Reproducibility of Three-Dimensional Density Measurements in Teeth Using Micro-Computed Tomography: An Image Acquisition Protocol
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Composition and Scanning
2.2. Dental Mineral Density Measurement Protocol
2.2.1. Segments and Thresholds
2.2.2. Reference Points
2.2.3. Angles and Distances
2.2.4. Mineral Density Calculation
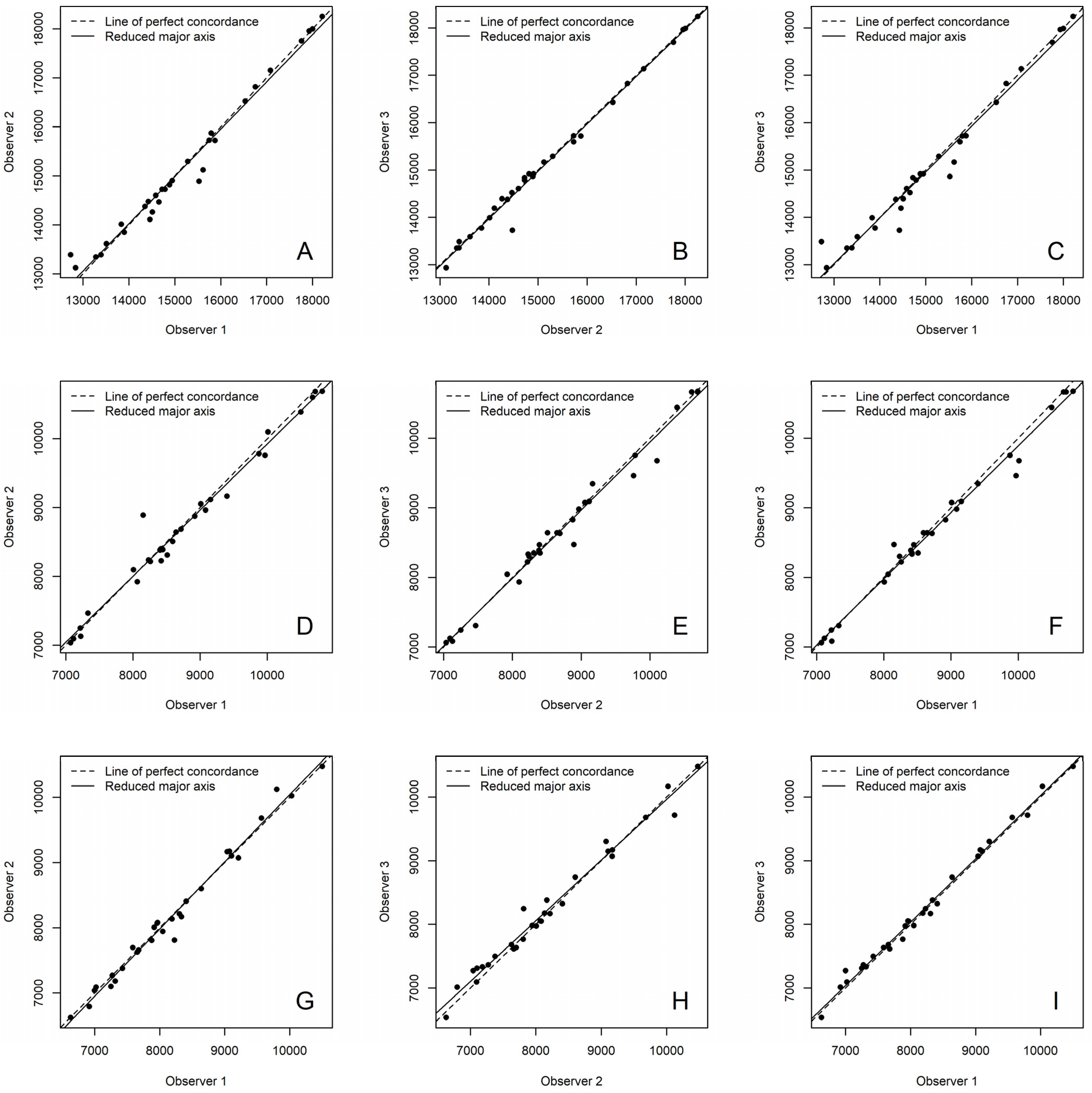
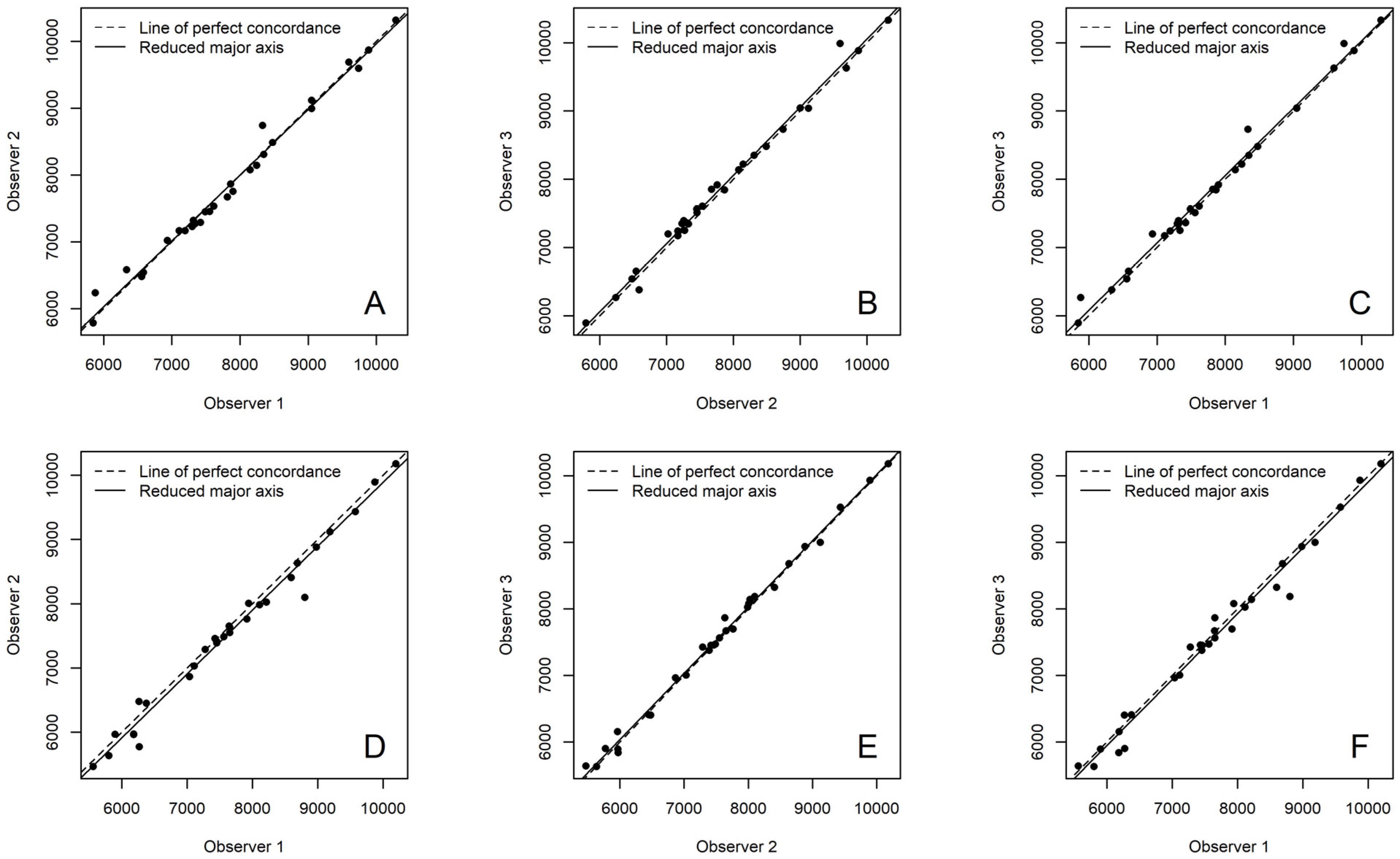
2.3. Statistical Analysis
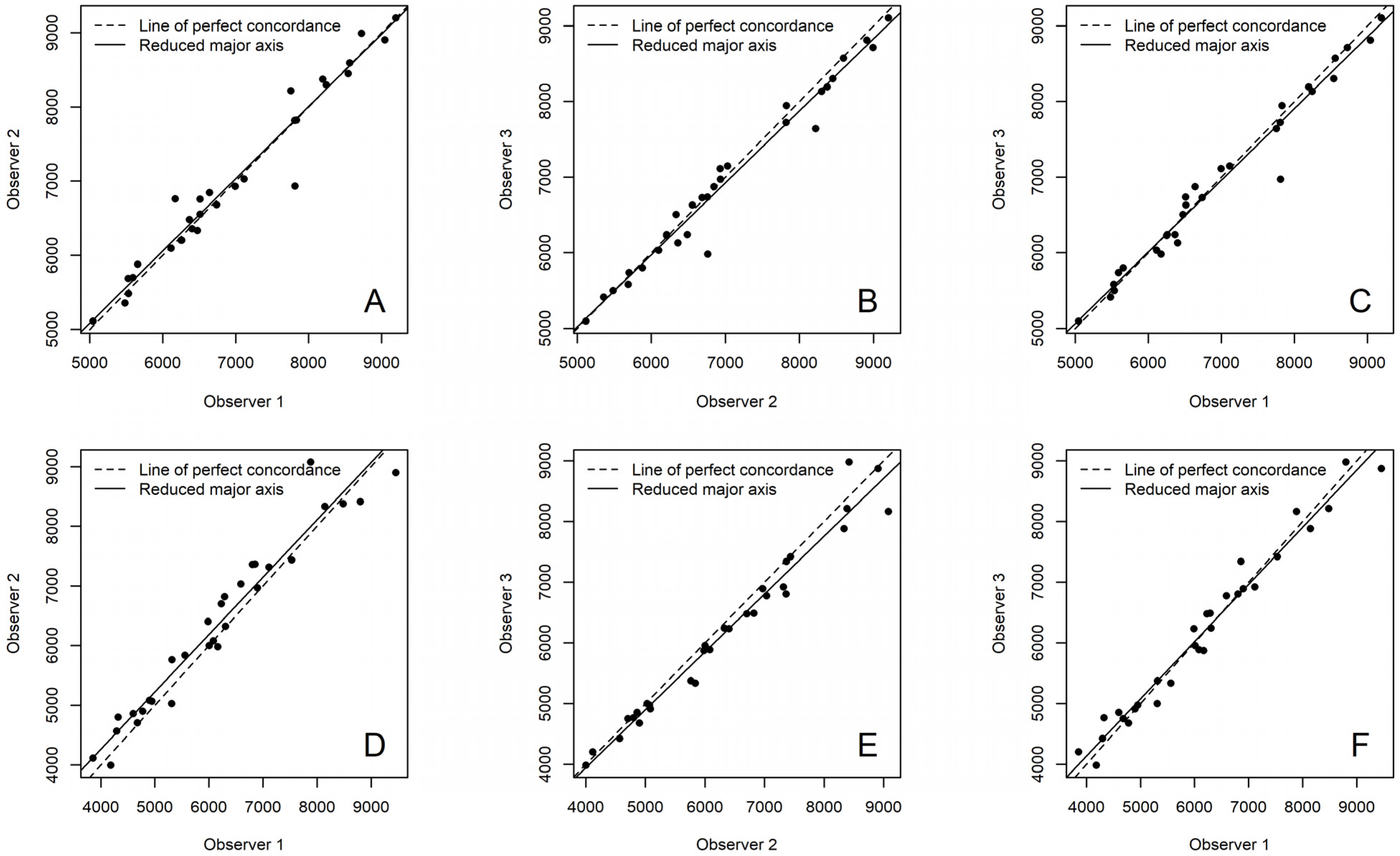
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holmes, J.; Akkinepalli, R. Computed Tomography versus Plain Radiography to Screen for Cervical Spine Injury: A Meta-Analysis. J. Trauma Inj. Infect. Crit. Care 2005, 58, 902–905. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.; Evershed, A.; Mills, D. Quantitative High Contrast X-Ray Microtomography for Dental Research. J. Dent. 2013, 41, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Beck, J. What Is the Future of Imaging in Forensic Practice? Radiography 2011, 17, 212–217. [Google Scholar] [CrossRef]
- Bibliowicz, J.; Khan, A.; Agur, A.; Singh, K. High-Precision Surface Reconstruction of Human Bones from Point-Sampled Data; Autodesk Inc.: San Francisco, CA, USA, 2011; pp. 1–10. [Google Scholar]
- Kim, I.; Paik, K.; Lee, S. Quantitative Evaluation of the Accuracy of Micro-Computed Tomography in Tooth Measurement. Clin. Anat. 2007, 20, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Balto, K.; Muller, R.; Carrington, D.; Dobeck, J.; Stashenko, P. Quantification of Periapical Bone Destruction in Mice by Micro-Computed Tomography. J. Dent. Res. 2000, 79, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Grande, N.; Plotino, G.; Gambarini, G.; Testarelli, L.; D’Ambrosio, F.; Pecci, R.; Bedini, R. Present and Future in the Use of Micro-CT Scanner 3D Analysis for the Study of Dental and Root Canal Morphology. Ann. Ist. Super. Sanità 2012, 48, 26–34. [Google Scholar]
- Marca, C.; Dummer, P.; Bryant, S.; Vier-Pelisser, F.; Só, M.; Fontanella, V.; Dutra, V.; de Figueiredo, J. Three-Rooted Premolar Analyzed by High-Resolution and Cone Beam CT. Clin. Oral Investig. 2013, 17, 1535–1540. [Google Scholar] [CrossRef]
- Shaheen, E.; Khalil, W.; Ezeldeen, M.; Van de Casteele, E.; Sun, Y.; Politis, C.; Jacobs, R. Accuracy of Segmentation of Tooth Structures Using 3 Different CBCT Machines. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 123, 123–128. [Google Scholar] [CrossRef]
- Paqué, F.; Laib, A.; Gautschi, H.; Zehnder, M. Hard-Tissue Debris Accumulation Analysis by High-Resolution Computed Tomography Scans. J. Endod. 2009, 35, 1044–1047. [Google Scholar] [CrossRef]
- Swain, M.; Xue, J. State of the Art of Micro-CT Applications in Dental Research. Int. J. Oral Sci. 2009, 1, 177–188. [Google Scholar] [CrossRef]
- Martin-Frances, L.; Martinoon-Torres, M.; Martínez de Pinillos, M.; Garcia-Campos, C.; Modesto-Mata, M.; Zanolli, C.; Rodrguez, L.; De Castro, J.M.B. Tooth Crown Tissue Proportions and Enamel Thickness in Early Pleistocene Homo Antecessor Molars (Atapuerca, Spain). PLoS ONE 2018, 13, e0203334. [Google Scholar] [CrossRef] [PubMed]
- Fernée, C.; Zakrzewski, S.; Robson Brown, K. Dimorphism in Dental Tissues: Sex Differences in Archaeological Individuals for Multiple Tooth Types. Am. J. Phys. Anthropol. 2021, 175, 106–127. [Google Scholar] [CrossRef] [PubMed]
- García-Campos, C.; Martinón-Torres, M.; Martín-Francés, L.; Martínez de Pinillos, M.; Modesto-Mata, M.; Perea-Pérez, B.; Zanolli, C.; Labajo González, E.; Sánchez Sánchez, J.A.; Ruiz Mediavilla, E.; et al. Contribution of Dental Tissues to Sex Determination in Modern Human Populations. Am. J. Phys. Anthropol. 2018, 166, 459–472. [Google Scholar] [CrossRef] [PubMed]
- García-Campos, C.; Martinón-Torres, M.; Martínez de Pinillos, M.; Modesto-Mata, M.; Martín-Francés, L.; Perea-Pérez, B.; Zanolli, C.; Bermúdez de Castro, J.M. Modern Humans Sex Estimation through Dental Tissue Patterns of Maxillary Canines. Am. J. Phys. Anthropol. 2018, 167, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Vázquez, R.; Urriolagoitia-Sosa, G.; Marquet-Rivera, R.; Romero-Ángeles, B.; Mastache-Miranda, O.; Vázquez-Feijoo, J.; Urriolagoitia-Calderón, G. High-Biofidelity Biomodel Generated from Three-Dimensional Imaging (Cone-Beam Computed Tomography): A Methodological Proposal. Comput. Math. Methods Med. 2020, 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.P.; Bowles, W.R.; Fok, A.; McClanahan, S.B. An Anatomic Investigation of the Mandibular First Molar Using Micro-Computed Tomography. J. Endod. 2013, 39, 1374–1378. [Google Scholar] [CrossRef]
- Tomaszewska, I.M.; Leszczyński, B.; Wróbel, A.; Gładysz, T.; Duncan, H.F. A Micro-Computed Tomographic (Micro-CT) Analysis of the Root Canal Morphology of Maxillary Third Molar Teeth. Ann. Anat. 2018, 215, 83–92. [Google Scholar] [CrossRef]
- Puwanun, S.; Delaine-Smith, R.; Colley, H.; Yates, J.; MacNeil, S.; Reilly, G. A Simple Rocker-Induced Mechanical Stimulus Upregulates Mineralization by Human Osteoprogenitor Cells in Fibrous Scaffolds. J. Tissue Eng. Regen. Med. 2018, 12, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Kim, U.; Yeo, I. Comparison of Three-Dimensional Digital Analyses and Two-Dimensional Histomorphometric Analyses of the Bone-Implant Interface. PLoS ONE 2022, 17, e0276269. [Google Scholar] [CrossRef]
- Rawson, S.; Maksimcuka, J.; Withers, P.; Cartmell, S. X-Ray Computed Tomography in Life Sciences. BMC Biol. 2020, 18, 21. [Google Scholar] [CrossRef]
- Dowker, S.; Davis, G.; Elliott, J. X-Ray Microtomography: Nondestructive Three-Dimensional Imaging for in Vitro Endodontic Studies. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1997, 83, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Efeoglu, N.; Wood, D.; Efeoglu, C. Microcomputerised Tomography Evaluation of 10% Carbamide Peroxide Applied to Enamel. J. Dent. 2005, 33, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Efeoglu, N.; Wood, D.J.; Efeoglu, C. Thirty-Five Percent Carbamide Peroxide Application Causes in Vitro Demineralization of Enamel. Dent. Mater. 2007, 23, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Peariasamy, K.; Anderson, P.; Brook, A. A Quantitative Study of the Effect of Pumicing and Etching on the Remineralisation of Enamel Opacities. Int. J. Paediatr. Dent. 2001, 11, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Naitoh, M.; Hirukawa, A.; Katsumata, A.; Ariji, E. Evaluation of Voxel Values in Mandibular Cancellous Bone: Relationship between Cone-Beam Computed Tomography and Multislice Helical Computed Tomography. Clin. Oral Implant. Res. 2009, 20, 503–506. [Google Scholar] [CrossRef]
- Silva, I.M.; De Freitas, D.; Ambrosano, G.; Bóscolo, F.; Almeida, S. Bone Density: Comparative Evaluation of Hounsfield Units in Multislice and Cone-Beam Computed Tomography. Braz. Oral Res. 2012, 26, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Heddleston, J.M.; Aaron, J.S.; Khuon, S.; Chew, T.L. A Guide to Accurate Reporting in Digital Image Acquisition—Can Anyone Replicate Your Microscopy Data? J. Cell Sci. 2021, 134, jcs254144, Erratum in J. Cell Sci. 2021, 134, jcs258933. [Google Scholar] [CrossRef] [PubMed]
- Kupitz, D.; Wissel, H.; Wuestemann, J.; Bluemel, S.; Pech, M.; Amthauer, H.; Kreissl, M.C.; Grosser, O.S. Optimization of SPECT/CT Imaging Protocols for Quantitative and Qualitative 99mTc SPECT. EJNMMI Phys. 2021, 8, 1–14. [Google Scholar] [CrossRef]
- Arango-Santander, S.; Montoya, C.; Pelaez-Vargas, A.; Ossa, E. Chemical, Structural and Mechanical Characterization of Bovine Enamel. Arch. Oral Biol. 2020, 109, 104573. [Google Scholar] [CrossRef]
- Camargo, M.; Marques, M.; de Cara, A. Morphological Analysis of Human and Bovine Dentine by Scanning Electron Microscope Investigation. Arch. Oral Biol. 2008, 53, 105–108. [Google Scholar] [CrossRef]
- Costa, B.; Iwamoto, A.; Puppin-Rontani, R.; Pascon, F. Comparative Analysis of Root Dentin Morphology and Structure of Human Versus Bovine Primary Teeth. Microsc. Microanal. 2015, 21, 689–694. [Google Scholar] [CrossRef]
- Soares, F.; Follak, A.; da Rosa, L.; Montagner, A.; Lenzi, T.; Rocha, R. Bovine Tooth Is a Substitute for Human Tooth on Bond Strength Studies: A Systematic Review and Meta-Analysis of in Vitro Studies. Dent. Mater. 2016, 32, 1385–1393. [Google Scholar] [CrossRef]
- Laurance-Young, P.; Bozec, L.; Gracia, L.; Rees, G.; Lippert, F.; Lynch, R.; Knowles, J. A Review of the Structure of Human and Bovine Dental Hard Tissues and Their Physicochemical Behaviour in Relation to Erosive Challenge and Remineralisation. J. Dent. 2011, 39, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Ruiz, A.; Teruel-Fernández, J.; Alcolea-Rubio, L.; Hernández-Fernández, A.; Martínez-Beneyto, Y.; Gispert-Guirado, F. Structural Differences in Enamel and Dentin in Human, Bovine, Porcine, and Ovine Teeth. Ann. Anat. 2018, 218, 7–17. [Google Scholar] [CrossRef]
- Lin, L. A Concordance Correlation Coefficient to Evaluate Reproducibility. Biometrics 1989, 45, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Barnhart, H.; Haber, M.; Song, J. Overall Concordance Correlation Coefficient for Evaluating Agreement among Multiple Observers. Biometrics 2002, 58, 1020–1027. [Google Scholar] [CrossRef]
- McBride, G. A Proposal for Strength-of-Agreement Criteria for Lin’s Concordance Correlation Coefficient. NIWA Client Rep. 2005, 45, 307–310. [Google Scholar]
- Slagter, K.; Raghoebar, G.; Vissink, A.; Meijer, H. Inter- and Intraobserver Reproducibility of Buccal Bone Measurements at Dental Implants with Cone Beam Computed Tomography in the Esthetic Region. Int. J. Implant Dent. 2015, 1, 1–5. [Google Scholar] [CrossRef]
- Curi, J.; Beaini, T.; da Silva, R.H.; Melani, R.; Chilvarquer, I.; Crosato, E. Guidelines for Reproducing Geometrical Aspects of Intra-Oral Radiographs Images on Cone-Beam Computed Tomography. Forensic Sci. Int. 2017, 271, 68–74. [Google Scholar] [CrossRef]
- Kochhar, A.; Sidhu, M.; Prabhakar, M.; Bhasin, R.; Kochhar, G.; Dadlani, H.; Spagnuolo, G.; Mehta, V. Intra- And Interobserver Reliability of Bone Volume Estimation Using OsiriX Software in Patients with Cleft Lip and Palate Using Cone Beam Computed Tomography. Dent. J. 2021, 9, 14. [Google Scholar] [CrossRef]
- Eguren, M.; Holguin, A.; Diaz, K.; Vidalon, J.; Linan, C.; Pacheco-Pereira, C.; Vich, M.O.L. Can Gray Values Be Converted to Hounsfield Units? A Systematic Review. Dentomaxillofacial Radiol. 2022, 51, 20210140. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, A.; Hirukawa, A.; Okumura, S.; Naitoh, M.; Fujishita, M.; Ariji, E.; Langlais, R.P. Effects of Image Artifacts on Gray-Value Density in Limited-Volume Cone-Beam Computerized Tomography. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2007, 104, 829–836. [Google Scholar] [CrossRef]
- Genisa, M.; Shuib, S.; Rajion, Z.; Arief, E.; Hermana, M. Density Estimation Based on the Hounsfield Unit Value of Cone Beam Computed Tomography Imaging of the Jawbone System. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2018, 232, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Mjör, I.; Nordahl, I. The Density and Branching of Dentinal Tubules in Human Teeth. Arch. Oral Biol. 1996, 41, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Komabayashi, T.; Nonomura, G.; Watanabe, L.; Marshall, G.; Marshall, S. Dentin Tubule Numerical Density Variations below the CEJ. J. Dent. 2008, 36, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Towle, I.; Salem, A.; Veneziano, A.; Loch, C. Variation in Enamel and Dentine Mineral Concentration and Density in Primate Molars. Arch. Oral Biol. 2023, 153, 105752. [Google Scholar] [CrossRef]
- Campioni, I.; Pecci, R.; Bedini, R. Ten Years of Micro-CT in Dentistry and Maxillofacial Surgery: A Literature Overview. Appl. Sci. 2020, 10, 4328. [Google Scholar] [CrossRef]
- Posada, M.C.; Sánches, C.F.; Gallego, G.J.; Vargas, A.P.; Restrepo, L.F.; López, J.D. Dientes de Bovino Como Sustituto de Dientes Humanos Para Su Uso En La Odontología. Revisión de Literatura. CES Odontol. 2006, 19, 63–68. [Google Scholar]
- Habel, R.E.; St. Clair, L.E. Digestive System of the Ox. In The Anatomy of the Domestic Animals; Sisson, S., Grossman, J., Getty, R., Eds.; Saunders Co: Centennial, CO, USA, 1975; pp. 444–469. [Google Scholar]
- Nakamichi, I.; Iwaku, M.; Fusayama, T. Bovine Teeth as Possible Substitutes in the Adhesion Test. J. Dent. Res. 1983, 62, 1076–1081. [Google Scholar] [CrossRef]
- Schilke, R.; Lisson, J.A.; Bauß, O.; Geurtsen, W. Comparison of the Number and Diameter of Dentinal Tubules in Human and Bovine Dentine by Scanning Electron Microscopic Investigation. Arch. Oral Biol. 2000, 45, 355–361. [Google Scholar] [CrossRef]
- ten Cate, J.M.; Rempt, H.E. Comparison of the in Vivo Effect of a 0 and 1,500 PpmF MFP Toothpaste on Fluoride Uptake, Acid Resistance and Lesion Remineralization. Caries Res. 1986, 20, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Camargo, C.H.R.; Siviero, M.; Camargo, S.E.A.; de Oliveira, S.H.G.; Carvalho, C.A.T.; Valera, M.C. Topographical, Diametral, and Quantitative Analysis of Dentin Tubules in the Root Canals of Human and Bovine Teeth. J. Endod. 2007, 33, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Attin, T.; Wegehaupt, F.; Gries, D.; Wiegand, A. The Potential of Deciduous and Permanent Bovine Enamel as Substitute for Deciduous and Permanent Human Enamel: Erosion-Abrasion Experiments. J. Dent. 2007, 35, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Turssi, C.P.; Messias, D.F.; Corona, S.M.; Serra, M.C. Viability of Using Enamel and Dentin from Bovine Origin as a Substitute for Human Counterparts in an Intraoral Erosion Model. Braz. Dent. J. 2010, 21, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Wegehaupt, F.; Gries, D.; Wiegand, A.; Attin, T. Is Bovine Dentine an Appropriate Substitute for Human Dentine in Erosion/Abrasion Tests? J. Oral Rehabil. 2008, 35, 390–394. [Google Scholar] [CrossRef]
- Dettwiler, C.A.; Walter, M.; Zaugg, L.K.; Lenherr, P.; Weiger, R.; Krastl, G. In Vitro Assessment of the Tooth Staining Potential of Endodontic Materials in a Bovine Tooth Model. Dent. Traumatol. 2016, 32, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Otsuki, M.; Tagami, J. Effect of PH Conditioners on Tooth Bleaching. Clin. Exp. Dent. Res. 2019, 5, 212–218. [Google Scholar] [CrossRef]
- Wiegand, A.; Vollmer, D.; Foitzik, M.; Attin, R.; Attin, T. Efficacy of Different Whitening Modalities on Bovine Enamel and Dentin. Clin. Oral Investig. 2005, 9, 91–97. [Google Scholar] [CrossRef]
- Asaka, Y.; Yamaguchi, K.; Inage, H.; Takamizawa, T.; Kurokawa, H.; Rikuta, A.; Kuroda, T.; Miyazaki, M. Effect of Thermal Cycling on Bond Strengths of Single-Step Self-Etch Adhesives to Bovine Dentin. J. Oral Sci. 2006, 48, 63–69. [Google Scholar] [CrossRef]
- Krifka, S.; Börzsönyi, A.; Koch, A.; Hiller, K.A.; Schmalz, G.; Friedl, K.H. Bond Strength of Adhesive Systems to Dentin and Enamel-Human vs. Bovine Primary Teeth in Vitro. Dent. Mater. 2008, 24, 888–894. [Google Scholar] [CrossRef]
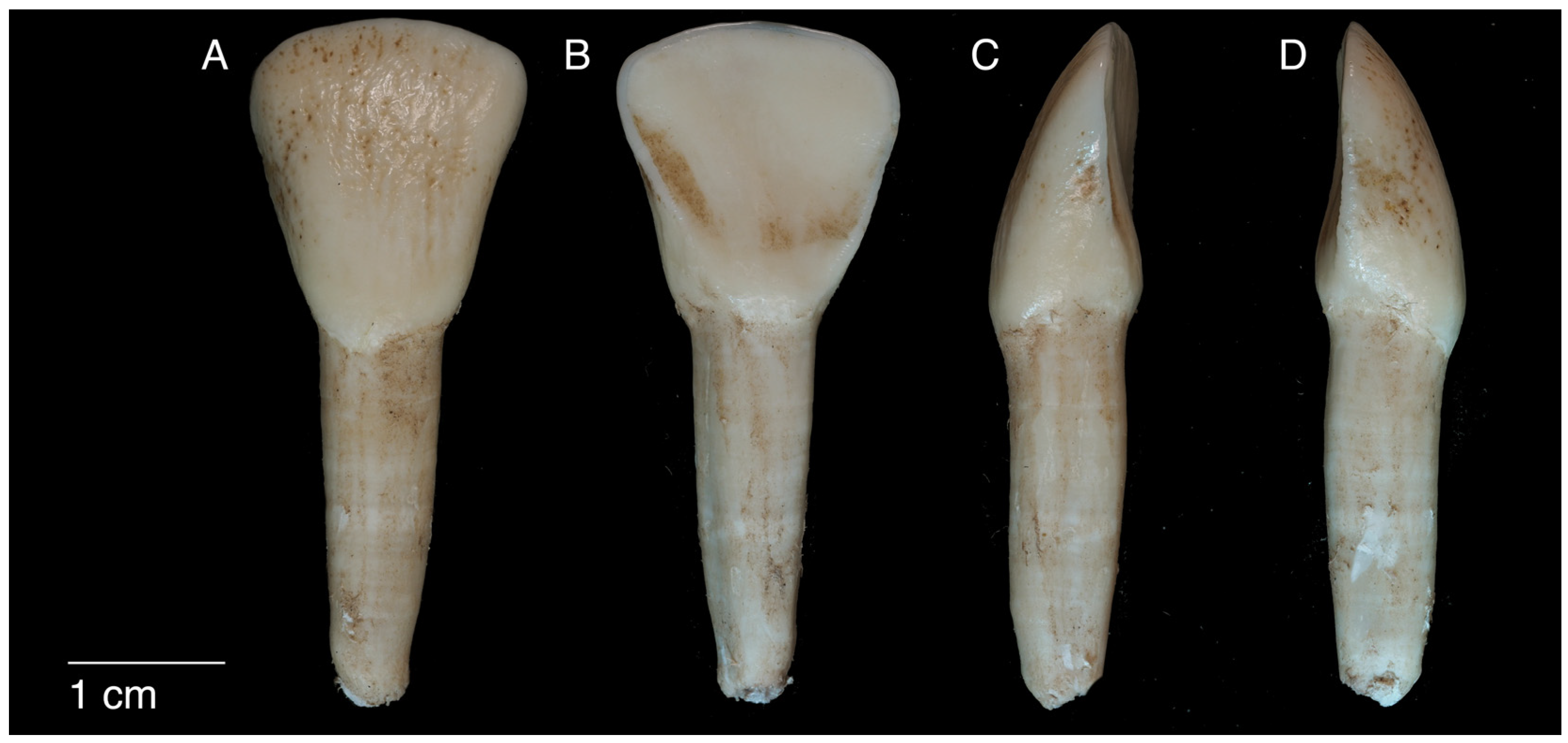
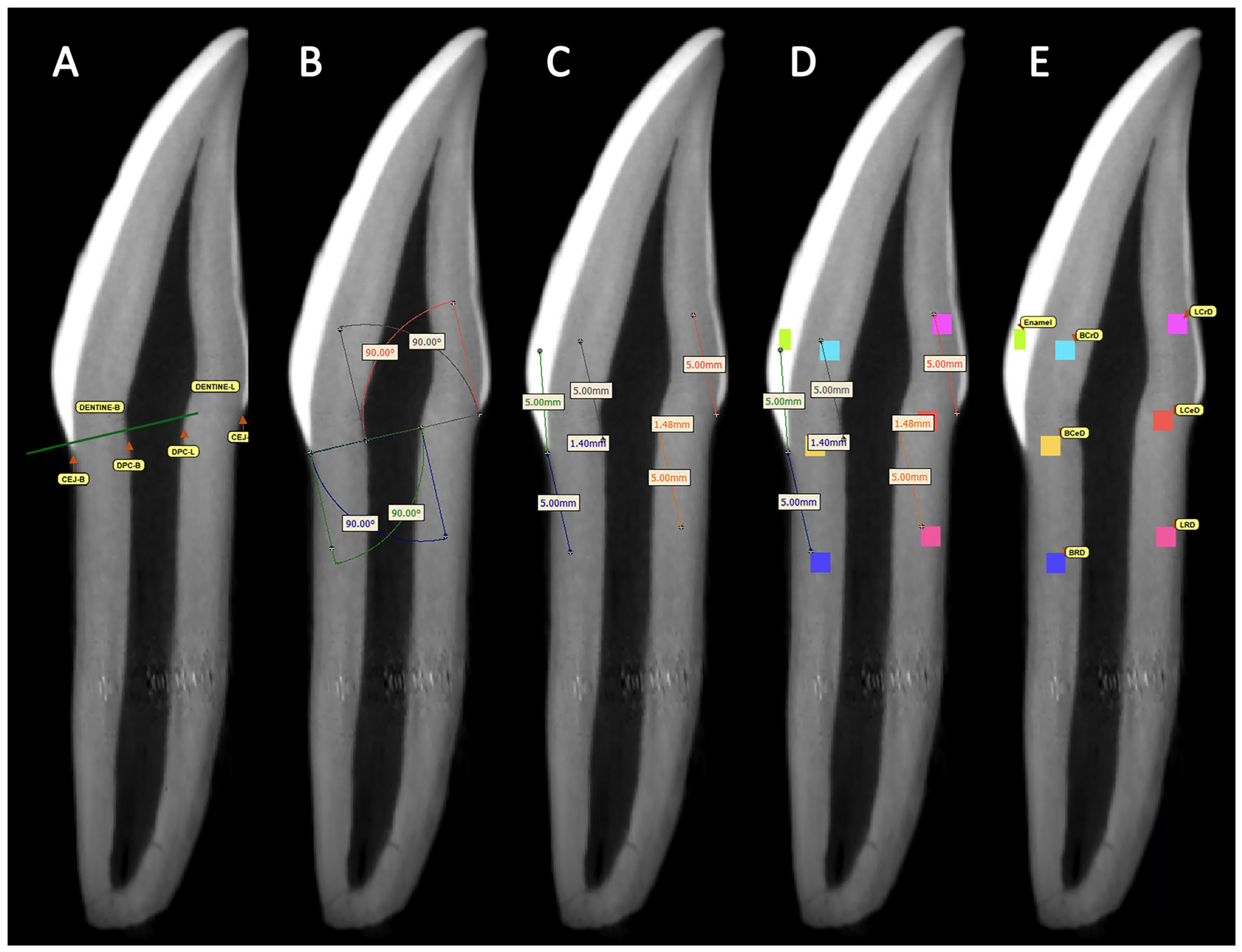

| Reference Point | Prisms | Abbreviation |
|---|---|---|
| Cemento-enamel junction to buccal surface | CEJ-B | |
| Cemento-enamel junction to lingual surface | CEJ-L | |
| Dentine pulp chamber to buccal surface | DPC-B | |
| Dentine pulp chamber to lingual surface | DPC-L | |
| Dentine to buccal surface | Dentine-B | |
| Dentine to lingual surface | Dentine-L | |
| Buccal crown dentine | BCrD | |
| Lingual crown dentine | LCrD | |
| Buccal cervical dentine | BCeD | |
| Lingual cervical dentine | LCeD | |
| Buccal root dentine | BRD | |
| Lingual root dentine | LRD | |
| Lingual root dentine | CEJ-B |
| Dentine Section | Reference Points for the Angles |
|---|---|
| Buccal crown dentine | From CEJ-L to DPC-B |
| Buccal root dentine | From DPC-L to CEJ-B |
| Lingual crown dentine | From DPC-B to CEJ-L |
| Lingual root dentine | From CEJ-B to DPC-L |
| Lingual root dentine | From CEJ-B to DPC-L |
| Sampling | Observer 1 vs. 2 | Observer 2 vs. 3 | Observer 1 vs. 3 |
|---|---|---|---|
| Enamel | 0.989 | 0.995 | 0.986 |
| Buccal crown dentine | 0.987 | 0.991 | 0.991 |
| Lingual crown dentine | 0.991 | 0.987 | 0.995 |
| Buccal cervical dentine | 0.993 | 0.995 | 0.993 |
| Lingual cervical dentine | 0.987 | 0.997 | 0.989 |
| Buccal root dentine | 0.979 | 0.982 | 0.985 |
| Lingual root dentine | 0.965 | 0.977 | 0.985 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huaiquin-Zúñiga, M.; Castillo-Alonso, C.; Fonseca, G.M.; López-Lázaro, S. Reproducibility of Three-Dimensional Density Measurements in Teeth Using Micro-Computed Tomography: An Image Acquisition Protocol. Appl. Sci. 2024, 14, 6334. https://doi.org/10.3390/app14146334
Huaiquin-Zúñiga M, Castillo-Alonso C, Fonseca GM, López-Lázaro S. Reproducibility of Three-Dimensional Density Measurements in Teeth Using Micro-Computed Tomography: An Image Acquisition Protocol. Applied Sciences. 2024; 14(14):6334. https://doi.org/10.3390/app14146334
Chicago/Turabian StyleHuaiquin-Zúñiga, Mary, Camila Castillo-Alonso, Gabriel M. Fonseca, and Sandra López-Lázaro. 2024. "Reproducibility of Three-Dimensional Density Measurements in Teeth Using Micro-Computed Tomography: An Image Acquisition Protocol" Applied Sciences 14, no. 14: 6334. https://doi.org/10.3390/app14146334
APA StyleHuaiquin-Zúñiga, M., Castillo-Alonso, C., Fonseca, G. M., & López-Lázaro, S. (2024). Reproducibility of Three-Dimensional Density Measurements in Teeth Using Micro-Computed Tomography: An Image Acquisition Protocol. Applied Sciences, 14(14), 6334. https://doi.org/10.3390/app14146334






