Influence of Treatment with Natural Phytoregulators on Purple Carrots (Daucus carota L.) during Cold Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Samples
2.3. Treatments
2.4. Physicochemical Parameters
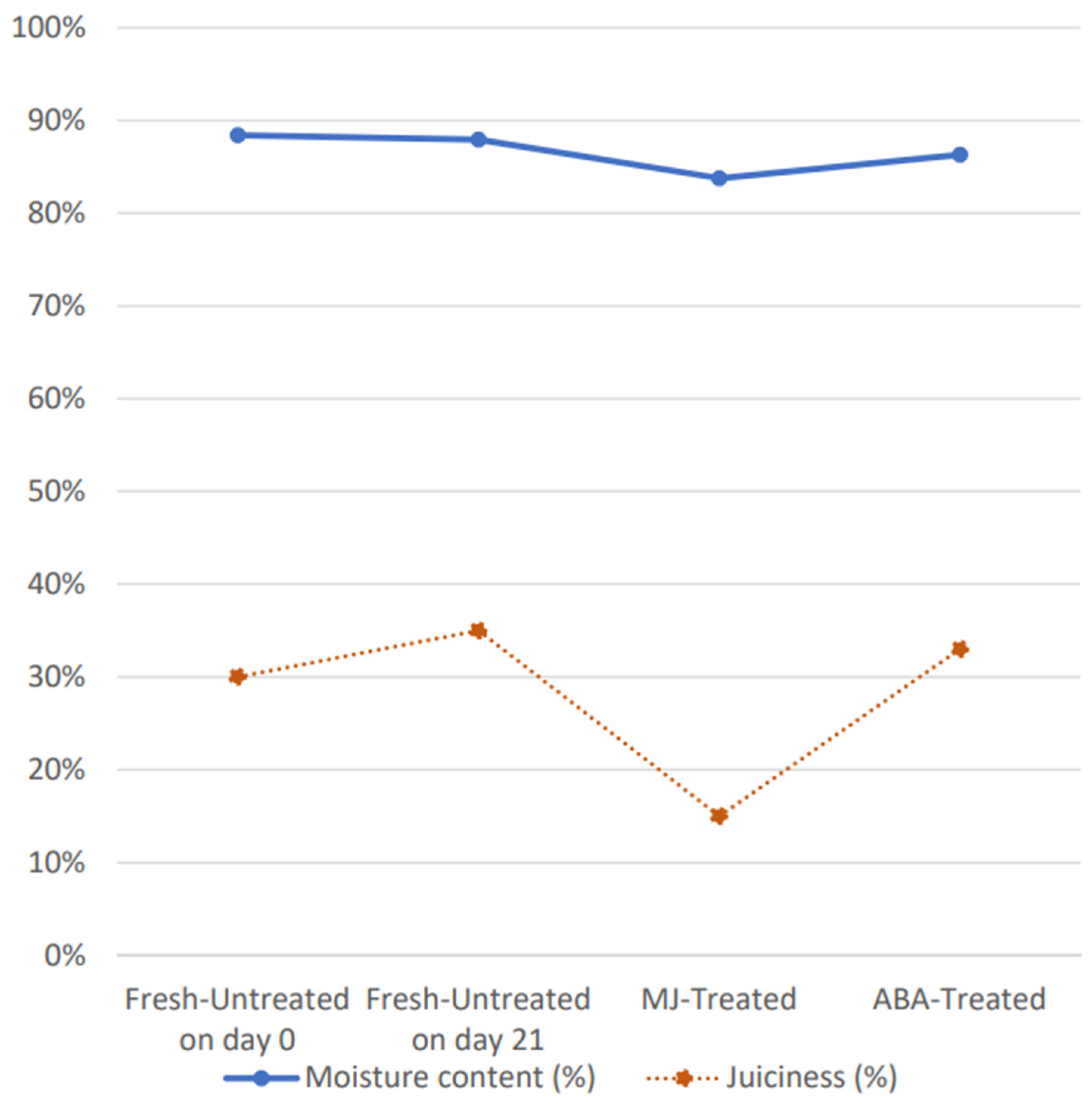
2.4.1. Juiciness
2.4.2. Moisture Content
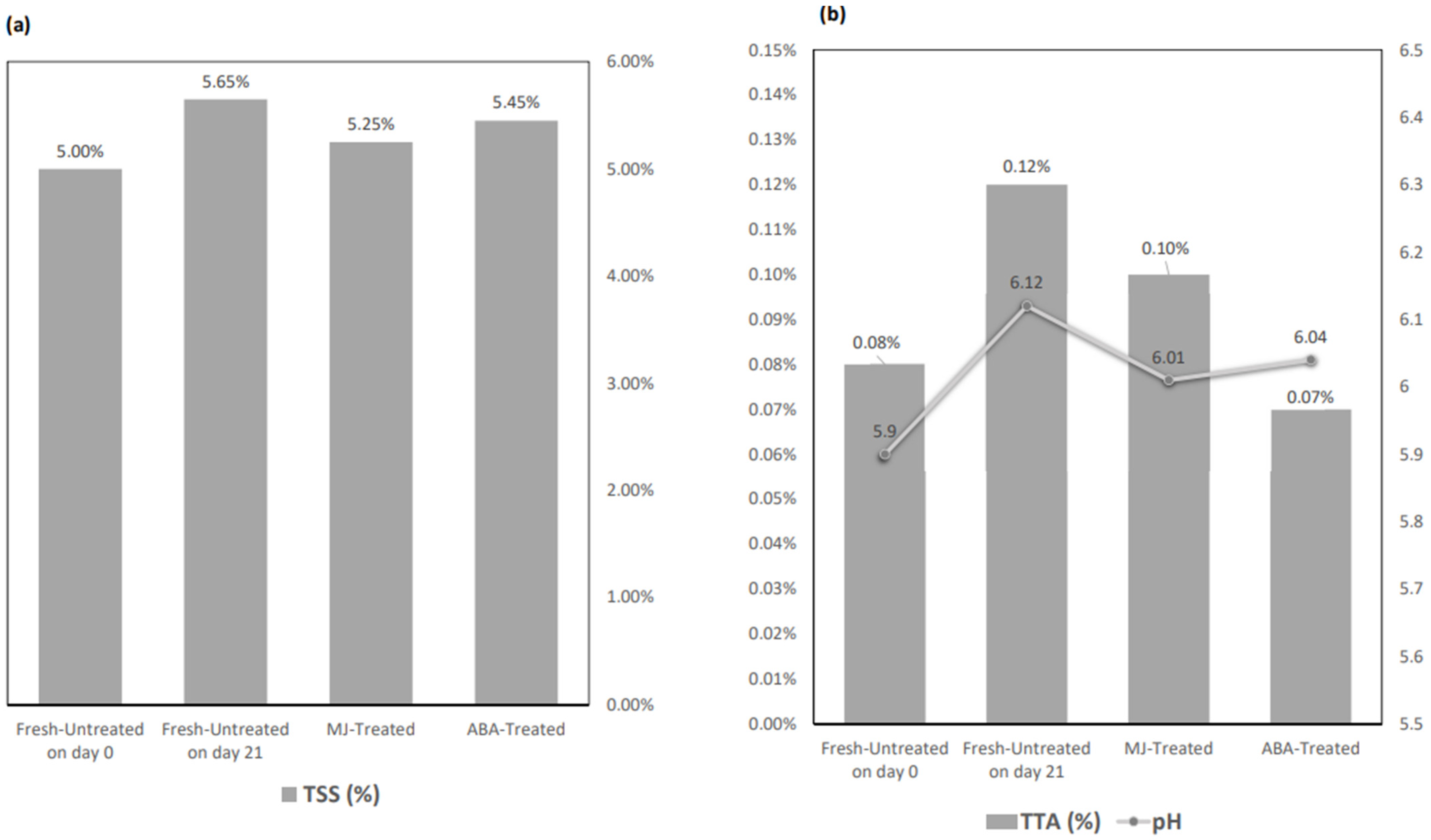
2.4.3. Total Soluble Solids (TSS)
2.4.4. Acidity
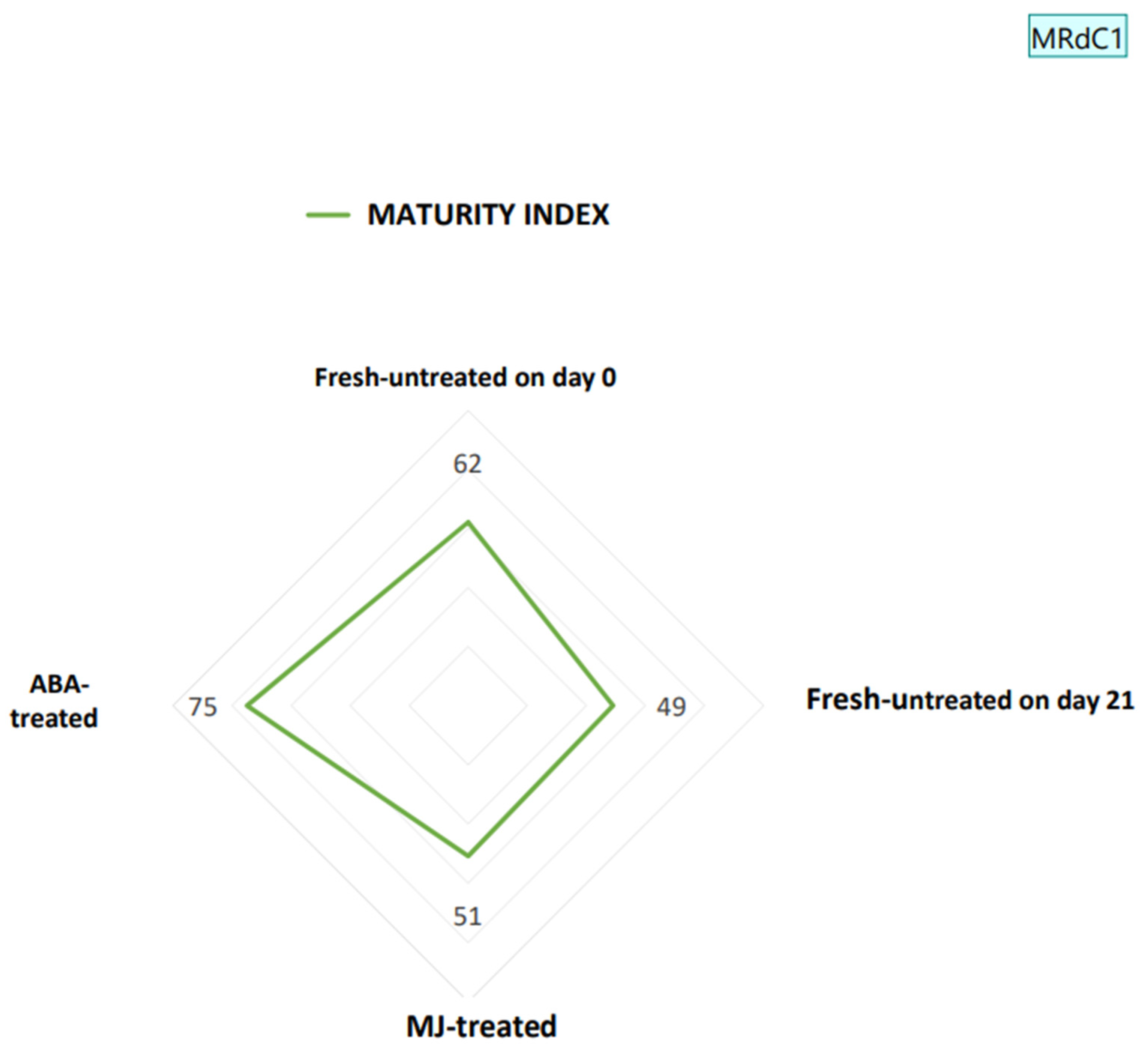
2.4.5. Maturity Index
2.5. Health-Related Quality
2.5.1. Determination of Carotenoids
2.5.2. Anthocyanins
2.5.3. Antioxidant Activity
2.6. Statistical Study
3. Results and Discussion
3.1. Physicochemical Quality
3.2. Health-Related Quality
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ikram, A.; Rasheed, A.; Khana, A.A.; Khana, R.; Ahmada, M.; Bashirb, R.; Mohamed, M.H. Exploring the health benefits and utility of carrots and carrot pomace: A systematic review. Int. J. Food Prop. 2024, 27, 180–193. [Google Scholar] [CrossRef]
- Mohammadi, N.; Farrell, M.; O’Sullivan, L.; Langan, A.; Franchin, M.; Azevedo, L.; Granato, D. Effectiveness of anthocyanin-containing foods and nutraceuticals in mitigating oxidative stress, inflammation, and cardiovascular health-related biomarkers: A systematic review of animal and human interventions. Food Funct. 2024, 15, 3274–3299. [Google Scholar] [CrossRef] [PubMed]
- Roy, V.C.; Haq, M.; Ho, T.C.; Park, J.-S.; Chamika, W.A.S.; Ali, M.S.; Haque, A.R.; Zhang, W.; Chun, B.-S. Important carotenoids derived from marine biomass: Extraction, stabilization, and potentiality in foods, cosmetics, and pharmaceutical application. Food Biosci. 2024, 60, 104421–104436. [Google Scholar] [CrossRef]
- Enaru, B.; Dretcanu, G.; Pop, T.D.; Stanila, A.; Diaconeasa, Z. Anthocyanins: Factors affecting their stability and degradation. Antioxidants 2021, 10, 1967–1991. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.H.; Shin, C.H.; Chang, C.H. Effect of adding ascorbic acid and glucose on the antioxidant properties during of dried carrots. Food Chem. 2008, 107, 265–272. [Google Scholar] [CrossRef]
- Hagen, S.F.; Schmidt, G.; Borge, G.I. Impact of industrial freezing on quality attributes of carrot slices. Acta Hortic. 2022, 1353, 301–308. [Google Scholar] [CrossRef]
- Parreno, W.C.; Torres, M.D.A. Quality and safety of frozen vegetables. In Handbook of Frozen Food Processing and Packaging; CRC Press: Boca Raton, FL, USA, 2005; Volume 377. [Google Scholar]
- Hellström, J.; Granato, D.; Mattila, P.H. Accumulation of phenolic acids during storage over differently handled fresh carrots. Foods 2020, 9, 1515–1528. [Google Scholar] [CrossRef] [PubMed]
- Hammaz, F.; Charles, F.; Kopec, R.E.; Halimi, C.; Fgaier, S.; Aarrouf, J.; Urban, L.; Borel, P. Temperature and storage time increase provitamin A carotenoid concentrations and bioaccessibility in post-harvest carrots. Food Chem. 2021, 338, 12004–12013. [Google Scholar] [CrossRef]
- Özen, G.; Akbulut, M.; Artik, N. Stability of black carrot anthocyanins in the Turkish delight (Lokum) during storage. J. Food Process Eng. 2011, 34, 1282–1297. [Google Scholar] [CrossRef]
- Opoku, A.; Meda, V.; Wahab, J. Effects of storage methods on quality characteristics of carrots grown under organic and conventional management. In Proceedings of the the Canadian Society of Bioengineering, CSBE/SCGAB, Annual Conference, Saskatchewan, PE, Canada, 12–15 July 2009. Paper No. CSBE09-702. [Google Scholar]
- Humbal, A.; Pathak, B. Influence of exogenous elicitors on the production of secondary metabolite in plants: A review (“VSI: Secondary metabolites”). Plant Stress 2023, 8, 100166–100179. [Google Scholar] [CrossRef]
- Flores, G.; Ruiz del Castillo, M.L. Accumulation of anthocyanins and flavonols in black currant (Ribes nigrum L.) by pre-harvest methyl jasmonate treatments. J. Sci. Food Agric. 2016, 96, 4026–4031. [Google Scholar] [CrossRef] [PubMed]
- Blanch, G.P.; Gómez-Jiménez, M.C.; Ruiz del Castillo, M.L. Exogenous salicylic acid improves phenolic content and antioxidant activity in table grapes. Plant Foods Hum. Nutr. 2020, 75, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Barba-Espín, G.; Lütken, H.; Glied, S.; Crocoll, C.; Joernsgaard, B.; Müller, R. Anthocyanin elicitation for bio-sustainable colourant production in carrot. Acta Hortic. 2019, 1242, 87–92. [Google Scholar] [CrossRef]
- Barba-Espín, G.; Martínez-Jiménez, C.; Izquierdo-Martínez, A.; Acosta-Motos, J.R.; Hernández, J.A.; Díaz-Vivancos, P. H2O2-elicitation of black carrot hairy roots induces a controlled oxidative burst leading to increased anthocyainin production. Plants 2021, 10, 2753–2768. [Google Scholar] [CrossRef] [PubMed]
- Flores, G.; Blanch, G.P.; Ruiz del Castillo, M.L. Abscisic acid treated olive seeds as a natural source of bioactive compounds. LWT-Food Technol. Biol. 2018, 90, 556–561. [Google Scholar] [CrossRef]
- Ruiz del Castillo, M.L.; Flores, G.; Blanch, G.P. Exogenous methyl jasmonate diminishes the formation of lipid-derived compounds in boiled potatoes (Solanum tuberosum L.). J. Sci. Food Agric. 2010, 90, 2263–2267. [Google Scholar] [CrossRef] [PubMed]
- IFU. Determination of Soluble Solids (Indirect Method by Refractometry) (Revised in 2005). International Federation of Fruit Juice Producers, Paris, 2000. Available online: https://www.scirp.org/reference/referencespapers?referenceid=1739546 (accessed on 17 July 2024).
- Kultys, E.; Kurek, M.A. Green extraction of carotenoids from fruit and vegetable byproducts: A review. Molecules 2022, 27, 518–532. [Google Scholar] [CrossRef] [PubMed]
- Britton, G. Carotenoids. In Methods in Plant Biochemistry, Volume 7. Terpenoids; Dey, P.M., Harborne, J.B., Eds.; Academic Press: New York, NY, USA, 1998; pp. 473–518. [Google Scholar]
- Zelenkova, E.N.; Yegorova, Z.E.; Shabunya, P.S.; Fatykhava, S.A. HPLC analysis of carotenoids in particular carrot (Daucus carota L.) cultivars. UDC 2015, 4, 9–15. [Google Scholar]
- Algarra, M.; Fernandes, A.; Mateus, N.; de Freitas, V.; Esteves de Silva, J.C.G.; Casado, J. Anthocyanin profile and antioxidant activity of black carrots (Daucus carota L. ssp sativus var. atrorubens Alef.) from Cuevas Bajas, Spain. J. Food Compos. Anal. 2014, 33, 71–76. [Google Scholar]
- Giusti, M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV visible spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Smith, R.C.; Reeves, J.C.; Dage, R.C.; Schnettler, R.A. Antioxidant properties of 2-imidazolones and 2-imidazolthiones. Biochem. Pharmacol. 1987, 36, 1457–1460. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Balk, M.; Sofia, P.; Neffe, A.T.; Tirelli, N. Lignin, the lignification process, and advanced, lignin-based materials. Intern. J. Mol. Sci. 2023, 24, 11668–11715. [Google Scholar] [CrossRef] [PubMed]
- da Graca, G.A.; Fontes, P.T.N.; Soares, A.C.; de Jesus, M.S.; Matos, P.N.; Neta, M.T.S.L.; Simoes, A.N.; de Carvalho, L.M.; de Olivera Junior, L.F.G.; Carnelossi, M.A.G.; et al. Characterization of growth and development of pumpkin cv Mini Jack fruits. Food Chem. Adv. 2024, 5, 100743–100765. [Google Scholar] [CrossRef]
- Haider, M.W.; Nafees, M.; Iqbal, R.; Asad, H.U.; Azeem, F.; Ali, B.; Shaheen, G.; Iqbal, J.; Vyas, S.; Arslan, M.; et al. Postharvest starch and sugars adjustment in potato tubers of wide-ranging dormancy genotypes subjected to various sprout forcing techniques. Sci. Rep. 2023, 13, 14845–14867. [Google Scholar] [CrossRef] [PubMed]
- Al-Dairi, M.; Pathare, P.B.; Al-Yahayi, R. Chemical and nutritional quality changes of tomato during postharvest transportation and storage. J. Saudi Soc. Agric. Sci. 2021, 20, 401–408. [Google Scholar] [CrossRef]
- Munhuewyi, K. Postharvest Losses and Changes in Quality of Vegetables from Retail to Consumer: A Case Study of Tomato, Cabbage and Carrot. Master’s Thesis, Stellenbosch University, Stellenbosch, South Africa, 2012. [Google Scholar]
- Blando, G.; Marchello, S.; Maiorano, G.; Durante, M.; Signore, A.; Laus, M.N.; Soccio, M.; Mita, G. Bioactive compounds and antioxidant capacity in anthocyanin-rich carrots: A comparison between the black carrot and the Apulian Landrace “Polignano” carrot. Plants 2021, 10, 564–579. [Google Scholar] [CrossRef] [PubMed]
- Howard, I.A.; Wong, A.D.; Perry, A.K.; Klein, B.P. Beta-carotene and ascorbic acid retention in fresh and processed vegetables. J. Food Sci. 1999, 64, 929–936. [Google Scholar] [CrossRef]
- Lee, C.Y. Changes in carotenoid content of carrots during growth and post-harvest storage. Food Chem. 1986, 20, 285–293. [Google Scholar] [CrossRef]
- Berger, M.; Kuchler, T.; Maasen, A.; Busch-Stockfisch, M.; Steinhart, M. Correlation of carotene with sensory attributes in carrots under different storage conditions. Food Chem. 2008, 106, 235–240. [Google Scholar] [CrossRef]
- Imsic, M.; Winkler, S.; Tomkins, B.; Jones, R. Effect of storage and cooking on beta-carotene isomers in carrots (Daucus carota L. cv Stefano). J. Agric. Food Chem. 2010, 58, 5109–5113. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.-S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Brenesa, P.; O’Hareb, T. Effect of freezing and cool storage on carotenoid content and quality of zeaxanthin-biofortified and standard yellow sweet-corn (Zea mays L.). J. Food Compos. Anal. 2020, 86, 103353–103360. [Google Scholar] [CrossRef]
- Haq, R.; Prasad, K. Antioxidant activity, phenolic, carotenoid and color changes in packaged fresh carrots stored under refrigeration temperature. J. Food Meas. Charact. 2017, 11, 1542–1549. [Google Scholar] [CrossRef]
- Hager, T.J.; Howard, L.R. Processing effects on carrots phytonutrients. Hort. Sci. 2006, 41, 74–79. [Google Scholar] [CrossRef]
- Galani, J.H.; Pater, J.S.; Patel, N.J.; Talati, J.G. Storage of fruits and vegetables in refrigerator increases their phenolic acids but decreases the total phenolics, anthocyanins and vitamin C with subsequent loss of their antioxidant capacity. Antioxidants 2017, 6, 58–59. [Google Scholar] [CrossRef]
- Macura, R.; Michalczyk, M.; Fiutak, G.; Maciejaszek, I. Effect of freeze-drying and air drying on the content of carotenoids and anthocyanins in stored purple carrot. Acta Sci. Pol. Technol. Alim. 2019, 18, 135–142. [Google Scholar]
- Pérez, M.B.; Carvajal, S.; Beretta, V.; Bannoud, F.; Fangio, M.F.; Berli, F.; Fontana, A.; Salomón, M.V.; González, R.; Valerga, L.; et al. Characterization of purple carrot germplasm for antioxidant capacity and root concentration of anthocyanins, phenolics, and carotenoids. Plants 2023, 12, 1796–1822. [Google Scholar] [CrossRef]

| Fresh-Untreated | MJ Treatment | AA Treatment | ||||
|---|---|---|---|---|---|---|
| On Day 0 | On Day 21 | Control | Treated | Control | Treated | |
| TCC (mg EβC g−1 DW) | 1.41 ± 0.03 a | 3.79 ± 0.05 b | 1.59 ± 0.02 a | 1.61 ± 0.03 a | 2.06 ± 0.04 c | 2.15 ± 0.03 c |
| TAC (mg ECGg−1 DW) | 2.23 ± 0.05 a | 2.18 ± 0.04 a | 0.43 ± 0.02 b | 0.37 ± 0.01 b | 0.26 ± 0.01 b | 0.30 ± 0.02 b |
| Carotenoids | Fresh-Untreated | MJ Treatment | ABA Treatment | |
|---|---|---|---|---|
| On Day 0 | On Day 21 | |||
| LUTEIN (mgEβC g−1 DW) | 0.23 ± 0.01 a | 0.68 ± 0.03 b | 0.18 ± 0.01 a | 0.16 ± 0.02 a |
| β-CAROTENE (mgEβC g−1 DW) | 4.87 ± 0.07 a | 28.16 ± 0.10 b | 2.06 ± 0.05 c | 4.93 ± 0.09 a |
| Assay | Fresh-Untreated | MJ Treatment | ABA Treatment | |||
|---|---|---|---|---|---|---|
| On Day 0 | On Day 21 | Control | Treated | Control | Treated | |
| DPPH (mg Trolox g−1 DW) | 12.24 ± 0.06 a | 12.71 ± 0.05 a | 4.73 ± 0.02 b | 4.09 ± 0.03 b | 4.25 ± 0.02 b | 4.89 ± 0.02 b |
| FRAP (mg Trolox g−1 DW) | 17.57 ± 0.08 a | 30.03 ± 0.07 a | 7.38 ± 0.02 b | 6.02 ± 0.02 b | 6.37 ± 0.04 b | 7.02 ± 0.03 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sáez-Escudero, L.; Blanch, G.P.; Ruiz del Castillo, M.L. Influence of Treatment with Natural Phytoregulators on Purple Carrots (Daucus carota L.) during Cold Storage. Appl. Sci. 2024, 14, 6359. https://doi.org/10.3390/app14146359
Sáez-Escudero L, Blanch GP, Ruiz del Castillo ML. Influence of Treatment with Natural Phytoregulators on Purple Carrots (Daucus carota L.) during Cold Storage. Applied Sciences. 2024; 14(14):6359. https://doi.org/10.3390/app14146359
Chicago/Turabian StyleSáez-Escudero, Laura, Gracia Patricia Blanch, and María Luisa Ruiz del Castillo. 2024. "Influence of Treatment with Natural Phytoregulators on Purple Carrots (Daucus carota L.) during Cold Storage" Applied Sciences 14, no. 14: 6359. https://doi.org/10.3390/app14146359
APA StyleSáez-Escudero, L., Blanch, G. P., & Ruiz del Castillo, M. L. (2024). Influence of Treatment with Natural Phytoregulators on Purple Carrots (Daucus carota L.) during Cold Storage. Applied Sciences, 14(14), 6359. https://doi.org/10.3390/app14146359







