. Mater. Technol. 2017, 2, 1600251. [Google Scholar] [CrossRef]
- Bihar, E.; Roberts, T.; Zhang, Y.; Ismailova, E.; Hervé, T.; Malliaras, G.G.; De Graaf, J.B.; Inal, S.; Saadaoui, M. Fully printed all-polymer tattoo/textile electronics for electromyography. Flex. Print. Electron. 2018, 3, 034004. [Google Scholar] [CrossRef]
- Casson, A.J.; Saunders, R.; Batchelor, J.C. Five Day Attachment ECG Electrodes for Longitudinal Bio-Sensing Using Conformal Tattoo Substrates. IEEE Sens. J. 2017, 17, 2205–2214. [Google Scholar] [CrossRef]
- Deng, J.; Yuk, H.; Wu, J.; Varela, C.E.; Chen, X.; Roche, E.T.; Guo, C.F.; Zhao, X. Electrical bioadhesive interface for bioelectronics. Nat. Mater. 2020, 20, 229–236. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, L.; Bai, Y.; Liu, S.; Wang, L.; Zhou, Y.; Hou, C.; Yang, Z.; Wu, H.; Ma, J.; et al. Electrically compensated, tattoo-like electrodes for epidermal electrophysiology at scale. Sci. Adv. 2020, 6, eabd0996. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, G.; Yuan, X.; Li, C.; Liu, L.; You, H. Substrate-free, ultra-conformable PEDOT: PSS E-tattoo achieved by energy regulation on skin. Biosens. Bioelectron. 2022, 206, 114118. [Google Scholar] [CrossRef]
- Jeong, J.-W.; Shin, G.; Park, S.I.; Yu, K.J.; Xu, L.; Rogers, J.A. Soft Materials in Neuroengineering for Hard Problems in Neuroscience. Neuron 2015, 86, 175–186. [Google Scholar] [CrossRef]
- Qiao, Y.; Li, X.; Jian, J.; Wu, Q.; Wei, Y.; Shuai, H.; Hirtz, T.; Zhi, Y.; Deng, G.; Wang, Y.; et al. Substrate-Free Multilayer Graphene Electronic Skin for Intelligent Diagnosis. ACS Appl. Mater. Interfaces 2020, 12, 49945–49956. [Google Scholar] [CrossRef]
- Aguzin, A.; Dominguez-Alfaro, A.; Criado-Gonzalez, M.; Velasco-Bosom, S.; Picchio, M.L.; Casado, N.; Mitoudi-Vagourdi, E.; Minari, R.J.; Malliaras, G.G.; Mecerreyes, D. Direct ink writing of PEDOT eutectogels as substrate-free dry electrodes for electromyography. Mater. Horiz. 2023, 10, 2516–2524. [Google Scholar] [CrossRef]
- Ferrari, L.M.; Sudha, S.; Tarantino, S.; Esposti, R.; Bolzoni, F.; Cavallari, P.; Cipriani, C.; Mattoli, V.; Greco, F. Ultraconformable Temporary Tattoo Electrodes for Electrophysiology. Adv. Sci. 2018, 5, 1700771. [Google Scholar] [CrossRef] [PubMed]
- Singh, O.; Sunkaria, R.K. ECG signal denoising via empirical wavelet transform. Australas. Phys. Eng. Sci. Med. 2016, 40, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Singh, O.; Sunkaria, R.K. Powerline interference reduction in ECG signals using empirical wavelet transform and adaptive filtering. J. Med. Eng. Technol. 2014, 39, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Kalra, A.; Lowe, A.; Al-Jumaily, A. Critical review of electrocardiography measurement systems and technology. Meas. Sci. Technol. 2018, 30, 012001. [Google Scholar] [CrossRef]
- Taji, B.; Shirmohammadi, S.; Groza, V.; Batkin, I. Impact of Skin–Electrode Interface on Electrocardiogram Measurements Using Conductive Textile Electrodes. IEEE Trans. Instrum. Meas. 2013, 63, 1412–1422. [Google Scholar] [CrossRef]
- Oliveira, C.C.; Machado Da Silva, J.; Trindade, I.G.; Martins, F. Characterization of the electrode-skin impedance of textile electrodes. In Proceedings of the Design of Circuits and Integrated Systems, Madrid, Spain, 26–28 November 2014; pp. 1–6. [Google Scholar]
- Gan, Y.; Rahajandraibe, W.; Vauche, R.; Ravelo, B.; Lorriere, N.; Bouchakour, R. A new method to reduce motion artifact in electrocardiogram based on an innovative skin-electrode impedance model. Biomed. Signal Proces. 2022, 76, 103640. [Google Scholar] [CrossRef]
- Luna, J.L.V.; Krenn, M.; Ramírez, J.A.C.; Mayr, W. Dynamic Impedance Model of the Skin-Electrode Interface for Transcutaneous Electrical Stimulation. PLoS ONE 2015, 10, e0125609. [Google Scholar] [CrossRef]
- Huigen, E.; Peper, A.; Grimbergen, C.A. Investigation into the origin of the noise of surface electrodes. Med. Biol. Eng. Comput. 2002, 40, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Sim, K.S.; Kim, K.K.; Lim, Y.G.; Park, K.S. Thin and flexible active electrodes with shield for capacitive electrocardiogram measurement. Med. Biol. Eng. Comput. 2010, 48, 447–457. [Google Scholar] [CrossRef]
- Beckmann, L.; Neuhaus, C.; Medrano, G.; Jungbecker, N.; Walter, M.; Gries, T.; Leonhardt, S. Characterization of textile electrodes and conductors using standardized measurement setups. Physiol. Meas. 2010, 31, 233–247. [Google Scholar] [CrossRef]
- Lempka, S.F.; Johnson, M.D.; Moffitt, M.A.; Otto, K.J.; Kipke, D.R.; McIntyre, C.C. Theoretical analysis of intracortical microelectrode recordings. J. Neural Eng. 2011, 8, 045006. [Google Scholar] [CrossRef] [PubMed]
- Berggren, M.; Malliaras, G.G. How conducting polymer electrodes operate. Science 2019, 364, 233–234. [Google Scholar] [CrossRef]
- Lu, B.; Yuk, H.; Lin, S.; Jian, N.; Qu, K.; Xu, J.; Zhao, X. Pure PEDOT:PSS hydrogels. Nat. Commun. 2019, 10, 1043. [Google Scholar] [CrossRef]
- Li, G.; Wang, S.; Duan, Y.Y. Towards conductive-gel-free electrodes: Understanding the wet electrode, semi-dry electrode and dry electrode-skin interface impedance using electrochemical impedance spectroscopy fitting. Sens. Actuators B Chem. 2018, 277, 250–260. [Google Scholar] [CrossRef]
- Kania, M.; Rix, H.; Fereniec, M.; Zavala-Fernandez, H.; Janusek, D.; Mroczka, T.; Stix, G.; Maniewski, R. The effect of precordial lead displacement on ECG morphology. Med. Biol. Eng. Comput. 2013, 52, 109–119. [Google Scholar] [CrossRef]
- Fasano, A.; Villani, V. ECG baseline wander removal and impact on beat morphology: A comparative analysis. In Proceedings of the Computing in Cardiology, Zaragoza, Spain, 22–25 September 2013; pp. 1167–1170. [Google Scholar]
- Appathurai, A.; Carol, J.J.; Raja, C.; Kumar, S.; Daniel, A.V.; Malar, A.J.G.; Fred, A.L.; Krishnamoorthy, S. A study on ECG signal characterization and practical implementation of some ECG characterization techniques. Measurement 2019, 147, 106384. [Google Scholar] [CrossRef]
- Aqil, M.; Jbari, A.; Bourouhou, A. ECG Signal Denoising by Discrete Wavelet Transform. Int. J. Online Eng. 2017, 13, 51–68. [Google Scholar] [CrossRef]
- Mani, R.; Oppenheim, A.V.; Willsky, A.S.; Nawab, S.H. Solutions Manual, Signals & Systems, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1997. [Google Scholar]
- Saleh, S.M.; Jusob, S.M.; Harun, F.K.C.; Yuliati, L.; Wicaksono, D.H.B. Optimization of Reduced GO-Based Cotton Electrodes for Wearable Electrocardiography. IEEE Sens. J. 2020, 20, 7774–7782. [Google Scholar] [CrossRef]
- Devi, R.; Tyagi, H.K.; Kumar, D. Performance Comparison and Applications of Sparsity Based Techniques for Denoising of ECG Signal. In Proceedings of the 2019 6th International Conference on Signal Processing and Integrated Networks (SPIN), Noida, India, 7–8 March 2019; pp. 346–351. [Google Scholar]
- Mateo, J.; Sánchez-Morla, E.; Santos, J. A new method for removal of powerline interference in ECG and EEG recordings. Comput. Electr. Eng. 2015, 45, 235–248. [Google Scholar] [CrossRef]
- Kuzilek, J.; Kremen, V.; Soucek, F.; Lhotska, L. Independent Component Analysis and Decision Trees for ECG Holter Recording De-Noising. PLoS ONE 2014, 9, e98450. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Xu, K.; Chen, Y. Study on the Anti-Interference Performance of Substrate-Free PEDOT:PSS ECG Electrodes. Appl. Sci. 2024, 14, 6367. https://doi.org/10.3390/app14146367
Li C, Xu K, Chen Y. Study on the Anti-Interference Performance of Substrate-Free PEDOT:PSS ECG Electrodes. Applied Sciences. 2024; 14(14):6367. https://doi.org/10.3390/app14146367
Chicago/Turabian StyleLi, Chunlin, Ke Xu, and Yuanfen Chen. 2024. "Study on the Anti-Interference Performance of Substrate-Free PEDOT:PSS ECG Electrodes" Applied Sciences 14, no. 14: 6367. https://doi.org/10.3390/app14146367
APA StyleLi, C., Xu, K., & Chen, Y. (2024). Study on the Anti-Interference Performance of Substrate-Free PEDOT:PSS ECG Electrodes. Applied Sciences, 14(14), 6367. https://doi.org/10.3390/app14146367





