Structure and Optoelectronic Properties of Perovskite-like (PEA)2PbBr3Cl on AlN/Sapphire Substrate Heterostructure
Abstract
1. Introduction
2. Materials and Methods
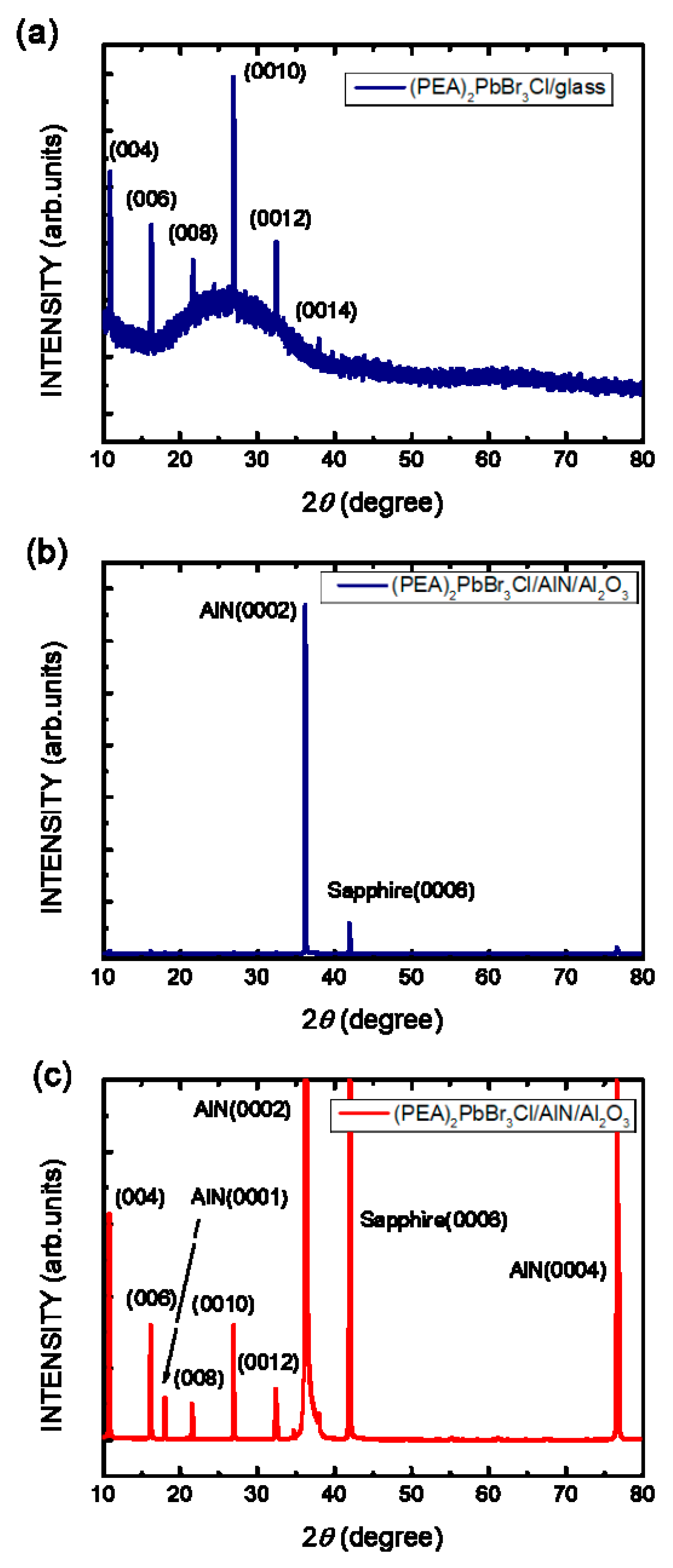
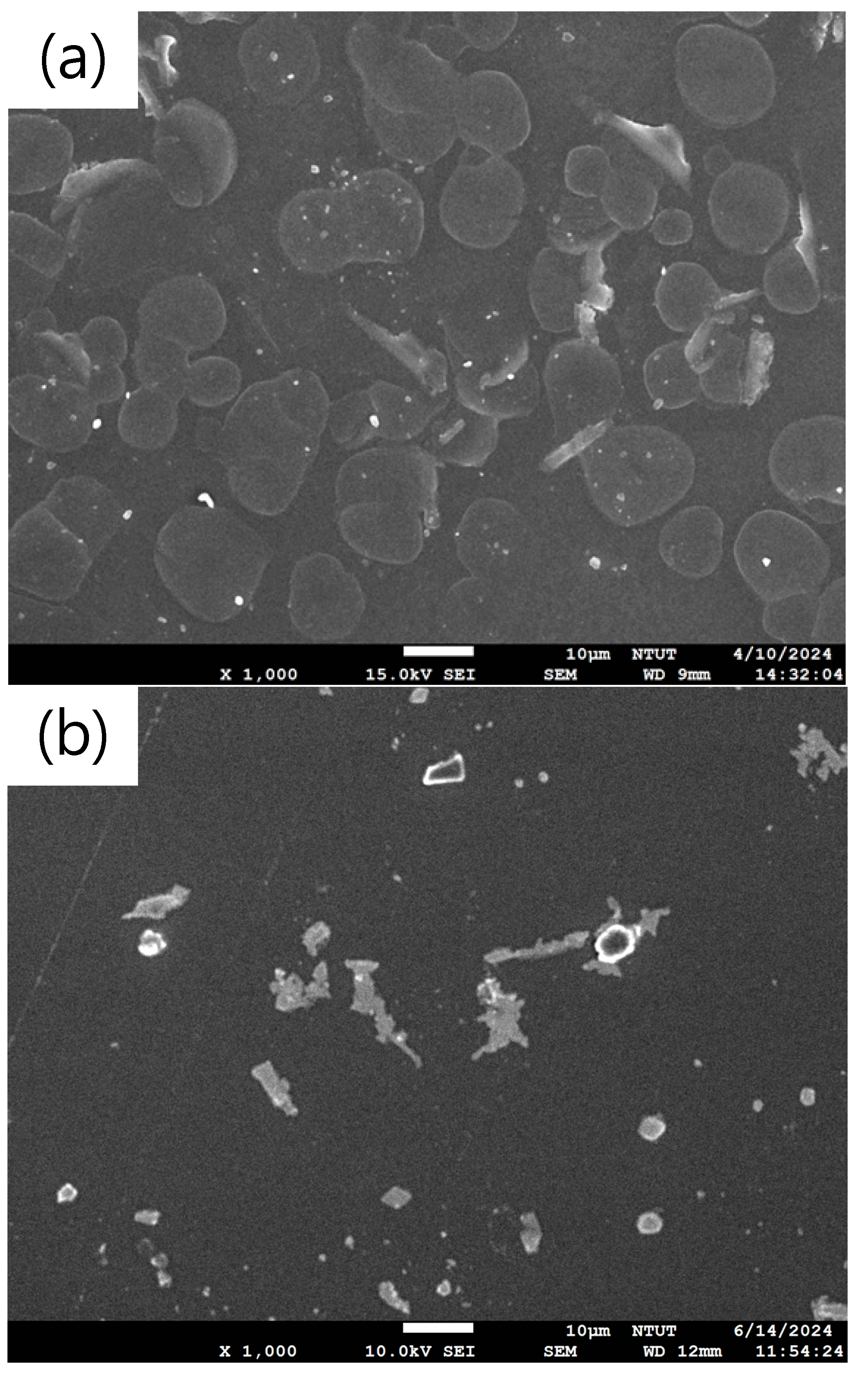
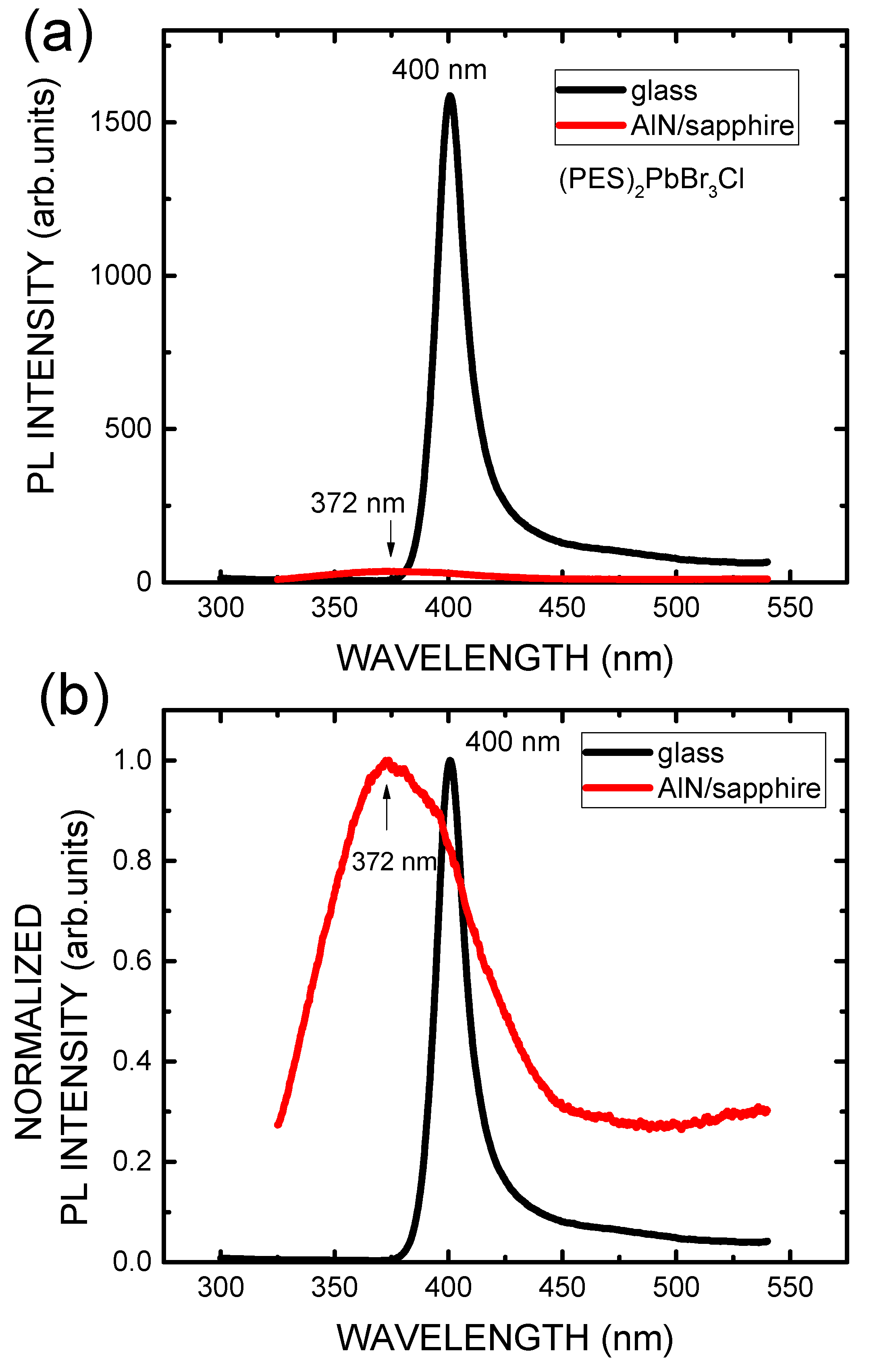
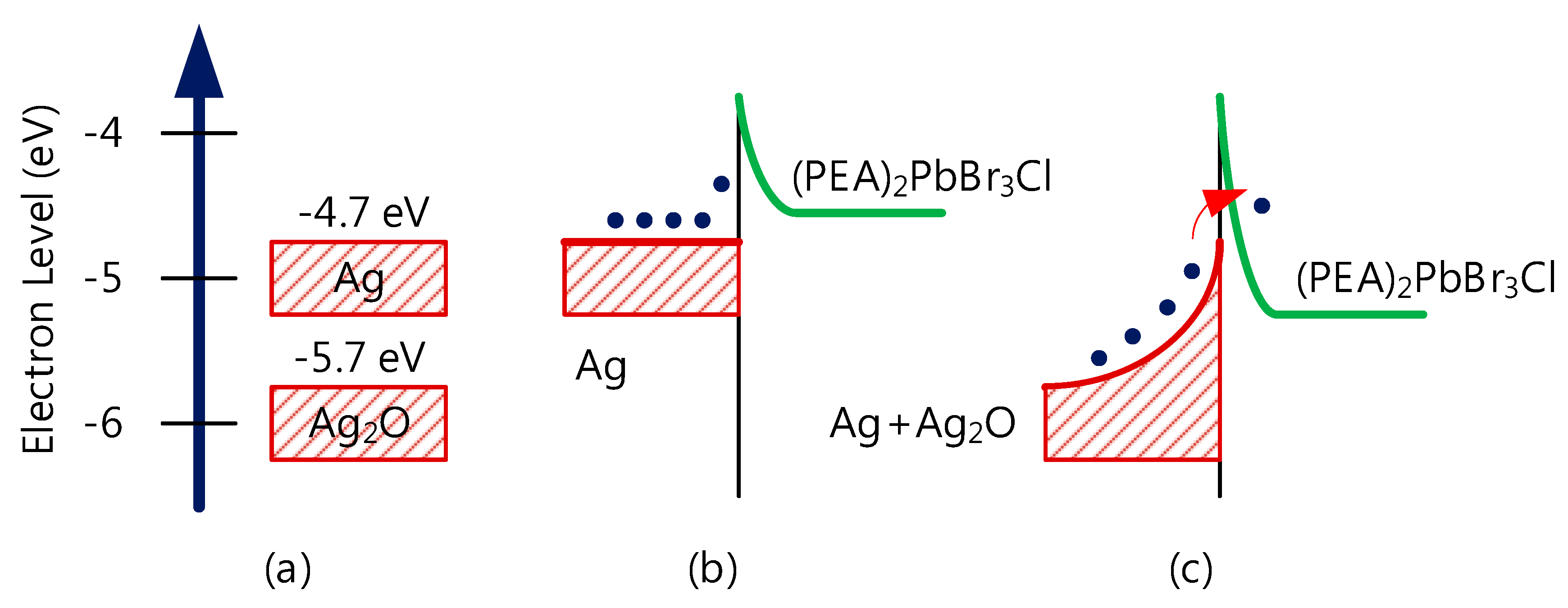
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, J.C.; Park, J.H.; Lee, S.Y.; Song, M.H. Effect of perovskite film morphology on device performance of perovskite light-emitting diodes. Nanoscale 2019, 11, 1505–1514. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, Y.; Wang, S.; Guo, J.; Yu, W.W.; Rogach, A.L. Metal halide perovskite light-emitting devices: Promising technology for next-generation displays. Adv. Funct. Mater. 2019, 29, 1902008. [Google Scholar] [CrossRef]
- Quan, L.N.; Rand, B.P.; Friend, R.H.; Mhaisalkar, S.G.; Lee, T.W.; Sargent, E.H. Perovskites for next-generation optical sources. Chem. Rev. 2019, 119, 7444–7477. [Google Scholar] [CrossRef]
- Hassan, Y.; Park, J.H.; Crawford, M.L.; Sadhanala, A.; Lee, J.; Sadighian, J.C.; Mosconi, E.; Shivanna, R.; Radicchi, E.; Jeong, M.; et al. Ligand-engineered bandgap stability in mixed-halide perovskite LEDs. Nature 2021, 591, 72–77. [Google Scholar] [CrossRef]
- Tan, Z.K.; Moghaddam, R.S.; Lai, M.L.; Docampo, P.; Higler, R.; Deschler, F.; Price, M.; Sadhanala, A.; Pazos, L.M.; Credgington, D.; et al. Bright light-emitting diodes based on organometal halide perovskite. Nat. Nanotechnol. 2014, 9, 687–692. [Google Scholar] [CrossRef]
- Bai, W.; Xuan, T.; Zhao, H.; Dong, H.; Cheng, X.; Wang, L.; Xie, R.J. Perovskite light-emitting diodes with an external quantum efficiency exceeding 30%. Adv. Mater. 2023, 35, 2302283. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Kumar, S.; Bär, J.; Bertolotti, F.; Masciocchi, N.; Guagliardi, A.; Grotevent, M.; Shorubalko, I.; Bodnarchuk, M.I.; et al. Dismantling the “Red Wall” of Colloidal Perovskites: Highly Luminescent Formamidinium and Formamidinium–Cesium Lead Iodide Nanocrystals. ACS Nano 2017, 11, 3119–3134. [Google Scholar] [CrossRef]
- Kovalenko, M.V.; Protesescu, L.; Bodnarchuk, M.I. Properties and potential optoelectronic applications of lead halide perovskite nanocrystals. Science 2017, 358, 745–750. [Google Scholar] [CrossRef]
- Yu, Y.; Tang, Y.; Wang, B.; Zhang, K.; Tang, J.X.; Li, Y.Q. Red Perovskite Light-Emitting Diodes: Recent Advances and Perspectives. Laser Photo. Rev. 2023, 17, 2200608. [Google Scholar] [CrossRef]
- Rahil, M.; Ansari, R.M.; Prakash, C.; Islam, S.S.; Dixit, A.; Ahmad, S. Ruddlesden–Popper 2D perovskites of type (C6H9C2H4NH3)2(CH3NH3)n−1PbnI3n+1 (n = 1–4) for optoelectronic applications. Sci. Rep. 2022, 12, 2176. [Google Scholar] [CrossRef]
- Chen, L.C.; Lee, K.L.; Wu, W.T.; Hsu, C.F.; Tseng, Z.L.; Sun, X.H.; Kao, Y.T. Effect of Different CH3NH3PbI3 Morphologies on Photovoltaic Properties of Perovskite Solar Cells. Nanoscale Res. Lett. 2018, 13, 140. [Google Scholar] [CrossRef]
- Mandal, S.; Khan, B.A.; Sarkar, P. 2D lead free Ruddlesden-Popper phase perovskites as efficient photovoltaic materials: A first-principles investigation. Comput. Mater. Sci. 2022, 211, 111545. [Google Scholar] [CrossRef]
- Chen, J.; Shi, Y.; He, Y.; Zhai, T. Two-dimensional Ruddlesden-Popper perovskite nanosheets: Synthesis, optoelectronic properties and miniaturized optoelectronic devices. FlatChem 2019, 17, 100116. [Google Scholar] [CrossRef]
- Chen, L.C.; Chen, C.C.; Chang, S.H.; Chen, S.H.; Kuo, H.C. Formation and characterization of preferred oriented perovskite thin films on single-crystalline substrates. Mater. Res. Express 2018, 5, 066403. [Google Scholar] [CrossRef]
- Hickman, A.L.; Chaudhuri, R.; Bader, S.J.; Nomoto, K.; Li, L.; Hwang, J.C.M.; Xing, H.G.; Jena, D. Next generation electronics on the ultrawide-bandgap aluminum nitride platform. Semicond. Sci. Technol. 2021, 36, 044001. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, H.; Dai, H.; Zhang, X.; Sun, X.W.; Zhang, Z. Exciton-Polariton Properties in Planar Microcavity of Millimeter-Sized Two-Dimensional Perovskite Sheet. ACS Appl. Mater. Interfaces 2020, 12, 5081–5089. [Google Scholar] [CrossRef]
- Wu, S.C.; Wu, C.S.; Chien, C.H.; Zhang, Y.W.; Yang, C.X.; Liu, C.; Li, M.H.; Lin, C.F.; Wu, Y.H.; Lin, B.H.; et al. Carrier–Phonon Interaction Induced Large Negative Thermal-Optic Coefficient at Near Band Edge of Quasi-2D (PEA)2PbBr4 Perovskite. Adv. Funct. Mater. 2023, 33, 2213427. [Google Scholar] [CrossRef]
- Chen, L.C.; Wei, Y.S.; Zhang, M. Double buffer of MAPbBr3 QDs using a bulk crystal source by the LARP method and MABr modifier for CsPbBr3 perovskite light-emitting diodes. J. Phys. D Appl. Phys. 2023, 56, 465102. [Google Scholar] [CrossRef]
- Ge, C.; Zhai, W.; Tain, C.; Zhao, S.; Guo, T.; Sun, S.; Chen, W.; Ran, G. Centimeter-scale 2D perovskite (PEA)2PbBr4 single crystal plates grown by a seeded solution method for photodetectors. RSC Adv. 2019, 9, 16779–16783. [Google Scholar] [CrossRef]
- Wang, F.; Bai, S.; Tress, W.; Hagfeldt, A.; Gao, F. Defects engineering for high-performance perovskite solar cells. npj Flexible Electron. 2018, 2, 22. [Google Scholar] [CrossRef]
- Li, B.; Shen, T.; Yun, S. Recent progress of crystal orientation engineering in halide perovskite photovoltaics. Mater. Horiz. 2023, 10, 13–40. [Google Scholar] [CrossRef] [PubMed]
- Abid, I.; Mehta, J.; Cordier, Y.; Derluyn, J.; Degroote, S.; Miyake, H.; Medjdoub, F. AlGaN Channel High Electron Mobility Transistors with Regrown Ohmic Contacts. Electronics 2021, 10, 635. [Google Scholar] [CrossRef]
- Yang, L.; Wang, J.; Sheng, S.; Zhang, B.; Sheng, B.; Wang, T.; Liu, F.; Tao, R.; Xu, F.; Yang, X.; et al. Low-Resistive Ohmic Contacts in High-Electron-Mobility AlN/GaN Heterostructures by Suppressing the Oxygen Incorporation. ACS Appl. Electron. Mater. 2022, 4, 3632–3639. [Google Scholar] [CrossRef]
- Magari, Y.; Makino, H.; Hashimoto, S.; Furuta, M. Origin of work function engineering of silver oxide for an In–Ga–Zn–O Schottky diode. Appl. Sur. Sci. 2020, 512, 144519. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-H.; Ni, J.-S.; Chen, L.-C. Structure and Optoelectronic Properties of Perovskite-like (PEA)2PbBr3Cl on AlN/Sapphire Substrate Heterostructure. Appl. Sci. 2024, 14, 6096. https://doi.org/10.3390/app14146096
Lin Y-H, Ni J-S, Chen L-C. Structure and Optoelectronic Properties of Perovskite-like (PEA)2PbBr3Cl on AlN/Sapphire Substrate Heterostructure. Applied Sciences. 2024; 14(14):6096. https://doi.org/10.3390/app14146096
Chicago/Turabian StyleLin, Yu-Hsien, Jing-Suei Ni, and Lung-Chien Chen. 2024. "Structure and Optoelectronic Properties of Perovskite-like (PEA)2PbBr3Cl on AlN/Sapphire Substrate Heterostructure" Applied Sciences 14, no. 14: 6096. https://doi.org/10.3390/app14146096
APA StyleLin, Y.-H., Ni, J.-S., & Chen, L.-C. (2024). Structure and Optoelectronic Properties of Perovskite-like (PEA)2PbBr3Cl on AlN/Sapphire Substrate Heterostructure. Applied Sciences, 14(14), 6096. https://doi.org/10.3390/app14146096







