Evaluating Commercial Electrical Neuromodulation Devices with Low-Cost Neural Phantoms
Abstract
1. Introduction
1.1. Overview
1.2. Background
2. Materials and Methods
2.1. Overview
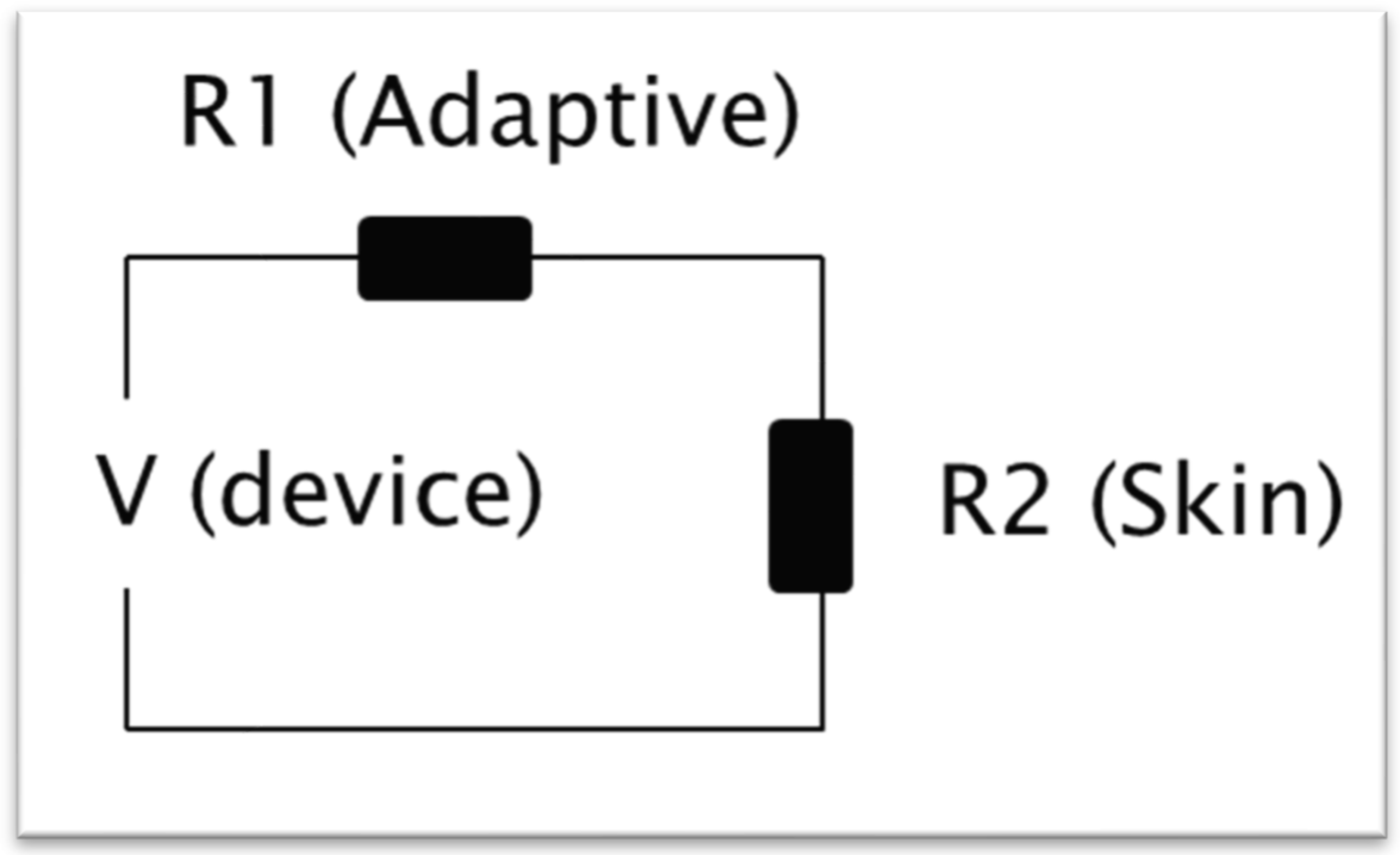
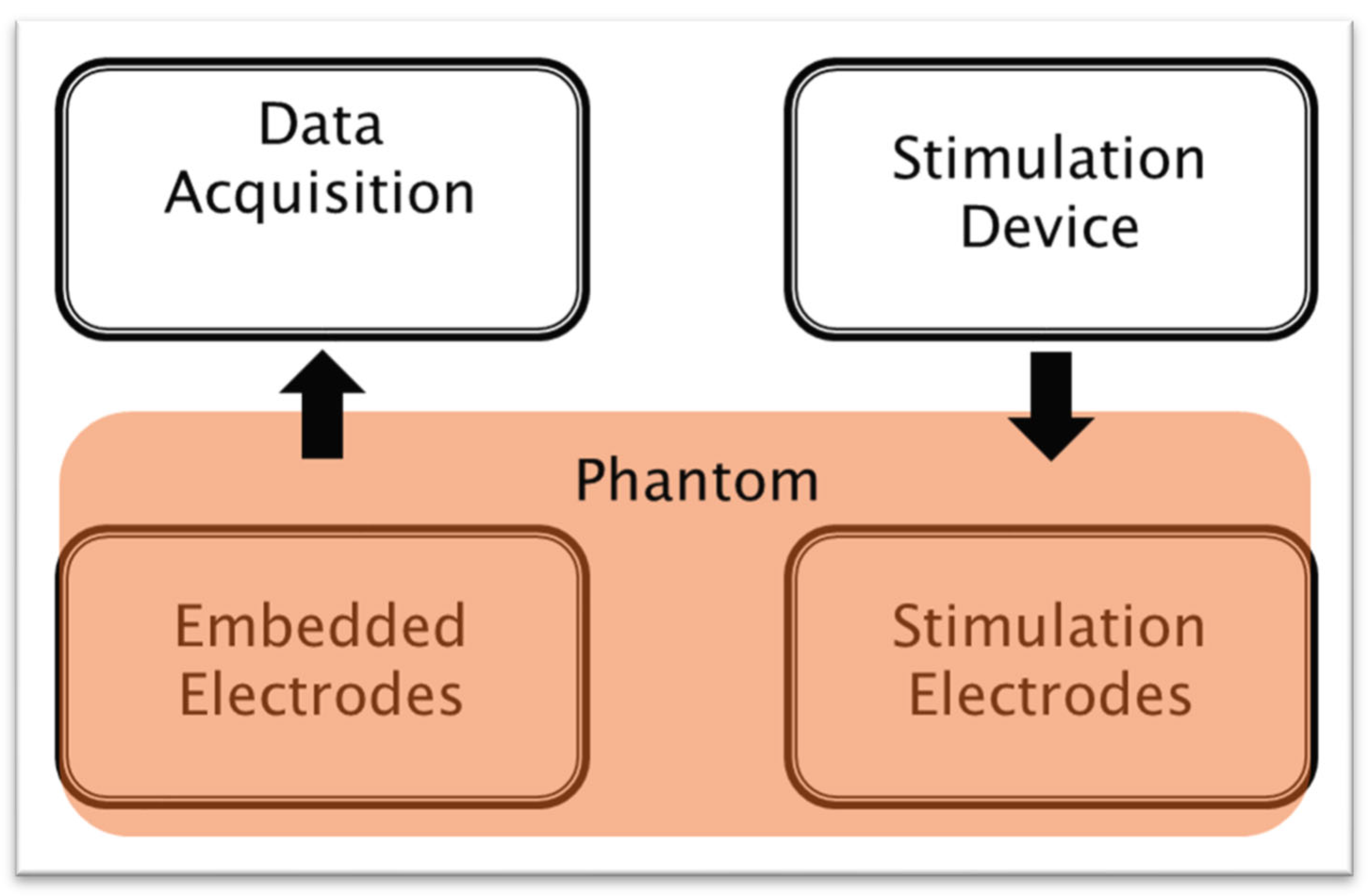
2.2. Phantoms
2.3. Device Characteristics
2.4. Calculations
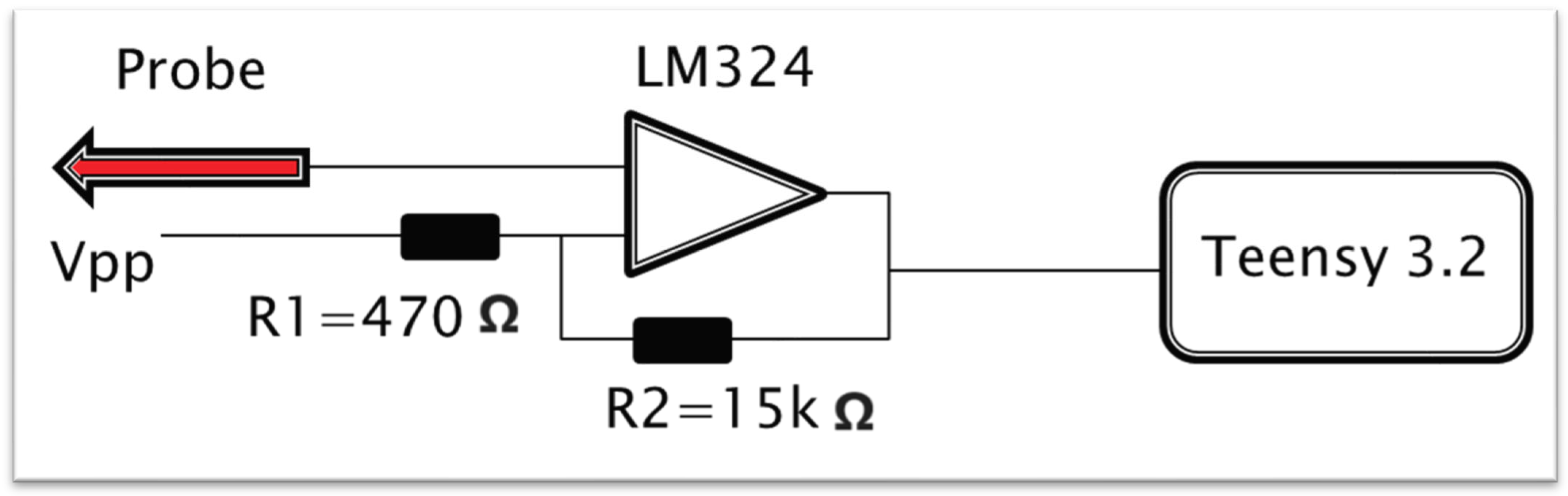
2.5. Measurement Systems
2.6. Measurement Steps
2.7. Hypotheses
3. Results
3.1. Overview
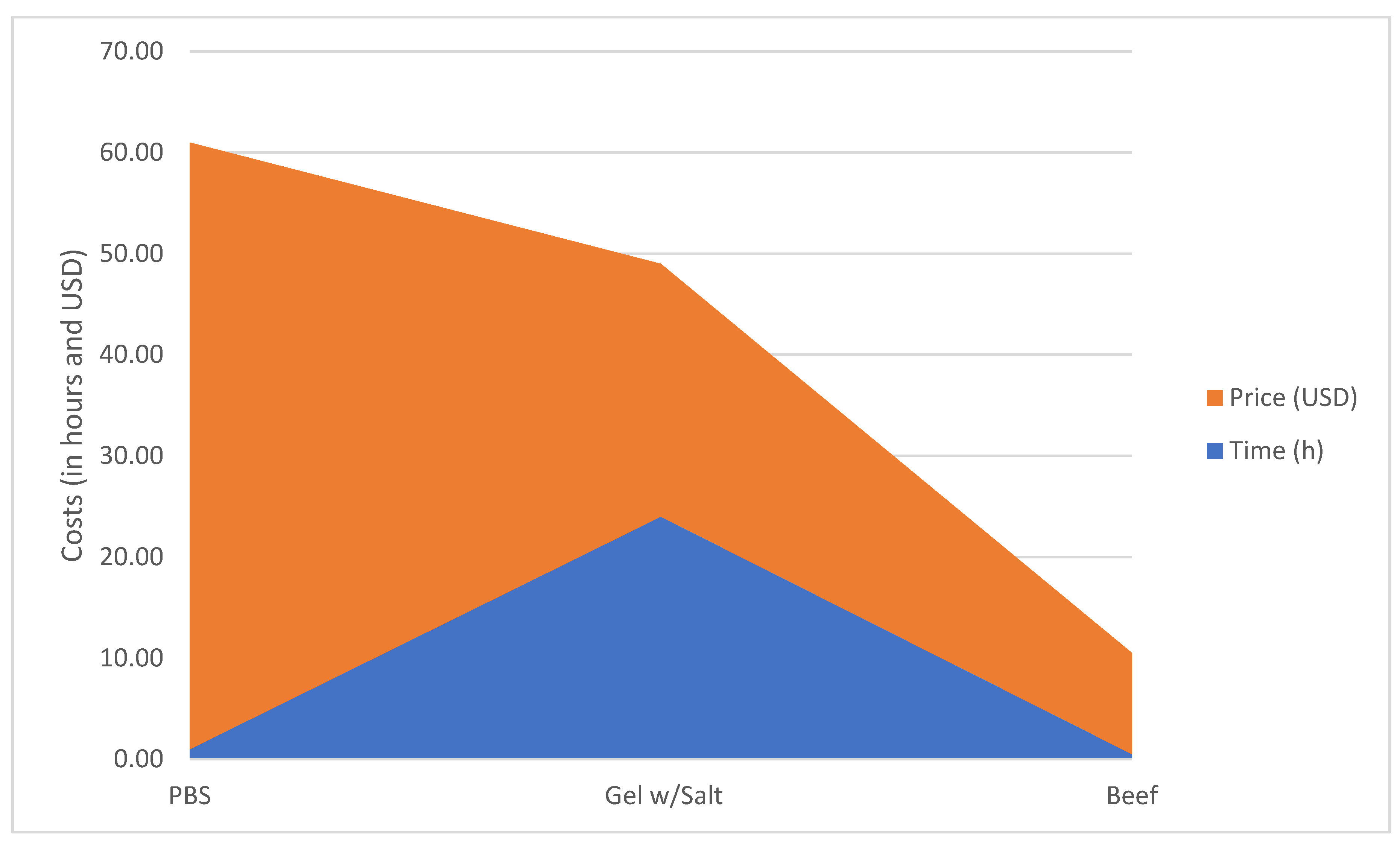
3.2. Phantom Costs
3.3. Experimental Measurements
3.4. Efficiency Factors
4. Discussion
4.1. Overview
4.2. Limitations
4.3. Future Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abouelsoud, M.; Kazadi, P.; Mishelevich, D.; Lubendo, M. Case Report of Transcranial Pulsed-Current Stimulation with Parkinson’s Disease. Clin. Exp. Psychol. 2023, 9, 1–4. [Google Scholar]
- Bradley, C.; Nydam, A.S.; Dux, P.E.; Mattingley, J.B. State-dependent effects of neural stimulation on brain function and cognition. Nat. Rev. Neurosci. 2022, 23, 459–475. [Google Scholar] [CrossRef]
- Elyamany, O.; Leicht, G.; Herrmann, C.S.; Mulert, C. Transcranial alternating current stimulation (tACS): From basic mechanisms towards first applications in psychiatry. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 135–156. [Google Scholar] [CrossRef]
- Lefaucheur, J.-P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 2017, 128, 56–92. [Google Scholar] [CrossRef] [PubMed]
- Malekahmad, M.; Frazer, A.; Zoghi, M.; Jaberzadeh, S. Transcranial pulsed current stimulation: A scoping review of the current literature on scope, nature, underlying mechanisms, and gaps. Psychophysiology 2024, 61, e14521. [Google Scholar] [CrossRef]
- Goering, S.; Klein, E.; Specker Sullivan, L.; Wexler, A.; Agüera y Arcas, B.; Bi, G.; Carmena, J.M.; Fins, J.J.; Friesen, P.; Gallant, J.; et al. Recommendations for responsible development and application of neurotechnologies. Neuroethics 2021, 14, 365–386. [Google Scholar] [CrossRef]
- Medeiros, W.; Barros, T.; Caixeta, F.V. Bibliometric mapping of non-invasive brain stimulation techniques (NIBS) for fluent speech production. Front. Hum. Neurosci. 2023, 17, 1164890. [Google Scholar] [CrossRef] [PubMed]
- Palm, U.; Hasan, A.; Strube, W.; Padberg, F. tDCS for the treatment of depression: A comprehensive review. Eur. Arch. Psychiatry Clin. Neurosci. 2016, 266, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Ugawa, Y. Adverse events of tDCS and tACS: A review. Clin. Neurophysiol. Pract. 2017, 2, 19–25. [Google Scholar] [CrossRef]
- Guido, K.; Kiourti, A. An in vivo-mimicking in vitro testbed for brain-computer interfaces. IEEE J. Electromagn. RF Microw. Med. Biol. 2019, 4, 240–246. [Google Scholar] [CrossRef]
- Guido, K.; Bringer, A.; Kiourti, A. Human Body Phantoms for Wideband Radiometry. In Proceedings of the 2020 IEEE International Symposium on Antennas and Propagation and North American Radio Science Meeting, Montreal, QC, Canada, 5–10 July 2020; Volume 2020, pp. 1709–1710. [Google Scholar]
- Owda, A.Y.; Casson, A.J. Investigating gelatine based head phantoms for electroencephalography compared to electrical and ex vivo porcine skin models. IEEE Access 2021, 9, 96722–96738. [Google Scholar] [CrossRef]
- Chew, K.M.; Sudirman, R.; Seman, N.; Yong, C.Y. Human brain phantom modeling based on relative permittivity dielectric properties. In Proceedings of the IEEE International Conference on Biomedical Engineering and Biotechnology, Macau, China, 28–30 May 2012; Volume 2012, pp. 817–820. [Google Scholar]
- Daru, R.R.; Rabby, M.M.; Ko, T.; Shinglot, Y.; Raihan, R.; Adnan, A. Electrically Equivalent Head Tissue Materials for Electroencephalogram Study on Head Surrogates. Appl. Sci. 2024, 14, 2495. [Google Scholar] [CrossRef]
- Mashayekhi, F.; Shanehsazzadeh, F.; Vazifeh, A.R.; Fardmanesh, M. Development of a Controllably Homogenous Conductive Ballistic Gelatin as a Realistic Spinal Cord Phantom. In Proceedings of the 2021 28th National and 6th International Iranian Conference, Proceedings of the International Iranian Conference on Biomedical Engineering, Tehran, Iran, 25–26 November 2021; Volume 2021, pp. 74–77.
- Tseghai, G.B.; Malengier, B.; Fante, K.A.; Van Langenhove, L. A long-lasting textile-based anatomically realistic head phantom for validation of EEG electrodes. Sensors 2021, 21, 4658. [Google Scholar] [CrossRef] [PubMed]
- Ramasubbu, R.; Lang, S.; Kiss, Z.H. Dosing of electrical parameters in deep brain stimulation (DBS) for intractable depression: A review of clinical studies. Front. Psychiatry 2018, 9, 302. [Google Scholar] [CrossRef] [PubMed]
- Lopes Alho, E.J.; Graciolli Cordeiro, J.; Assumpcao de Monaco, B.; Russell Jagid, J. Introduction and History of Neuromodulation for Pain. In Neuromodulation Techniques for Pain Treatment; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–21. [Google Scholar]
- Poreisz, C.; Boros, K.; Antal, A.; Paulus, W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res. Bull. 2007, 72, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Mikkonen, M.; Laakso, I.; Sumiya, M.; Koyama, S.; Hirata, A.; Tanaka, S. TMS motor thresholds correlate with TDCS electric field strengths in hand motor area. Front. Neurosci. 2018, 12, 426. [Google Scholar] [CrossRef]
- Chhatbar, P.Y.; Kautz, S.A.; Takacs, I.; Rowland, N.C.; Revuelta, G.J.; George, M.S.; Bikson, M.; Feng, W. Evidence of transcranial direct current stimulation generated electric fields at subthalamic level in human brain in vivo. Brain Stimul. 2018, 11, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Rawji, V.; Ciocca, M.; Zacharia, A.; Soares, D.; Truong, D.; Bikson, M.; Rothwell, J.; Bestmann, S. tDCS changes in motor excitability are specific to orientation of current flow. Brain Stimul. 2018, 11, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Vöröslakos, M.; Takeuchi, Y.; Brinyiczki, K.; Zombori, T.; Oliva, A.; Fernández-Ruiz, A.; Kozák, G.; Kincses, Z.T.; Iványi, B.; Buzsáki, G.; et al. Direct effects of transcranial electric stimulation on brain circuits in rats and humans. Nat. Commun. 2018, 9, 483. [Google Scholar] [CrossRef]
- Solomons, C.D.; Slovak, M.; Heller, B.; Barker, A.T. Reducing the sensation of electrical stimulation with dry electrodes by using an array of constant current sources. Med. Eng. Phys. 2018, 51, 91–95. [Google Scholar] [CrossRef]
- Brancucci, A.; Rivolta, D.; Nitsche, M.A.; Manippa, V. The effects of transcranial random noise stimulation on motor function: A comprehensive review of the literature. Physiol. Behav. 2023, 261, 114073. [Google Scholar] [CrossRef] [PubMed]
- Calancie, B.; Harris, W.; Broton, J.G.; Alexeeva, N.; Green, B.A. Threshold-level multiThreshold-level multipulse transcranial electrical stimulation of motor cortex for intraoperative monitoring of spinal motor tracts: Description of method and comparison to somatosensory evoked potential monitoring. J. Neurosurg. 1998, 88, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Liu, T.; Wang, J. Improving the effect of transcranial alternating current stimulation (tACS): A systematic review. Front. Hum. Neurosci. 2021, 15, 652393. [Google Scholar] [CrossRef] [PubMed]
- Jaberzadeh, S.; Bastani, A.; Zoghi, M. Anodal transcranial pulsed current stimulation: A novel technique to enhance corticospinal excitability. Clin. Neurophysiol. 2014, 125, 344–351. [Google Scholar] [CrossRef]
- U: The Mind Company. Axis Operation Manual; U: The Mind Company: Columbus, OH, USA, 2021. [Google Scholar]
- Sieni, E.; Sgarbossa, P.; Mognaschi, M.E.; Forzan, M.; Parupudi, T.; Mittal, L.; Camarillo, I.G.; Sundararajan, R. Electric field distribution study in inhomogeneous biological tissues. Int. J. Numer. Model. Electron. Netw. Devices Fields 2020, 33, e2699. [Google Scholar] [CrossRef]
- Sel, D.; Mazeres, S.; Teissie, J.; Miklavcic, D. Finite-element modeling of needle electrodes in tissue from the perspective of frequent model computation. IEEE Trans. Biomed.Eng. 2003, 50, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Al-Arifin, A. Development of a Realistic Tissue Mimicking Phantom Using Finite Element Method. Master’s Thesis, Department of Electrical and Electronic Engineering, Islamic University of Technology (IUT), Gazipur, Bangladesh, 2021. [Google Scholar]
- Bikson, M.; Grossman, P.; Thomas, C.; Zannou, A.L.; Jiang, J.; Adnan, T.; Mourdoukoutas, A.P.; Kronberg, G.; Truong, D.; Boggio, P.; et al. Safety of transcranial direct current stimulation: Evidence based update since 2016. Brain Stimul. 2016, 9, 641–661. [Google Scholar] [CrossRef] [PubMed]
- Koponen, L.M.; Stenroos, M.; Nieminen, J.O.; Jokivarsi, K.; Gröhn, O.; Ilmoniemi, R.J. Individual head models for estimating the TMS-induced electric field in rat brain. Sci. Rep. 2020, 10, 17397. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.-D.; Lisanby, S.H.; Peterchev, A.V. Electric field strength and focality in electroconvulsive therapy and magnetic seizure therapy: A finite element simulation study. J. Neural Eng. 2011, 8, 016007. [Google Scholar] [CrossRef]
- Fregni, F.; Nitsche, M.A.; Loo, C.K.; Brunoni, A.R.; Marangolo, P.; Leite, J.; Carvalho, S.; Bolognini, N.; Caumo, W.; Paik, N.J.; et al. Regulatory considerations for the clinical and research use of transcranial direct current stimulation (tDCS): Review and recommendations from an expert panel. Clin. Res. Regul. Aff. 2015, 32, 22–35. [Google Scholar] [CrossRef]
- Zell, M.; Lyng, J.G.; Cronin, D.A.; Morgan, D.J. Ohmic heating of meats: Electrical conductivities of whole meats and processed meat ingredients. Meat Sci. 2009, 83, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Koessler, L.; Colnat-Coulbois, S.; Cecchin, T.; Hofmanis, J.; Dmochowski, J.P.; Norcia, A.M.; Maillard, L.G. In-vivo measurements of human brain tissue conductivity using focal electrical current injection through intracerebral multicontact electrodes. Hum. Brain Mapp. 2017, 38, 974–986. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-C.; Guido, K.; Kiourti, A. Passive impedance matching for implanted brain–electrode interfaces. IEEE J. Electromagn. RF Microw. Med. Biol. 2019, 3, 233–239. [Google Scholar] [CrossRef]
- Nwanya, A.C.; Amaechi, C.I.; Udounwa, A.E.; Osuji, R.U.; Maaza, M.; Ezema, F.I. Complex impedance and conductivity of agar-based ion-conducting polymer electrolytes. Appl. Phys. A 2015, 119, 387–396. [Google Scholar] [CrossRef]
- TheBrainDriver tDCS Instruction Manual. TheBrainDriver; LLC. Available online: https://thebraindriver.com/pages/thebraindriver-tdcs-instruction-manual (accessed on 1 March 2024).
- Kim, S.H. Effects of dual transcranial direct current stimulation and modified constraint-induced movement therapy to improve upper-limb function after stroke: A double-blinded, pilot randomized controlled trial. J. Stroke Cerebrovasc. Dis. 2021, 30, 105928. [Google Scholar] [CrossRef]
- Vlach, J.; Singhal, K. Computer Methods for Circuit Analysis and Design; Springer Science & Business Media: New York, NY, USA, 1983. [Google Scholar]

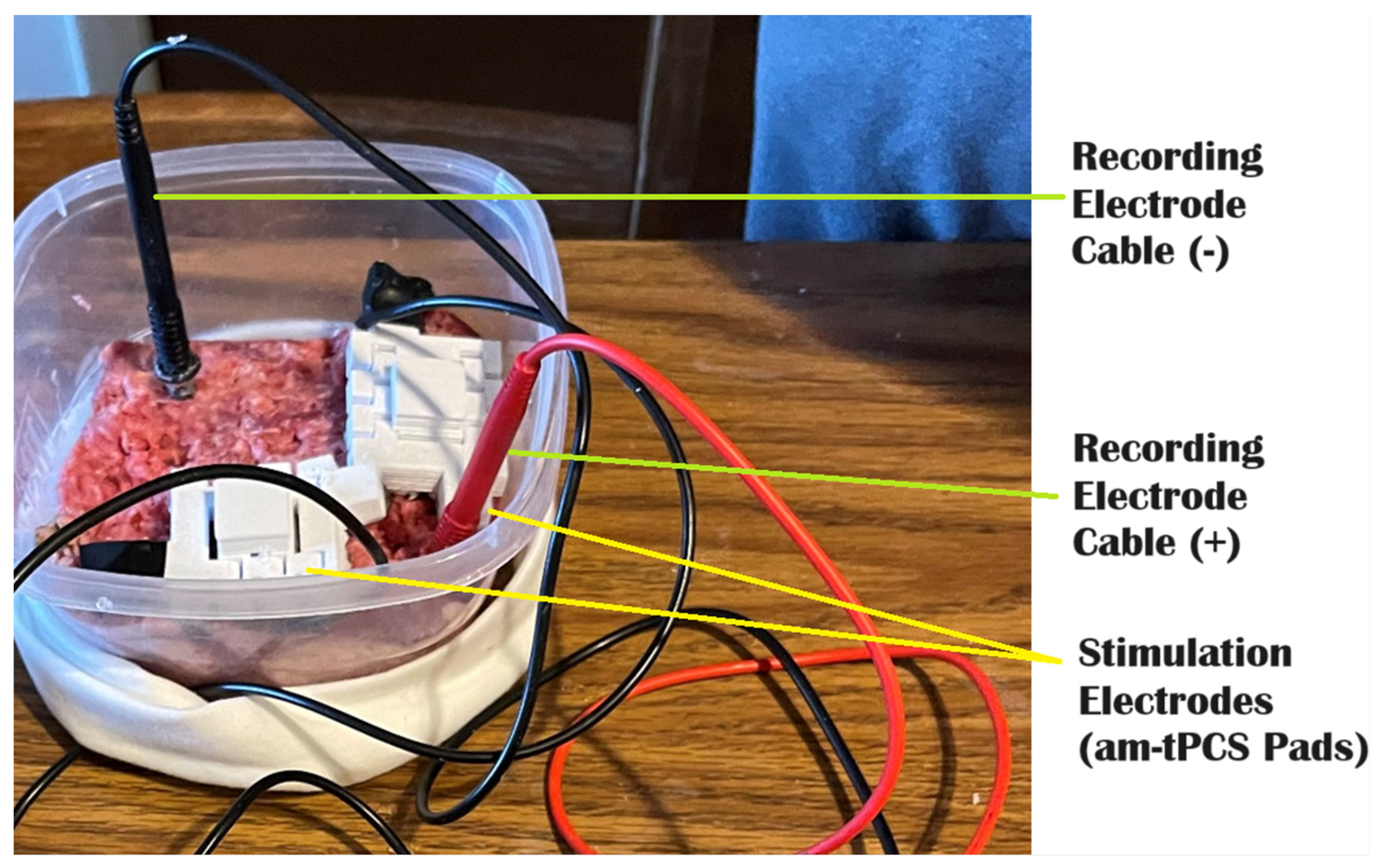
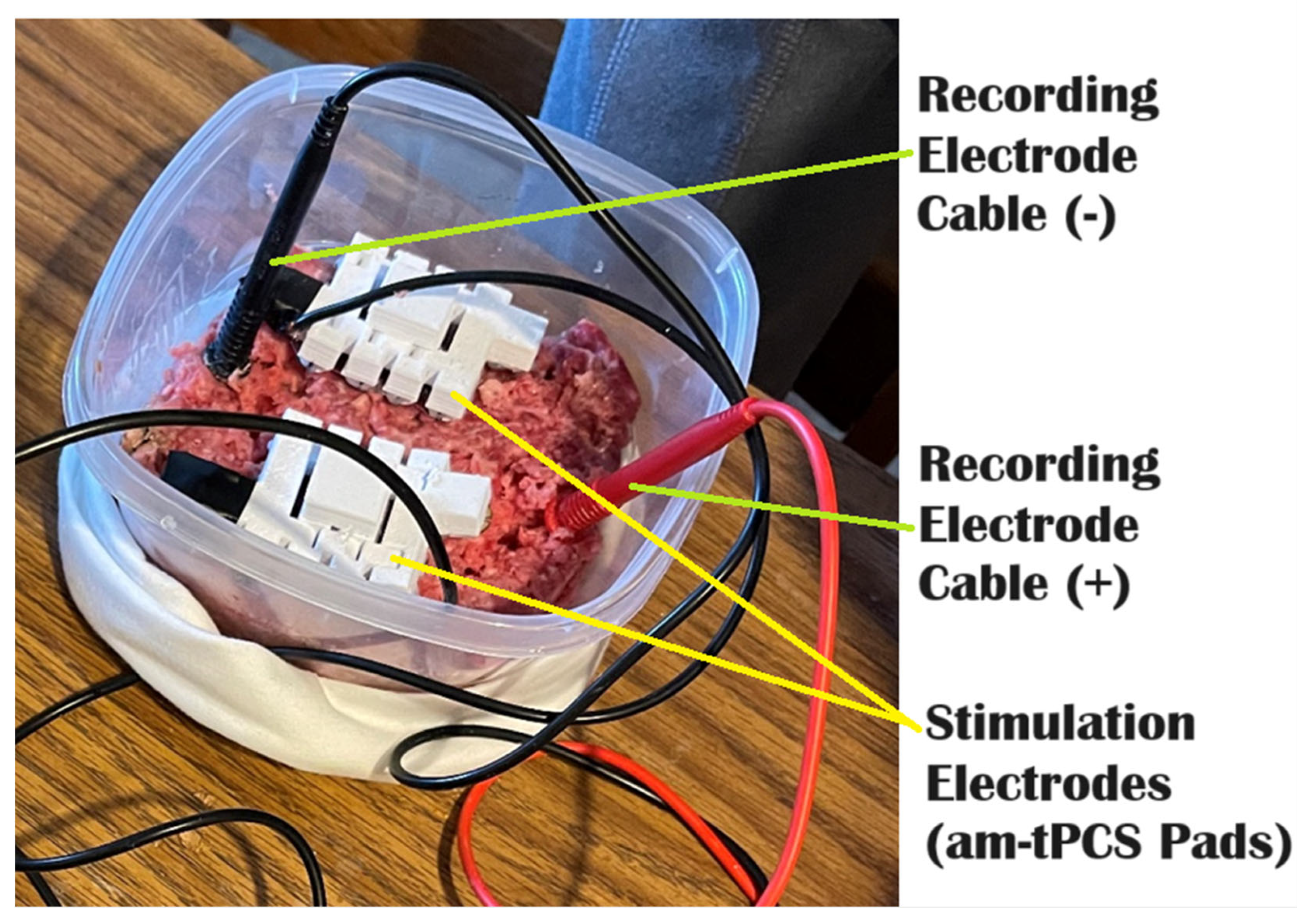

| Agar Gel w/Salt | PBS | Beef | This | |
|---|---|---|---|---|
| Reference | Owda (2021) [12] | Guido (2019) [10] | Guido (2020) [11] | N/A |
| Type | -Electric Stim | -DBS Testing | -RF Heating | -Electric Stim |
| Time (h) | 24 | 1 | <1 | 1–24 |
| Cost (USD) | USD 25 | USD 60 | USD 10 | USD 10–25 |
| Measure | Voltage (V) | Voltage (V) | Thermal, Temp | Voltage (V), EF |
| Limits | -Long prep time | -Costly | -Different domain | -Purpose-built |
| Benefits | -Customizable | -Chemically close | -Cheap, fast setup | -Cheap, fast setup |
| Current (A) | Voltage (V) | Power (W) | Time (min) | Time (s) | Dose (W-s) | |
|---|---|---|---|---|---|---|
| am-tPCS | 0.004 | 5 | 0.02 | 15 | 900 | 18 |
| tDCS | 0.002 | 9 | 0.018 | 20 | 1200 | 21.6 |
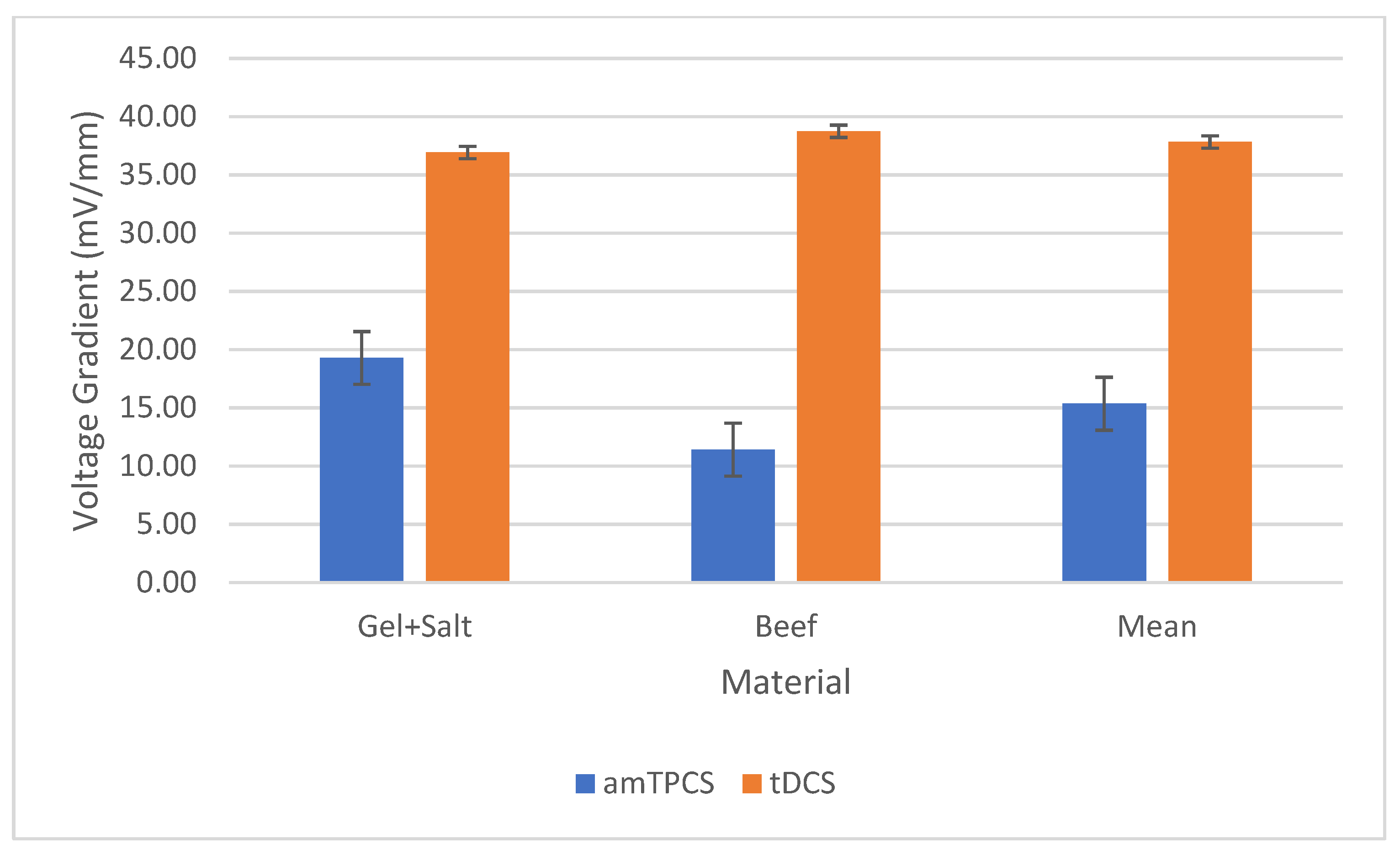
| Current (A) | Vg (mV/mm) | Dose Observed (W-s) | Dose Expected (W-s) | EF (%) | |
|---|---|---|---|---|---|
| am-tPCS | 0.004 | 15 | 0.55 | 18.00 | 0.031 |
| tDCS | 0.002 | 38 | 0.91 | 21.60 | 0.042 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
LaRocco, J.; Eom, T.; Seth, E.; Gandhi, V.; Bontempo, A.; Zachariah, E. Evaluating Commercial Electrical Neuromodulation Devices with Low-Cost Neural Phantoms. Appl. Sci. 2024, 14, 6328. https://doi.org/10.3390/app14146328
LaRocco J, Eom T, Seth E, Gandhi V, Bontempo A, Zachariah E. Evaluating Commercial Electrical Neuromodulation Devices with Low-Cost Neural Phantoms. Applied Sciences. 2024; 14(14):6328. https://doi.org/10.3390/app14146328
Chicago/Turabian StyleLaRocco, John, Taeyoon Eom, Ekansh Seth, Vania Gandhi, Anna Bontempo, and Eric Zachariah. 2024. "Evaluating Commercial Electrical Neuromodulation Devices with Low-Cost Neural Phantoms" Applied Sciences 14, no. 14: 6328. https://doi.org/10.3390/app14146328
APA StyleLaRocco, J., Eom, T., Seth, E., Gandhi, V., Bontempo, A., & Zachariah, E. (2024). Evaluating Commercial Electrical Neuromodulation Devices with Low-Cost Neural Phantoms. Applied Sciences, 14(14), 6328. https://doi.org/10.3390/app14146328







