From Feed to Fish—Nutrients’ Fate in Aquaculture Systems
Abstract
1. Introduction
2. Materials and Methods
3. Results
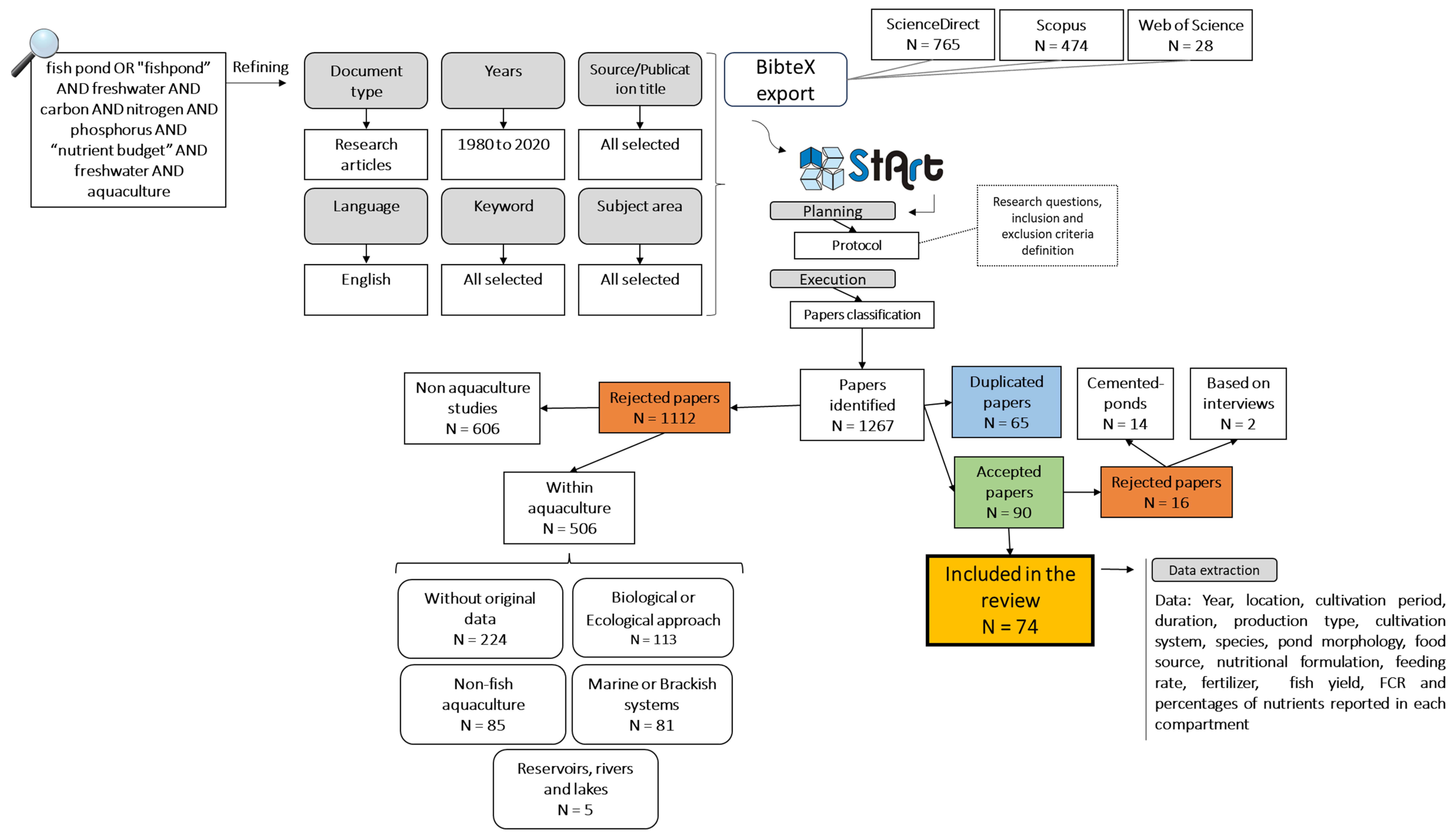
3.1. Paper Selection and Nutrient Budget
3.2. Fishpond and Cultivation Characteristics
3.3. Nutrient Input in Fishponds
3.3.1. Precipitation
3.3.2. Inlet Water
3.3.3. Gas Influx
3.3.4. Stocked Fish
3.3.5. Feed and Fertilizers
3.4. Nutrient Output in Fishponds
3.4.1. Infiltration
3.4.2. Outlet Water
3.4.3. Gas Efflux, Ammonia Volatilization and Denitrification
3.4.4. Harvested Fish
3.4.5. Sedimentation
3.5. Gaps in the Nutrient Budget
4. Discussion
4.1. Inputs
4.2. Outputs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture—Towards Blue Transformation; FAO: Rome, Italy, 2022; p. 236. [Google Scholar]
- Ahmed, N.; Thompson, S. The blue dimensions of aquaculture: A global synthesis. Sci. Total Environ. 2019, 652, 851–861. [Google Scholar] [CrossRef]
- de Boer, J.; Schösler, H.; Aikinga, H. Fish as an alternative protein—A consumer-oriented perspective on its role in a transition towards more healthy and sustainable diets. Appetite 2020, 152, 104721. [Google Scholar] [CrossRef]
- Fry, J.P.; Mailloux, N.A.; Love, D.C.; Milli, M.C.; Cao, L. Feed conversion efficiency in aquaculture: Do we measure it correctly? Environ. Res. Lett. 2018, 13, 024017. [Google Scholar] [CrossRef]
- Talbot, C.; Hole, R. Fish diets and the control of eutrophication resulting from aquaculture. J. Appl. Ichthyol. 1994, 10, 258–270. [Google Scholar] [CrossRef]
- Montanhini Neto, R.; Ostrensky, A. Nutrient load estimation in the waste of Nile tilapia Oreochromis niloticus (L.) reared in cages in tropical climate conditions. Aquac. Res. 2015, 46, 1309–1322. [Google Scholar] [CrossRef]
- Lin, C.K.; Yi, Y. Minimizing environmental impacts of freshwater aquaculture and reuse of pond effluents and mud. Aquaculture 2003, 226, 57–68. [Google Scholar] [CrossRef]
- Valenti, W.C.; Kimpara, J.M.; Preto, B.d.L. Measuring aquaculture sustainability. World Aquac. 2011, 42, 26–30. [Google Scholar]
- Kosten, S.; Almeida, R.M.; Barbosa, I.; Mendonca, R.; Santos Muzitano, I.; Sobreira Oliveira-Junior, E.; Vroom, R.J.E.; Wang, H.J.; Barros, N. Better assessments of greenhouse gas emissions from global fish ponds needed to adequately evaluate aquaculture footprint. Sci. Total Environ. 2020, 748, 141247. [Google Scholar] [CrossRef]
- Yuan, J.; Xiang, J.; Liu, D.; Kang, H.; He, T.; Kim, S.; Lin, Y.; Freeman, C.; Ding, W. Rapid growth in greenhouse gas emissions from the adoption of industrial-scale aquaculture. Nat. Clim. Chang. 2019, 9, 318–322. [Google Scholar] [CrossRef]
- Soto, D.; Aguilar-Manjarrez, J.; Brugère, C.; Angel, D.; Bailey, C.; Black, K.; Edwards, P.; Costa-Pierce, B.; Chopin, T.; Deudero, S.; et al. Applying an Ecosystem-Based Approach to Aquaculture: Principles, Scales and Some Management Measures; FAO Fisheries and Aquaculture Proceedings: Palma de Mallorca, Spain, 2007; pp. 15–35. [Google Scholar]
- Yaobin, L.; Lin, Q.; Fengbo, L.; Xiyue, Z.; Chunchun, X.; Long, J.; Zhongdu, C.; Jinfei, F.; Fuping, F. Impact of Rice-Catfish/Shrimp Co-culture on Nutrients Fluxes across Sediment-Water Interface in Intensive Aquaculture Ponds. Rice Sci. 2019, 26, 416–424. [Google Scholar] [CrossRef]
- Nhan, D.K.; Verdegem, M.C.J.; Milstein, A.; Verreth, J.A.V. Water and nutrient budgets of ponds in integrated agriculture-aquaculture systems in the Mekong Delta, Vietnam. Aquac. Res. 2008, 39, 1216–1228. [Google Scholar] [CrossRef]
- Ofor, C.O. A comparison of the yield and yield economics of three types of semi-intensive grow out systems, in the production of Heterobranchus longifilis (Teleostei: Clariidae) (Val. 1840), in Southeast Nigeria. Aquaculture 2007, 269, 402–413. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, M.; Kong, C.; Tang, H.; Gan, L.; Zhang, M. Enclosure experiment of effects of dietary phosphorus level on water quality, phosphorus budget, and plankton composition in intensive culture of crucian carp. Aquac. Int. 2016, 25, 1145–1158. [Google Scholar] [CrossRef]
- Brown, T.W.; Boyd, C.E.; Chappell, J.A. Organic Carbon and Dissolved Oxygen Budgets for a Commercial-Size, In-pond Raceway System. J. World Aquac. Soc. 2015, 46, 539–548. [Google Scholar] [CrossRef]
- Adhikari, S.; Sahu, B.C.; Dey, L. Nutrients budget and effluents characteristics in polyculture of scampi (Macrobrachium rosenbergii) and Indian major carps ponds using organic inputs. Water Sci. Technol. 2012, 66, 1540–1548. [Google Scholar] [CrossRef]
- Sahu, B.C.; Adhikari, S.; Mahapatra, A.S.; Dey, L. Nitrogen, Phosphorus, and Carbon Budgets in Polyculture Ponds of Indian Major Carps and Giant Freshwater Prawn in Orissa State, India. J. Appl. Aquac. 2015, 27, 365–376. [Google Scholar] [CrossRef]
- Costa-Pierce, B.A.; Clay, L.; Malecha, S.R.; Laws, E.A. Polyculture of Chinese carps and prawns at commercial densities and reduced feeding rates I. Water and sediment microbial ecology. J. World Aquac. Soc. 1984, 15, 367–390. [Google Scholar] [CrossRef]
- Uddin, M.S.; Azim, M.E.; Wahab, M.A.; Verdegem, M.C.J. Effects of substrate addition and supplemental feeding on plankton composition and production in tilapia (Oreochromis niloticus) and freshwater prawn (Macrobrachium rosenbergii) polyculture. Aquaculture 2009, 297, 99–105. [Google Scholar] [CrossRef]
- David, F.S.; Proença, D.C.; Valenti, W.C. Nitrogen budget in integrated aquaculture systems with Nile tilapia and Amazon River prawn. Aquac. Int. 2017, 25, 1733–1746. [Google Scholar] [CrossRef]
- David, F.S.; Proença, D.C.; Valenti, W.C. Phosphorus Budget in Integrated Multitrophic Aquaculture Systems with Nile Tilapia, Oreochromis niloticus, and Amazon River Prawn, Macrobrachium amazonicum. J. World Aquac. Soc. 2017, 48, 402–414. [Google Scholar] [CrossRef]
- Rodrigues, C.G.; Garcia, B.F.; Verdegem, M.; Santos, M.R.; Amorim, R.V.; Valenti, W.C. Integrated culture of Nile tilapia and Amazon river prawn in stagnant ponds, using nutrient-rich water and substrates. Aquaculture 2019, 503, 111–117. [Google Scholar] [CrossRef]
- Flickinger, D.L.; Costa, G.A.; Dantas, D.P.; Moraes-Valenti, P.; Valenti, W.C. The budget of nitrogen in the grow-out of the Amazon river prawn (Macrobrachium amazonicum Heller) and tambaqui (Colossoma macropomum Cuvier) farmed in monoculture and in integrated multitrophic aquaculture systems. Aquac. Res. 2019, 50, 3444–3461. [Google Scholar] [CrossRef]
- Flickinger, D.L.; Costa, G.A.; Dantas, D.P.; Proença, D.C.; David, F.S.; Durborow, R.M.; Moraes-Valenti, P.; Valenti, W.C. The budget of carbon in the farming of the Amazon river prawn and tambaqui fish in earthen pond monoculture and integrated multitrophic systems. Aquac. Rep. 2020, 17, 100340. [Google Scholar] [CrossRef]
- Flickinger, D.L.; Dantas, D.P.; Proença, D.C.; David, F.S.; Valenti, W.C. Phosphorus in the culture of the Amazon river prawn (Macrobrachium amazonicum) and tambaqui (Colossoma macropomum) farmed in monoculture and in integrated multitrophic systems. J. World Aquac. Soc. 2019, 51, 1002–1023. [Google Scholar] [CrossRef]
- Pouil, S.; Samsudin, R.; Slembrouck, J.; Sihabuddin, A.; Sundari, G.; Khazaidan, K.; Kristanto, A.H.; Pantjara, B.; Caruso, D. Nutrient budgets in a small-scale freshwater fish pond system in Indonesia. Aquaculture 2019, 504, 267–274. [Google Scholar] [CrossRef]
- Gross, A.; Boyd, C.E.; Wood, C.W. Nitrogen transformations and balance in channel catfish ponds. Aquac. Eng. 2000, 24, 1–14. [Google Scholar] [CrossRef]
- Green, B.W.; Boyd, C.E. Chemical budgets for organically fertilized fish ponds in the dry tropics. J. World Aquac. Soc. 1995, 26, 284–296. [Google Scholar] [CrossRef]
- Zhang, K.; Xie, J.; Yu, D.-G.; Wang, G.-J.; Yu, E.-M.; Gong, W.-B.; Li, Z.-F.; Wang, C.-C.; Xia, Y. A comparative study on the budget of nitrogen and phosphorus in polyculture systems of snakehead with bighead carp. Aquaculture 2018, 483, 69–75. [Google Scholar] [CrossRef]
- Zhang, K.; Yu, D.; Li, Z.; Xie, J.; Wang, G.; Gong, W.; Yu, E.; Tian, J. Influence of eco-substrate addition on organic carbon, nitrogen and phosphorus budgets of intensive aquaculture ponds of the Pearl River, China. Aquaculture 2020, 520, 734868. [Google Scholar] [CrossRef]
- Potužák, J.; Duras, J.; Drozd, B. Mass balance of fishponds: Are they sources or sinks of phosphorus? Aquac. Int. 2016, 24, 1725–1745. [Google Scholar] [CrossRef]
- Hlaváč, D.; Anton-Pardo, M.; Másílko, J.; Hartman, P.; Regenda, J.; Vejsada, P.; Baxa, M.; Pechar, L.; Valentová, O.; Všetičková, L.; et al. Supplementary feeding with thermally treated cereals in common carp (Cyprinus carpio L.) pond farming and its effects on water quality, nutrient budget and zooplankton and zoobenthos assemblages. Aquac. Int. 2016, 24, 1681–1697. [Google Scholar] [CrossRef]
- Cang, P.; Zhang, M.; Qiao, G.; Sun, Q.; Xu, D.; Li, Q.; Yuan, X.; Liu, W. Analysis of Growth, Nutrition and Economic Profitability of Gibel Carp (Carassius auratus gibelio ♀ × C. carpio ♂) Cultured in Zero-water Exchange System. Pak. J. Zool. 2019, 51, 619–630. [Google Scholar] [CrossRef]
- Yi, Y.; Kwei Lina, C.; Diana, J.S. Hybrid catfish (Clarias macrocephalus × C. gariepinus) and Nile tilapia (Oreochromis niloticus) culture in an integrated pen-cum-pond system: Growth performance and nutrient budgets. Aquaculture 2003, 217, 395–408. [Google Scholar] [CrossRef]
- Hlaváč, D.; Másílko, J.; Hartman, P.; Bláha, M.; Pechar, L.; Anton-Pardo, M.; Adámek, Z. Effects of common carp (Cyprinus carpio Linnaeus, 1758) supplementary feeding with modified cereals on pond water quality and nutrient budget. J. Appl. Aquac. 2015, 31, 30–37. [Google Scholar] [CrossRef]
- Azim, M.E.; Verdegem, M.C.J.; Khatoon, H.; Wahab, M.A. A comparison of fertilization, feeding and three periphyton substrates for increasing fish production in freshwater pond aquaculture in Bangladesh. Aquaculture 2002, 212, 227–243. [Google Scholar] [CrossRef]
- Zur, O. Primary production in intensive fish ponds and a complete organic carbon balance in the ponds. Aquaculture 1981, 23, 197–210. [Google Scholar] [CrossRef]
- Banas, D.; Masson, G.; Leglize, L.; Usseglio-Polatera, P.; Boyd, C.E. Assessment of sediment concentration and nutrient loads in effluents drained from extensively managed fishponds in France. Environ. Pollut. 2008, 152, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.F.; Boyd, C.E. Channel Catfish Pond Effluents. Prog. Fish-Cult. 1994, 56, 273–281. [Google Scholar] [CrossRef]
- Schroeder, G.L.; Wohlfarth, G.; Alkon, A.; Halevy, A.; Krueger, H. The dominance of algal-based food webs in fish ponds receiving chemical fertilizers plus organic manures. Aquaculture 1990, 86, 219–229. [Google Scholar] [CrossRef]
- Chary, K.; van Riel, A.J.; Muscat, A.; Wilfart, A.; Harchaoui, S.; Verdegem, M.; Filgueira, R.; Troell, M.; Henriksson, P.J.G.; de Boer, I.J.M.; et al. Transforming sustainable aquaculture by applying circularity principles. Rev. Aquac. 2023, 16, 656–673. [Google Scholar] [CrossRef]
- Mishra, B.; Ayyappan, S.; Jena, J.K.; Priyambada, P.; Behera, P.K.; Aravindakshan, P.K.; Purushothaman, C.S. Carp polyculture in aquaculture sewage treatment plant and its impact on soil and water characteristics. Indian. J. Fish. 2009, 56, 271–276. [Google Scholar]
- Knud-Hansen, C.F.; Batterson, T.R.; McNabb, C.D.; Harahatb, I.S.; Sumantadinatab, K.; Eidmanb, H.M. Nitrogen input, primary productivity and fish yield in fertilized freshwater ponds in Indonesia. Aquaculture 1991, 94, 49–63. [Google Scholar] [CrossRef]
- Kause, A.; Nousiainen, A.; Koskinen, H. Improvement in feed efficiency and reduction in nutrient loading from rainbow trout farms: The role of selective breeding. J. Anim. Sci. 2022, 100, skac214. [Google Scholar] [CrossRef] [PubMed]
- d’Orbcastel, E.R.; Blancheton, J.-P.; Aubin, J. Towards environmentally sustainable aquaculture: Comparison between two trout farming systems using Life Cycle Assessment. Aquac. Eng. 2009, 40, 113–119. [Google Scholar] [CrossRef]
- Dróżdż, D.; Malińska, K.; Mazurkiewicz, J.; Kacprzak, M.; Mrowiec, M.; Szczypiór, A.; Postawa, P.; Stachowiak, T. Fish pond sediment from aquaculture production—Current practices and the potential for nutrient recovery: A Review. Int. Agrophys. 2020, 34, 33–41. [Google Scholar] [CrossRef]




| Reference | Location | C (%) | kg ha−1 d−1 | N (%) | kg ha−1 d−1 | P (%) | kg ha−1 d−1 |
|---|---|---|---|---|---|---|---|
| [15] | China | n.r. | n.r. | n.r. | n.r. | 0.09 | 0.001 |
| [16] | USA | 0 | 0 | n.r. | n.r. | n.r. | n.r. |
| [18] | India | 0.5 | 0.06 | 0.08 | 0.001 | 0.07 | 0.003 |
| [21] | Brazil | n.r. | n.r. | 0.06 | 0.002 | n.r. | n.r. |
| [22] | Brazil | n.r. | n.r. | n.r. | n.r. | 0.5 | 0.004 |
| [24] | Brazil | n.r. | n.r. | 0.9 | 0.02 | n.r. | n.r. |
| [25] | Brazil | 0.6 | 0.19 | n.r. | n.r. | n.r. | n.r. |
| [26] | Brazil | n.r. | n.r. | n.r. | n.r. | 0.5 | 0.002 |
| [28] | USA | n.r. | n.r. | 4 | 0.07 | n.r. | n.r. |
| [29] | Honduras | n.r. | n.r. | 0.6 | 0.01 | 0.1 | 0.001 |
| [30] | China | n.r. | n.r. | 0.5 | 0.009 | 0.05 | 0.0002 |
| [31] | China | 0.06 | 0.05 | 0.11 | 0.01 | 0.08 | 0.001 |
| Reference | Water sources | C (%) | kg ha−1 d−1 | N (%) | kg ha−1 d−1 | P (%) | kg ha−1 d−1 |
|---|---|---|---|---|---|---|---|
| [13] | Irrigation canals, precipitation, and infiltration * | 7 | 4.3 | 54 | 4.6 | 10 | 0.3 |
| [14] | Stream | n.r. | n.r. | n.r. | n.r. | 0.3 | 0.001 |
| [16] | Precipitation, groundwater, and runoff | 0 | 0 | n.r. | n.r. | n.r. | n.r. |
| [17] | n.r. | 0.5 | 0.06 | 0.1 | 0.0009 | 0.07 | 0.0003 |
| [21] | Reservoir | n.r. | n.r. | 29 | 1 | n.r. | n.r. |
| [22] | Reservoir | n.r. | n.r. | n.r. | n.r. | 26 | 0.19 |
| [24] | Reservoir | n.r. | n.r. | 58 | 1 | n.r. | n.r. |
| [25] | Reservoir | 32 | 10 | n.r. | n.r. | n.r. | n.r. |
| [26] | Reservoir | n.r. | n.r. | n.r. | n.r. | 37 | 0.11 |
| [27] | Reservoir | n.r. | n.r. | 6 | 0.6 | 0.1 | 0.004 |
| [28] | Main source n.r. and precipitation | n.r. | n.r. | 7 | 0.12 | n.r. | n.r. |
| [29] | Main source n.r. and precipitation | n.r. | n.r. | 4 | 0.06 | 4 | 0.03 |
| [30] | Main source n.r. and precipitation | n.r. | n.r. | 1 | 0.01 | 0.4 | 0.001 |
| [31] | Main source n.r. and precipitation | 0.45 | 0.3 | 1.7 | 0.17 | 1.4 | 0.02 |
| [32] | Watershed | n.r. | n.r. | n.r. | n.r. | 72 | 0.0001 |
| [33] | Effluents from fish culture | n.r. | n.r. | 75 | 1.3 | 55 | 0.12 |
| [34] | Water from fishpond | n.r. | n.r. | n.r. | n.r. | 1 | 0.01 |
| [35] | Effluents from fish culture | n.r. | n.r. | 1 | 0.2 | 0.2 | 0.006 |
| Reference | Fish Species | Cultivation System | Gas | C (%) | kg ha−1 d−1 | N (%) | kg ha−1 d−1 |
|---|---|---|---|---|---|---|---|
| [16] | Ictalurus punctatus, hybrid channel catfish (Ictalurus punctatus × Ictalurus furcatus), Polyodon spathula, Oreochromis niloticus | I | CO2 | 3.5 | 8 | n.r. | n.r. |
| [21] | Oreochromis niloticus | I | N2 | n.r. | n.r. | 1 | 0.04 |
| [24] | Colossoma macropomum | I and M | N2 | n.r. | n.r. | 0.2 | 0.02 |
| [25] | Colossoma macropomum | I and M | CO2 CH4 | 12 0.2 | 3.5 0.08 | n.r. | n.r. |
| [28] | Ictalurus punctatus | M | N2 | n.r. | n.r. | 0.05 | 0.003 |
| [31] | Micropterus salmoides | M | N2 | n.r. | n.r. | 0.05 | 5.5 |
| Reference | Fish Species | C (%) | kg ha−1 d−1 | N (%) | kg ha−1 d−1 | P (%) | kg ha−1 d−1 |
|---|---|---|---|---|---|---|---|
| [14] | Heterobranchus longifilis and Oreochromis niloticus | n.r. | n.r. | n.r. | n.r. | 2 | 0.006 |
| [15] | Carassius carassius | n.r. | n.r. | n.r. | n.r. | 26 | 0.5 |
| [16] | Ictalurus punctatus, hybrid channel catfish, hybrid channel catfish (Ictalurus punctatus × Ictalurus furcatus), Polyodon spathula, and Oreochromis niloticus | 3.5 | 8 | n.r. | n.r. | n.r. | n.r. |
| [21] | Oreochromis niloticus | n.r. | n.r. | 1 | 0.04 | n.r. | n.r. |
| [22] | Oreochromis niloticus | n.r. | n.r. | n.r. | n.r. | 1.3 | 0.01 |
| [24] | Colossoma macropomum | n.r. | n.r. | 0.2 | 0.02 | n.r. | n.r. |
| [25] | Colossoma macropomum | 0.10 | 0.03 | n.r. | n.r. | n.r. | n.r. |
| [26] | Colossoma macropomum | n.r. | n.r. | n.r. | n.r. | 0.2 | 0.001 |
| [27] | Osphronemus goramy | n.r. | n.r. | 3 | 0.3 | 0.7 | 0.02 |
| [28] | Ictalurus punctatus | n.r. | n.r. | 2 | 0.04 | n.r. | n.r. |
| [29] | Oreochromis niloticus | n.r. | n.r. | 5 | 0.05 | 2 | 0.02 |
| [30] | Channa arguss and Hypothalmichthys nobilis | n.r. | n.r. | 0.2 | 0.003 | 0.1 | 0.0004 |
| [31] | Micropterus salmoides | 0.3 | 0.2 | 0.7 | 0.1 | 0.5 | 0.01 |
| [33] | Cyprinus carpio | n.r. | n.r. | 36 | 0.05 | 28 | 0.01 |
| [35] | Hybrid catfish (Clarias macrocephalus × Clarias gariepinus) and Oreochromis niloticus | n.r. | n.r. | 3 | 0.5 | 2 | 0.06 |
| [36] | Cyprinus carpio | n.r. | n.r. | 11 | 0.2 | 26 | 0.06 |
| Reference | Fish Species | Cultivation System | Period (Days) | Feed/Fertilizer Type | C (%) | kg ha−1 d−1 | N (%) | kg ha−1 d−1 | P (%) | kg ha−1 d−1 |
|---|---|---|---|---|---|---|---|---|---|---|
| [13] | Barboides gonionotus, Cirrhinus mrigala, Cyprinus carpio, Hypophthalmichthys molitrix, Oreochromis niloticus, Helostoma temminckii, Osphronemus goramy, Pangasius hypophthalmus, and hybrid catfish (Clarias macrocephalus × Clarias gariepinus) | I | 342 | Rice bran, fish powder, and commercial feed Cow manure, human excreta, crop residues | 21 65 | 5.7 19 | 3 37 | 0.2 2.2 | 12 64 | 0.1 1 |
| [14] | Heterobranchus longifilis and Oreochromis niloticus | I and P | 365 | Formulated feed Chicken manure, lime, and plant wastes | n.r. n.r. | n.r. n.r. | n.r. n.r. | n.r. n.r. | 49 44 | 0.1 0.1 |
| [15] | Carassius carassius | M | 73 | Formulated feed | n.r. | n.r. | n.r. | n.r. | 73 | 1.5 |
| [16] | Ictalurus punctatus, hybrid channel catfish (Ictalurus punctatus × Ictalurus furcatus), Polyodon spathula, and Oreochromis niloticus | I | 250 | Commercial feed | 22 | 51 | n.r. | n.r. | n.r. | n.r. |
| [17] | Catla catla, Labeo rohita, and Cirrhinus mrigala | I | 292 | Commercial feed Cow manure and fermented rice straw | 95 4 | 11 0.5 | 97 2 | 0.9 0.007 | 97 2 | 0.4 0.02 |
| [18] | Catla catla, Labeo rohita, and Cirrhinus mrigala | I | 280 | Commercial feed Cow manure, urea, and simple superphosphate | 95 4 | 12 0.5 | 82 17 | 1 0.22 | 92 7 | 0.4 0.03 |
| [21] | Oreochromis niloticus | I | 140 | Commercial feed Urea and simple superphosphate | n.r. n.r. | n.r. n.r. | 67 0.4 | 2.5 0.01 | n.r. n.r. | n.r. n.r. |
| [22] | Oreochromis niloticus | I | 140 | Commercial feed Urea and simple superphosphate | n.r. n.r. | n.r. n.r. | n.r. n.r. | n.r. n.r. | 54 6 | 0.06 0.5 |
| [24] | Colossoma macropomum | I and M | 171 | Commercial feed | n.r. | n.r. | 28 | 1.2 | n.r. | n.r. |
| [25] | Colossoma macropomum | I and M | 171 | Commercial feed | 27 | 9 | n.r. | n.r. | n.r. | n.r. |
| [26] | Colossoma macropomum | I and M | 171 | Commercial feed | n.r. | n.r. | n.r. | n.r. | 61 | 0.26 |
| [27] | Osphronemus goramy | M | 152 | Commercial feed Chicken manure and rice bran | n.r. n.r. | n.r. n.r. | 56 34 | 6.5 4 | 60 38 | 1.8 1.1 |
| [28] | Ictalurus punctatus | M | 133 | Commercial feed | n.r. | n.r. | 88 | 1.7 | n.r. | n.r. |
| [29] | Oreochromis niloticus | M | 151 | Chicken litter | n.r. | n.r. | 93 | 1.6 | 94 | 0.8 |
| [30] | Channa arguss and Hypothalmichthys nobilis | P | 120 | Commercial feed | n.r. | n.r. | 98 | 1.5 | 99 | 0.3 |
| [31] | Micropterus salmoides | M | 240 | Fish | 34 | 28 | 97 | 9 | 98 | 1.3 |
| [33] | Cyprinus carpio | M | 163 | Wheat | n.r. | n.r. | 69 | 0.1 | 70 | 0.02 |
| [35] | Hybrid catfish (Clarias macrocephalus × Clarias gariepinus) and Oreochromis niloticus | I | 87 | Commercial feed Urea and TSP | n.r. n.r. | n.r. n.r. | 63 5.7 | 12 1.2 | 83 8.7 | 2.8 0.3 |
| [37] | Catla catla, Labeo rohita, and Labeo calbasu | P | 135 | Rice bran and mustard oil cake Cow manure, urea, and TSP | n.r. n.r. | n.r. n.r. | 25 95 | 4.5 1.5 | n.r. n.r. | n.r. n.r. |
| [38] | Cyprinus carpio and hybrid tilapia (Oreochromis niloticus × Oreochromis aureus) | P | 53 | Commercial feed Chicken manure | 47 6 | 36 3.5 | n.r. n.r. | n.r. n.r. | n.r. n.r. | n.r. n.r. |
| Reference | Methods | C (%) | kg ha−1 d−1 | N (%) | kg ha−1 d−1 | P (%) | kg ha−1 d−1 |
|---|---|---|---|---|---|---|---|
| [16] | Same concentration as pond water | 0.01 | 0.03 | n.r. | n.r. | n.r. | n.r. |
| [28] | Sediment cores | n.r. | n.r. | 0.5 | 0.01 | n.r. | n.r. |
| [29] | Same concentration as pond water | n.r. | n.r. | 3 | 1.7 | 14 | 0.9 |
| [31] | Sediment cores | 0.2 | 0.2 | 0.4 | 0.04 | 0.5 | 0.01 |
| Reference | Pond Hydrology | C (%) | kg ha−1 d−1 | N (%) | kg ha−1 d−1 | P (%) | kg ha−1 d−1 |
|---|---|---|---|---|---|---|---|
| [13] | Flushed | 16 | 6 | 61 | 7 | 38 | 0.8 |
| [15] | Not flushed | n.r. | n.r. | n.r. | n.r. | 4 | 0.08 |
| [16] | Not flushed | 0.1 | 0.3 | n.r. | n.r. | n.r. | n.r. |
| [17] | Not flushed | 1.2 | 0.14 | 1 | 0.01 | 0.6 | 0.002 |
| [18] | Not flushed | 1 | 0.15 | 0.9 | 0.01 | 0.6 | 0.003 |
| [21] | Not flushed | n.r. | n.r. | 5 | 0.2 | n.r. | n.r. |
| [22] | Not flushed | n.r. | n.r. | n.r. | n.r. | 8 | 0.07 |
| [24] | Not flushed | n.r. | n.r. | 12 | 0.5 | n.r. | n.r. |
| [25] | Not flushed | 7 | 2 | n.r. | n.r. | n.r. | n.r. |
| [26] | Not flushed | n.r. | n.r. | n.r. | n.r. | 9.5 | 0.03 |
| [27] | Flushed | n.r. | n.r. | 5 | 0.4 | 0.4 | 0.01 |
| [28] | n.r. | n.r. | n.r. | 14 | 0.3 | n.r. | n.r. |
| [29] | n.r. | n.r. | n.r. | 8 | 0.1 | 33 | 0.3 |
| [30] | Not flushed | n.r. | n.r. | 0.2 | 3.2 | 0.3 | 1.1 |
| [31] | Flushed | 0.8 | 0.05 | 7.3 | 0.6 | 4.5 | 0.06 |
| [32] | Flushed | n.r. | n.r. | n.r. | n.r. | 13 | 0.01 |
| [36] | Flushed | n.r. | n.r. | 76 | 1.3 | 55 | 0.1 |
| [35] | Flushed | n.r. | n.r. | 13 | 1.3 | 9 | 0.06 |
| Reference | Pathway | C (%) | kg ha−1 d−1 | N (%) | kg ha−1 d−1 |
|---|---|---|---|---|---|
| [16] | Respiration | 26 | 62 | n.r. | n.r. |
| [21] | Diffusion + ebullition | n.r. | n.r. | 32 | 1.2 |
| [24] | Diffusion | n.r. | n.r. | 0.3 | 0.01 |
| Ebullition | n.r. | n.r. | 52 | 2 | |
| [25] | Diffusion CO2 | 1.4 | 0.4 | n.r. | n.r. |
| Diffusion CH4 | 0 | 0.01 | n.r. | n.r. | |
| Ebullition CO2 | 0.2 | 0.1 | n.r. | n.r. | |
| Ebullition CH4 | 1.3 | 0.3 | n.r. | n.r. | |
| [28] | NH3 volatilization | n.r. | n.r. | 12.5 | 0.25 |
| Denitrification | n.r. | n.r. | 17 | 0.34 | |
| [30] | NH3 volatilization | n.r. | n.r. | 0.8 | 0.1 |
| [31] | NH3 volatilization | n.r. | n.r. | 2.7 | 0.25 |
| [38] | Respiration | 40 | 20 | n.r. | n.r. |
| Reference | Fish Species | Cultivation System | Period (Days) | FCR | Yield | C (%) | kg ha−1 d−1 | N (%) | kg ha−1 d−1 | P (%) | kg ha−1 d−1 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [13] | Barboides gonionotus, Cirrhinus mrigala, Cyprinus carpio, Hypophthalmichthys molitrix, Oreochromis niloticus, Helostoma temminckii, Osphronemus goramy, Pangasius hypophthalmus, and hybrid catfish (Clarias macrocephalus × Clarias gariepinus) | I | 342 | n.r. | 4.9 ton ha−1 | 6 | 1.7 | 4 | 0.3 | 4 | 0.07 |
| [14] | Heterobranchus longifilis and Oreochromis niloticus | I and P | 365 | n.r. | 8 ton ha−1 | n.r. | n.r. | n.r. | n.r. | 11 | 0.04 |
| [15] | Carassius carassius | M | 73 | n.r. | 53 kg ha−1 | n.r. | n.r. | n.r. | n.r. | 41 | 0.6 |
| [16] | Ictalurus punctatus, hybrid channel catfish (Ictalurus punctatus × Ictalurus furcatus), Polyodon spathula, and Oreochromis niloticus | P | 250 | 1.5 | 20 ton ha−1 | 11 | 25 | n.r. | n.r. | n.r. | n.r. |
| [17] | Catla catla, Labeo rohita, and Cirrhinus mrigala | I | 292 | 1.8 | 4.6 ton ha−1 | 9 | 2.10 | 34 | 0.4 | 10 | 0.07 |
| [18] | Catla catla, Labeo rohita, and Cirrhinus mrigala | I | 280 | 1.7 | 2.7 ton ha−1 | 19 | 2.5 | 44 | 0.5 | 19 | 0.08 |
| [21] | Oreochromis niloticus | I | 140 | n.r. | 4.8 ton ha−1 | n.r. | n.r. | 21 | 0.7 | n.r. | n.r. |
| [22] | Oreochromis niloticus | I | 140 | n.r. | 4.8 ton ha−1 | n.r. | n.r. | n.r. | n.r. | 21 | 0.2 |
| [24] | Colossoma macropomum | I and M | 171 | n.r. | 4.6 ton ha−1 | n.r. | n.r. | 12 | 0.5 | n.r. | n.r. |
| [25] | Colossoma macropomum | I and M | 171 | n.r. | 4.6 ton ha−1 | 9 | 3 | n.r. | n.r. | n.r. | n.r. |
| [26] | Colossoma macropomum | I and M | 171 | n.r. | 4.6 ton ha−1 | n.r. | n.r. | n.r. | n.r. | 23 | n.r. |
| [27] | Osphronemus goramy | M | 152 | n.r. | 8 ton ha−1 | n.r. | n.r. | 18 | 1.7 | 3 | 0.1 |
| [28] | Ictalurus punctatus | M | 133 | 1.4 | 3.5 ton ha−1 | n.r. | n.r. | 31 | 0.6 | n.r. | n.r. |
| [29] | Oreochromis niloticus | M | 151 | n.r. | n.r. | n.r. | n.r. | 18 | 0.3 | 15 | 0.1 |
| [30] | Channa arguss and Hypophthalmichthys nobilis | P | 120 | 1.3 | 20 ton ha−1 | n.r. | n.r. | 39 | 0.5 | 14 | 0.5 |
| [31] | Micropterus salmoides | M | 240 | 3.9 | 43 ton ha−1 | 14 | 10 | 44 | 3.7 | 25 | 0.3 |
| [32] | Cyprinus carpio | M | 365 | n.r. | 664 kg ha−1 | n.r. | n.r. | n.r. | n.r. | 6 | 0.01 |
| [35] | Hybrid catfish (Clarias macrocephalus × Clarias gariepinus) and Oreochromis niloticus | I | 87 | n.r. | 1.9 ton ha−1 | n.r. | n.r. | 40 | 7 | 59 | 1.7 |
| [36] | Cyprinus carpio (scaled common carp) | M | 120 | n.r. | >450 kg ha−1 | n.r. | n.r. | 23 | 0.4 | 44 | 0.1 |
| [37] | Catla catla, Labeo rohita, and Labeo calbasu | P | 135 | 12.4 | 623 kg ha−1 | n.r. | n.r. | 7 | 0.3 | n.r. | n.r. |
| [38] | Cyprinus carpio and hybrid tilapia (Oreochromis niloticus × Oreochromis aureus) | P | 53 | 2.9 | 8.1 ton ha−1 | 12 | 5 | n.r. | n.r. | n.r. | n.r. |
| [41] | Hybrid tilapia, Oreochromis niloticus, Hypophthalmichthys molitrix, and Ctenopharyngodon idella | P | 98 | n.r. | 2.6 ton ha−1 | 5 | 1.5 | 13 | 3.7 | n.r. | n.r. |
| Reference | Cultivation System | Fish Species | C (%) | kg ha−1 d−1 | N (%) | kg ha−1 da−1 | P (%) | kg ha−1 d−1 |
|---|---|---|---|---|---|---|---|---|
| [13] | I | Barboides gonionotus, Cirrhinus mrigala, Cyprinus carpio, Hypophthalmichthys molitrix, Oreochromis niloticus, Helostoma temminckii, Osphronemus goramy, Pangasius hypophthalmus, and hybrid catfish (Clarias macrocephalus × Clarias gariepinus) | 81 | 23 | 29 | 1.9 | 48 | 0.7 |
| [15] | M | Carassius carassius | n.r. | n.r. | n.r. | n.r. | 55 | 1 |
| [16] | I | Ictalurus punctatus, hybrid channel catfish (Ictalurus punctatus × Ictalurus furcatus), Polyodon spathula, and Oreochromis niloticus | 9 | 21 | n.r. | n.r. | n.r. | n.r. |
| [17] | I | Catla catla, Labeo rohita, and Cirrhinus mrigala | 68 | 8 | 38 | 0.4 | 72 | 0.2 |
| [18] | I | Catla catla, Labeo rohita, and Cirrhinus mrigala | 69 | 9 | 46 | 0.5 | 70 | 0.3 |
| [21] | I | Oreochromis niloticus | n.r. | n.r. | 31 | 1.2 | n.r. | n.r. |
| [22] | I | Oreochromis niloticus | n.r. | n.r. | n.r. | n.r. | 64 | 0.6 |
| [24] | I and M | Colossoma macropomum | n.r. | n.r. | 19 | 0.7 | n.r. | n.r. |
| [25] | I and M | Colossoma macropomum | 70 | 24 | n.r. | n.r. | n.r. | n.r. |
| [26] | I and M | Colossoma macropomum | n.r. | n.r. | n.r. | n.r. | 51 | 0.2 |
| [27] | M | Osphronemus goramy | n.r. | n.r. | 60 | 6 | 77 | 2 |
| [28] | M | Ictalurus punctatus | n.r. | n.r. | 23 | 0.45 | n.r. | n.r. |
| [30] | P | Channa arguss and Hypophthalmichthys nobilis | n.r. | n.r. | 60 | 0.7 | 82 | 0.2 |
| [31] | M | Micropterus salmoides | 16 | 11 | 45 | 3 | 69 | 0.09 |
| [35] | I | Hybrid catfish (Clarias macrocephalus × Clarias gariepinus) and Oreochromis niloticus | n.r. | n.r. | 40 | 8 | 24 | 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalbem Barbosa, A.P.; Kosten, S.; Muniz, C.C.; Oliveira-Junior, E.S. From Feed to Fish—Nutrients’ Fate in Aquaculture Systems. Appl. Sci. 2024, 14, 6056. https://doi.org/10.3390/app14146056
Dalbem Barbosa AP, Kosten S, Muniz CC, Oliveira-Junior ES. From Feed to Fish—Nutrients’ Fate in Aquaculture Systems. Applied Sciences. 2024; 14(14):6056. https://doi.org/10.3390/app14146056
Chicago/Turabian StyleDalbem Barbosa, Ana Paula, Sarian Kosten, Claumir Cesar Muniz, and Ernandes Sobreira Oliveira-Junior. 2024. "From Feed to Fish—Nutrients’ Fate in Aquaculture Systems" Applied Sciences 14, no. 14: 6056. https://doi.org/10.3390/app14146056
APA StyleDalbem Barbosa, A. P., Kosten, S., Muniz, C. C., & Oliveira-Junior, E. S. (2024). From Feed to Fish—Nutrients’ Fate in Aquaculture Systems. Applied Sciences, 14(14), 6056. https://doi.org/10.3390/app14146056






