Effect of Sterilization Methods on Collagen Hydrolysate Obtained from Tuna Tendon
Abstract
1. Introduction
2. Materials and Methods
2.1. Collagen Hydrolysate from Tuna Tendon
2.2. Ethylene Oxide Sterilization of Collagen Hydrolysate
2.3. Beta and Gamma Radiation Sterilization of Collagen Hydrolysate
2.4. Physical Appearances and Image Analysis of Collagen Hydrolysate
2.5. Nuclear Magnetic Resonance Spectroscopy (NMR) Characterization
2.6. Sample Preparation and Generation of MALDI-TOF Mass Spectra
2.7. Differential Scanning Calorimetry Analysis
3. Results and Discussion
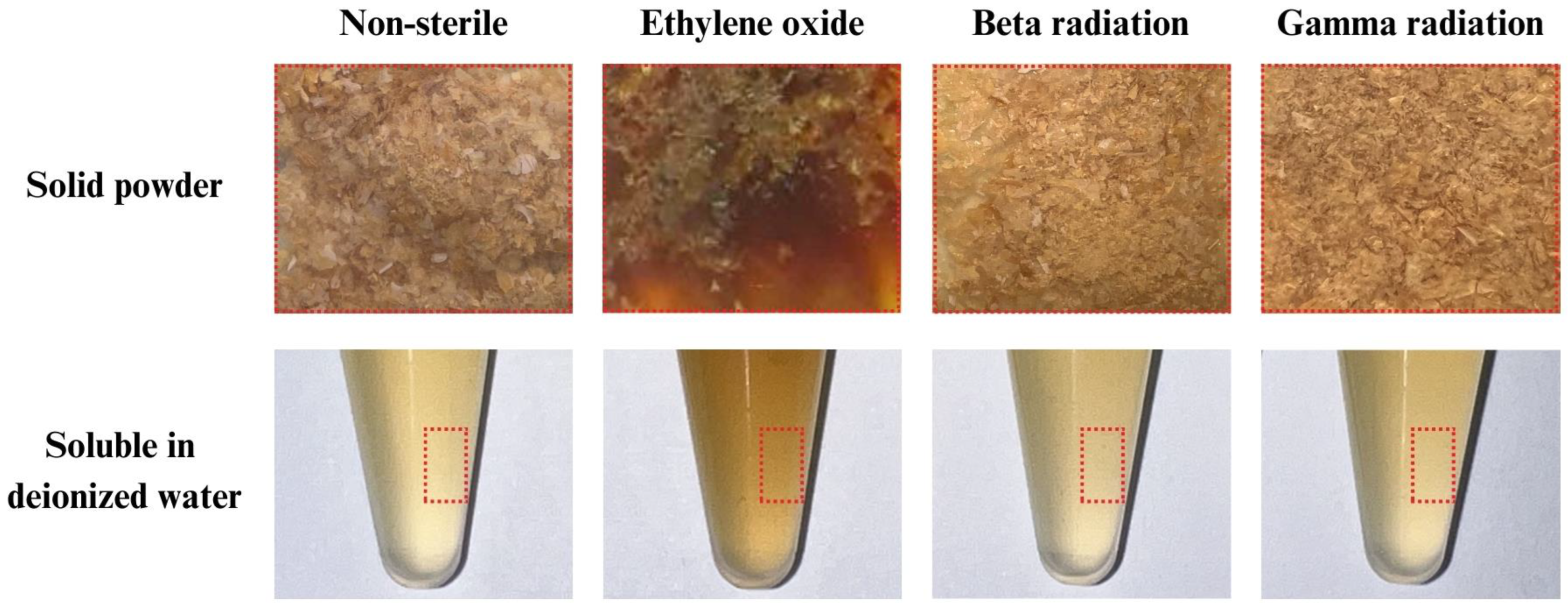
3.1. Physical Appearances of Collagen Hydrolysate before and after Sterilization
3.2. NMR Characterization of the Chemical Structure of Collagen Hydrolysate before and after Sterilization
3.3. MALDI-TOF-MS Characterization of the Molecular Weight of Collagen Hydrolysate before and after Sterilization
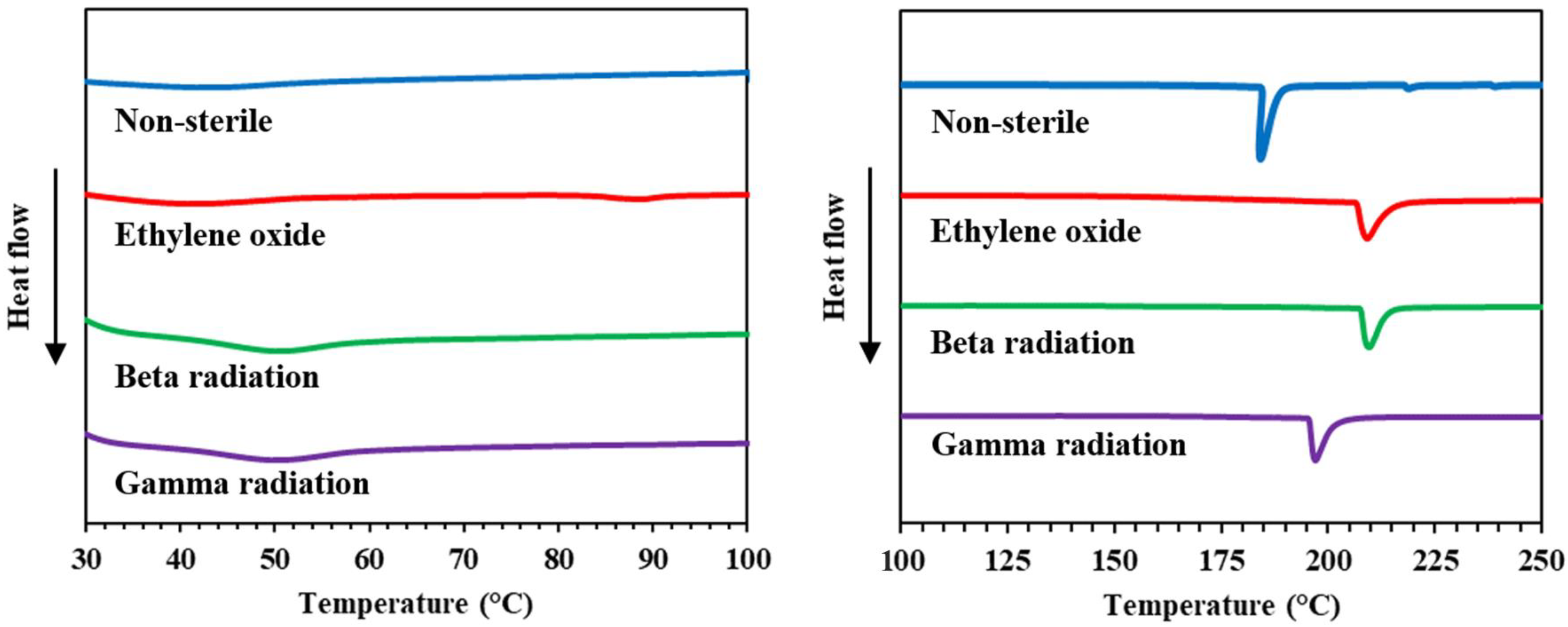
3.4. Characterization of Collagen Hydrolysate before and after Sterilization by DSC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bolke, L.; Schlippe, G.; Gerß, J.; Voss, W. A Collagen Supplement Improves Skin Hydration, Elasticity, Roughness, and Density: Results of a Randomized, Placebo-Controlled, Blind Study. Nutrients 2019, 11, 2494. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Tarnutzer, K.; Siva Sankar, D.; Dengjel, J.; Ewald, C.Y. Collagen constitutes about 12% in females and 17% in males of the total protein in mice. Sci. Rep. 2023, 13, 4490. [Google Scholar] [CrossRef] [PubMed]
- León-López, A.; Morales-Peñaloza, A.; Martínez-Juárez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Álvarez, G. Hydrolyzed Collagen-Sources and Applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.; Potgieter, J.; Ishfaq, K.; Shahzad, M. Developments for Collagen Hydrolysate in Biological, Biochemical, and Biomedical Domains: A Comprehensive Review. Materials 2021, 14, 2806. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.S.; Ok, Y.J.; Hwang, S.Y.; Kwak, J.Y.; Yoon, S. Marine Collagen as a Promising Biomaterial for Biomedical Applications. Mar. Drugs 2019, 17, 467. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Pohan, G.; Mattiassi, S.; Yao, Y.; Zaw, A.M.; Anderson, D.E.J.; Cutiongco, M.F.A.; Hinds, M.T.; Yim, E.K.F. Effect of Ethylene Oxide Sterilization on Polyvinyl Alcohol Hydrogel Compared with Gamma Radiation. Tissue Eng. Part A 2020, 26, 1077–1090. [Google Scholar] [CrossRef] [PubMed]
- Gorham, S.D.; Srivastava, S.; French, D.A.; Scott, R. The effect of gamma-ray and ethylene oxide sterilization on collagen-based wound-repair materials. J. Mater. Sci. Mater. Med. 1993, 4, 40–49. [Google Scholar] [CrossRef]
- Faraj, K.; Brouwer, K.; Geutjes, P.; Versteeg, E.; Wismans, R.; Deprest, J.; Chajra, H.; Tiemessen, D.; Feitz, W.; Oosterwijk, E.; et al. The effect of ethylene oxide sterilisation, beta irradiation and gamma irradiation on collagen fibril-based scaffolds. Evaluation of biological, physical and chemical parameters. Tissue Eng. Regen. Med. 2011, 8, 460–470. [Google Scholar]
- Stanca, M.; Gaidau, C.; Zaharescu, T.; Balan, G.A.; Matei, I.; Precupas, A.; Leonties, A.R.; Ionita, G. Physico-Chemical Changes Induced by Gamma Irradiation on Some Structural Protein Extracts. Biomolecules 2023, 13, 774. [Google Scholar] [CrossRef] [PubMed]
- Nimptsch, A.; Schibur, S.; Ihling, C.; Sinz, A.; Riemer, T.; Huster, D.; Schiller, J. Quantitative analysis of denatured collagen by collagenase digestion and subsequent MALDI-TOF mass spectrometry. Cell Tissue Res. 2011, 343, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Hema, G.S.; Joshy, C.G.; Shyni, K.; Chatterjee, N.S.; Ninan, G.; Mathew, S. Optimization of process parameters for the production of collagen peptides from fish skin (Epinephelus malabaricus) using response surface methodology and its characterization. J. Food Sci. Technol. 2017, 54, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Fontoura, A.; Vidal, A.; Prestes Dornelles, R.; Kubota, E.; Mello, R.; Cansian, R.; Demiate, I.; Soltovski, C. Characterization of hydrolysates of collagen from mechanically separated chicken meat residue. Food Sci. Technol. 2020, 40, 355–362. [Google Scholar] [CrossRef]
- Chanmangkang, S.; Maneerote, J.; Surayot, U.; Panya, A.; You, S.; Wangtueai, S. Physicochemical and Biological Properties of Collagens Obtained from Tuna Tendon by Using the Ultrasound-Assisted Extraction. J. Agric. Food Res. 2024, 15, 100984. [Google Scholar] [CrossRef]
- ISO 11135:2014; Sterilization of Health-Care Products—Ethylene Oxide—Requirements for the Development, Validation and Routine Control of a Sterilization Process for Medical Devices. The International Organization for Standardization (ISO): Geneva, Switzerland, 2014; 78p.
- Ruiz, C.; Vera, M.; Rivas, B.; Sánchez, S.; Urbano, B. Magnetic methacrylated gelatin-g-polyelectrolyte for methylene blue sorption. RSC Adv. 2020, 10, 43799–43810. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Sun, J.; Dan, W.; Yuan, L.; Yang, J.; Guo, L.; Fan, H.; Zhang, X. Photo-crosslinked Mono-component Type II Collagen Hydrogel as Matrix to Induce Chondrogenic Diffrentiation of Bone Marrow Mesenchymal Stem Cells. J. Mater. Chem. B 2017, 5, 8707–8718. [Google Scholar] [CrossRef] [PubMed]
- Mendes, G.C.C.; Brandão, T.R.S.; Silva, C.L.M. Ethylene oxide sterilization of medical devices: A review. Am. J. Infect. Control 2007, 35, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Monaco, G.; Cholas, R.; Salvatore, L.; Madaghiele, M.; Sannino, A. Sterilization of collagen scaffolds designed for peripheral nerve regeneration: Effect on microstructure, degradation and cellular colonization. Mater. Sci. Eng. C 2017, 71, 335–344. [Google Scholar] [CrossRef] [PubMed]
- deWit, J.N.; Klarenbeek, G. Effects of Various Heat Treatments on Structure and Solubility of Whey Proteins. J. Dairy Sci. 1984, 67, 2701–2710. [Google Scholar] [CrossRef]
- Windmueller, H.G. The Reaction of Ethylene Oxide with Some Proteins, Amino Acids and Vitamins. Ph.D. Dissertation, Virginia Polytechnic Institute, Blacksburg, VA, USA, 1958; 120p. [Google Scholar]
- Ye, M.P.; Zhou, R.; Shi, Y.R.; Chen, H.C.; Du, Y. Effects of heating on the secondary structure of proteins in milk powders using mid-infrared spectroscopy. J. Dairy Sci. 2016, 100, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tian, Y.; Liu, C.; Dou, Z.; Diao, J. Effects of Heat Treatment on the Structural and Functional Properties of Phaseolus vulgaris L. Protein. Foods 2023, 12, 2869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, R.; Zhang, J.; Zhou, P. Heat-induced denaturation and bioactivity changes of whey proteins. Int. Dairy J. 2021, 123, 105175. [Google Scholar] [CrossRef]
- Wiegand, C.; Abel, M.; Ruth, P.; Wilhelms, T.; Schulze, D.; Norgauer, J.; Hipler, U.-C. Effect of the sterilization method on the performance of collagen type I on chronic wound parameters in vitro. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90B, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Chun, B.-S. Method for Obtaining Low-Molecular Weight Collagen Peptides from Fish Bones and Shells Using Pressurized Hydrothermal Hydrolysis. WIPO Patent WO2015182859A1.
- Reisz, J.A.; Bansal, N.; Qian, J.; Zhao, W.; Furdui, C.M. Effects of ionizing radiation on biological molecules—Mechanisms of damage and emerging methods of detection. Antioxid. Redox Signal. 2014, 21, 260–292. [Google Scholar] [CrossRef] [PubMed]
- Golz, A.-L.; Bradshaw, C. Gamma Radiation Induced Changes in the Biochemical Composition of Aquatic Primary Producers and Their Effect on Grazers. Front. Environ. Sci. 2019, 7, 100. [Google Scholar] [CrossRef]
- Hassan, A.B.; Mahmoud, N.S.; Elmamoun, K.; Adiamo, O.Q.; Mohamed Ahmed, I.A. Effects of gamma irradiation on the protein characteristics and functional properties of sesame (Sesamum indicum L.) seeds. Radiat. Phys. Chem. 2018, 144, 85–91. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Liu, X.; Shi, J.; Chen, H.; Gong, Y. SEM, FTIR and DSC Investigation of Collagen Hydrolysate Treated Degraded Leather. J. Cult. Herit. 2021, 48, 205–210. [Google Scholar] [CrossRef]



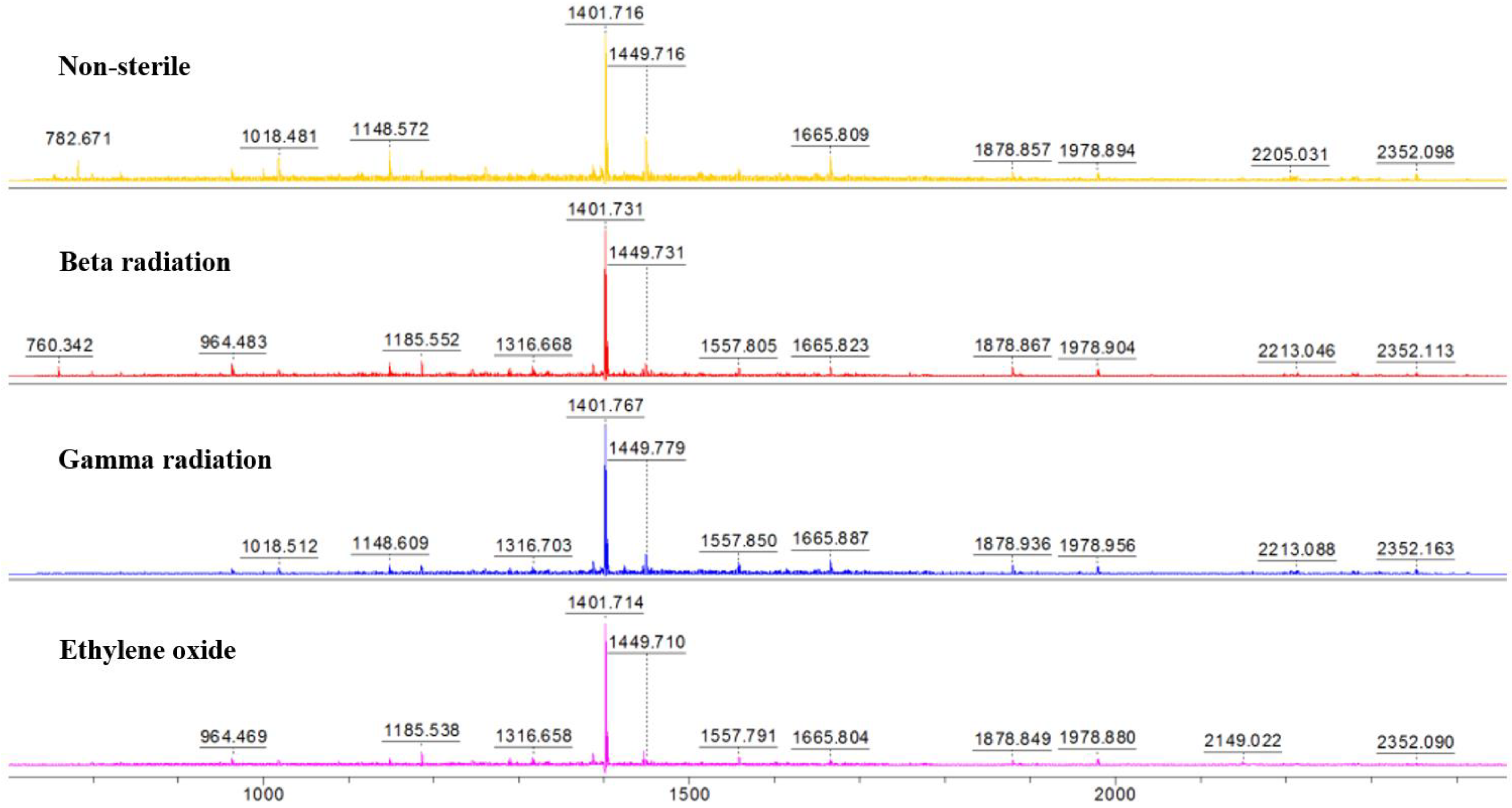
| Sample | m/z | % Difference from the control group |
| Non-sterile | 782.671 + 1018.481 + 1148.572 + 1401.716 + 1449.716 + 1665.809 + 1878.857 + 1978.894 + 2205.031 + 2352.098 = 15,881.845 | |
| Beta | 760.342 + 964.483 + 1185.552 + 1316.668 + 1401.731 + 1449.731 + 1557.805 + 1665.823 + 1878.867 + 1978.904 + 2213.046 + 2352.113 = 15,725.065 | −0.987% |
| Gamma | 1018.512 + 1148.609 + 1316.703 + 1401.767 + 1449.779 + 1557.850 + 1665.887 + 1878.936 + 1978.956 + 2213.088 + 2352.163 = 15,981.250 | +0.626% |
| Ethylene oxide | 964.469 + 1185.538 + 1316.658 + 1401.714 + 1449.710 + 1557.791 + 1665.804 + 1878.849 + 1978.880 + 2149.022 + 2352.090 = 15,900.525 | +0.118% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasanaphong, K.; Jittrontrum, P.; Srikaew, N.; Boonyagul, S.; Wangtueai, S.; Jantanasakulwong, K.; Rachtanapun, P.; Tawonsawatruk, T.; Tanadchangsaeng, N. Effect of Sterilization Methods on Collagen Hydrolysate Obtained from Tuna Tendon. Appl. Sci. 2024, 14, 6201. https://doi.org/10.3390/app14146201
Pasanaphong K, Jittrontrum P, Srikaew N, Boonyagul S, Wangtueai S, Jantanasakulwong K, Rachtanapun P, Tawonsawatruk T, Tanadchangsaeng N. Effect of Sterilization Methods on Collagen Hydrolysate Obtained from Tuna Tendon. Applied Sciences. 2024; 14(14):6201. https://doi.org/10.3390/app14146201
Chicago/Turabian StylePasanaphong, Kitipong, Paisal Jittrontrum, Narongrit Srikaew, Sani Boonyagul, Sutee Wangtueai, Kittisak Jantanasakulwong, Pornchai Rachtanapun, Tulyapruek Tawonsawatruk, and Nuttapol Tanadchangsaeng. 2024. "Effect of Sterilization Methods on Collagen Hydrolysate Obtained from Tuna Tendon" Applied Sciences 14, no. 14: 6201. https://doi.org/10.3390/app14146201
APA StylePasanaphong, K., Jittrontrum, P., Srikaew, N., Boonyagul, S., Wangtueai, S., Jantanasakulwong, K., Rachtanapun, P., Tawonsawatruk, T., & Tanadchangsaeng, N. (2024). Effect of Sterilization Methods on Collagen Hydrolysate Obtained from Tuna Tendon. Applied Sciences, 14(14), 6201. https://doi.org/10.3390/app14146201










