A Comprehensive Review on Brain–Computer Interface (BCI)-Based Machine and Deep Learning Algorithms for Stroke Rehabilitation
Abstract
1. Introduction
2. Background
- Unlocking the potential of brain–computer interfaces (BCIs) for brain rehabilitation goes hand in hand with the remarkable power of machine learning. These intricate algorithms act as digital guides, navigating the complex landscape of brain signals to reveal the hidden pathways to recovery. By learning to recognize the unique patterns within each person’s neural code, machine learning techniques like support vector machines, deep neural networks, and random forests become adept at deciphering intentions and translating them into tangible actions. This remarkable synergy opens a world of possibilities for individualized rehabilitation, allowing us to harness the power of our own minds to regain control and rebuild skills [21,22,23,24].
- Predicting success: Machine learning can look at brain activity and predict how well someone will do in rehab. This helps doctors make better treatment plans and obtain better results [20].
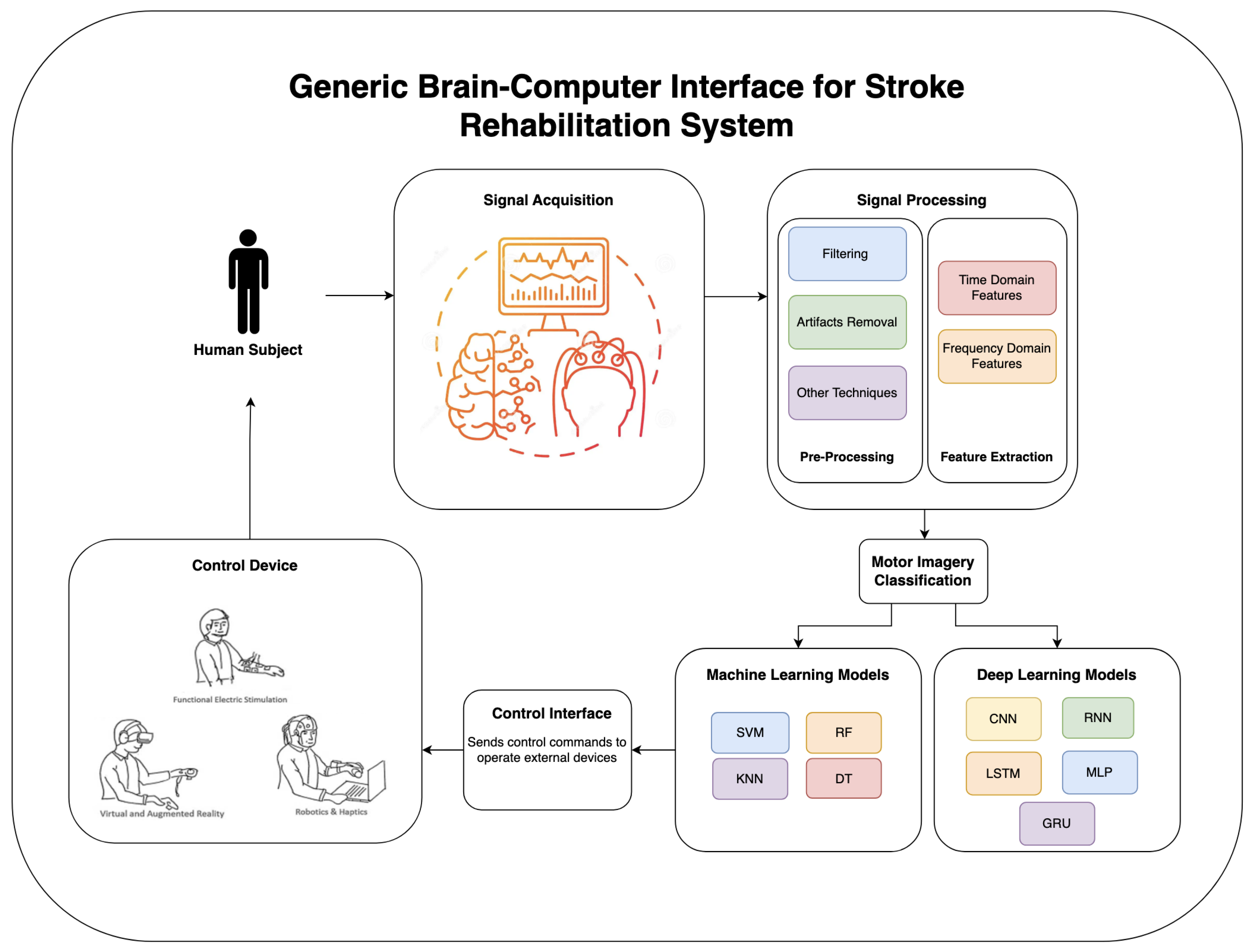
3. Diverse Applications of EEG, Machine Learning, and Deep Learning in Rehabilitation
- Motor Rehabilitation: The utilization of machine learning algorithms in decoding EEG signals during motor imagery tasks opens avenues for controlling external devices, including robotic exoskeletons and prosthetics [13,19,31,33]. Systems providing feedback in real time not only instruct users through precise motor tasks but also contribute significantly to motor skill relearning and the promotion of neuroplasticity [14,19,32,33].
- Cognitive Rehabilitation: EEG signals play a pivotal role in gauging and enhancing attention stages using Neurofeedback techniques [8,11,13,34]. The adaptability of machine learning facilitates the tailoring of training procedures tailored to individual mental states, while the nuanced capabilities of deep learning contribute to the design of personalized memory training tasks [7,8,13,34].
- Neuropsychiatric Rehabilitation: EEG neurotraining emerges as a beneficial instrument for handling stress and anxiety, utilizing artificial intelligence to recognize patterns associated with stress and triggering interventions for relaxation [10,28,35,36]. Moreover, neurotraining based on EEG assists those with ADHD in refining concentration and controlling attention by reinforcing preferred brain activity patterns [13].
4. EEG Signal Acquisition and Motor Imagery Training
4.1. EEG-Based Signal Acquisition
4.2. Patient Training on Motor Imagery Tasks
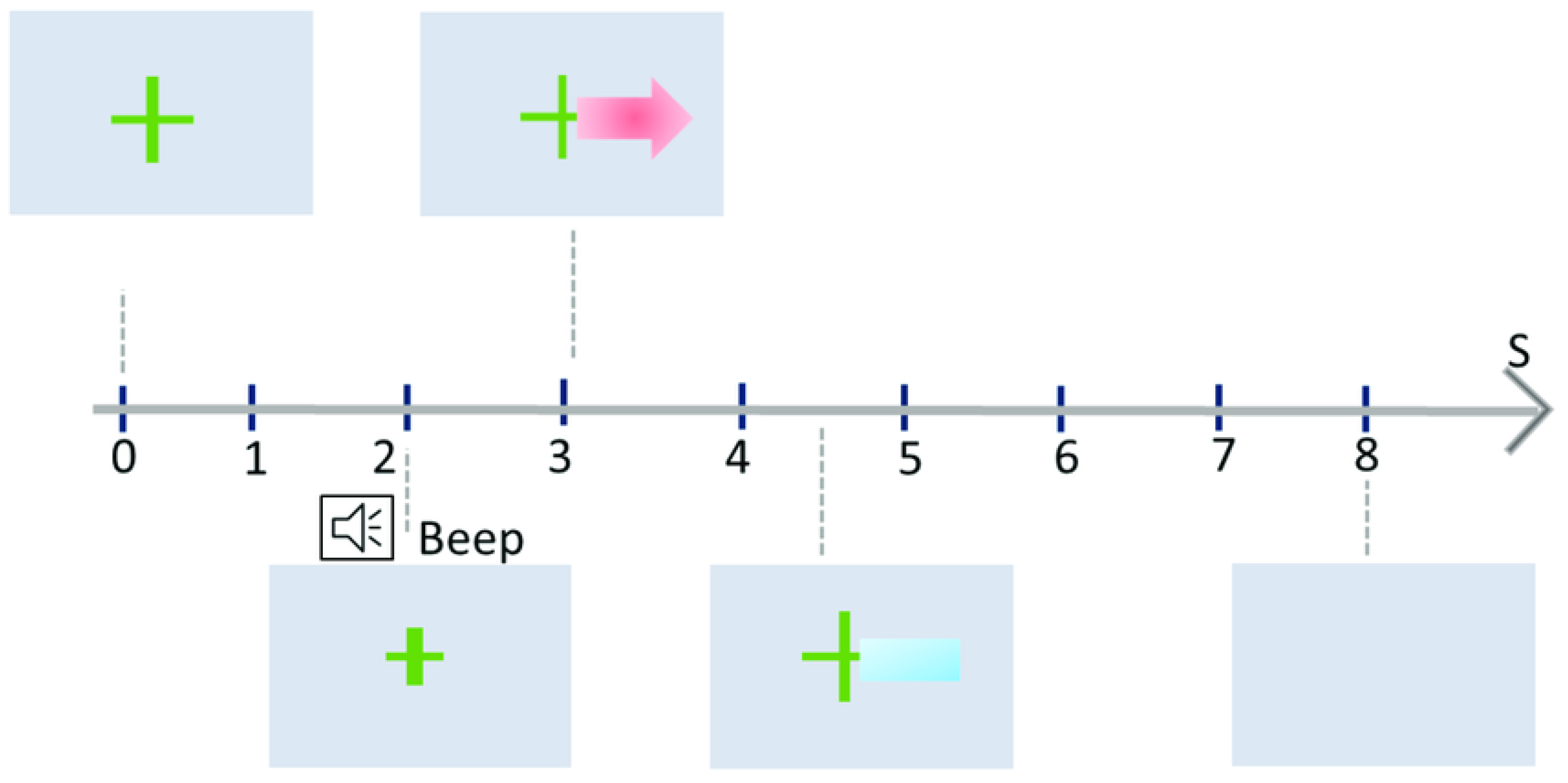
- Calibration Phase:
- Warm-up (t = 0 s): The user focuses on a fixation point or cue to settle their mind and prepare for the upcoming task. MI Task: A visual cue, such as an arrow, instructs the user to perform a specific MI task (e.g., imagine left-hand movement).
- Data Collection (t = 3 s): During the task period, EEG sensors capture the user’s brain activity, recording the unique electrical signature of the imagined movement.
- Machine Learning (t = 3–7 s): The collected data are then analyzed by a machine learning algorithm. This algorithm identifies the key features that distinguish different MI tasks from the EEG signals.
- No Feedback: In the initial phase, no feedback is provided to the user. This allows the machine learning algorithm to focus solely on understanding the user’s individual EEG patterns.
- Feedback Phase:
- MI Task Repetition: Once the system has been calibrated, users repeat the MI tasks. Real-time Feedback: This time, the system provides feedback in real time. For example, a bar might grow longer or change color based on the system’s confidence in recognizing the current MI task.
- Refinement and Repetition: With each trial, the user receives feedback and can adjust their mental strategies to produce clearer EEG patterns. This iterative process strengthens the connection between the imagined movement and the corresponding EEG signature.
- Multiple Sessions:
- Gradual Improvement: The Graz training paradigm involves multiple training sessions, spread over days or weeks. With each session, the user’s ability to generate distinct EEG patterns for different MI tasks improves, leading to more accurate recognition by the system.
- Customization: The training protocol can be adapted to individual needs and goals. The specific MI tasks, cues, and feedback types can be tailored to suit different applications, such as controlling a prosthetic limb or navigating a virtual environment.
5. EEG Signal Processing and Classification Techniques in Rehabilitation Research
5.1. Feature and Channel Selection
- Filter Approach: Initiating with the full set of features, filter methods meticulously identify the optimal subset through dedicated selection criteria. These criteria often revolve around key characteristics like information gain, dependency, consistency, correlation, and distance measures [57]. A significant advantage of filter methods lies in their minimal computational requirements. Additionally, the feature selection process operates independently of the chosen classifier, providing greater flexibility. Widely utilized filter methods include correlation criteria and mutual information techniques, both meticulously honing in on the most informative features within the data landscape.
- Wrapper Approach: Distinct from filter methods, wrapper approaches forge a direct partnership with the classifier to select features meticulously. They iteratively present candidate feature subsets to the classifier, diligently evaluating its performance. This feedback loop guides the selection process, prompting either acceptance of a subset based on established criteria or the proposal of new combinations for further evaluation. Algorithms within this realm encompass searching algorithms and evolutionary algorithms. The former embarks on their quest with an empty set, strategically adding or removing features until the classifier’s performance peaks. Their journey typically concludes when a designated maximum feature subset size is attained. Meanwhile, evolutionary algorithms, such as particle swarm optimization (PSO) [58], and artificial bee colony (ABC) [59,60], harness nature-inspired optimization techniques to uncover optimal subsets. While wrapper methods excel at identifying feature combinations that yield superior classifier performance compared to filter methods, their computational demands are considerable, rendering them less suitable for handling vast datasets.
5.2. EEG-Based Machine Learning and Deep Learning Algorithms
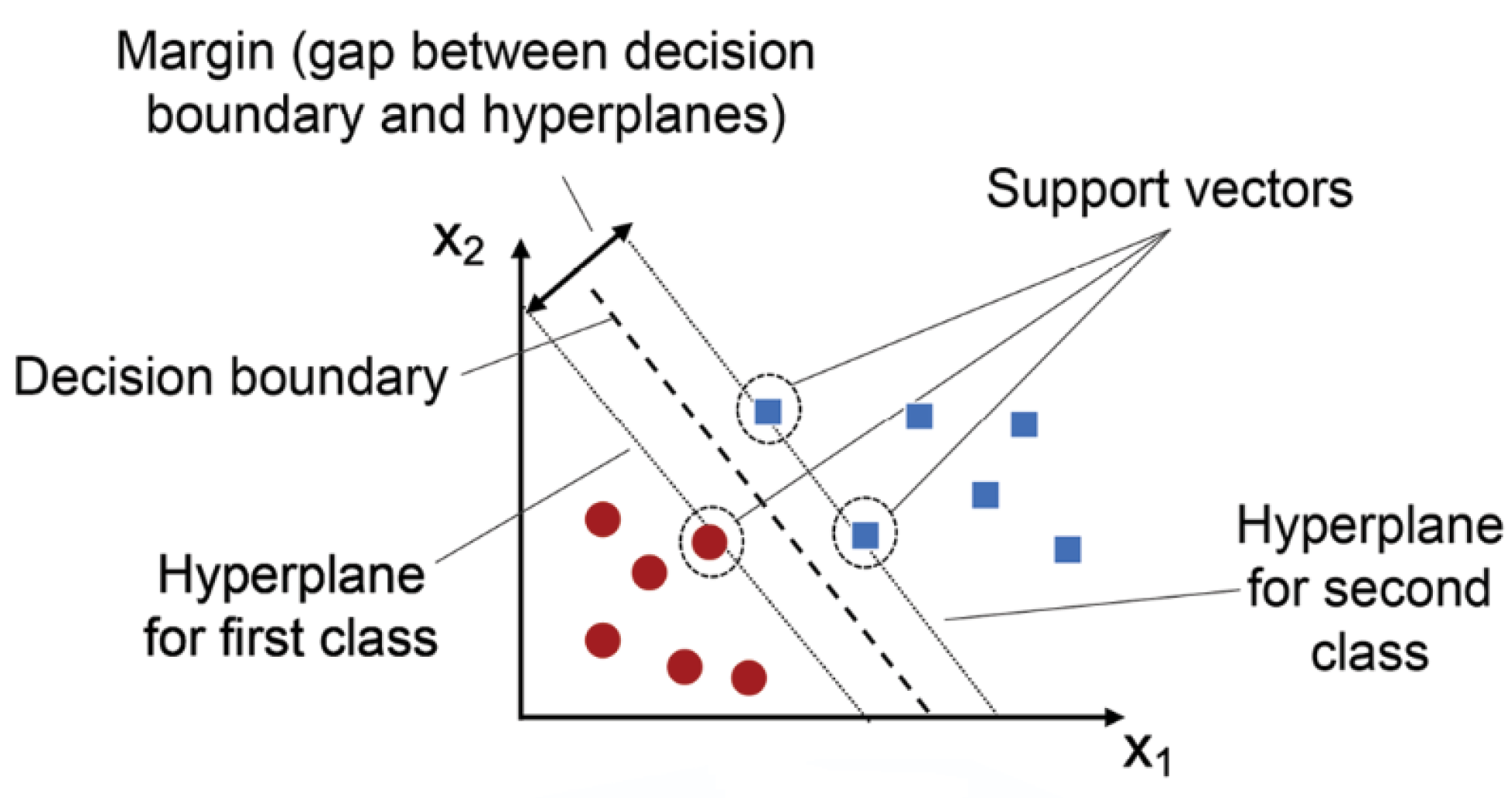
- Machine Learning Techniques:Machine learning techniques like support vector machines (SVM) and k-nearest neighbors (k-NN) have been effectively utilized in EEG-based rehabilitation.Support Vector Machines (SVM):SVMs are robust classifiers known for their precision in distinguishing between different motor tasks or targets based on EEG data. They are particularly effective with small-to-medium-sized datasets due to their ability to find the optimal hyperplane that separates different classes.
- Sample Size: Effective with small-to-medium-sized datasets.
- Accuracy: High accuracy in binary and multi-class classification problems as Figure 4.
- Training Cost: Moderate, with a need for tuning hyperparameters.
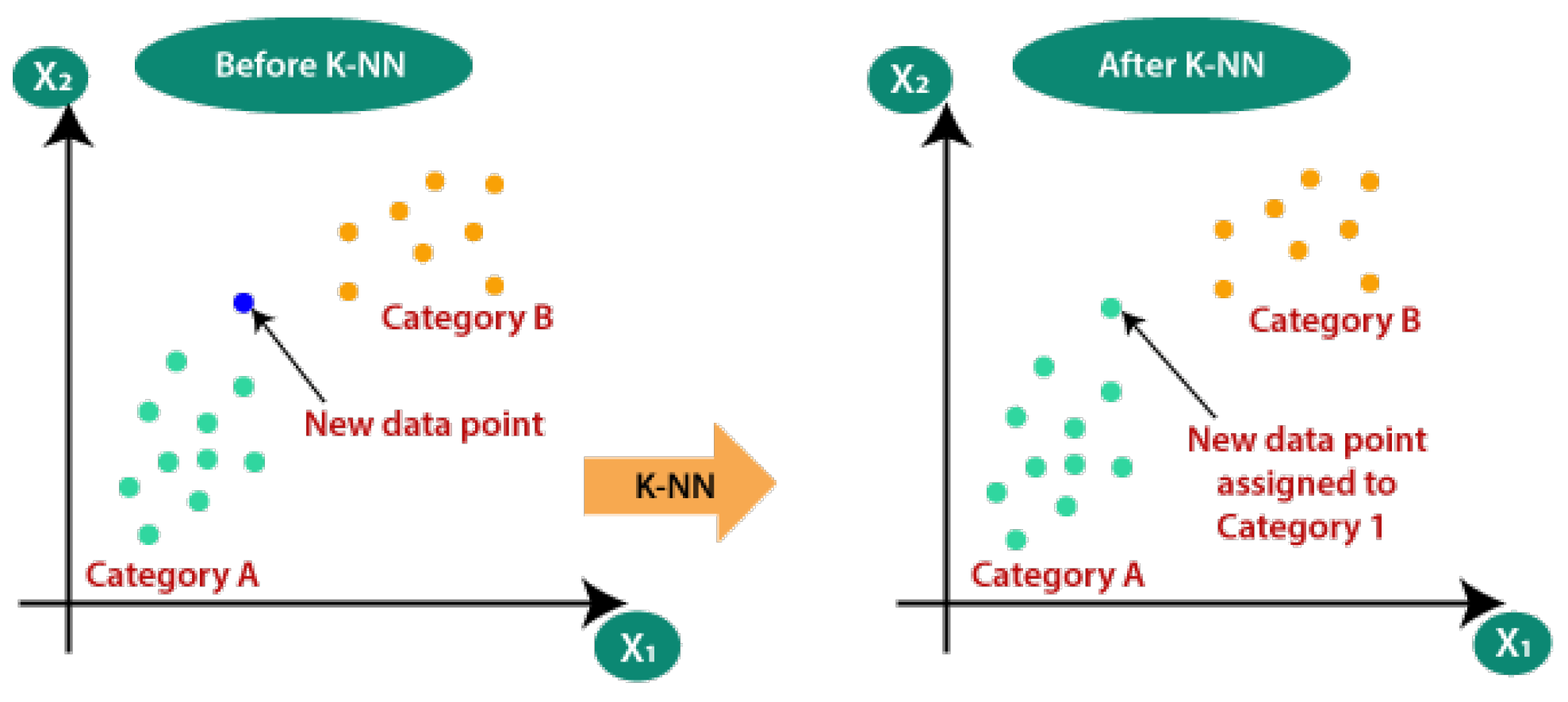
Pseudocode for SVM:Initialize parameters and load EEG datasetPreprocess the data (filtering, normalization)Define kernel function (linear, RBF, etc.)Split data into training and testing setsTrain SVM model on training dataEvaluate model on testing dataTune hyperparameters for optimal performanceOutput classification accuracyk-Nearest Neighbors (k-NN):k-NN algorithms are simple yet powerful tools for identifying patterns in brain activity related to cognitive performance. They work by comparing a new data point to the k-nearest data points in the training set, illustrated in Figure 5.- Sample Size: Suitable for small datasets.
- Accuracy: Good for pattern recognition tasks.
- Training Cost: Low, as k-NN is a lazy learner.
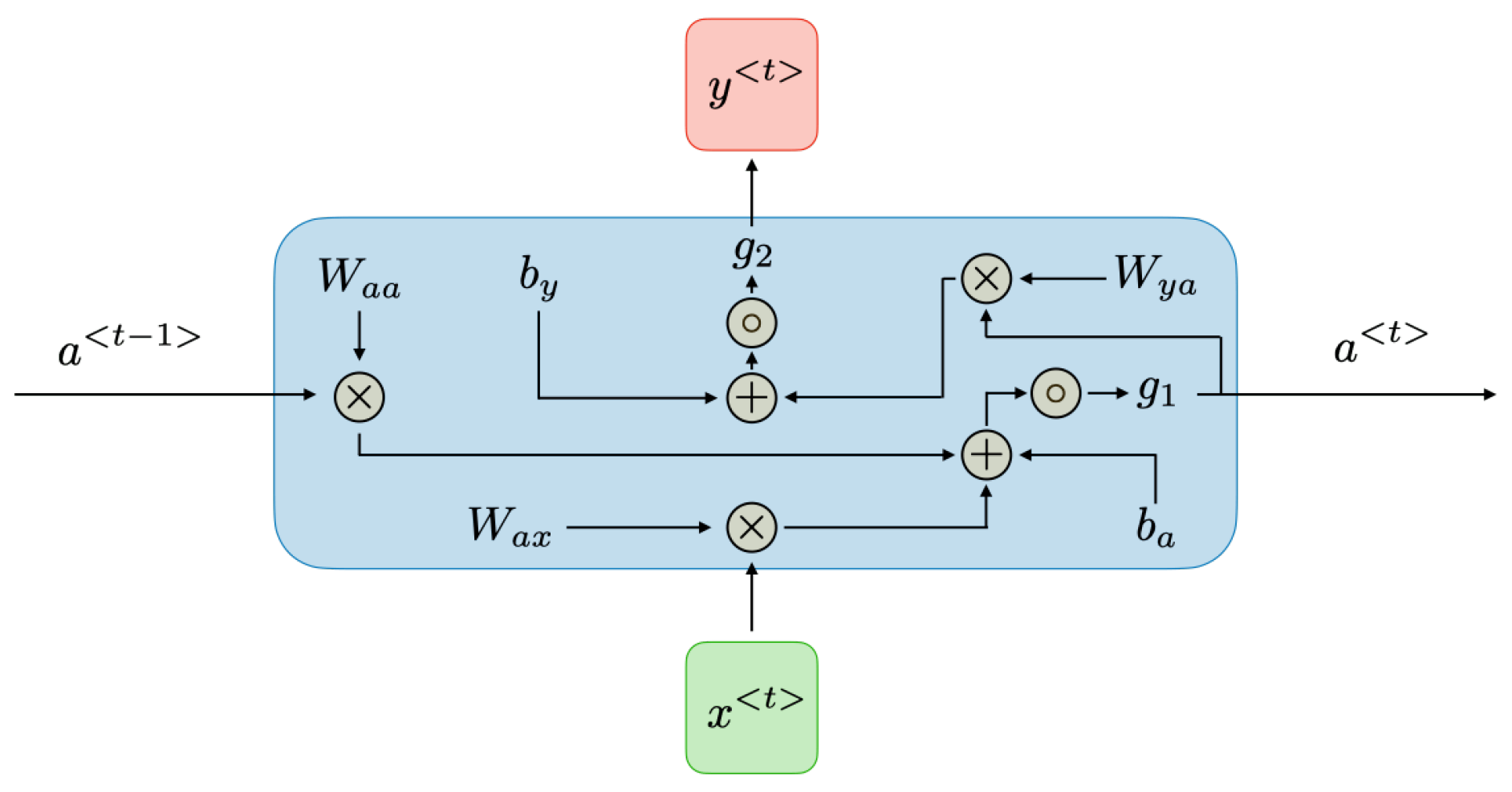
Pseudocode for k-NN:Initialize parameters and load EEG datasetPreprocess the data (filtering, normalization)Define value of kSplit data into training and testing setsFor each data point in the testing set:Calculate distance to all points in the training setIdentify k-nearest neighborsAssign class label based on majority voteEvaluate accuracy of the classifier - Deep Learning Techniques:Deep learning techniques such as Convolutional Neural Networks (CNNs), Recurrent Neural Networks (RNNs), Long Short-Term Memory networks (LSTMs), and Multi-Layer Perceptrons (MLPs) are employed in EEG-based rehabilitation for their superior ability to automatically extract features and handle complex data.Convolutional Neural Networks (CNNs):CNNs are particularly effective in spatial data analysis (see Figure 6) and have been successfully applied to EEG signal classification by extracting spatial features from EEG recordings.
- Sample Size: Requires a large dataset.
- Accuracy: High for spatial feature extraction and classification.
- Training Cost: High due to the need for extensive computational resources.
Pseudocode for CNN:Initialize parameters and load EEG datasetPreprocess the data (filtering, normalization)Define CNN architecture (number of layers, filters)Split data into training and testing setsTrain CNN model on training dataEvaluate model on testing dataTune hyperparameters for optimal performanceOutput classification accuracyRecurrent Neural Networks (RNNs):RNNs as Figure 7, especially those using LSTM or GRU units, are suitable for temporal sequence prediction, making them ideal for processing sequential EEG data.- Sample Size: Requires a large dataset.
- Accuracy: High for sequential data analysis.
- Training Cost: High due to recurrent nature and complex computations.
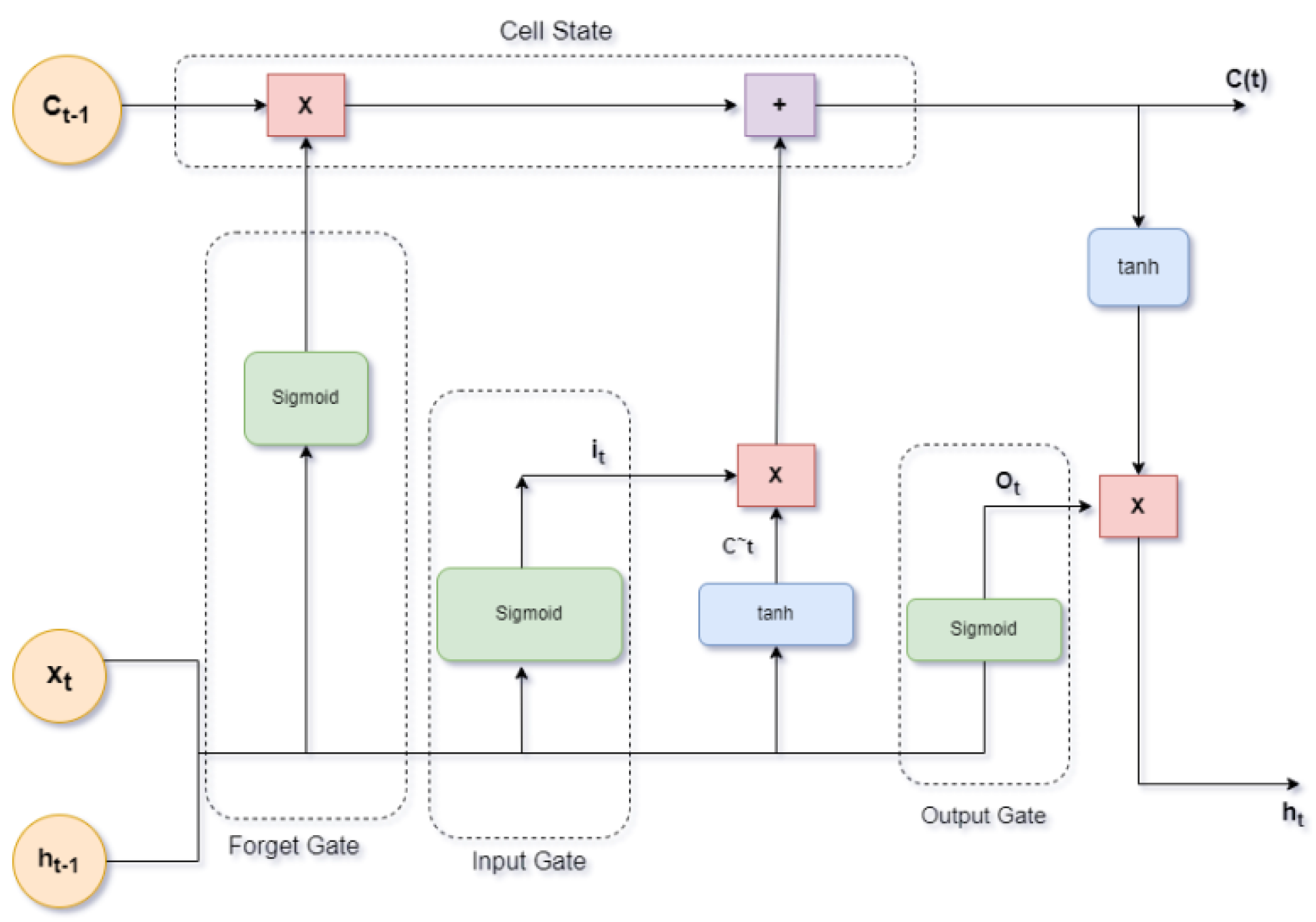
Pseudocode for RNN:Initialize parameters and load EEG datasetPreprocess the data (filtering, normalization)Define RNN architecture (number of layers, units)Split data into training and testing setsTrain RNN model on training data (sequence data)Evaluate model on testing dataTune hyperparameters for optimal performance{Output sequence prediction accuracy}Long Short-Term Memory Networks (LSTMs):LSTMs are a type of recurrent neural network capable of learning long-term dependencies, depicted in Figure 8. They excel in tasks that involve sequential data, such as learning motor sequences from EEG signals.- Sample Size: Requires a large dataset.
- Accuracy: High for temporal pattern recognition.
- Training Cost: High due to complex architecture.
Pseudocode for LSTM:Initialize parameters and load EEG datasetPreprocess the data (filtering, normalization)Define LSTM architecture (number of layers, units)Split data into training and testing setsTrain LSTM model on training data (sequence data)Evaluate model on testing dataTune hyperparameters for optimal performanceOutput sequence prediction accuracyMulti-Layer Perceptrons (MLPs):MLPs are a type of feedforward neural network that can be used for classification tasks, explained in Figure 9. They are simpler compared to CNNs and RNNs but still effective for basic EEG data classification.- Sample Size: Effective with medium to large datasets.
- Accuracy: Moderate to high, depending on the complexity of the task.
- Training Cost: Moderate.
Pseudocode for MLP:Initialize parameters and load EEG datasetPreprocess the data (filtering, normalization)Define MLP architecture (number of layers, neurons)Split data into training and testing setsTrain MLP model on training dataEvaluate model on testing dataTune hyperparameters for optimal performanceOutput classification accuracy
5.3. Performance Indicators and Metrics in Evaluating the Effectiveness of Methods
- Classification Accuracy: The efficacy of EEG-based models is scrutinized through the lens of classification accuracy, providing insights into their ability to distinguish between different classes or states in activities like differentiating motor imagery or identifying cognitive states [11,13,14,19,31,38,38].
6. Present Constraints in the Ongoing Research on Rehabilitation Utilizing EEG with ML and DL Methods
- Noise and Artifacts: Concerns surrounding data quality, preprocessing methodologies, and the standardization of data gathering protocols cast a spotlight on the imperative need to address these aspects to ensure the reliability and consistency of results.
- Small Sample Sizes: The challenges when acquiring high-quality EEG data from groups of patients contribute to the prevalence of small sample sizes, potentially leading to model overfitting and hindering the generalizability of findings.
- Longitudinal EEG Datasets: The scarcity of longitudinal EEG datasets poses a significant hurdle in monitoring progress during neural rehabilitation. A dedicated focus on long-term research is indispensable for comprehensively understanding the effectiveness of diverse approaches and customizing interventions and treatments accordingly.
- Interpretability of Deep Learning: The opaque nature of deep learning models poses challenges in interpreting results, necessitating research that seamlessly combines deep learning methodologies with insights from neuroscience. This integration is crucial for gaining a deeper understanding of the fundamental neurophysiological mechanisms associated with brain rehabilitation.
- Ethical Concerns in Real-Time Applications: While offline analysis dominates several EEG-based brain rehabilitation techniques, the shift towards real-time applications introduces ethical considerations related to patient consent, data ownership, and privacy. Meticulous attention is required to ensure that these issues are addressed with the utmost care, respecting patients’ rights.
- Bridging the Gap Between Research and Clinical Implementation: Despite the strides made in research in neurological rehabilitation utilizing EEG signals, there remains a discernible discrepancy between academic research and the practical implementation of clinical solutions. Efforts to bridge this gap are essential for the seamless translation of research findings into real-world clinical practices.
7. Comparison of EEG Headsets for Rehabilitation Purposes with Various Datasets
8. Most Significant EEG- and Motor Imagery-Based Studies in the Last 7 Years
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McFarland, D.J.; Wolpaw, J.R. Brain-computer interfaces for the operation of robotic and prosthetic devices. In Advances in Computers; Elsevier: Amsterdam, The Netherlands, 2010; Volume 79, pp. 169–187. [Google Scholar] [CrossRef]
- Dobkin, B.H. Brain-computer interface technology as a tool to augment plasticity and outcomes for neurological rehabilitation. J. Physiol. 2007, 579, 637–642. [Google Scholar] [CrossRef]
- Dodd, K.C.; Nair, V.A.; Prabhakaran, V. Role of the contralesional vs. ipsilesional hemisphere in stroke recovery. Front. Hum. Neurosci. 2017, 11, 469. [Google Scholar] [CrossRef]
- Fouad, M.M.; Amin, K.M.; El-Bendary, N.; Hassanien, A.E. Brain computer interface: A review. In Brain-Computer Interfaces: Current Trends and Applications; Springer: Berlin/Heidelberg, Germany, 2015; Volume 74, pp. 3–30. [Google Scholar] [CrossRef]
- Birbaumer, N.; Ghanayim, N.; Hinterberger, T.; Iversen, I.; Kotchoubey, B.; Kübler, A.; Perelmouter, J.; Taub, E.; Flor, H. A spelling device for the paralyzed. Nature 1999, 398, 297–298. [Google Scholar] [CrossRef]
- Mondal, D.; Alagirisamy, M. Brain computer interface (BCI): Mechanism and challenges–A survey. Int. J. Pharm. Res. 2020, 12, 2200–2208. [Google Scholar] [CrossRef]
- Wilson, J.A.; Felton, E.A.; Garell, P.C.; Schalk, G.; Williams, J.C. ECoG factors underlying multimodal control of a brain-computer interface. IEEE Trans. Neural Syst. Rehabil. Eng. 2006, 14, 246–250. [Google Scholar] [CrossRef]
- Rezeika, A.; Benda, M.; Stawicki, P.; Gembler, F.; Saboor, A.; Volosyak, I. Brain–computer interface spellers: A review. Brain Sci. 2018, 8, 57. [Google Scholar] [CrossRef]
- Hwang, H.J.; Kwon, K.; Im, C.H. Neurofeedback-based motor imagery training for brain–computer interface (BCI). J. Neurosci. Methods 2009, 179, 150–156. [Google Scholar] [CrossRef]
- Remsik, A.; Young, B.; Vermilyea, R.; Kiekhoefer, L.; Abrams, J.; Evander Elmore, S.; Schultz, P.; Nair, V.; Edwards, D.; Williams, J.; et al. A review of the progression and future implications of brain–computer interface therapies for restoration of distal upper extremity motor function after stroke. Expert Rev. Med. Devices 2016, 13, 445–454. [Google Scholar] [CrossRef]
- Ramos-Murguialday, A.; Broetz, D.; Rea, M.; Läer, L.; Yilmaz, O.; Brasil, F.L.; Liberati, G.; Curado, M.R.; Garcia-Cossio, E.; Vyziotis, A.; et al. Brain-machine interface in chronic stroke rehabilitation: A controlled study. Ann. Neurol. 2013, 74, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Kitchenham, B.; Brereton, O.P.; Budgen, D.; Turner, M.; Bailey, J.; Linkman, S. Systematic literature reviews in software engineering—A systematic literature review. Inf. Softw. Technol. 2009, 51, 7–15. [Google Scholar] [CrossRef]
- Demers, L.; Fuhrer, M.J.; Jutai, J.; Lenker, J.; Depa, M.; De Ruyter, F. A conceptual framework of outcomes for caregivers of assistive technology users. Am. J. Phys. Med. Rehabil. 2009, 88, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.; Zimmermann, J.B. Neural interfaces for the brain and spinal cord—Restoring motor function. Nat. Rev. Neurol. 2012, 8, 690–699. [Google Scholar] [CrossRef] [PubMed]
- López-Larraz, E.; Sarasola-Sanz, A.; Irastorza-Landa, N.; Birbaumer, N.; Ramos-Murguialday, A. Brain-machine interfaces for rehabilitation in stroke: A review. NeuroRehabilitation 2018, 43, 77–97. [Google Scholar] [CrossRef] [PubMed]
- Coscia, M.; Wessel, M.J.; Chaudary, U.; Millán, J.d.R.; Micera, S.; Guggisberg, A.; Vuadens, P.; Donoghue, J.; Birbaumer, N.; Hummel, F.C. Neurotechnology-aided interventions for upper limb motor rehabilitation in severe chronic stroke. Brain 2019, 142, 2182–2197. [Google Scholar] [CrossRef]
- Oguntosin, V.W.; Mori, Y.; Kim, H.; Nasuto, S.J.; Kawamura, S.; Hayashi, Y. Design and validation of exoskeleton actuated by soft modules toward neurorehabilitation—Vision-based control for precise reaching motion of upper limb. Front. Neurosci. 2017, 11, 352. [Google Scholar] [CrossRef]
- Cheng, N.; Phua, K.S.; Lai, H.S.; Tam, P.K.; Tang, K.Y.; Cheng, K.K.; Yeow, R.C.; Ang, K.K.; Guan, C.; Lim, J.H. Brain–computer interface-based soft robotic glove rehabilitation for stroke. IEEE Trans. Biomed. Eng. 2020, 67, 3339–3351. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.; Höller, M.K.; Pereira, J.; Ofner, P.; Müller-Putz, G.R. Decoding hand movements from human EEG to control a robotic arm in a simulation environment. J. Neural Eng. 2020, 17, 036010. [Google Scholar] [CrossRef] [PubMed]
- Diekelmann, S.; Born, J. The memory function of sleep. Nat. Rev. Neurosci. 2010, 11, 114–126. [Google Scholar] [CrossRef]
- Meng, J.; Zhang, S.; Bekyo, A.; Olsoe, J.; Baxter, B.; He, B. Non-invasive electroencephalogram based control of a robotic arm for reach and grasp tasks. Sci. Rep. 2016, 6, 38565. [Google Scholar] [CrossRef]
- Akbari, H.; Sadiq, M.T.; Payan, M.; Esmaili, S.S.; Baghri, H.; Bagheri, H. Depression detection based on geometrical features extracted from SODP shape of EEG signals and binary PSO. Trait. Signal 2021, 38, 13–26. [Google Scholar] [CrossRef]
- Sadiq, M.T.; Yu, X.; Yuan, Z.; Zeming, F.; Rehman, A.U.; Ullah, I.; Li, G.; Xiao, G. Motor imagery EEG signals decoding by multivariate empirical wavelet transform-based framework for robust brain–computer interfaces. IEEE Access 2019, 7, 171431–171451. [Google Scholar] [CrossRef]
- Sharma, R.; Kim, M.; Gupta, A. Motor imagery classification in brain-machine interface with machine learning algorithms: Classical approach to multi-layer perceptron model. Biomed. Signal Process. Control 2022, 71, 103101. [Google Scholar] [CrossRef]
- Antony, J.W.; Piloto, L.; Wang, M.; Pacheco, P.; Norman, K.A.; Paller, K.A. Sleep spindle refractoriness segregates periods of memory reactivation. Current Biol. 2018, 28, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Akbari, H.; Sadiq, M.T.; Jafari, N.; Too, J.; Mikaeilvand, N.; Cicone, A.; Serra-Capizzano, S. Recognizing seizure using Poincaré plot of EEG signals and graphical features in DWT domain. Bratisl. Med. J. 2022, 124, 12–24. [Google Scholar] [CrossRef]
- Sadiq, M.T.; Yu, X.; Yuan, Z.; Aziz, M.Z. Motor imagery BCI classification based on novel two-dimensional modelling in empirical wavelet transform. Electron. Lett. 2020, 56, 1367–1369. [Google Scholar] [CrossRef]
- LaFleur, K.; Cassady, K.; Doud, A.; Shades, K.; Rogin, E.; He, B. Quadcopter control in three-dimensional space using a noninvasive motor imagery-based brain–computer interface. J. Neural Eng. 2013, 10, 046003. [Google Scholar] [CrossRef] [PubMed]
- Wodlinger, B.; Downey, J.E.; Tyler-Kabara, E.C.; Schwartz, A.B.; Boninger, M.L.; Collinger, J.L. Ten-dimensional anthropomorphic arm control in a human brain–machine interface: Difficulties, solutions, and limitations. J. Neural Eng. 2015, 12, 016011. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Xu, H.; Yuan, M.; Aziz, M.Z.; Yu, X. Exploiting asymmetric EEG signals with EFD in deep learning domain for robust BCI. Symmetry 2022, 14, 2677. [Google Scholar] [CrossRef]
- Cypher, D.; Chevrollier, N.; Montavont, N.; Golmie, N. Prevailing over wires in healthcare environments: Benefits and challenges. IEEE Commun. Mag. 2006, 44, 56–63. [Google Scholar] [CrossRef]
- Sarasola-Sanz, A.; López-Larraz, E.; Irastorza-Landa, N.; Klein, J.; Valencia, D.; Belloso, A.; Morin, F.O.; Spüler, M.; Birbaumer, N.; Ramos-Murguialday, A. An EEG-based brain-machine interface to control a 7-degrees of freedom exoskeleton for stroke rehabilitation. Converging Clin. Eng. Res. Neurorehabilit. II (Biosyst. Biorobot.) 2017, 15, 1127–1131. [Google Scholar] [CrossRef]
- Escolano, C.; Navarro-Gil, M.; Garcia-Campayo, J.; Congedo, M.; Ridder, D.D.D.; Minguez, J. A controlled study on the cognitive effect of alpha neurofeedback training in patients with major depressive disorder. Front. Behav. Neurosci. 2014, 8, 296. [Google Scholar] [CrossRef] [PubMed]
- Mortenson, W.; Demers, L.; Fuhrer, M.; Jutai, J.; Lenker, J.; DeRuyter, F. Development and preliminary evaluation of the caregiver assistive technology outcome measure. J. Rehabil. Med. 2015, 47, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.S.; Hsiao, K.L.; Liu, L.C. EEG-based detection model for evaluating and improving learning attention. J. Med. Biol. Eng. 2018, 38, 847–856. [Google Scholar] [CrossRef]
- Iturrate, I.; Chavarriaga, R.; Montesano, L.; Minguez, J.; Millán, J.d.R. Teaching brain-machine interfaces as an alternative paradigm to neuroprosthetics control. Sci. Rep. 2015, 5, 13893. [Google Scholar] [CrossRef] [PubMed]
- Hamid, H.; Naseer, N.; Nazeer, H.; Khan, M.J.; Khan, R.A.; Khan, U.S. Analyzing classification performance of fNIRS-BCI for gait rehabilitation using deep neural networks. Sensors 2022, 22, 1932. [Google Scholar] [CrossRef] [PubMed]
- Buch, E.; Weber, C.; Cohen, L.G.; Braun, C.; Dimyan, M.A.; Ard, T.; Mellinger, J.; Caria, A.; Soekadar, S.; Fourkas, A.; et al. Think to move: A neuromagnetic brain–computer interface (BCI) system for chronic stroke. Stroke 2008, 39, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Nakagome, S.; Luu, T.P.; He, Y.; Ravindran, A.S.; Contreras-Vidal, J.L. An empirical comparison of neural networks and machine learning algorithms for EEG gait decoding. Sci. Rep. 2020, 10, 4372. [Google Scholar] [CrossRef]
- Tortora, S.; Ghidoni, S.; Chisari, C.; Micera, S.; Artoni, F. Deep learning-based BCI for gait decoding from EEG with LSTM recurrent neural network. J. Neural Eng. 2020, 17, 046011. [Google Scholar] [CrossRef] [PubMed]
- Martini, M.L.; Oermann, E.K.; Opie, N.L.; Panov, F.; Oxley, T.; Yaeger, K. Sensor modalities for brain-computer interface technology: A comprehensive literature review. Neurosurgery 2020, 86, E108–E117. [Google Scholar] [CrossRef]
- Alotaiby, T.; El-Samie, F.E.A.; Alshebeili, S.A.; Ahmad, I. A review of channel selection algorithms for EEG signal processing. EURASIP J. Adv. Signal Process. 2015, 2015, 66. [Google Scholar] [CrossRef]
- Wan, X.; Zhang, K.; Ramkumar, S.; Deny, J.; Emayavaramban, G.; Ramkumar, M.S.; Hussein, A.F. A review on electroencephalogram based brain computer interface for elderly disabled. IEEE Access 2019, 7, 36380–36387. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Neuper, C. Motor imagery and direct brain-computer communication. Proc. IEEE 2001, 89, 1123–1134. [Google Scholar] [CrossRef]
- Jeunet, C.; Jahanpour, E.; Lotte, F. Why standard brain-computer interface (BCI) training protocols should be changed: An experimental study. J. Neural Eng. 2016, 13, 036024. [Google Scholar] [CrossRef] [PubMed]
- Farnsworth, B. Eeg (Electroencephalography): The Complete Pocket Guide; IMotions, Global HQ: Copenhagen, Denmark, 2019. [Google Scholar]
- Shute, V.J.; Zapata-Rivera, D. Adaptive technologies. ETS Res. Rep. Ser. 2007, 2007, 34. [Google Scholar] [CrossRef]
- Norman, S.L.; McFarland, D.J.; Miner, A.; Cramer, S.C.; Wolbrecht, E.T.; Wolpaw, J.R.; Reinkensmeyer, D.J. Controlling pre-movement sensorimotor rhythm can improve finger extension after stroke. J. Neural Eng. 2018, 15, 056026. [Google Scholar] [CrossRef]
- Sadiq, M.T.; Akbari, H.; Siuly, S.; Li, Y.; Wen, P. Alcoholic EEG signals recognition based on phase space dynamic and geometrical features. Chaos Solitons Fractals 2022, 158, 112036. [Google Scholar] [CrossRef]
- Sadiq, M.T.; Yu, X.; Yuan, Z.; Aziz, M.Z.; Rehman, N.u.; Ding, W.; Xiao, G. Motor imagery BCI classification based on multivariate variational mode decomposition. IEEE Trans. Emerg. Top. Comput. Intell. 2022, 6, 1177–1189. [Google Scholar] [CrossRef]
- Yu, X.; Aziz, M.Z.; Sadiq, M.T.; Fan, Z.; Xiao, G. A new framework for automatic detection of motor and mental imagery EEG signals for robust BCI systems. IEEE Trans. Instrum. Meas. 2021, 70, 1–12. [Google Scholar] [CrossRef]
- Altaheri, H.; Muhammad, G.; Alsulaiman, M.; Amin, S.; Altuwaijri, G.; Abdul, W.; Bencherif, M.; Faisal, M. Deep learning techniques for classification of electroencephalogram (EEG) motor imagery (MI) signals: A review. Neural Comput. Appl. 2023, 35, 14681–14722. [Google Scholar] [CrossRef]
- Escolano, C.; Navarro-Gil, M.; Garcia-Campayo, J.; Congedo, M.; Minguez, J. The effects of individual upper alpha neurofeedback in ADHD: An open-label pilot study. Appl. Psychophysiol. Biofeedback 2014, 39, 193–202. [Google Scholar] [CrossRef]
- Ketz, N.; Jones, A.P.; Bryant, N.B.; Clark, V.P.; Pilly, P.K. Closed-loop slow-wave tACS improves sleep-dependent long-term memory generalization by modulating endogenous oscillations. J. Neurosci. 2018, 38, 7314–7326. [Google Scholar] [CrossRef] [PubMed]
- Acı, Ç.İ.; Kaya, M.; Mishchenko, Y. Distinguishing mental attention states of humans via an EEG-based passive BCI using machine learning methods. Expert Syst. Appl. 2019, 134, 153–166. [Google Scholar] [CrossRef]
- Kamble, A.; Ghare, P.H.; Kumar, V. Deep-learning-based BCI for automatic imagined speech recognition using SPWVD. IEEE Trans. Instrum. Meas. 2023, 72, 1–10. [Google Scholar] [CrossRef]
- Venkatesh, B.; Anuradha, J. A review of feature selection and its methods. Cybern. Inf. Technol. 2019, 19, 3–26. [Google Scholar] [CrossRef]
- Atyabi, A.; Shic, F.; Naples, A. Mixture of autoregressive modeling orders and its implication on single trial EEG classification. Expert Syst. Appl. 2016, 65, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Karaboga, D. An Idea Based on Honey Bee Swarm for Numerical Optimization; Technical Report, Technical Report-tr06; Erciyes University, Engineering Faculty, Computer Engineering Department: Kayseri, Turkey, 2005. [Google Scholar]
- Rakshit, P.; Bhattacharyya, S.; Konar, A.; Khasnobish, A.; Tibarewala, D.N.; Janarthanan, R. Artificial Bee Colony Based Feature Selection for Motor Imagery EEG Data. In Proceedings of the Seventh International Conference on Bio-Inspired Computing: Theories and Applications (BIC-TA 2012); Bansal, J.C., Singh, P., Deep, K., Pant, M., Nagar, A., Eds.; Springer: Gwalior, India, 2013; pp. 127–138. [Google Scholar]
- Proulx, C.E.; Beaulac, M.; David, M.; Deguire, C.; Haché, C.; Klug, F.; Kupnik, M.; Higgins, J.; Gagnon, D.H. Review of the effects of soft robotic gloves for activity-based rehabilitation in individuals with reduced hand function and manual dexterity following a neurological event. J. Rehabil. Assistive Technol. Eng. 2020, 7, 205566832091813. [Google Scholar] [CrossRef] [PubMed]
- Tabar, Y.R.; Halici, U. A novel deep learning approach for classification of EEG motor imagery signals. J. Neural Eng. 2016, 14, 016003. [Google Scholar] [CrossRef]
- Tang, Z.; Li, C.; Sun, S. Single-trial EEG classification of motor imagery using deep convolutional neural networks. Optik 2017, 130, 11–18. [Google Scholar] [CrossRef]
- Dose, H.; Møller, J.S.; Iversen, H.K.; Puthusserypady, S. An end-to-end deep learning approach to MI-EEG signal classification for BCIs. Expert Syst. Appl. 2018, 114, 532–542. [Google Scholar] [CrossRef]
- Olivas-Padilla, B.E.; Chacon-Murguia, M.I. Classification of multiple motor imagery using deep convolutional neural networks and spatial filters. Appl. Soft Comput. 2019, 75, 461–472. [Google Scholar] [CrossRef]
- Tayeb, Z.; Fedjaev, J.; Ghaboosi, N.; Richter, C.; Everding, L.; Qu, X.; Wu, Y.; Cheng, G.; Conradt, J. Validating Deep Neural Networks for Online Decoding of Motor Imagery Movements from EEG Signals. Sensors 2019, 19, 210. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhao, J.; Fu, W.; Pan, J.; Zhou, H. A novel classification algorithm for MI-EEG based on deep learning. In Proceedings of the 2019 IEEE 8th Joint International Information Technology and Artificial Intelligence Conference (ITAIC), Chongqing, China, 24–26 May 2019; pp. 606–611. [Google Scholar]
- Qiao, W.; Bi, X. Deep spatial-temporal neural network for classification of EEG-based motor imagery. In Proceedings of the 2019 International Conference on Artificial Intelligence and Computer Science, Wuhan, China, 12–13 July 2019; pp. 265–272. [Google Scholar]
- Song, Y.; Wang, D.; Yue, K.; Zheng, N.; Shen, Z.J.M. EEG-based motor imagery classification with deep multi-task learning. In Proceedings of the 2019 International Joint Conference on Neural Networks (IJCNN), Budapest, Hungary, 14–19 July 2019; pp. 1–8. [Google Scholar]
- Amin, S.U.; Alsulaiman, M.; Muhammad, G.; Mekhtiche, M.A.; Shamim Hossain, M. Deep Learning for EEG motor imagery classification based on multi-layer CNNs feature fusion. Future Gener. Comput. Syst. 2019, 101, 542–554. [Google Scholar] [CrossRef]
- Alazrai, R.; Abuhijleh, M.; Alwanni, H.; Daoud, M.I. A Deep Learning Framework for Decoding Motor Imagery Tasks of the Same Hand Using EEG Signals. IEEE Access 2019, 7, 109612–109627. [Google Scholar] [CrossRef]
- Li, F.; He, F.; Wang, F.; Zhang, D.; Xia, Y.; Li, X. A Novel Simplified Convolutional Neural Network Classification Algorithm of Motor Imagery EEG Signals Based on Deep Learning. Appl. Sci. 2020, 10, 1605. [Google Scholar] [CrossRef]
- Miao, M.; Hu, W.; Yin, H.; Zhang, K. Spatial-Frequency Feature Learning and Classification of Motor Imagery EEG Based on Deep Convolution Neural Network. Comput. Math. Methods Med. 2020, 2020, 1981728. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ma, Z.; Wang, J.; Fu, Y. A Novel Deep Learning Scheme for Motor Imagery EEG Decoding Based on Spatial Representation Fusion. IEEE Access 2020, 8, 202100–202110. [Google Scholar] [CrossRef]
- Ieracitano, C.; Morabito, F.C.; Hussain, A.; Mammone, N. A hybrid-domain deep learning-based BCI for discriminating hand motion planning from EEG sources. Int. J. Neural Syst. 2021, 31, 2150038. [Google Scholar] [CrossRef]
- Zhang, C.; Kim, Y.K.; Eskandarian, A. EEG-inception: An accurate and robust end-to-end neural network for EEG-based motor imagery classification. J. Neural Eng. 2021, 18, 046014. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhang, B.; Yu, N.; Liu, K.; Sun, K. Advanced TSGL-EEGNet for Motor Imagery EEG-Based Brain-Computer Interfaces. IEEE Access 2021, 9, 25118–25130. [Google Scholar] [CrossRef]
- Cho, J.H.; Jeong, J.H.; Lee, S.W. NeuroGrasp: Real-Time EEG Classification of High-Level Motor Imagery Tasks Using a Dual-Stage Deep Learning Framework. IEEE Trans. Cybern. 2022, 52, 13279–13292. [Google Scholar] [CrossRef]
- Tiwari, S.; Goel, S.; Bhardwaj, A. MIDNN-a classification approach for the EEG based motor imagery tasks using deep neural network. Appl. Intell. 2022, 52, 4824–4843. [Google Scholar] [CrossRef]
- Huang, E.; Zheng, X.; Fang, Y.; Zhang, Z. Classification of Motor Imagery EEG Based on Time-Domain and Frequency-Domain Dual-Stream Convolutional Neural Network. IRBM 2022, 43, 107–113. [Google Scholar] [CrossRef]
- Huang, W.; Chang, W.; Yan, G.; Yang, Z.; Luo, H.; Pei, H. EEG-based motor imagery classification using convolutional neural networks with local reparameterization trick. Expert Syst. Appl. 2022, 187, 115968. [Google Scholar] [CrossRef]
- Zhu, H.; Forenzo, D.; He, B. On the Deep Learning Models for EEG-Based Brain-Computer Interface Using Motor Imagery. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 2283–2291. [Google Scholar] [CrossRef]
- Mattioli, F.; Porcaro, C.; Baldassarre, G. A 1D CNN for high accuracy classification and transfer learning in motor imagery EEG-based brain-computer interface. J. Neural Eng. 2022, 18, 066053. [Google Scholar] [CrossRef]
- Du, Y.; Liu, J. IENet: A robust convolutional neural network for EEG based brain-computer interfaces. J. Neural Eng. 2022, 19, 036031. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Chen, W. Adaptive CNN for EEG classification in stroke rehabilitation. J. Neurosci. Methods 2023, 275, 112–120. [Google Scholar]
- Wang, Z.; Liu, S.; Zhou, Y. Attention-based CNN for EEG motor imagery classification. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 1365–1373. [Google Scholar]
- Zhang, L.; Wang, H.; Li, J. Graph-CNN for spatial feature learning in EEG classification. Med. Biol. Eng. Comput. 2023, 61, 451–459. [Google Scholar]
- Xu, Y.; Zhang, W.; Chen, S. Hybrid CNN-RNN model for EEG motor imagery classification. Front. Neurosci. 2024, 18, 127–135. [Google Scholar]
- Chen, L.; Liu, Q.; Yang, H. Transformer-based model for EEG classification in stroke rehabilitation. IEEE Trans. Biomed. Eng. 2024, 71, 1461–1470. [Google Scholar]



| Brand | Model | Number of Channels | Intended Use |
|---|---|---|---|
| Emotiv | Emotiv EPOCH | 5–14 channels | Used in research and for personal use |
| Emotiv Insight | |||
| BIOPAC systems | EEG100C | 16 channels | Used in sleep studies and evoked responses |
| OpenBCI | OpenBCI 32-bit | 4–21 channels | Used in BCI and biosensing |
| OpenBCI Cyton | |||
| OpenBCI Ganglion | |||
| Ultracortex BCI | |||
| Narosky | Brainwave | 1 channel | Used in neurogaming and meditation |
| Mindflex | |||
| Mindwave |
| References | Year | Dataset | DL Model | Classification Results |
|---|---|---|---|---|
| [62] | 2016 | BCI competition IV dataset 2b | CNN+SAE | 72.40% |
| [63] | 2017 | Collected (109 subjects) | CNN | 86.41% |
| [64] | 2018 | Physionet EEG MI Dataset | CNN | 80.38% |
| [65] | 2019 | BCI competition IV dataset 2a | CNN | 82.09% |
| [66] | 2019 | BCI competition IV dataset 2b | CNN | 77.72% |
| [67] | 2019 | BCI competition data IV 2a | CNN+SAE | 79.90% |
| [68] | 2019 | BCI competition data IV 2a | CNN+Bi-GRU | 76.62% |
| [69] | 2019 | BCI competition data IV 2a | CNN | 73.40% |
| [70] | 2019 | BCI competition data IV 2a | CNN | 75.7% |
| [71] | 2019 | Collected (22 subjects) | CNN | 73.70% |
| [72] | 2020 | BCI Competition IV 2b | CNN | 83.20% |
| [73] | 2020 | BCI competition IVa, right index finger MI dataset | CNN | 90.00% |
| [74] | 2020 | BCI competition IV dataset 1 | CNN | 86.40% |
| [75] | 2021 | 15 subjects | CNN | 76.21% |
| [76] | 2021 | BCI Competition IV dataset 2a and 2b | CNN | 88.40% |
| [77] | 2021 | BCI Competition IV 2a, III | CNN | 85.30% |
| [78] | 2021 | Collected (12 subjects) | Bi-LSTM | 68.00% |
| [79] | 2021 | BCI competition V dataset, Emotiv dataset | CNN | 72.51% and 72% |
| [80] | 2021 | BCI competition IV dataset 2a | CNN | 90.00% |
| [81] | 2022 | PhysioNet dataset | CNN | 92.00% |
| [82] | 2022 | Med-62 | ConvNet | 72.66% |
| [83] | 2022 | EEG Motor Movement Dataset V 1.0.0 | CNN | 99.38% |
| [84] | 2022 | MRCP | CNN | 91.00% |
| [85] | 2023 | BCI competition IV dataset 2a | Adaptive CNN | 93.20% |
| [86] | 2023 | Collected (30 subjects) | Attention-based CNN | 88.75% |
| [87] | 2023 | BCI competition IV dataset 2b | Graph-CNN | 89.60% |
| [88] | 2024 | PhysioNet EEG MI Dataset | Hybrid CNN-RNN | 94.50% |
| [89] | 2024 | BCI competition IV dataset 2a | Transformer-based Model | 92.30% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elashmawi, W.H.; Ayman, A.; Antoun, M.; Mohamed, H.; Mohamed, S.E.; Amr, H.; Talaat, Y.; Ali, A. A Comprehensive Review on Brain–Computer Interface (BCI)-Based Machine and Deep Learning Algorithms for Stroke Rehabilitation. Appl. Sci. 2024, 14, 6347. https://doi.org/10.3390/app14146347
Elashmawi WH, Ayman A, Antoun M, Mohamed H, Mohamed SE, Amr H, Talaat Y, Ali A. A Comprehensive Review on Brain–Computer Interface (BCI)-Based Machine and Deep Learning Algorithms for Stroke Rehabilitation. Applied Sciences. 2024; 14(14):6347. https://doi.org/10.3390/app14146347
Chicago/Turabian StyleElashmawi, Walaa H., Abdelrahman Ayman, Mina Antoun, Habiba Mohamed, Shehab Eldeen Mohamed, Habiba Amr, Youssef Talaat, and Ahmed Ali. 2024. "A Comprehensive Review on Brain–Computer Interface (BCI)-Based Machine and Deep Learning Algorithms for Stroke Rehabilitation" Applied Sciences 14, no. 14: 6347. https://doi.org/10.3390/app14146347
APA StyleElashmawi, W. H., Ayman, A., Antoun, M., Mohamed, H., Mohamed, S. E., Amr, H., Talaat, Y., & Ali, A. (2024). A Comprehensive Review on Brain–Computer Interface (BCI)-Based Machine and Deep Learning Algorithms for Stroke Rehabilitation. Applied Sciences, 14(14), 6347. https://doi.org/10.3390/app14146347







