Biocontrol of Aflatoxin-Producing Aspergillus flavus ATCC 22546 by a Non-Aflatoxigenic Aspergillus flavus ATCC 9643
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains Used in This Study
2.2. Culture Conditions of Fungal Strains
2.3. Anti-Aflatoxin Activity Measurement
2.3.1. Comparison of the Expression Levels of Genes Involved in Aflatoxin Synthesis
2.3.2. Determination of In Vitro Anti-Aflatoxin Activity Using Dominance Difference
2.3.3. Determination of In Vitro Anti-Aflatoxin Activity on Rice Using Dominance Difference
2.4. Aflatoxin Content Determination
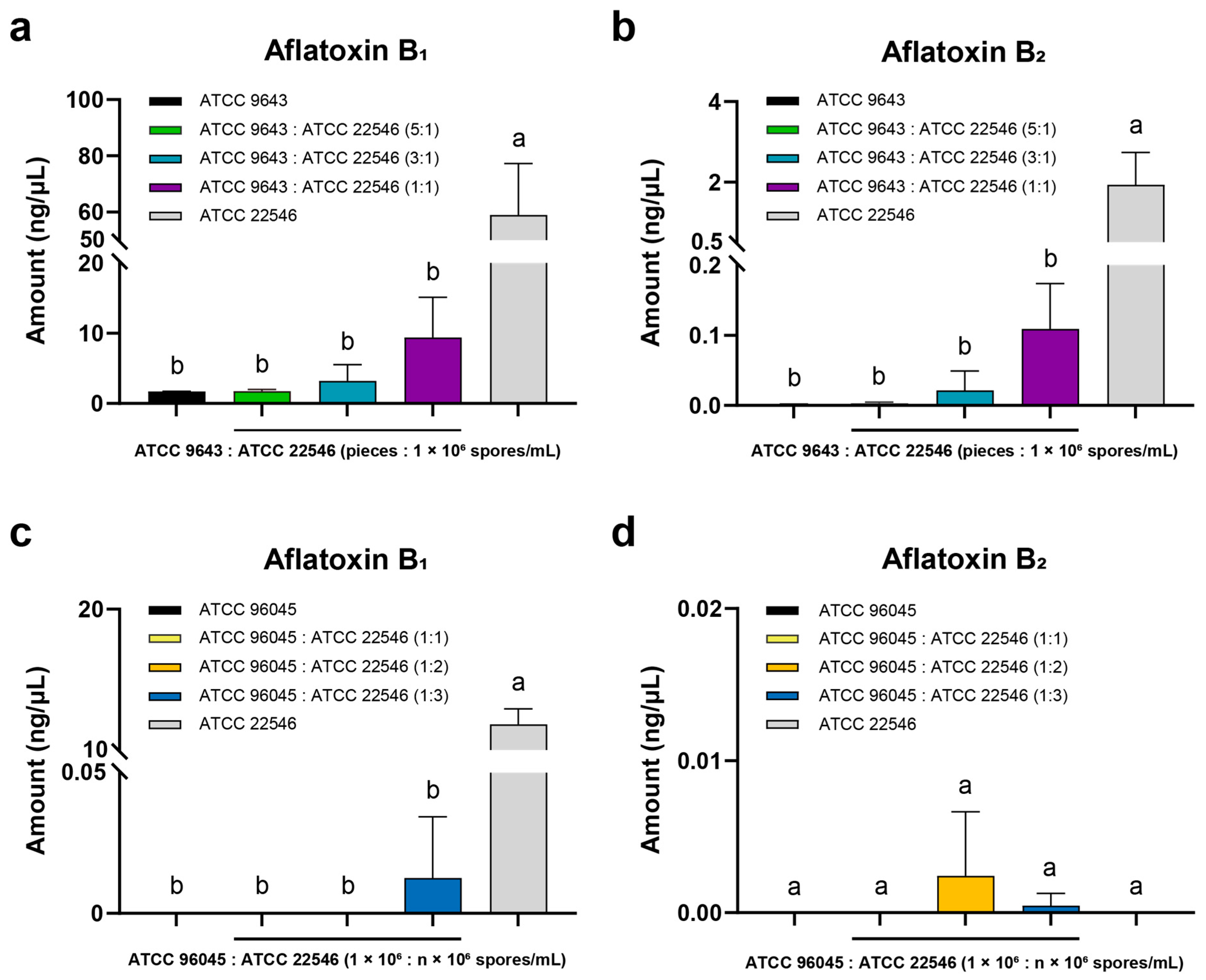
3. Results
3.1. Gene Expression in AFB1-Producing and Non-AFB1-Producing A. flavus
3.2. Suppressive Effect of Non-AFB1-Producing A. flavus ATCC 9643 on ATCC 22546 Growth
3.3. Antiaflatoxigenic Effect of Non-AFB1-Producing A. flavus ATCC 9643 on ATCC 22546
4. Discussion
4.1. Presence of Aflatoxin-Producing Genes in Wild Aspergillus sp. Isolates
4.2. Growth Competition between ATCC 22546 and ATCC 9643
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A global concern for food safety, human health and their management. Front. Microbiol. 2017, 7, 2170. [Google Scholar] [CrossRef]
- Marchese, S.; Polo, A.; Ariano, A.; Velotto, S.; Costantini, S.; Severino, L. Aflatoxin B1 and M1: Biological properties and their involvement in cancer development. Toxins 2018, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Nazhand, A.; Durazzo, A.; Lucarini, M.; Souto, E.B.; Santini, A. Characteristics, occurrence, detection and detoxification of aflatoxins in foods and feeds. Foods 2020, 9, 644. [Google Scholar] [CrossRef] [PubMed]
- Ali, N. Aflatoxins in rice: Worldwide occurrence and public health perspectives. Toxicol. Rep. 2019, 6, 1188–1197. [Google Scholar] [CrossRef] [PubMed]
- Van de Perre, E.; Jacxsens, L.; Lachat, C.; Tahan, F.E.; De Meulenaer, B. Impact of maximum levels in European legislation on exposure of mycotoxins in dried products: Case of aflatoxin B1 and ochratoxin A in nuts and dried fruits. Food Chem. Toxicol. 2015, 75, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Miklós, G.; Angeli, C.; Ambrus, A.; Nagy, A.; Kardos, V.; Zentai, A.; Kerekes, K.; Farkas, Z.; Jóźwiak, A.; Bartók, T. Detection of aflatoxins in different matrices and food-chain positions. Front. Microbiol. 2020, 11, 1916. [Google Scholar] [CrossRef] [PubMed]
- Toregeani-Mendes, K.A.; Arroteia, C.C.; Kemmelmeier, C.; Dalpasquale, V.A.; Bando, E.; Alves, A.F.; Marques, O.J.; Nishiyama, P.; Mossini, S.A.G.; Machinski, M., Jr. Application of hazard analysis critical control points system for the control of aflatoxins in the Brazilian groundnut-based food industry. Int. J. Food Sci. Technol. 2011, 46, 2611–2618. [Google Scholar] [CrossRef]
- Kang, S.Y.; Woo, S.Y.; Tian, F.; Lee, S.Y.; Chun, H.S. Comparison of aflatoxins and ochratoxin A contaminated in homemade and commercial doenjang manufactured by traditional and modified methods. Food Control 2023, 151, 109796. [Google Scholar] [CrossRef]
- Woo, S.Y.; Ok, H.E.; Lee, S.Y.; Jeong, A.Y.; Jeong, T.K.; Chun, H.S. Simple chromatographic determination of aflatoxins in Korean fermented soybean products doenjang, ganjang, and gochujang, with comparison of derivatization methods. Food Sci. Biotechnol. 2022, 31, 475–482. [Google Scholar] [CrossRef]
- Park, D.L.; Lee, S.L.; Price, R.L.; Pohland, A.E. Review of the decontamination of aflatoxins by ammoniation: Current status and regulation. J. Assoc. Off. Anal. Chem. 1988, 71, 685–703. [Google Scholar] [CrossRef]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hoogenboom, L.; Leblanc, J.C.; Nebbia, C.S.; Nielsen, E.; et al. Assessment of an application on a detoxification process of groundnut press cake for aflatoxins by ammoniation. EFSA J. 2021, 19, 7035. [Google Scholar]
- Mahato, D.K.; Lee, K.E.; Kamle, M.; Devi, S.; Dewangan, K.N.; Kumar, P.; Kang, S.G. Aflatoxins in food and feed: An overview on prevalence, detection and control strategies. Front. Microbiol. 2019, 10, 2266. [Google Scholar] [CrossRef] [PubMed]
- Srinivason, B.; Li, W.; Ruth, C.J.; Herrman, T.J.; Erickson, D.; Mehta, S. Rapid quantification of aflatoxin in food at the point of need: A monitoring tool for food systems dashboards. Curr. Res. Biotechnol. 2023, 6, 100153. [Google Scholar] [CrossRef]
- Masiello, M.; Somma, S.; Ghionna, V.; Logrieco, A.F.; Moretti, A. In vitro and in field response of different fungicides against Aspergillus flavus and Fusarium species causing for ear rot disease of maize. Toxins 2019, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.S.; Kim, H.M.; Chun, H.S.; Lee, S.E. Organic acids suppress aflatoxin production via lowering expression of aflatoxin biosynthesis-related genes in Aspergillus flavus. Food Control 2018, 88, 207–216. [Google Scholar] [CrossRef]
- Kim, G.; Lee, S.E. Antifungal and antiaflatoxigenic properties of naphthoquinones toward Aspergillus flavus and their mode of inhibitory action on aflatoxin biosynthesis. Food Control 2021, 119, 107506. [Google Scholar] [CrossRef]
- Guan, Y.; Chen, J.; Nepovimova, E.; Long, M.; Wu, W.; Kuca, K. Aflatoxin detoxification using microorganisms and enzymes. Toxins 2021, 13, 46. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.T.; Rehman, A.U.; Naqvi, S.A.; Hasnain, A.; Umar, U.U.; Azeem, H.; Shahid, M.; Umair, M. Biological mediated management of bacterial diseases in crop plants: A review. Pak. J. Phytopathol. 2021, 33, 217. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Do, A.D. Biological control of Aspergillus flavus infection and growth promotion of peanut seedlings by Lactiplantibacillus plantarum and Levilactobacillus brevis. Egypt. J. Biol. Pest Control 2024, 34, 32. [Google Scholar] [CrossRef]
- Doster, M.A.; Cotty, P.J.; Michailides, T.J. Evaluation of the atoxigenic Aspergillus flavus strain AF36 in pistachio orchards. Plant Dis. 2014, 98, 948–956. [Google Scholar] [CrossRef]
- Garcia-Lopez, M.T.; Luo, Y.; Ortega-Beltran, A.; Jaime, R.; Moral, J.; Michailides, T.J. Quantification of the aflatoxin biocontrol strain Aspergillus flavus AF36 in soil and in nuts and leaves of pistachio by real-time PCR. Plant Dis. 2021, 105, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Cotty, P.J.; Mellon, J. Ecology of aflatoxin producing fungi and biocontrol of aflatoxin contamination. Mycotox. Res. 2006, 22, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Cotty, P.J. Virulence and cultural characteristics of two Aspergillus flavus strains pathogenic on cotton. Phytopathology 1989, 79, 808–814. [Google Scholar] [CrossRef]
- Ortega-Beltran, A.; Moral, J.; Picot, A.; Puckett, R.D.; Cotty, P.J.; Michailides, T.J. Atoxigenic Aspergillus flavus isolates endemic to almond, fig, and pistachio orchards in California with potential to reduce aflatoxin contamination in these crops. Plant Dis. 2019, 103, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Freimoser, F.M.; Rueda-Mejia, M.P.; Tilocca, B.; Migheli, Q. Biocontrol yeasts: Mechanisms and applications. World J. Microbiol. Biotechnol. 2019, 35, 154. [Google Scholar] [CrossRef] [PubMed]
- Pandin, C.; Le Coq, D.; Canette, A.; Aymerich, S.; Briandet, R. Should the biofilm mode of life be taken into consideration for microbial biocontrol agents? Microb. Biotechnol. 2017, 10, 719–734. [Google Scholar] [CrossRef] [PubMed]
- Rossouw, D.; Meiring, S.P.; Bauer, F.F. Modifying Saccharomyces cerevisiae adhesion properties regulates yeast ecosystem dynamics. Appl. Environ. Sci. 2018, 3, e00383-18. [Google Scholar] [CrossRef] [PubMed]
- Podgórska-Kryszczuk, I. Biological control of Aspergillus flavus by the yeast Aureobasidium pullulans in vitro and on tomato fruit. Plants 2023, 12, 236. [Google Scholar] [CrossRef] [PubMed]
- Dikmetas, D.N.; Özer, H.; Karbancioglu-Guler, F. Biocontrol potential of antagonistic yeasts on in vitro and in vivo Aspergillus growth and its AFB1 production. Toxins 2023, 15, 402. [Google Scholar] [CrossRef]
- Fakruddin, M.; Chowdhury, A.; Hossain, M.N.; Ahmed, M.M. Characterization of aflatoxin producing Aspergillus flavus from food and feed samples. SpringerPlus 2015, 4, 159. [Google Scholar] [CrossRef]
- Yu, J. Current understanding on aflatoxin biosynthesis and future perspective in reducing aflatoxin contamination. Toxins 2012, 4, 1024–1057. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.M.; Korman, T.P.; Labonte, J.W.; Vagstad, A.L.; Hill, E.A.; Kamari-Bidkorpeh, O.; Tsai, S.C.; Townsend, C.A. Structural basis for biosynthesis programming of fungal aromatic polyketide cyclization. Nature 2009, 461, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Kwon, H.; Kim, K.; Lee, S.E. Antifungal and antiaflatoxigenic activities of 1,8-cineole and t-cinnamaldehyde on Aspergillus flavus. Appl. Sci. 2018, 8, 1655. [Google Scholar] [CrossRef]
- Podgórska-Kryszczuk, I.; Pankiewicz, U.; Sas-Paszt, L. Biological control of Aspergillus parasiticus and Aspergillus ochraceus and reductions in the amount of ochratoxin A and aflatoxins in bread by selected non-conventional yeast. Foods 2023, 12, 3871. [Google Scholar] [CrossRef] [PubMed]
- Loc, N.H.; Huy, N.D.; Quang, H.T.; Lan, T.T.; Ha, T.T.T. Characterisation and antifungal activity of extracellular chitinase from a biocontrol fungus, Trichoderma asperellum PQ34. Mycology 2020, 11, 38–48. [Google Scholar] [CrossRef]
- Ueki, A.; Takehara, T.; Ishioka, G.; Kaku, N.; Ueki, K. β-1,3-glucanase production as an antifungal enzyme by phylogenetically different strains of the genus Clostridium isolated from anoxic soil that underwent biological disinfestation. Appl. Microbiol. Biotechnol. 2020, 104, 5563–5578. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Cheng, W.; Jiang, K.; Jiao, Z.; Luo, H.; Yang, C.; Pang, M.; Lu, L. The antifungal activity of a serine protease and the enzyme production of characteristics of Bacillus licheniformis TG116. Arch. Microbiol. 2022, 204, 601. [Google Scholar] [CrossRef]
- Malik, M.S.; Rehman, A.; Khan, I.U.; Khan, T.A.; Jamil, M.; Rha, E.S.; Anees, M. Thermo-neutrophilic cellulases and chitinases characterized from a novel putative antifungal biocontrol agent: Bacillus subtilis TD11. PLoS ONE 2023, 18, e0281102. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, D.F.; Wu, H.; Zhang, L.; Xu, Y. Inhibition of endogenous α-amylase and protease of Aspergillus flavus by trypsin inhibitor from cultivated and wild-type soybean. Ann. Microbiol. 2010, 60, 405–414. [Google Scholar] [CrossRef]
- Ehrlich, K. Effect on aflatoxin production of competition between wild type and mutant strains of Aspergillus parasiticus. Mycopathologia 1987, 97, 93–96. [Google Scholar] [CrossRef]
- Cotty, P.J. Aflatoxin-producing potential of communities of Aspergillus section flavi from cotton-producing areas in the United States. Mycol. Res. 1997, 101, 698–704. [Google Scholar] [CrossRef]
- Dorner, J.W.; Cole, R.J.; Connick, W.J.; Daigle, D.L.; McGuire, M.R.; Shasha, B.S. Evaluation of biological control formulations to reduce aflatoxin contamination in peanuts. Biol. Control 2003, 26, 318–324. [Google Scholar] [CrossRef]
- Cotty, P.J.; Bhatnagar, D. Variability among atoxigenic Aspergillus flavus strains in the ability to prevent aflatoxin contamination and production of aflatoxin biosynthetic pathway enzymes. Appl. Environ. Microbiol. 1994, 60, 2248–2251. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.K.; Accinelli, C.; Shier, W.T. Biological control of Aflatoxin Contamination in U.S crops and the use of bioplasticformulations of Aspergillus flavus biocontrol strains to optimize application strategies. J. Agric. Food Chem. 2017, 65, 7081–7087. [Google Scholar] [CrossRef] [PubMed]
- Kabak, B.; Dobson, A.D. Biological strategies to counteract the effects of mycotoxins. J. Food Prot. 2009, 72, 2006–2016. [Google Scholar] [CrossRef]
- Mamo, F.T.; Selvaraj, J.N.; Wang, Y.; Liu, Y. Recent developments in the screening of atoxigenic Aspergillus flavus towards aflatoxin biocontrol. J. Appl. Environ. Microbiol. 2017, 5, 20–30. [Google Scholar]





| Gene Symbol | Gene Function | Primer Sequences (5′-3′) | |
|---|---|---|---|
| 18S rRNA | Housekeeping gene | F | ATGGCCGTTCTTAGTTGGTG |
| R | GTACAAAGGGCAGGGACGTA | ||
| aflC | Polyketide synthase | F | ACTGGCAACTGCAAACCCTA |
| R | CCAGCCGTTTGATGAACACC | ||
| aflD | Reductase | F | CCAACATGCACGACTATGCG |
| R | GCCGTGAGCCATTTGTTCTC | ||
| aflE | NOR reductase | F | CGTCTCTCAGTCAAGGCCAG |
| R | TCGCATCACTTCCTCCACAC | ||
| aflG | P450 monooxygenase | F | GCATCTTCCACCCTTCCACA |
| R | GAAAAGGCCAACAGTCGTCG | ||
| aflK | VERB synthase | F | ATGCAGGGAAAGACCTTGGG |
| R | AACTATCGTCGCCAACGTGA | ||
| aflL | Desaturase | F | GCAACAGTTTGTGGCCGATT |
| R | ATGAACTTGTCGGCGTGAGT | ||
| aflO | O-methyltransferase | F | AATTCCCCGCTCCTGACAAG |
| R | CGACCAGGAAGGTTGGGAAA | ||
| aflP | O-methyltransferase | F | CTTTCTCATTGGCATTTGCGC |
| R | CGCGTTTGCGRCAACAACTTG | ||
| aflQ | Oxidoreductase | F | GATAACCCGGACGACCTTCG |
| R | CTCATCTTTTCCATGCGGCG | ||
| aflR | transcription regulator | F | TGCAGTCAATGGAACACGGA |
| R | TGGGGGTCCCTACTTCCAAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, K.-S.; Kim, H.-M.; Lee, J.; Ganbat, D.; Lee, S.-E. Biocontrol of Aflatoxin-Producing Aspergillus flavus ATCC 22546 by a Non-Aflatoxigenic Aspergillus flavus ATCC 9643. Appl. Sci. 2024, 14, 6142. https://doi.org/10.3390/app14146142
Jung K-S, Kim H-M, Lee J, Ganbat D, Lee S-E. Biocontrol of Aflatoxin-Producing Aspergillus flavus ATCC 22546 by a Non-Aflatoxigenic Aspergillus flavus ATCC 9643. Applied Sciences. 2024; 14(14):6142. https://doi.org/10.3390/app14146142
Chicago/Turabian StyleJung, Kwang-Soo, Hyeong-Mi Kim, Jieun Lee, Dariimaa Ganbat, and Sung-Eun Lee. 2024. "Biocontrol of Aflatoxin-Producing Aspergillus flavus ATCC 22546 by a Non-Aflatoxigenic Aspergillus flavus ATCC 9643" Applied Sciences 14, no. 14: 6142. https://doi.org/10.3390/app14146142
APA StyleJung, K.-S., Kim, H.-M., Lee, J., Ganbat, D., & Lee, S.-E. (2024). Biocontrol of Aflatoxin-Producing Aspergillus flavus ATCC 22546 by a Non-Aflatoxigenic Aspergillus flavus ATCC 9643. Applied Sciences, 14(14), 6142. https://doi.org/10.3390/app14146142







