NOx Formation Mechanism and Emission Prediction in Turbulent Combustion: A Review
Abstract
1. Introduction
2. NOx Formation Mechanism
2.1. Thermal NOx Route
2.2. Prompt NO Route
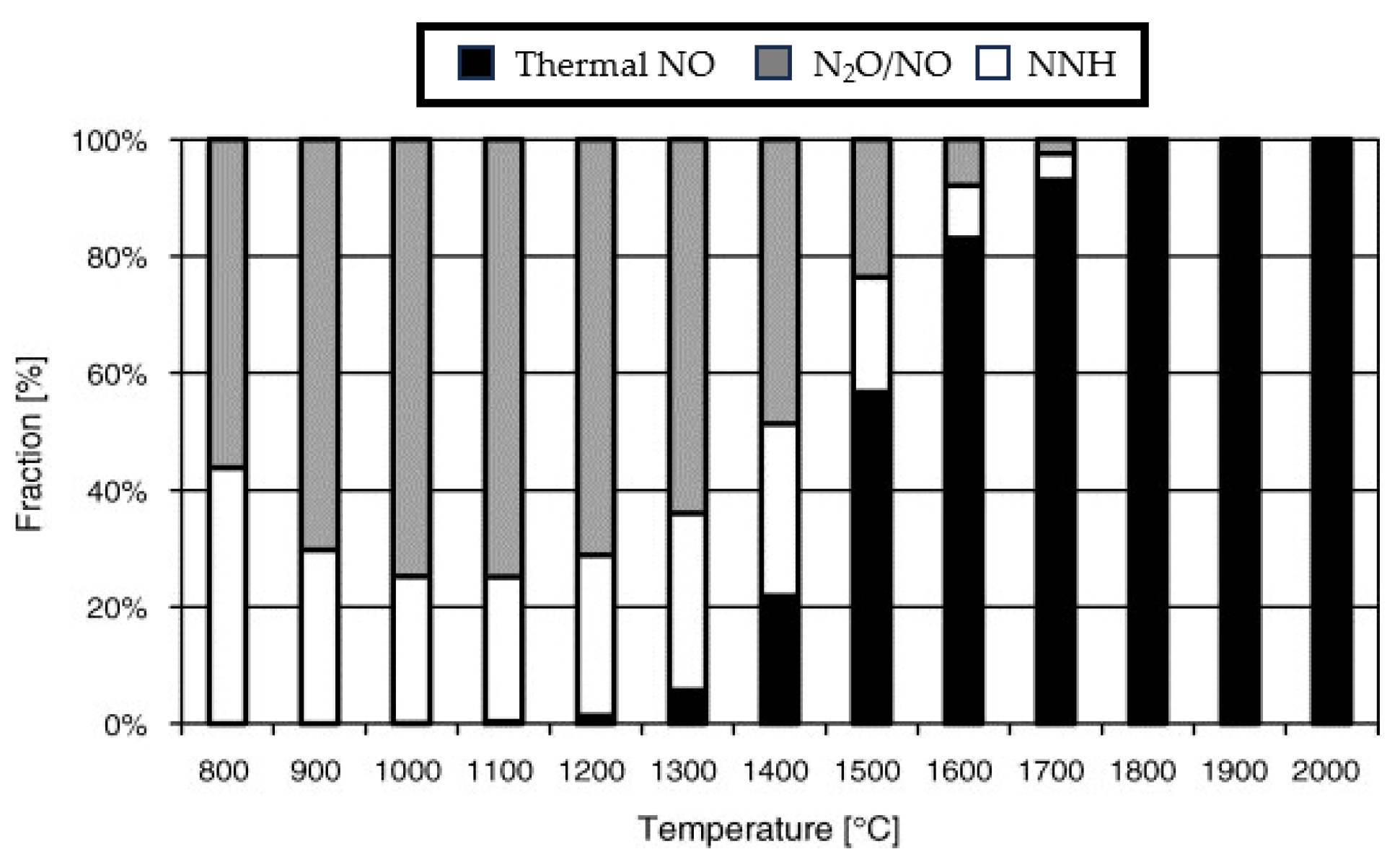
2.3. N2O Route
2.4. NNH Route
2.5. Fuel-N Route
3. NOx Formation-Kinetics Approach
3.1. Simplified Kinetics
3.2. Detailed Mechanisms
4. The Empirical Formula Approach
5. The Chemical Reaction Network Method
6. Turbulent Combustion Simulation Approach
6.1. Direct Numerical Simulation Method
6.2. CFD with Different Combustion Models
6.2.1. Combustion Models with Fast Chemistry Hypothesis
6.2.2. Combustion Models with Detailed Mechanisms
6.2.3. Combustion Models with CRNs Method
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, P.; Li, Q.; Feng, X.; Liang, H.; Suo, J.; Liu, Z. Experimental and Simulation Study on the Emissions of a Multi-Point Lean Direct Injection Combustor. J. Appl. Fluid Mech. 2023, 16, 1938–1950. [Google Scholar] [CrossRef]
- Peters, N. Multiscale combustion and turbulence. Proc. Combust. Inst. 2009, 32, 1–25. [Google Scholar] [CrossRef]
- Khelil, A.; Naji, H.; Loukarfi, L.; Mompean, G. Prediction of a high swirled natural gas diffusion flame using a PDF model. Fuel 2009, 88, 374–381. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Wang, Z.C.; Yang, X.Y. Emission characteristics of cellulosic jet biofuel blend under laminar and turbulent combustion. Biotechnol. Biofuels Bioprod. 2023, 16, 196. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Wang, Z.C.; Gu, X.Y.; Liu, H.B.; Yang, L.H.; Yang, J.Y.; Yang, X.Y. Intelligent quantitative assessment on the spray performance of alternative aviation fuel. Front. Energy Res. 2022, 10, 944668. [Google Scholar] [CrossRef]
- Hung, W.S.Y. An Experimentally Verified NOx Emission Model for Gas Turbine Combustors. Am. Soc. Mech. Eng. 1975, 79771, V01BT02A009. [Google Scholar]
- Wei, Z.B.; Li, X.L.; Xu, L.J.; Cheng, Y.T. Comparative study of computational intelligence approaches for NOx reduction of coal-fired boiler. Energy 2013, 55, 683–692. [Google Scholar] [CrossRef]
- Liou, J.L.; Liao, K.C.; Wen, H.T.; Wu, H.Y. A study on nitrogen oxide emission prediction in Taichung thermal power plant using artificial intelligence (AI) model. Int. J. Hydrogen Energy 2024, 63, 1–9. [Google Scholar] [CrossRef]
- Wu, W.; Lin, Y.T.; Liao, P.H.; Aziz, M.; Kuo, P.C. Prediction of CO-NOx Emissions from a Natural Gas Power Plant Using Proper Machine Learning Models. Energy Technol. 2023, 11, 2300041. [Google Scholar] [CrossRef]
- Lim, J.Z.F.; Mazlan, N.M. Analysing NOx and soot formations of an annular chamber with various types of biofuels. Adv. Aircr. Spacecr. Sci. 2022, 9, 537–551. [Google Scholar] [CrossRef]
- Safarzadeh, M.; Heidarinejad, G.; Pasdarshahri, H. A Study on turbulence-combustion interaction and Sub-grid Scale model in the simulation of Methane pool fire using LES. Sci. Iran. 2021, 28, 2133–2149. [Google Scholar] [CrossRef]
- Pashchenko, D. Hydrogen-rich fuel combustion in a swirling flame: CFD-modeling with experimental verification. Int. J. Hydrogen Energy 2020, 45, 19996–20003. [Google Scholar] [CrossRef]
- Fan, H.B.; Feng, J.X.; Bai, W.; Wang, H.; Akkurt, N.; Li, W.D.; Gao, J.; Aliakbaria, O.; Xu, Q. A study on the combustion performance and NOx emissions characteristics of a novel U-type radiant tube based on MILD combustion theory. Fuel 2023, 354, 128321. [Google Scholar] [CrossRef]
- Bhargava, A.; Kendrick, D.W.; Colket, M.B.; Sowa, W.A.; Maloney, D.J. Pressure Effect on NOx and CO Emissions in Industrial Gas Turbines. In Proceedings of the ASME Turbo Expo 2000: Power for Land, Sea, and Air, Munich, Germany, 8–11 May 2000. [Google Scholar]
- Mohamed, H.; Ben Ticha, H.; Mohamed, S. Simulation of pollutant emissions from a gas-turbine combustor. Combust. Sci. Technol. 2004, 176, 819–834. [Google Scholar] [CrossRef]
- Kaluri, A.; Malte, P.; Novosselov, I. Real-time prediction of lean blowout using chemical reactor network. Fuel 2018, 234, 797–808. [Google Scholar] [CrossRef]
- Gupta, S.; Malte, P.; Brunton, S.L.; Novosselov, I. Prevention of lean flame blowout using a predictive chemical reactor network control. Fuel 2019, 236, 583–588. [Google Scholar] [CrossRef]
- Khodayari, H.; Ommi, F.; Saboohi, Z. Multi-objective optimization of a lean premixed laboratory combustor through CFD-CRN approach. Therm. Sci. Eng. Prog. 2021, 25, 101014. [Google Scholar] [CrossRef]
- Russo, C.; Mori, G.; Anisimov, V.V.; Parente, J. Micro gas turbine combustor emissions evaluation using the chemical reactor modelling approach. In Proceedings of the 52nd ASME Turbo Expo, Montreal, QC, Canada, 14–17 May 2007; pp. 531–542. [Google Scholar]
- Benedetto, D.; Pasini, S.; Falcitelli, M.; La Marca, C.; Tognotti, L. NOx emission prediction from 3D complete modelling to reactor network analysis. Combust. Sci. Technol. 2000, 153, 279–294. [Google Scholar] [CrossRef]
- Zeldovitch, J. The oxidation of nitrogen in combustion and explosions. Acta Physicochim. 1946, 21, 577–628. [Google Scholar]
- Löffler, G.; Sieber, R.; Harasek, M.; Hofbauer, H.; Hauss, R.; Landauf, J. NOx formation in natural gas combustion—A new simplified reaction scheme for CFD calculations. Fuel 2006, 85, 513–523. [Google Scholar] [CrossRef]
- Mancini, M.; Weber, R.; Bollettini, U. Predicting NOx emissions of a burner operated in flameless oxidation mode. Proc. Combust. Inst. 2002, 29, 1155–1163. [Google Scholar] [CrossRef]
- Kang, Y.; Lu, X.; Wang, Q.; Ji, X.; Miao, S.; Zong, C.; Luo, G.; Liu, H. An experimental and modeling study of NOx and CO emission behaviors of dimethyl ether (DME) in a boiler furnace. Fuel Process. Technol. 2014, 122, 129–140. [Google Scholar] [CrossRef]
- Emami, M.D.; Shahbazian, H.; Sunden, B. Effect of Operational Parameters on Combustion and Emissions in an Industrial Gas Turbine Combustor. J. Energy Resour. Technol-Trans. ASME 2019, 141, 012202. [Google Scholar] [CrossRef]
- Hwang, C.H.; Hyun, S.H.; Lee, C.E. Effects of heat loss on NOx emission in the postflame region of premixed CH4—Air combustion. Energy Fuels 2008, 22, 996–1003. [Google Scholar] [CrossRef]
- Xue, H.S.; Aggarwal, S.K. NOx emissions in n-heptane/air partially premixed flames. Combust. Flame 2003, 132, 723–741. [Google Scholar] [CrossRef]
- Love, N.; Parthasarathy, R.N.; Gollahalli, S.R. Concentration Measurements of CH and OH Radicals in Laminar Biofuel Flames. Int. J. Green Energy 2011, 8, 113–120. [Google Scholar] [CrossRef]
- Versailles, P.; Watson, G.M.G.; Durocher, A.; Bourque, G.; Bergthorson, J.M. Thermochemical Mechanism Optimization for Accurate Predictions of CH Concentrations in Premixed Flames of C1-C3 Alkane Fuels. J. Eng. Gas Turbines Power-Trans. ASME 2018, 140, 061508. [Google Scholar] [CrossRef]
- Xi, Z.Y.; Liu, Z.D.; Shi, X.X.; Lian, T.Y.; Li, Y.Y. Numerical investigation on flow characteristics and emissions under varying swirler vane angle in a lean premixed combustor. Case Stud. Therm. Eng. 2022, 31, 101800. [Google Scholar] [CrossRef]
- Li, P.; Wang, F.; Mi, J.; Dally, B.B.; Mei, Z.; Zhang, J.; Parente, A. Mechanisms of NO formation in MILD combustion of CH4/H2 fuel blends. Int. J. Hydrogen Energy 2014, 39, 19187–19203. [Google Scholar] [CrossRef]
- Jiang, X.D.; Li, P.F.; Guo, J.J.; Hu, F.; Wang, F.F.; Mi, J.C.; Liu, Z.H. Detailed investigation of NO mechanism in non-premixed oxy-fuel jet flames with CH4/H2 fuel blends. Int. J. Hydrogen Energy 2018, 43, 8534–8557. [Google Scholar] [CrossRef]
- Bozzelli, J.W.; Dean, A.M. O + NNH: A possible new route for NOx formation in flames. Int. J. Chem. Kinet. 1995, 27, 1097–1109. [Google Scholar] [CrossRef]
- Xu, S.T.; Tong, Y.H.; Jin, S.C.; Ren, H.; Tu, Y.J.; Zhang, S.H.; Liu, H. NO formation and reduction during methane/hydrogen MILD combustion over a wide range of hydrogen-blending ratios in a well-stirred reactor. Fuel 2023, 346, 128324. [Google Scholar] [CrossRef]
- Konnov, A.A.; Colson, G.; De Ruyck, J. NO formation rates for hydrogen combustion in stirred reactors. Fuel 2001, 80, 49–65. [Google Scholar] [CrossRef]
- Rutar, T.; Malte, P.C. NOx Formation in High-Pressure Jet-Stirred Reactors With Significance to Lean-Premixed Combustion Turbines. J. Eng. Gas Turbines Power 2002, 124, 776–783. [Google Scholar] [CrossRef]
- Soete, G.G.D. Overall reaction rates of NO and N2 formation from fuel nitrogen. Symp. Combust. 1975, 15, 1093–1102. [Google Scholar] [CrossRef]
- Waluyo, R.; Aziz, M. Advanced numerical simulation of hydrogen/air turbulent non-premixed flame on model burner. Therm. Sci. Eng. Prog. 2024, 49, 102467. [Google Scholar] [CrossRef]
- Tasdemir, S.; Karyeyen, S. Colourless distributed combustion effects on a pre-mixed coke oven gas flame. Can. J. Chem. Eng. 2024, 102, 1726–1742. [Google Scholar] [CrossRef]
- Lefebvre, A.H. Fuel effects on gas turbine combustion—Liner temperature, pattern factor, and pollutant emissions. J. Aircr. 1984, 21, 887–898. [Google Scholar] [CrossRef]
- Odgers, J.; Kretschmer, D. The Prediction of Thermal NOx in Gas Turbines. Int. J. Turbo Jet Engines 1988, 5, 225–232. [Google Scholar] [CrossRef]
- Rizk, N.; Mongia, H. Emissions predictions of different gas turbine combustors. In Proceedings of the 32nd Aerospace Sciences Meeting & Exhibit, Reno, NV, USA, 10–12 January 1994. [Google Scholar]
- Laviolette, M.; Perez, R. On the Prediction of Pollutant Emission Indices From Gas Turbine Combustion Chambers. In Proceedings of the ASME Turbo Expo: Turbine Technical Conference & Exposition, Copenhagen, Denmark, 11–15 June 2012. [Google Scholar]
- Saravanan, S.; Nagarajan, G.; Rao, G.L.N.; Sampath, S. Theoretical and experimental investigation on effect of injection timing on NOx emission of biodiesel blend. Energy 2014, 66, 216–221. [Google Scholar] [CrossRef]
- Iavarone, S.; Cafiero, M.; Ferrarotti, M.; Contino, F.; Parente, A. A multiscale combustion model formulation for NOx predictions in hydrogen enriched jet flames. Int. J. Hydrogen Energy 2019, 44, 23436–23457. [Google Scholar] [CrossRef]
- Shen, Q.Q.; Wang, G.Y.; Wang, Y.H.; Zeng, B.S.; Yu, X.; He, S.C. Prediction Model for Transient NOx Emission of Diesel Engine Based on CNN-LSTM Network. Energies 2023, 16, 5347. [Google Scholar] [CrossRef]
- Park, J.J.; Lee, S.; Shin, S.; Kim, M.; Park, J. Development of a Light and Accurate NOx Prediction Model for Diesel Engines Using Machine Learning and Xai Methods. Int. J. Automot. Technol. 2023, 24, 559–571. [Google Scholar] [CrossRef]
- Han, Z.Z.; Xie, Y.; Hossain, M.M.; Xu, C.L. A hybrid deep neural network model for NOx emission prediction of heavy oil-fired boiler flames. Fuel 2023, 333, 126419. [Google Scholar] [CrossRef]
- Savarese, M.; Cuoci, A.; De Paepe, W.; Parente, A. Machine learning clustering algorithms for the automatic generation of chemical reactor networks from CFD simulations. Fuel 2023, 343, 127945. [Google Scholar] [CrossRef]
- Sharma, S.; Savarese, M.; Coussement, A.; Parente, A. Decarbonisation potential of dimethyl ether/hydrogen mixtures in a flameless furnace: Reactive structures and pollutant emissions. Int. J. Hydrogen Energy 2023, 48, 2401–2427. [Google Scholar] [CrossRef]
- Liu, Z.G.; Xiong, Y.; Zhu, Z.R.; Zhang, Z.D.; Liu, Y. Effects of hydrogen addition on combustion characteristics of a methane fueled MILD model combustor. Int. J. Hydrogen Energy 2022, 47, 16309–16320. [Google Scholar] [CrossRef]
- Liu, H.; Qian, W.; Zhu, M.; Li, S. Kinetics Modeling on NOx Emissions of a Syngas Turbine Combustor Using Rich-Burn, Quick-Mix, Lean-Burn Combustion Method. J. Eng. Gas Turbines Power-Trans. ASME 2020, 142, 021005. [Google Scholar] [CrossRef]
- Xiao, Y.L.; Lai, Z.X.; Wang, Z.P.; Chen, K.F. Predicting Lean Blowout and Emissions of Aircraft Engine Combustion Chamber Based on CRN. Int. J. Turbo Jet-Engines 2019, 36, 147–156. [Google Scholar] [CrossRef]
- Xu, K.; Shen, S.; Li, C.; Zheng, L. A New Procedure for Predicting Nox Emission in Preliminary Gas Turbine Combustor Design. In Proceedings of the ASME Turbo Expo: Turbine Technical Conference and Exposition, San Antonio, TX, USA, 3–7 June 2013. [Google Scholar]
- Rizk, N.K.; Chin, J.S.; Marshall, A.W.; Razdan, M.K. Predictions of NOx formation under combined droplet and partially premixed reaction of diffusion flame combustors. J. Eng. Gas Turbines Power-Trans. ASME 2002, 124, 31–38. [Google Scholar] [CrossRef]
- Andreini, A.; Facchini, B. Gas turbines design and off-design performance analysis with emissions evaluation. J. Eng. Gas Turbines Power-Trans. ASME 2004, 126, 83–91. [Google Scholar] [CrossRef]
- Villette, S.; Adam, D.; Alexiou, A.; Aretakis, N.; Mathioudakis, K. A Simplified Chemical Reactor Network Approach for Aeroengine Combustion Chamber Modeling and Preliminary Design. Aerospace 2024, 11, 22. [Google Scholar] [CrossRef]
- Fichet, V.; Kanniche, M.; Plion, P.; Gicquel, O. A reactor network model for predicting NOx emissions in gas turbines. Fuel 2010, 89, 2202–2210. [Google Scholar] [CrossRef]
- Khodayari, H.; Ommi, F.; Saboohi, Z. A review on the applications of the chemical reactor network approach on the prediction of pollutant emissions. Aircr. Eng. Aerosp. Technol. 2020, 92, 551–570. [Google Scholar] [CrossRef]
- Liu, F.; Mu, Y.; Liu, C.; Yang, J.; Mao, Y.; Xu, G.; Zhu, J. Effect of Fuel Staged Proportion on NOx Emission Performance of Centrally Staged Combustor. In Proceedings of the ASME Turbo Expo: Turbine Technical Conference and Exposition, San Antonio, TX, USA, 3–7 June 2013. [Google Scholar]
- Kim, S.K.; Kim, Y. Assessment of the Eulerian particle flamelet model for non-premixed turbulent jet flames. Combust. Flame 2008, 154, 232–247. [Google Scholar] [CrossRef]
- Domingo, P.; Vervisch, L. Recent developments in DNS of turbulent combustion. Proc. Combust. Inst. 2023, 39, 2055–2076. [Google Scholar] [CrossRef]
- Trisjono, P.; Pitsch, H. Systematic Analysis Strategies for the Development of Combustion Models from DNS: A Review. Flow Turbul. Combust. 2015, 95, 231–259. [Google Scholar] [CrossRef]
- Bédat, B.; Egolfopoulos, F.N.; Poinsot, T. Direct numerical simulation of heat release and NOx formation in turbulent non-premixed flames. Combust. Flame 1999, 119, 69–83. [Google Scholar] [CrossRef]
- Ohta, T.; Hirata, R.; Sakai, Y. DNS predictions of NOx production in developing turbulent mixing layers with non-premixed hydrogen-air combustion. J. Turbul. 2022, 23, 636–654. [Google Scholar] [CrossRef]
- Dinesh, K.; van Oijen, J.A.; Luo, K.H.; Jiang, X. Nitric oxide pollutant formation in high hydrogen content (HHC) syngas flames. Int. J. Hydrogen Energy 2015, 40, 13621–13634. [Google Scholar] [CrossRef]
- Ren, J.; Wang, H.; Wang, C.; Luo, K.; Fan, J. A-priori and a-posterior studies of filtered probability density function models and NO formation prediction in turbulent stratified premixed combustion using machine learning. Fuel 2023, 333, 126358. [Google Scholar] [CrossRef]
- Luo, K.; Bai, Y.; Yang, J.S.; Wang, H.O.; Zhou, L.X.; Fan, J.R. A priori validation of a second-order moment combustion model via DNS database. Int. J. Heat Mass Transf. 2015, 86, 415–425. [Google Scholar] [CrossRef]
- da Silva, C.V.; Deon, D.L.; Centeno, F.R.; França, F.H.R.; Pereira, F.M. Assessment of combustion models for numerical simulations of a turbulent non-premixed natural gas flame inside a cylindrical chamber. Combust. Sci. Technol. 2018, 190, 1528–1556. [Google Scholar] [CrossRef]
- Choi, Y.C.; Li, X.Y.; Park, T.J.; Kim, J.H.; Lee, J.G. Numerical study on the coal gasification characteristics in an entrained flow coal gasifier. Fuel 2001, 80, 2193–2201. [Google Scholar] [CrossRef]
- Yan, Y.W.; Zhao, J.X.; Zhang, J.Z.; Liu, Y. Large-eddy simulation of two-phase spray combustion for gas turbine combustors. Appl. Therm. Eng. 2008, 28, 1365–1374. [Google Scholar] [CrossRef]
- Joung, D.; Huh, K.Y. 3D RANS Simulation of Turbulent Flow and Combustion in a 5 MW Reverse-Flow Type Gas Turbine Combustor. J. Eng. Gas Turbines Power 2010, 132, 111504. [Google Scholar] [CrossRef]
- Mobasheri, R.; Shahrokhi-Dehkordi, M.S. A Comparative Study of Combustion Models for Spark Ignition Engines Based on Experimentation and CFD Simulation. In Proceedings of the Fall Technical Conference of the ASME Internal Combustion Engine Division, SW Res Inst, San Antonio, TX, USA, 12–15 September 2010; pp. 289–299. [Google Scholar]
- Lyu, Z.H.; Li, G.S.; Song, X.Z.; Cui, L.; Ji, G.D.; Wang, Z.H.; Hu, X.D.; Xu, Z.P. Comparative numerical analysis and optimization in downhole combustion chamber of thermal spallation drilling. Appl. Therm. Eng. 2017, 119, 481–489. [Google Scholar] [CrossRef]
- Hosseini, A.A.; Ghodrat, M.; Moghiman, M.; Pourhoseini, S.H. Numerical study of inlet air swirl intensity effect of a Methane-Air Diffusion Flame on its combustion characteristics. Case Stud. Therm. Eng. 2020, 18, 100610. [Google Scholar] [CrossRef]
- Ribert, G.; Champion, M.; Gicquel, O.; Darabiha, N.; Veynante, D. Modeling nonadiabatic turbulent premixed reactive flows including tabulated chemistry. Combust. Flame 2005, 141, 271–280. [Google Scholar] [CrossRef]
- Robin, V.; Mura, A.; Champion, M.; Plion, P. A multi-dirac presumed PDF model for turbulent reactive flows with variable equivalence ratio. Combust. Sci. Technol. 2006, 178, 1843–1870. [Google Scholar] [CrossRef]
- Bazdidi-Tehrani, F.; Zeinivand, H. Presumed PDF modeling of reactive two-phase flow in a three dimensional jet-stabilized model combustor. Energy Convers. Manag. 2010, 51, 225–234. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Fattahi, A.; Sheikhzadeh, G.A.; Mehrabian, M.A. Investigation of the effect of air turbulence intensity on NOx emission in non-premixed hydrogen and hydrogen-hydrocarbon composite fuel combustion. Int. J. Hydrogen Energy 2011, 36, 10159–10168. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Fattahi, A.; Sheikhzadeh, G.A.; Hajialigol, N.; Nikfar, M. Numerical investigation of NOx reduction in a sudden-expansion combustor with inclined turbulent air jet. J. Mech. Sci. Technol. 2012, 26, 3723–3731. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Fattahi, A.; Sheikhzadeh, G.A.; Mehrabian, M.A. The effect of oxidant flow rate on a coaxial oxy-fuel flame. Heat Mass Transf. 2012, 48, 1615–1626. [Google Scholar] [CrossRef]
- Mohapatra, S.; Garnayak, S.; Lee, B.J.; Elbaz, A.M.; Roberts, W.L.; Dash, S.K.; Reddy, V.M. Numerical and chemical kinetic analysis to evaluate the effect of steam dilution and pressure on combustion of n-dodecane in a swirling flow environment. Fuel 2021, 288, 119710. [Google Scholar] [CrossRef]
- Sahin, M. Entrainment effects on combustion characteristics of various syngases using a perforated burner. Int. J. Hydrogen Energy 2022, 47, 15282–15295. [Google Scholar] [CrossRef]
- Tang, Q.; Xu, J.; Pope, S.B. Probability density function calculations of local extinction and no production in piloted-jet turbulent methane/air flames. Proc. Combust. Inst. 2000, 28, 133–139. [Google Scholar] [CrossRef]
- Paul, C.; Gao, J.; Jin, K.; Patel, D.; Roggendorf, K.; Fogla, N.; Parrish, S.E.; Wahiduzzaman, S. An indirect approach to optimize the reaction rates of thermal NO formation for diesel engines. Fuel 2023, 338, 127287. [Google Scholar] [CrossRef]
- Zhou, L.X. Developing unconditional moment models of turbulent combustion. Numer. Heat Transf. Part B-Fundam. 2020, 79, 16–28. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Zhou, Y.G.; Zhou, B.F. The effects of the initial NO volume fractions on the NOx generation and reduction routes under natural gas MILD combustion conditions. Fuel 2022, 328, 125175. [Google Scholar] [CrossRef]
- Albrecht, B.A.; Zahirovic, S.; Bastiaans, R.J.M.; van Oijen, J.A.; de Goey, L.P.H. A premixed flamelet-PDF model for biomass combustion in a grate furnace. Energy Fuels 2008, 22, 1570–1580. [Google Scholar] [CrossRef]
- Zhao, R.; Igie, U.; Abbott, D.; Amer Soc Mechanical, E. Hydrogen-Enriched Natural Gas Co-Firing: A Comparison of FGM and EDC Models. In Proceedings of the ASME Turbomachinery Technical Conference and Exposition (Turbo Expo) on Collaborate, Innovate and Empower-Propulsion and Power for a Sustainable Future, Boston, MA, USA, 26–30 June 2023. [Google Scholar]
- Qiao, L.; Zhou, L.X.; Chen, X.L.; Zhang, J. A Unified Second-Order Moment Model for Turbulent Combustion. J. Combust. Sci. Technol. 2002, 8, 297–301. [Google Scholar]
- Tao, L.; Kan, Z.; Kun, M.; Ren, F. Application of the Elliptic-Type Conditional Moment Closure Method to Turbulent Diffusion Bluff-Body Flames. J. Aerosp. Power 2003, 18, 761–767. [Google Scholar]
- Amzin, S.; Yasin, M.F.M. Modelling of a Bluff-Body Stabilised Premixed Flames Close to Blow-Off. Computation 2021, 9, 43. [Google Scholar] [CrossRef]
- Yue, C.; Wang, J.; Li, X. Modeling of Sandia Flame D with the non-adiabatic chemistry tabulation approach: The effects of different laminar flames on NOx prediction. RSC Adv. 2023, 13, 4590–4600. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Zhang, Y.; Wang, X.; Tian, Z.; Hu, G.; Du, W. Investigation of NOx emission under different burner structures with the optimized combustion model. Neurocomputing 2022, 482, 224–235. [Google Scholar] [CrossRef]
- Tian, L.; Sun, H.; Xu, Y.; Jiang, P.; Lu, H.; Hu, X. Numerical analysis on combustion flow characteristics of jet-stabilized combustor with different geometry. Case Stud. Therm. Eng. 2022, 32, 101885. [Google Scholar] [CrossRef]
- Guan, W.; Abdelsamie, A.; Chi, C.; He, Z.X.; Thévenin, D. A dedicated reduced kinetic model for ammonia/dimethyl-ether turbulent premixed flames. Combust. Flame 2023, 257, 113002. [Google Scholar] [CrossRef]
- Truc Huu, N.; Kim, S.; Park, J.; Jung, S.; Kim, S. CFD-CRN validation study for NOx emission prediction in lean premixed gas turbine combustor. J. Mech. Sci. Technol. 2017, 31, 4933–4942. [Google Scholar] [CrossRef]
- Monaghan, R.F.D.; Tahir, R.; Cuoci, A.; Bourque, G.; Füri, M.; Gordon, R.L.; Faravelli, T.; Frassoldati, A.; Curran, H.J. Detailed Multi-dimensional Study of Pollutant Formation in a Methane Diffusion Flame. Energy Fuels 2012, 26, 1598–1611. [Google Scholar] [CrossRef]
- Grimm, F. Low-Order Reactor-Network-Based Prediction of Pollutant Emissions Applied to FLOX(R) Combustion. Energies 2022, 15, 1740. [Google Scholar] [CrossRef]
- Xue, R.; Hu, C.; Nikolaidis, T.; Pilidis, P. Effect of Steam Addition on the Flow Field and NOx Emissions for Jet-A in an Aircraft Combustor. Int. J. Turbo Jet-Engines 2016, 33, 381–393. [Google Scholar] [CrossRef][Green Version]
- Ahmad, N.; Nairui, L.; Tariq, M.; Swati, R.F.; Anwar, M.B. NOx Emission Prediction Analysis and Comparison in Gas Turbine Combustor Utilizing CFD and CRN Combined Approach. In Proceedings of the 6th International Conference on Aerospace Science and Engineering (ICASE), Islamabad, Pakistan, 12–14 November 2019; pp. 44–52. [Google Scholar]
- Nguyen, T.H.; Park, J.; Jung, S.; Kim, S. A numerical study on NOx formation behavior in a lean-premixed gas turbine combustor using CFD-CRN method. J. Mech. Sci. Technol. 2019, 33, 5051–5060. [Google Scholar] [CrossRef]
- Chang, S.H.; Bak, H.S.; Yu, H.; Yoo, C.S. A numerical study of combustion and NOx emission characteristics of a lean premixed model gas turbine combustor. J. Mech. Sci. Technol. 2020, 34, 1795–1803. [Google Scholar] [CrossRef]
- Nguyen, T.H. Improved Chemical Reactor Network Application for Predicting the Emission of Nitrogen Oxides in a Lean Premixed Gas Turbine Combustor. Combust. Explos. Shock Waves 2019, 55, 267–273. [Google Scholar] [CrossRef]
- Zhang, Q.; Hai, H.; Li, C.; Wang, Y.; Zhang, P.; Wang, X. Predictions of NOx and CO emissions from a low-emission concentric staged combustor for civil aeroengines. Proc. Inst. Mech. Eng. Part G J. Aerosp. Eng. 2020, 234, 1075–1091. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Santhosh, R.; Mashruk, S.; Yadav, R.; Valera-Medina, A. Prediction of NOx emissions and pathways in premixed ammonia-hydrogen-air combustion using CFD-CRN methodology. J. Energy Inst. 2023, 111, 101406. [Google Scholar] [CrossRef]
- Trespi, S.; Nicolai, H.; Debiagi, P.; Janicka, J.; Dreizler, A.; Hasse, C.; Faravelli, T. Development and Application of an Efficient Chemical Reactor Network Model for Oxy-fuel Combustion. Energy Fuels 2021, 35, 7121–7132. [Google Scholar] [CrossRef]
- Perpignan, A.A.V.; Tomasello, S.G.; Rao, A.G. Evolution of Emission Species in an Aero-Engine Turbine Stator. Aerospace 2021, 8, 11. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Park, J.; Sin, C.; Jung, S.; Kim, S. Numerical Investigation of the Pressure Effect on the NOx Formation in a Lean-Premixed Gas Turbine Combustor. Energy Fuels 2021, 35, 6776–6784. [Google Scholar] [CrossRef]
- Khodayari, H.; Ommi, F.; Saboohi, Z. Uncertainty Analysis of the Chemical Reactor Network Approach for Predicting the Pollutant Emissions in a Double-Swirl Combustor. J. Energy Resour. Technol-Trans. ASME 2020, 142, 092302. [Google Scholar] [CrossRef]
- Perpignan, A.A.V.; Sampat, R.; Rao, A.G. Modeling Pollutant Emissions of Flameless Combustion with a Joint CFD and Chemical Reactor Network Approach. Front. Mech. Eng. 2019, 5, 63. [Google Scholar] [CrossRef]
| Fuel | Premixed/ Non-Premixed | Turbulence Model | Reaction Model | Mechanisms | Deviation | Reference |
|---|---|---|---|---|---|---|
| Ammonia/dimethyl-ether | Premixed | DNS | - | A reduced model with 48 species and 294 reactions | Close to experimental data | [96] |
| Hydrogen | Non-premixed | DNS | - | Konnov mechanism with 31 species and 241 reactions | - | [65] |
| CnHm | premixed | Standard | EDU | NOx equilibrium assumption | Only one point compared with the experimental data | [73] |
| Methane/air | Non-premixed | Standard | EDM | NOx equilibrium assumption | - | [75] |
| CH4/H2 | Non-premixed | Realizable | Presumed PDF | Thermal and prompt pathway | 19% approximately | [79] |
| Synthetic (hydrogen-rich) fuel | Premixed | RNG | Transported PDF | - | Root-mean-square error for NOx emission about 6% | [12] |
| Methane/air | Non-premixed | - | Transported PDF | 19 species, 15 reactions | Agreement with the experimental data | [84] |
| Natural gas | Non-premixed | standard model | EDC | GRI-Mech 2.2 | Small deviations with the experimental data | [87] |
| Fuel | Non-premixed | model | CMC | - | Reasonable agreement between experiments and predictions | [91] |
| CH4-air | Premixed | Standard | FLM | GRI-Mech 2.11 | 0.5 times overestimation referring to the experimental data | [93] |
| CRNs/CFD_CRNs | Fuel | Object | Mechanism | Units | Deviation | Reference |
|---|---|---|---|---|---|---|
| CRNs | Dimethyl ether and its mixtures with methane/hydrogen | Flameless furnace | NUIG-Mech 1.1 and CRECK kinetic schemes | 2 PSRs | 7.1 dry ppm @3% O2 to 7.0 of experimental data | [50] |
| CRNs | Hydrogen/methane blends | Moderate or intense low-oxygen dilution (MILD) combustion | GRI-Mech 3.0 | 1 Mixer and 1 PSR | Similar tendency to the experimental results | [51] |
| CRNs | Syngas-fueled | Gas turbine combustor with RQL | a dedicated syngas mechanism with 44 species and 251 reactions | 9 PSRs, 1 Mixer, and 1 PFR | - | [52] |
| CRNs | RP-3 kerosene | LESS combustor | a surrogate of Jet-A fuel mechanism | 10 PSRs, 2 PFRs | Maximum 10.13% | [54] |
| CFD_CRNs | Jet-A fuel | A model annular-type gas turbine combustor | Jet-A fuel | 10 PSRs, 1 PFR, and 3 Mixer | Maximum 10% | [100] |
| CFD_CRNs | Methane gas | Gas turbine ignition chamber | GRI-Mech 3.0 | 12 PSRs, 1 PFR, and 1 Mixer | Maximum 11.1% | [101] |
| CFD_CRNs | CH4 | Lean–premixed gas turbine combustors | 2-step model of CFD GRI-Mech 3.0 of CRNs | 39 PSRs, 1 PFR, and 1 Mixer | Similar tendency to the experimental results | [102] |
| CFD_CRNs | CH4/air | Gas turbine combustor | GRI-Mech 3.0 of CRNs | 17 PSRs, 2 PFRs, and 5 Mixers | The error estimate is smaller than 1.2% | [104] |
| CFD_CRNs | JP10 | Aero-engine combustor | detailed JP10 mechanism of CRNs | 15 PSRs, 1 PFR, and 3 Mixer | EI_NOx was 24 g/kg to 26.4 g/kg of the experiment at 100% load | [105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Yang, X. NOx Formation Mechanism and Emission Prediction in Turbulent Combustion: A Review. Appl. Sci. 2024, 14, 6104. https://doi.org/10.3390/app14146104
Wang Z, Yang X. NOx Formation Mechanism and Emission Prediction in Turbulent Combustion: A Review. Applied Sciences. 2024; 14(14):6104. https://doi.org/10.3390/app14146104
Chicago/Turabian StyleWang, Zhichao, and Xiaoyi Yang. 2024. "NOx Formation Mechanism and Emission Prediction in Turbulent Combustion: A Review" Applied Sciences 14, no. 14: 6104. https://doi.org/10.3390/app14146104
APA StyleWang, Z., & Yang, X. (2024). NOx Formation Mechanism and Emission Prediction in Turbulent Combustion: A Review. Applied Sciences, 14(14), 6104. https://doi.org/10.3390/app14146104







