Aflatoxin B1 Detoxification and Antioxidant Effect of Selected Omani Medicinal Plants against Aflatoxin B1-Induced Oxidative Stress Pathogenesis in the Mouse Liver
Abstract
1. Introduction
2. Materials and Methods
2.1. Aflatoxin
2.2. Plant Materials
2.3. Test for Detoxification of AFB1 Using Medicinal Plant Extracts
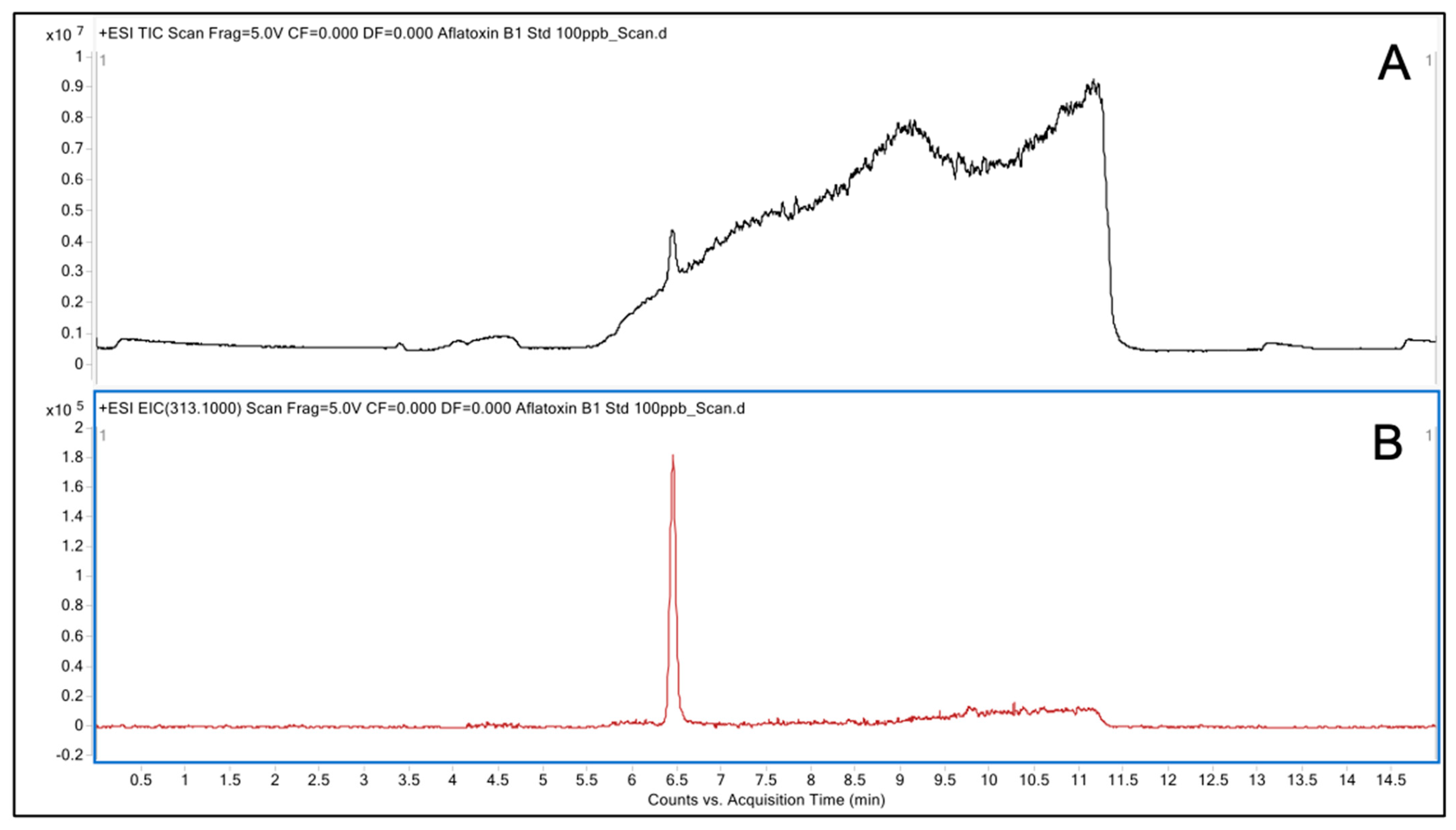
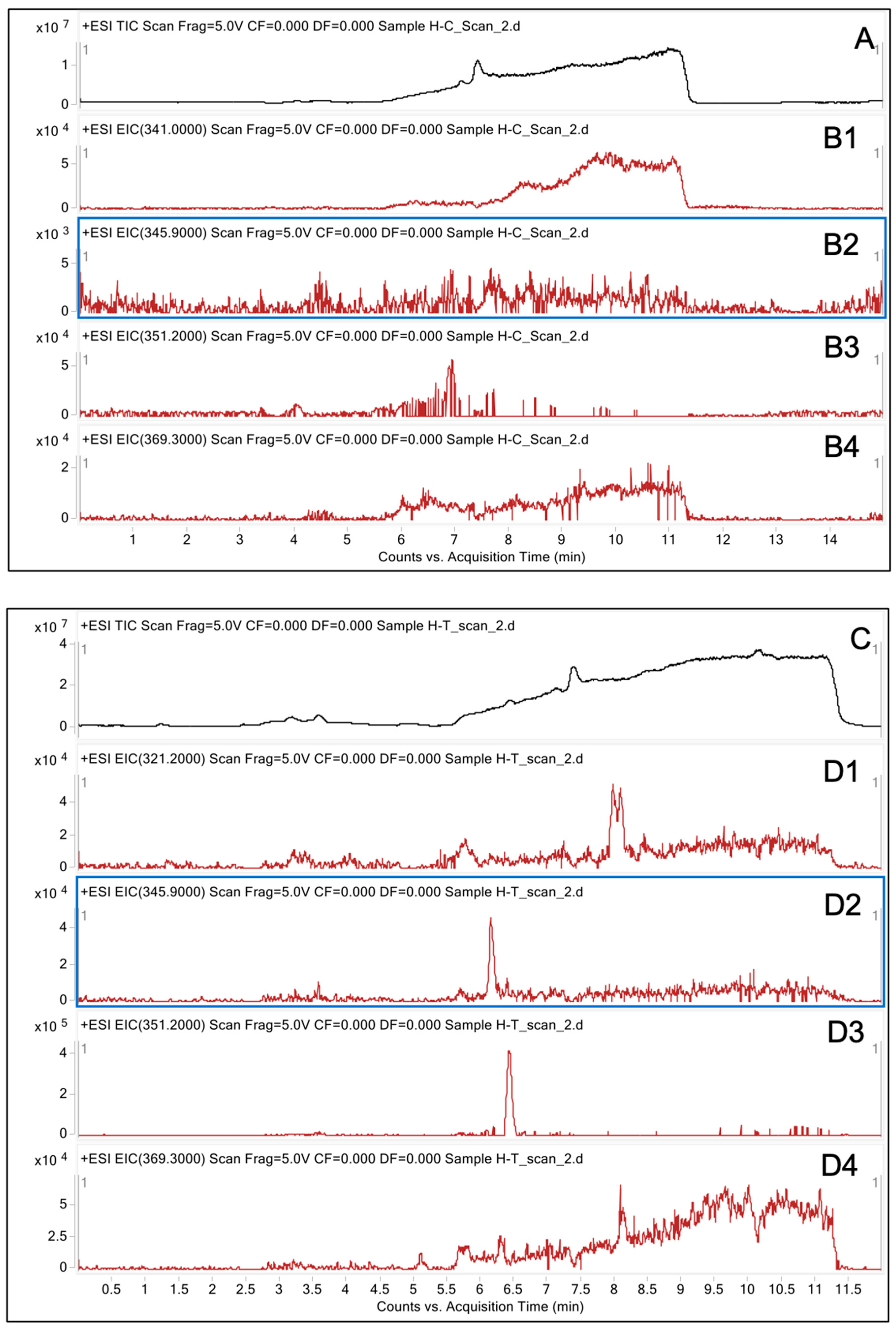
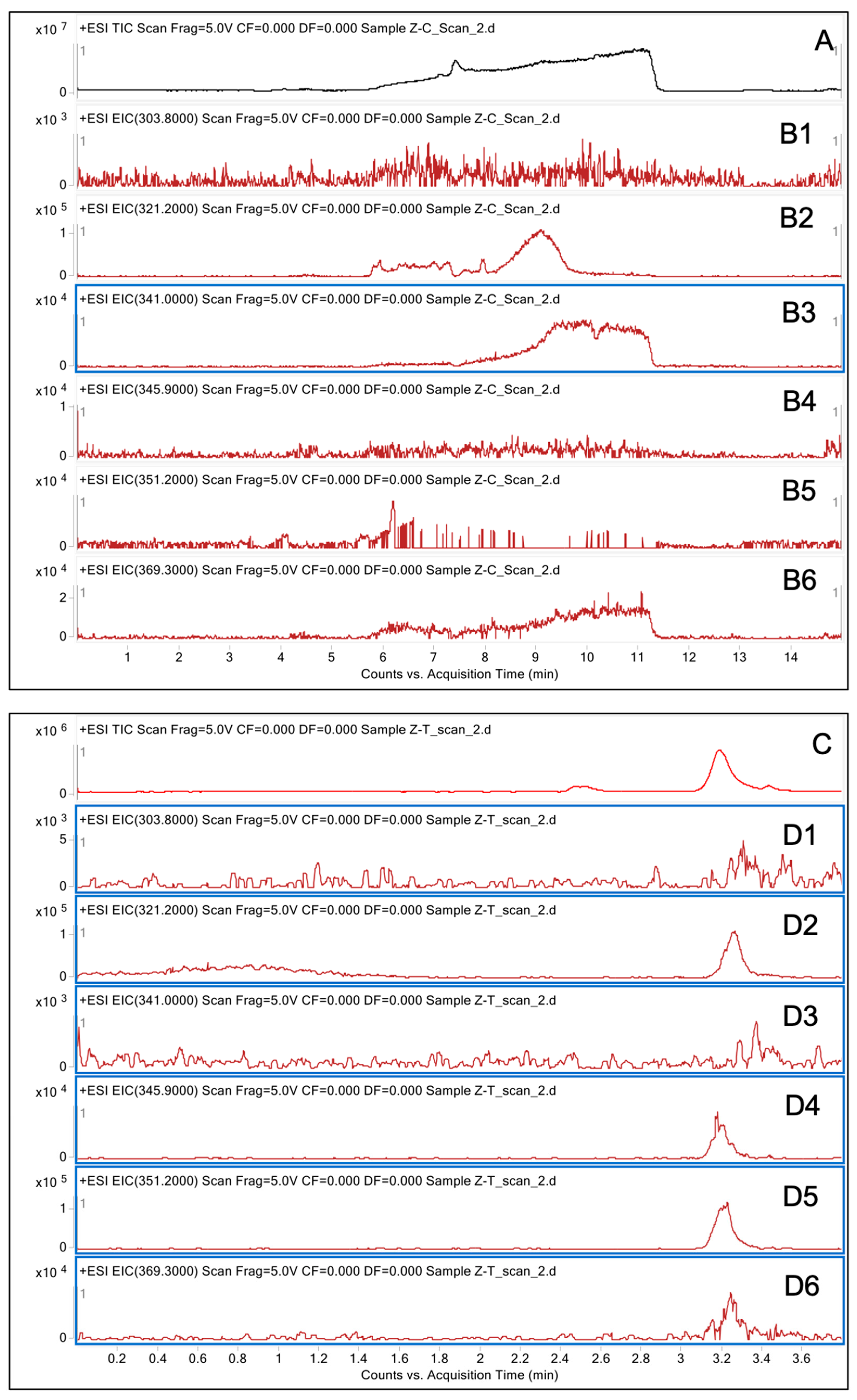
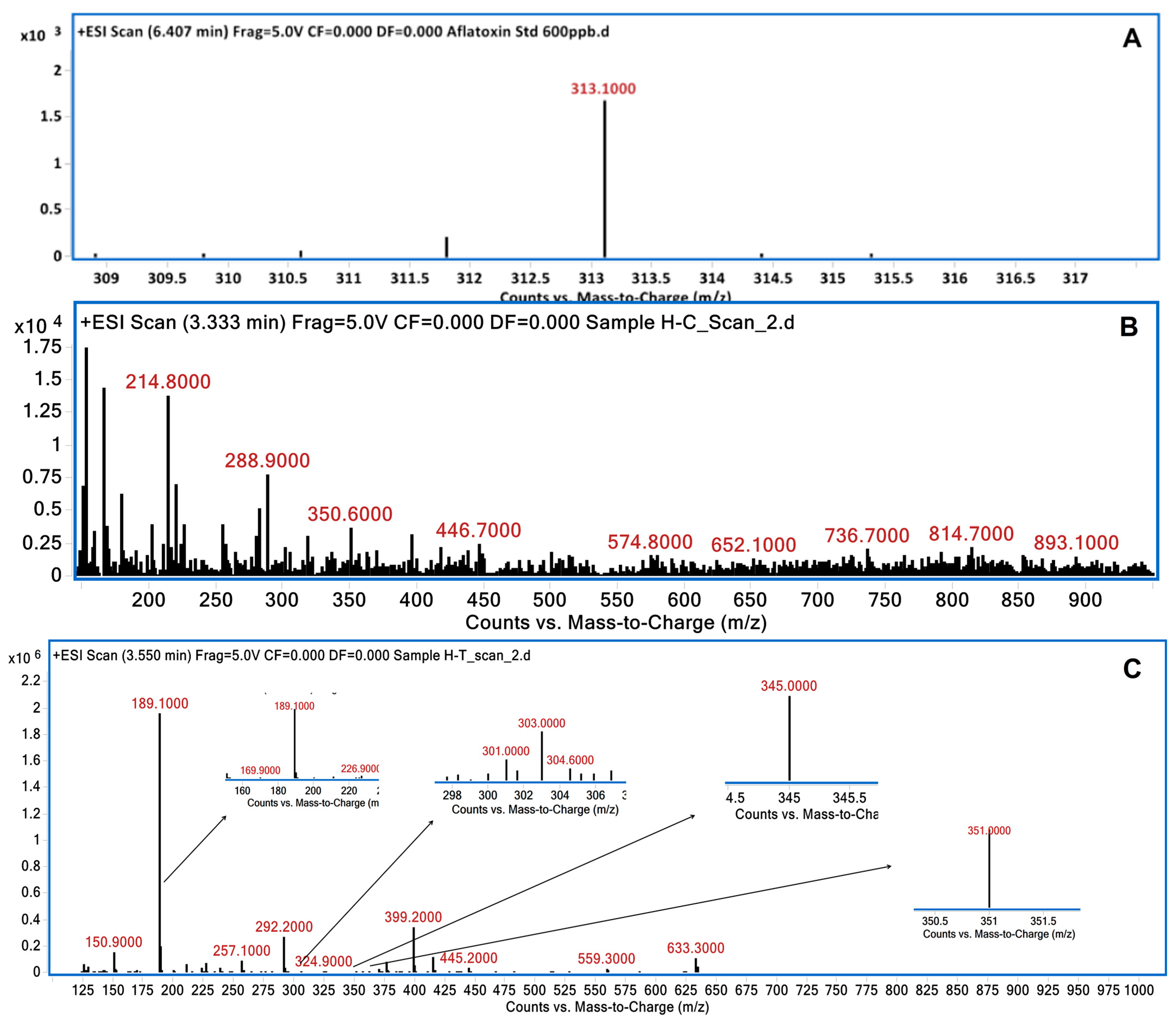
2.4. Analysis of Degraded AFB1 Products
2.5. Brine Shrimp Lethality Assay
2.6. Preparation of Plant Extracts
2.7. Antioxidant Potential of Plant Extracts
2.8. Mouse Assay Experiments
2.9. Mouse Sacrifice
2.10. Biochemical Tests
2.11. Histopathological Studies
2.12. Statistical Analysis
3. Results
3.1. Screening of Medicinal Plants for AFB1 Detoxification
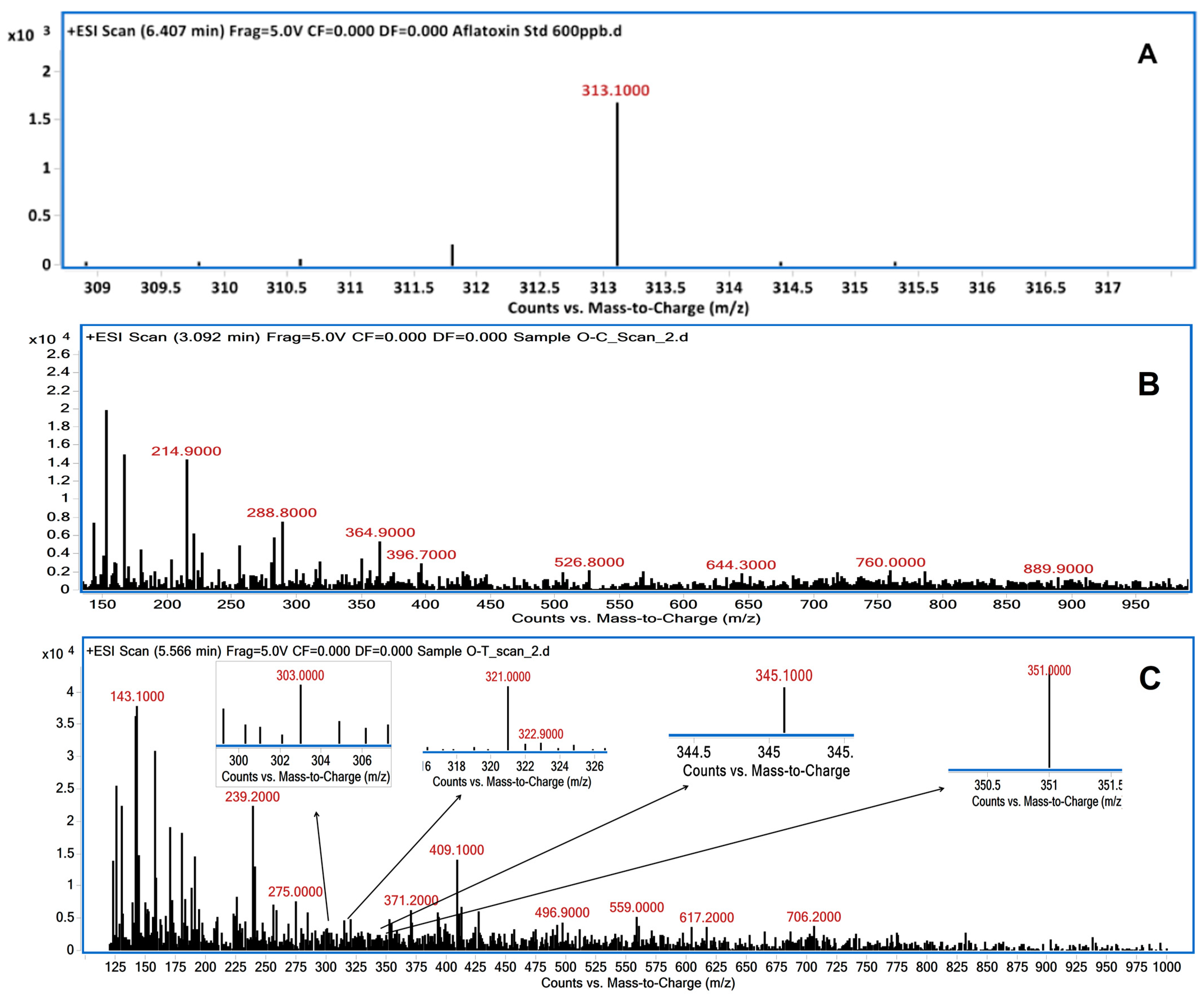
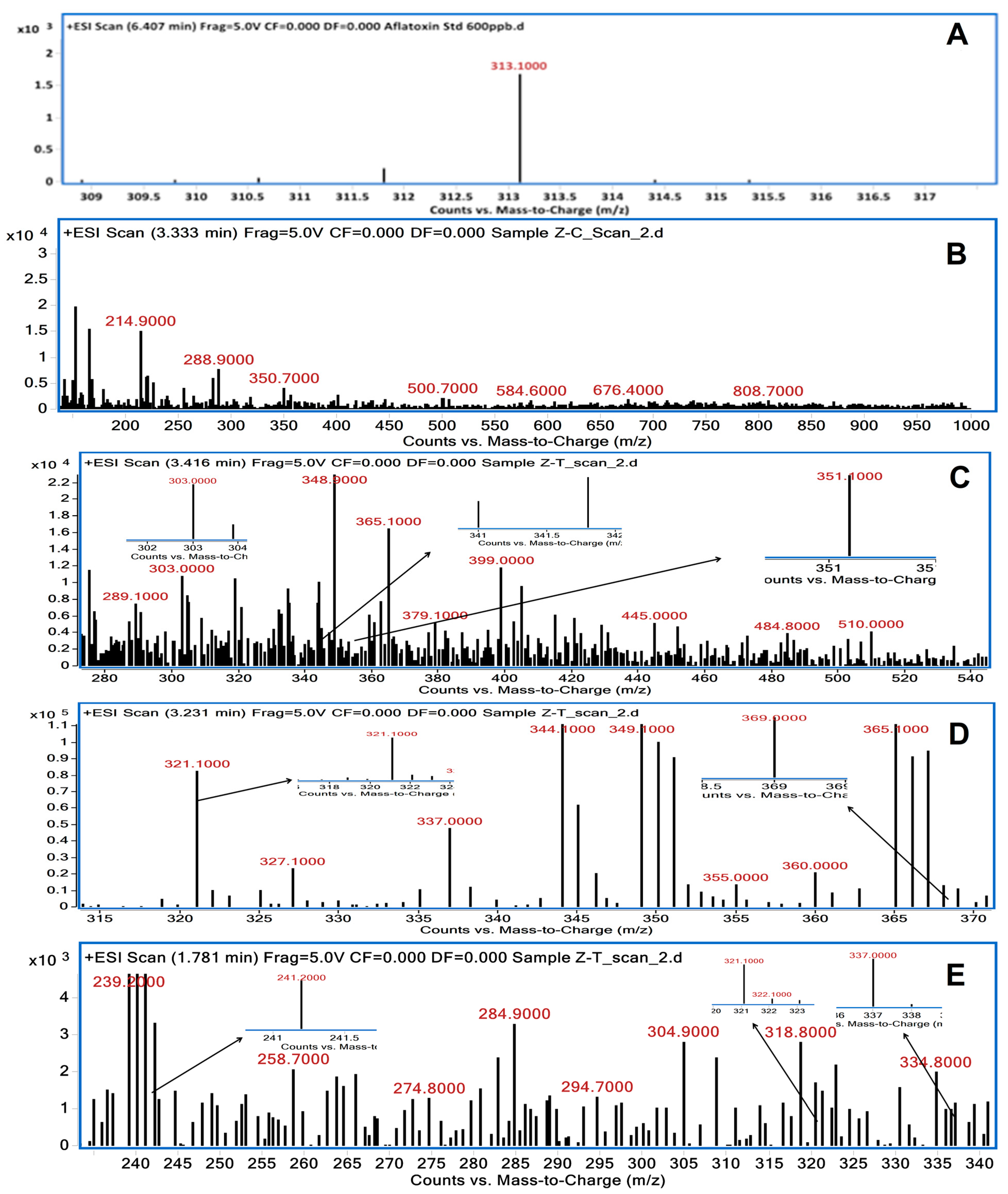
3.2. LC/MS Analysis of Degraded AFB1 Products
3.3. Brine Shrimp Lethality Assay
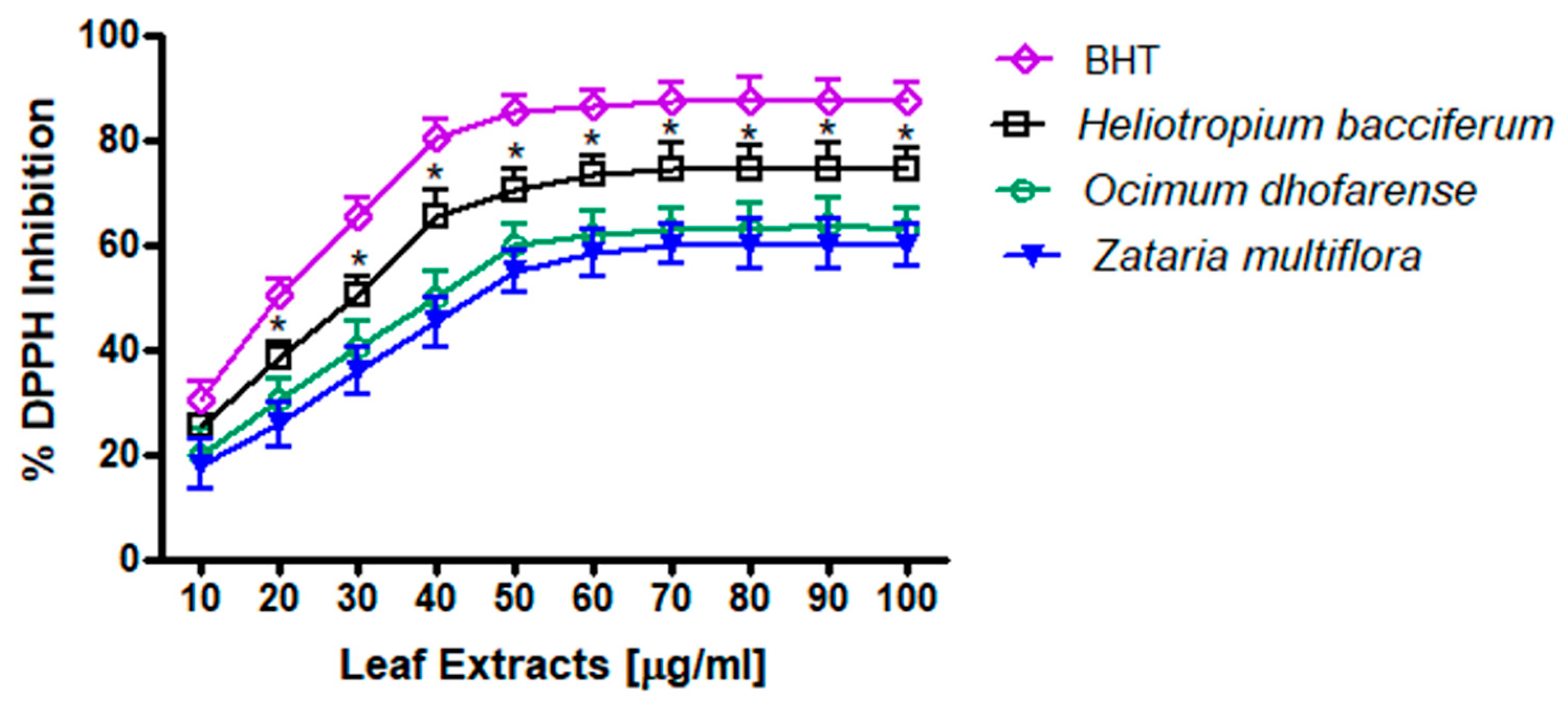
3.4. DPPH Measurements
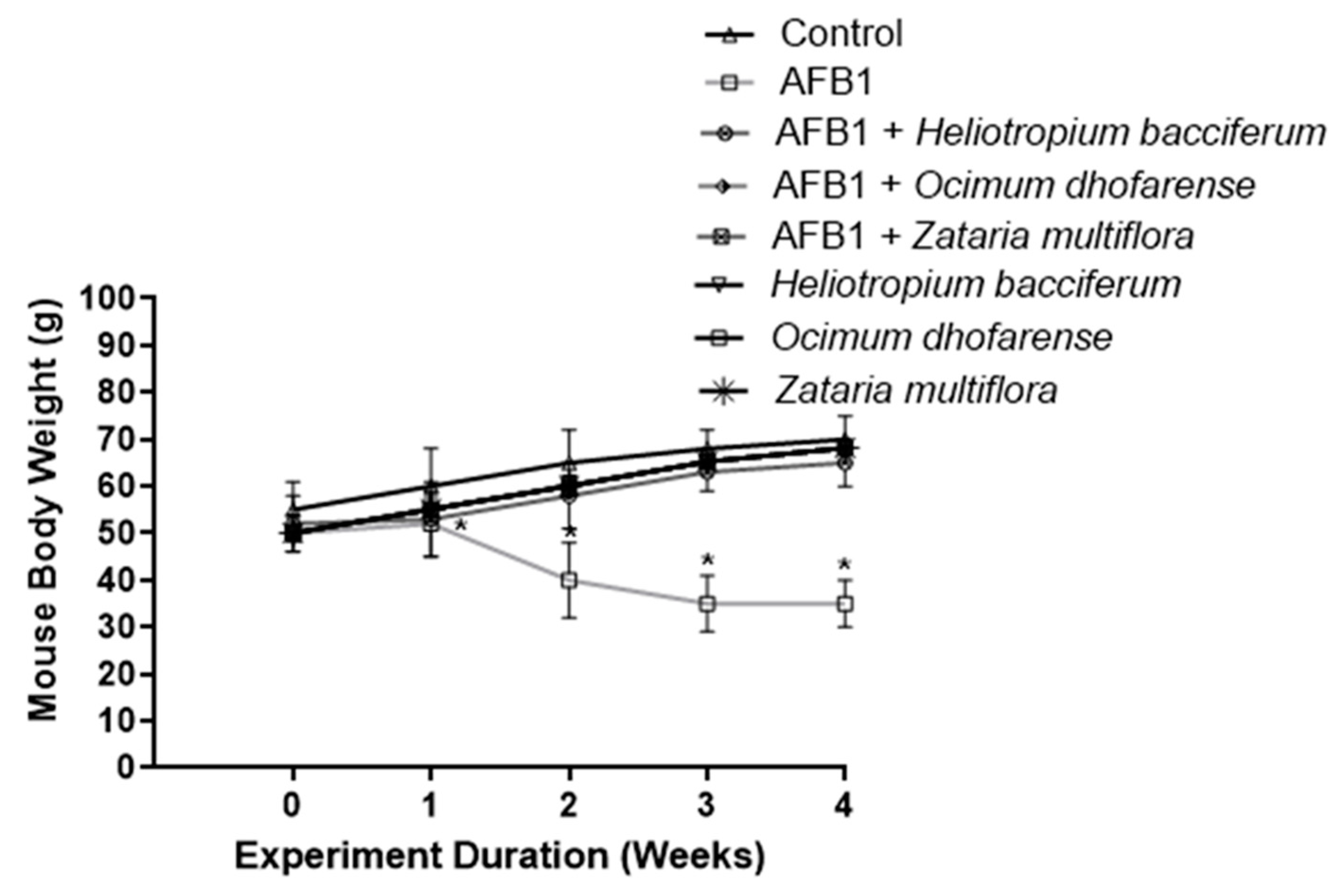
3.5. Body Weight of Mice
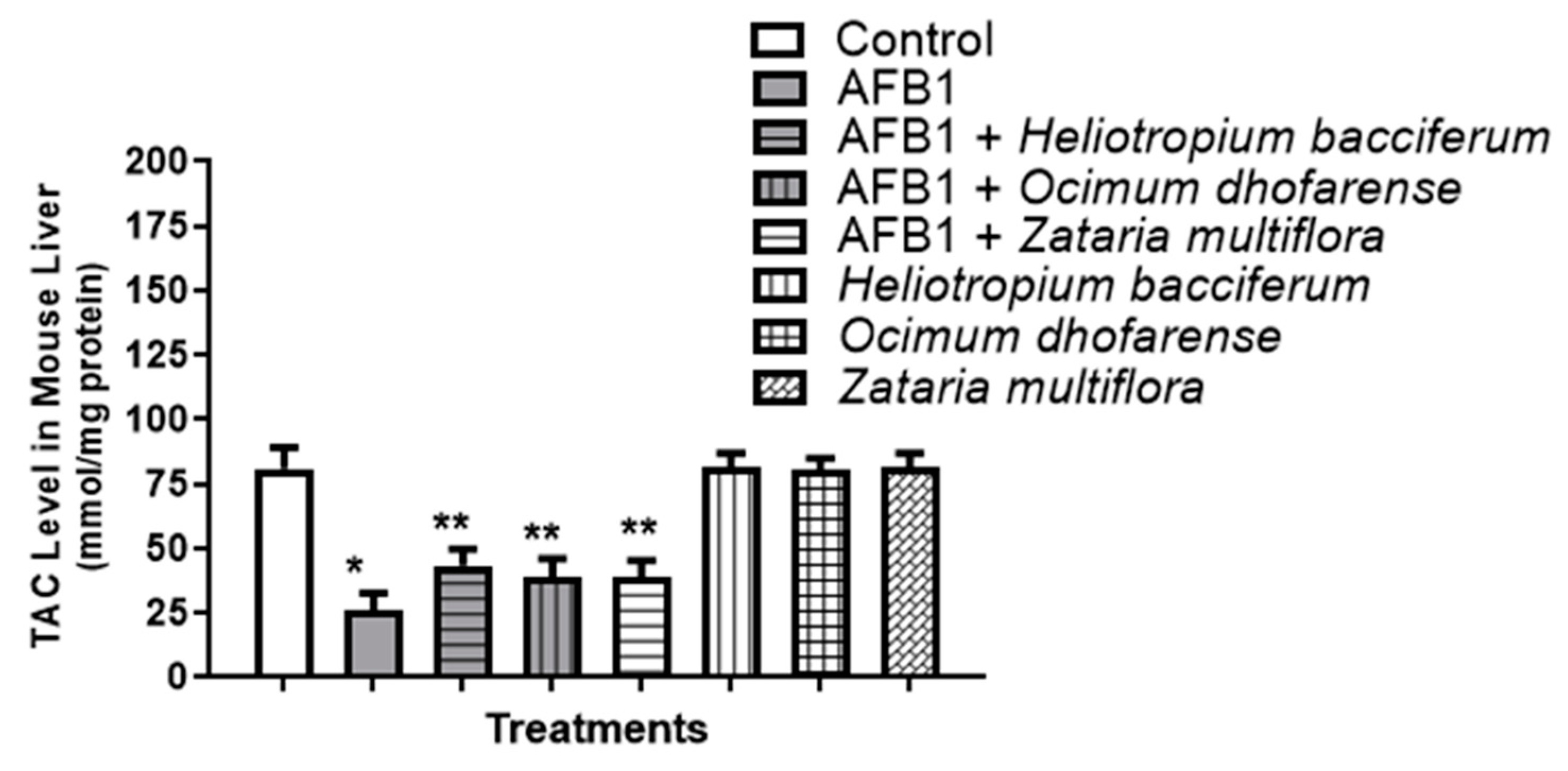
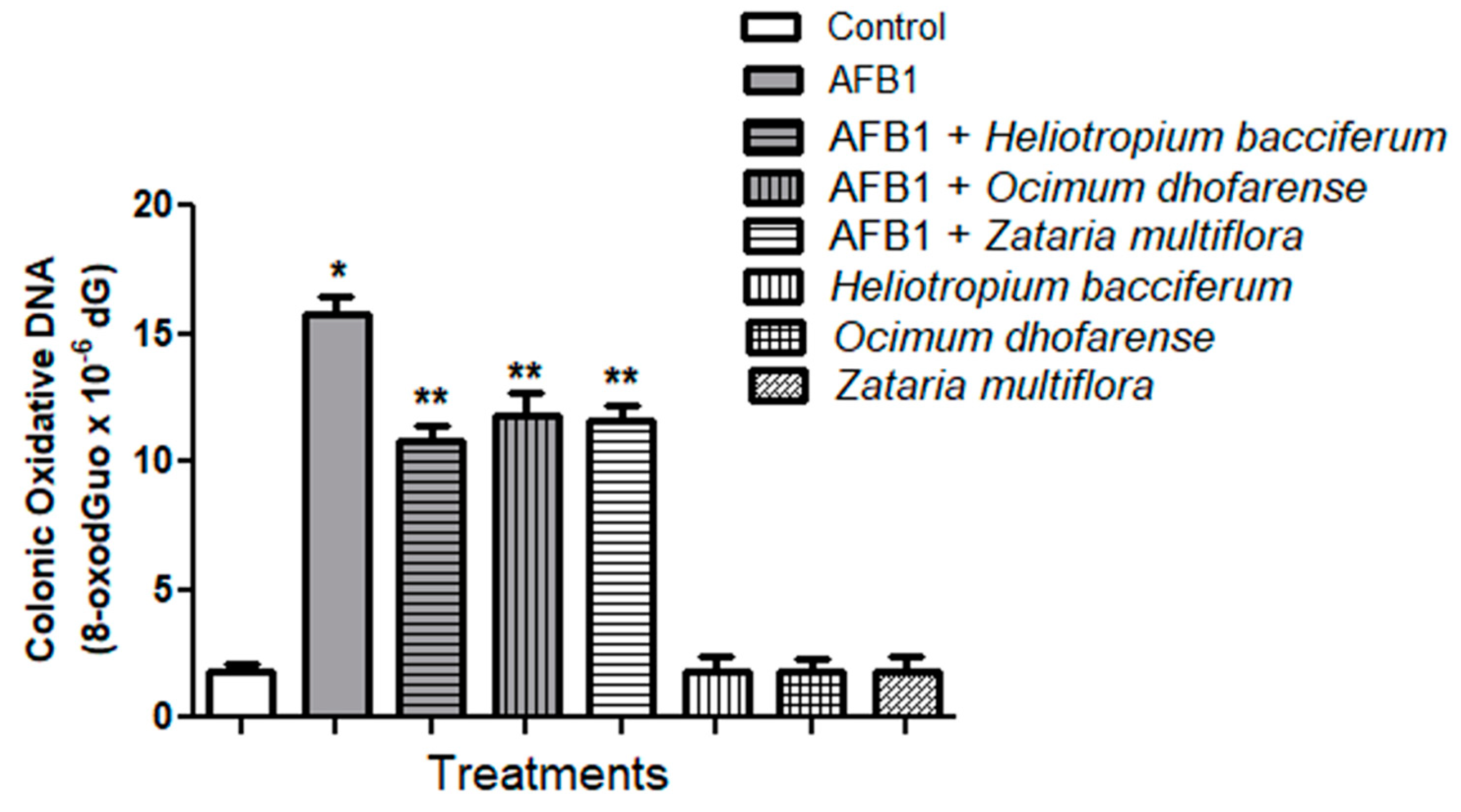
3.6. AFB1-Induced Oxidative Stress
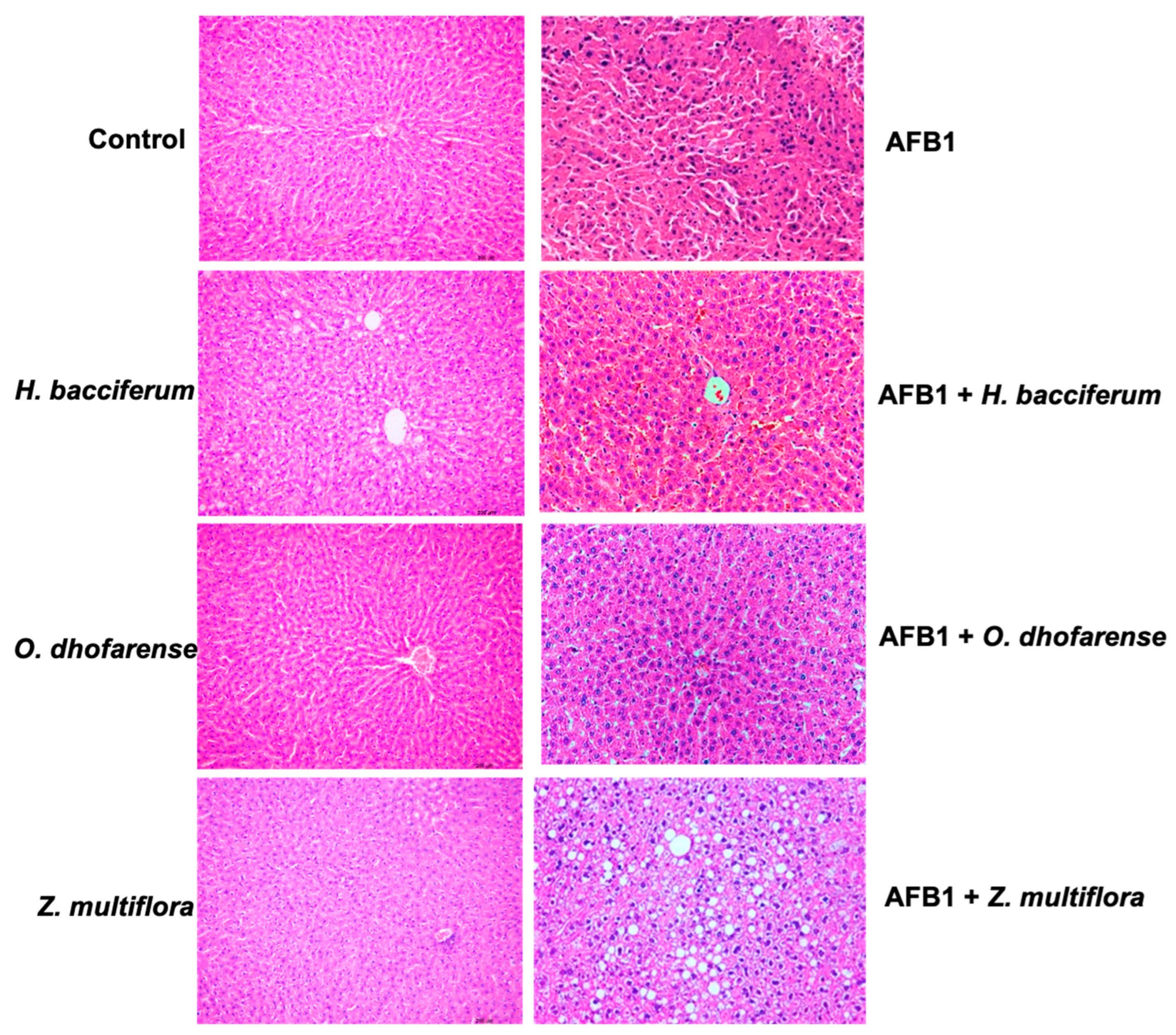
3.7. Histopathological Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 3, 529. [Google Scholar] [CrossRef] [PubMed]
- Adeyeye, S.A. Fungal mycotoxins in foods: A review. Cogent Food Agric. 2016, 2, 1213127. [Google Scholar] [CrossRef]
- Sarma, U.P.; Bhetaria, P.J.; Devi, P.; Varma, A. Aflatoxins: Implications on health. Indian J. Clin. Biochem. 2017, 32, 124–133. [Google Scholar] [CrossRef]
- Wu, F.; Groopman, J.D.; Pestka, J.J. Public health impacts of foodborne mycotoxins. Annu. Rev. Food Sci. Technol. 2014, 5, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A global concern for food safety, human health and their management. Front. Microbiol. 2017, 7, 2170. [Google Scholar] [CrossRef]
- Williams, J.H.; Phillips, T.D.; Jolly, P.E.; Stiles, J.K.; Jolly, C.M.; Aggarwal, D. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nutr. 2004, 80, 1106–1122. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Tian, E.; Hao, Z.; Tang, S.; Wang, Z.; Sharma, G.; Jiang, H.; Shen, J. Aflatoxin B1 toxicity and protective effects of curcumin: Molecular mechanisms and clinical implications. Antioxidants 2022, 11, 2031. [Google Scholar] [CrossRef] [PubMed]
- Jallow, A.; Xie, H.; Tang, X.; Qi, Z.; Li, P. Worldwide aflatoxin contamination of agricultural products and foods: From occurrence to control. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2332–2381. [Google Scholar] [CrossRef]
- Roze, L.V.; Hong, S.Y.; Linz, J.E. Aflatoxin biosynthesis: Current frontiers. Annu. Rev. Food Sci. Technol. 2013, 4, 293–311. [Google Scholar] [CrossRef]
- Adam, M.A.A.; Tabana, Y.M.; Musa, K.B.; Sandai, D.A. Effects of different mycotoxins on humans, cell genome and their involvement in cancer. Oncol. Rep. 2017, 37, 1321–1336. [Google Scholar] [CrossRef]
- van Egmond, H.P.; Schothorst, R.C.; Jonker, M.A. Regulations relating to mycotoxins in food. Anal. Bioanal. Chem. 2007, 389, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Kaale, L.D.; Kimanya, M.E.; Macha, I.J.; Mlalila, N. Aflatoxin contamination and recommendations to improve its control: A review. World Mycotoxin J. 2021, 14, 27–40. [Google Scholar] [CrossRef]
- Aiko, V.; Mehta, A. Occurrence, detection and detoxification of mycotoxins. J. Biosci. 2015, 40, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Velazhahan, R. Bioprospecting of medicinal plants for detoxification of aflatoxins. Int. J. Nutr. Pharmacol. Neurol. Dis. 2017, 7, 60–63. [Google Scholar] [CrossRef]
- Ismail, A.; Gonçalves, B.L.; de Neeff, D.V.; Ponzilacqua, B.; Coppa, C.F.S.C.; Hintzsche, H.; Sajid, M.; Cruz, A.G.; Corassin, C.H.; Oliveira, C.A.F. Aflatoxin in foodstuffs: Occurrence and recent advances in decontamination. Food Res. Int. 2018, 113, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Al-Mamari, A.; Al-Sadi, A.M.; Al-Harrasi, M.M.A.; Sathish Babu, S.P.; Al-Mahmooli, I.H.; Velazhahan, R. Biodegradation of aflatoxin B1 by Bacillus subtilis YGT1 isolated from yoghurt. Int. Food Res. J. 2023, 30, 142–150. [Google Scholar] [CrossRef]
- Velazhahan, R.; Vijayanandraj, S.; Vijayasamundeeswari, A.; Paranidharan, V.; Samiyappan, R.; Iwamoto, T.; Friebe, B.; Muthukrishnan, S. Detoxification of aflatoxins by seed extracts of the medicinal plant, Trachyspermum ammi (L.) Sprague ex Turrill-Structural analysis and biological toxicity of degradation product of aflatoxin G1. Food Control 2010, 21, 719–725. [Google Scholar] [CrossRef]
- Panda, P.; Mehta, A. Aflatoxin detoxification potential of Ocimum tenuiflorum. J. Food Saf. 2013, 33, 265–272. [Google Scholar] [CrossRef]
- Iram, W.; Anjum, T.; Iqbal, M.; Ghaffar, A.; Abbas, M.; Khan, A.M. Structural analysis and biological toxicity of aflatoxins B1 and B2 degradation products following detoxification of Ocimum basilicum and Cassia fistula aqueous extracts. Front. Microbiol. 2016, 7, 1105. [Google Scholar] [CrossRef]
- Sandosskumar, R.; Karthikeyan, M.; Mathiyazhagan, S.; Mohankumar, M.; Chandrasekar, G.; Velazhahan, R. Inhibition of Aspergillus flavus growth and detoxification of aflatoxin B1 by the medicinal plant zimmu (Allium sativum L. × Allium cepa L.). World J. Microbiol. Biotechnol. 2007, 23, 1007–1014. [Google Scholar] [CrossRef]
- Kannan, K.; Velazhahan, R. The potential of leaf extract of Barleria lupulina for detoxification of aflatoxins. Indian Phytopathol. 2014, 67, 298–302. [Google Scholar]
- Vijayanandraj, S.; Brinda, R.; Kannan, K.; Adhithya, R.; Vinothini, S.; Senthil, K.; Ramakoteswara Rao, C.; Paranidharan, V.; Velazhahan, R. Detoxification of aflatoxin B1 by an aqueous extract from leaves of Adhatoda vasica Nees. Microbiol. Res. 2014, 169, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Al-Owaisi, A.; Al-Sadi, A.M.; Al-Sabahi, J.N.; Sathish Babu, S.P.; Al-Harrasi, M.M.A.; Al-Mahmooli, I.H.; Abdel-Jalil, R.; Velazhahan, R. In vitro detoxification of aflatoxin B1 by aqueous extracts of medicinal herbs. All Life 2022, 15, 314–324. [Google Scholar] [CrossRef]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nichols, D.E.; McLaughlin, J.L. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Waly, M.I.; Al-Rawahi, A.S.; Al Riyami, M.; Al-Kindi, M.A.; Al-Issaei, H.K.; Farooq, S.A.; Al-Alawi, A.; Rahman, M.S. Amelioration of azoxymethane induced-carcinogenesis by reducing oxidative stress in rat colon by natural extracts. BMC Complement. Altern. Med. 2014, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Iram, W.; Anjum, T.; Iqbal, M.; Ghaffar, A.; Abbas, M. Structural elucidation and toxicity assessment of degraded products of aflatoxin B1 and B2 by aqueous axtracts of Trachyspermum ammi. Front. Microbiol. 2016, 7, 346. [Google Scholar]
- Ponzilacqua, B.; Rottinghaus, G.E.; Landers, B.R.; Oliveira, C.A.F. Effects of medicinal herb and Brazilian traditional plant extracts on in vitro mycotoxin decontamination. Food Control 2019, 100, 24–27. [Google Scholar] [CrossRef]
- Hamad, G.M.; Mohdaly, A.A.A.; El-Nogoumy, B.A.; Ramadan, M.F.; Hassan, S.A.; Zeitoun, A.M. Detoxification of aflatoxin B1 and ochratoxin A using Salvia farinacea and Azadirachta indica water extract and application in meat products. Appl. Biochem. Biotechnol. 2021, 193, 3098–3120. [Google Scholar] [CrossRef]
- Hajare, S.S.; Hajare, S.H.; Sharma, A. Aflatoxin inactivation using aqueous extract of Ajowan (Trachyspermum ammi) seeds. J. Food Sci. 2005, 70, 29–34. [Google Scholar] [CrossRef]
- Hammiche, V.; Maiza, K. Traditional medicine in Central Sahara: Pharmacopoeia of Tassili N’ajjer. J. Ethnopharmacol. 2006, 105, 358–367. [Google Scholar] [CrossRef]
- Ahmad, S.; Abdel-Salam, N.M.; Ullah, R. In vitro antimicrobial bioassays, DPPH radical scavenging activity, and FTIR spectroscopy analysis of Heliotropium bacciferum. BioMed Res. Int. 2016, 2016, 3818945. [Google Scholar] [CrossRef] [PubMed]
- Farrag, N.M.; Abdel-Aziz, E.M.; El-Shafae, A.M.; Ateya, A.M.; El Domiaty, M.M. Pyrrolizidine alkaloids of Heliotropium bacciferum Forssk from Egypt. Int. J. Pharmacogn. 1996, 34, 374–377. [Google Scholar] [CrossRef]
- Rizk, A.M.; Hammouda, F.M.; Roder, E.; Wiedenfeld, H.; Ismail, S.I.; Hassan, N.M.; Hosseiny, H.A. Constituents of plants growing in Qatar. XIII. Occurrence of pyrrolizidine alkaloids in Heliotropium bacciferum Forssk. Sci. Pharm. 1998, 56, 105–110. [Google Scholar]
- Aissaoui, H.; Mencherini, T.; Esposito, T.; De Tommasi, N.; Gazzerro, P.; Benayache, S.; Benayache, F.; Mekkiou, R. Heliotropium bacciferum Forssk. (Boraginaceae) extracts: Chemical constituents, antioxidant activity and cytotoxic effect in human cancer cell lines. Nat. Prod. Res. 2019, 33, 1813–1818. [Google Scholar] [CrossRef] [PubMed]
- Sharififar, F.; Moshafi, M.H.; Mansouri, S.H.; Khodashenas, M.; Khoshnoodi, M. In vitro evaluation of antibacterial and antioxidant activities of the essential oil and methanol extract of endemic Zataria multiflora Boiss. Food Control 2007, 18, 800–805. [Google Scholar] [CrossRef]
- Sajed, H.; Sahebkar, A.; Iranshahi, M. Zataria multiflora Boiss. (Shirazi thyme)- an ancient condiment with modern pharmaceutical uses. J. Ethnopharmacol. 2013, 145, 686–698. [Google Scholar] [CrossRef]
- Al-Balushi, Y.J.R.; Al-Sadi, A.M.; Al-Mahmooli, I.H.; Al-Harrasi, M.M.A.; Al-Sabahi, J.N.; Al-Alawi, A.K.S.; Al-Farsi, K.; Velazhahan, R. Antifungal activity of Shirazi thyme (Zataria multiflora Boiss.) essential oil against Hypomyces perniciosus, a causal agent of wet bubble disease of Agaricus bisporus. J. Agric. Mar. Sci. 2022, 27, 59–65. [Google Scholar]
- Gandomi, H.; Misaghi, A.; Basti, A.A.; Bokaei, S.; Khosravi, A.; Abbasifar, A.; Javan, A.J. Effect of Zataria multiflora Boiss. essential oil on growth and aflatoxin formation by Aspergillus flavus in culture media and cheese. Food Chem. Toxicol. 2009, 47, 2397–2400. [Google Scholar] [CrossRef]
- Gupta, S.; Mediratta, P.K.; Singh, S.; Sharma, K.K.; Shukla, R. Antidiabetic, antihypercholesterolaemic and antioxidant effect of Ocimum sanctum (Linn) seed oil. Indian J. Exp. Biol. 2006, 44, 300–304. [Google Scholar]
- Pattanayak, P.; Behera, P.; Das, D.; Panda, S.K. Ocimum sanctum Linn. A reservoir plant for therapeutic applications: An overview. Pharmacogn. Rev. 2010, 4, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Jaggi, R.K.; Madaan, R.; Singh, B. Anticonvulsant potential of holy basil, Ocimum sanctum Linn. and its cultures. Indian J. Exp. Biol. 2003, 41, 1329–1333. [Google Scholar] [PubMed]
- Kumar, A.; Shukla, R.; Singh, P.; Dubey, N.K. Chemical composition, antifungal and antiaflatoxigenic activities of Ocimum sanctum L. essential oil and its safety assessment as plant-based antimicrobial. Food Chem. Toxicol. 2010, 48, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Singh, P.; Tripathi, N.N. Chemistry and bioactivities of essential oils of some Ocimum species: An overview. Asian Pac. J. Trop. Biomed. 2014, 4, 682–694. [Google Scholar] [CrossRef]
- Al-Harrasi, M.M.A.; Al-Sadi, A.M.; Al-Sabahi, J.N.; Al-Farsi, K.; Waly, M.I.; Velazhahan, R. Essential oils of Heliotropium bacciferum, Ocimum dhofarense and Zataria multiflora exhibit aflatoxin B1 detoxification potential. All Life 2021, 14, 989–996. [Google Scholar] [CrossRef]
- Ventura, M.; Guillen, D.; Anaya, I.; Broto-Puig, F.; Lliberia, J.L.; Agut, M.; Comellas, L. Ultra-performance liquid chromatography/tandem mass spectrometry for the simultaneous analysis of aflatoxins B1, G1, B2, G2 and ochratoxin A in beer. Rapid Commun. Mass Spectrom. 2006, 20, 3199–3204. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, Q.; Wu, J.; Wu, W.; Jiang, J.; Yan, H.; Huang, J.; Sun, Y.; Deng, Y. The metabolism and biotransformation of AFB1: Key enzymes and pathways. Biochem. Pharmacol. 2022, 199, 115005. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Islam, M.B.; Biswas, M.; Khurshid Alam, A.H.M. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res. Notes 2015, 8, 621. [Google Scholar] [CrossRef] [PubMed]
- Benkerroum, N. Chronic and acute toxicities of aflatoxins: Mechanisms of action. Int. J. Environ. Res. Public Health 2020, 17, 423. [Google Scholar] [CrossRef]
- Abdel-Wahhab, M.A.; Aly, S.E. Antioxidants and radical scavenging properties of vegetable extracts in rats fed aflatoxin-contaminated diet. J. Agric. Food Chem. 2003, 51, 2409–2414. [Google Scholar] [CrossRef]
- Rastogi, S.; Shukla, Y.; Paul, B.N.; Chowdhuri, D.K.; Khanna, S.K.; Das, M. Protective effect of Ocimum sanctum on 3-methylcholanthrene, 7, 12-dimethylbenz (a) anthracene and aflatoxin B1 induced skin tumorigenesis in mice. Toxicol. Appl. Pharmacol. 2007, 224, 228–240. [Google Scholar] [CrossRef]
- Naiel, M.A.E.; Ismael, N.E.M.; Shehata, S.A. Ameliorative effect of diets supplemented with rosemary (Rosmarinus officinalis) on aflatoxin B1 toxicity in terms of the performance, liver histopathology, immunity and antioxidant activity of Nile Tilapia (Oreochromis niloticus). Aquaculture 2019, 511, 734264. [Google Scholar] [CrossRef]
- Yarru, L.P.; Settivari, R.S.; Gowda, N.K.S.; Antoniou, E.; Ledoux, D.R.; Rottinghaus, G.E. Effects of turmeric (Curcuma longa) on the expression of hepatic genes associated with biotransformation, antioxidant, and immune systems in broiler chicks fed aflatoxin. Poult. Sci. 2009, 88, 2620–2627. [Google Scholar] [CrossRef] [PubMed]
- Gyamfi, M.A.; Aniya, Y. Medicinal herb, Thonningia sanguinea protects against aflatoxin B1 acute hepatotoxicity in Fischer 344 rats. Hum. Exp. Toxicol. 1998, 17, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Brinda, R.; Vijayanandraj, S.; Uma, D.; Malathi, D.; Paranidharan, V.; Velazhahan, R. Role of Adhatoda vasica (L.) Nees leaf extract in the prevention of aflatoxin-induced toxicity in Wistar rats. J. Sci. Food. Agric. 2013, 93, 2743–2748. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.A.; Sun, L.; Zhang, N.Y.; Khalil, M.M.; Ling, Z.; Chong, L.; Wang, S.; Rajput, I.R.; Bloch, D.M.; Khan, F.A.; et al. Grape seed proanthocyanidin extract alleviates aflatoxinB1-induced immunotoxicity and oxidative stress via modulation of NF-κB and Nrf2 signaling pathways in broilers. Toxins 2019, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- El-Nekeety, A.A.; Mohamed, S.R.; Hathout, A.S.; Hassan, N.S.; Aly, S.E.; Abdel-Wahhab, M.A. Antioxidant properties of Thymus vulgaris oil against aflatoxin-induce oxidative stress in male rats. Toxicon 2011, 57, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.Y.; Qi, M.; Zhao, L.; Zhu, M.K.; Guo, J.; Liu, J.; Gu, C.Q.; Rajput, S.A.; Krumm, C.S.; Qi, D.S.; et al. Curcumin prevents aflatoxin B1 hepatoxicity by inhibition of cytochrome P450 isozymes in chick liver. Toxins 2016, 8, 327. [Google Scholar] [CrossRef]
- El-Mekkawy, H.I.; Al-Kahtani, M.A.; Shati, A.A.; Alshehri, M.A.; Al-Doaiss, A.A.; Elmansi, A.A.; Ahmed, A.E. Black tea and curcumin synergistically mitigate the hepatotoxicity and nephropathic changes induced by chronic exposure to aflatoxin-B1 in Sprague–Dawley rats. J. Food Biochem. 2020, 44, e13346. [Google Scholar] [CrossRef]
- Unsal, V.; Kurutas, E.B. Experimental hepatic carcinogenesis: Oxidative stress and natural antioxidants. Open Access Maced. J. Med. Sci. 2017, 5, 686–691. [Google Scholar] [CrossRef]
- Fathalipour-Rayeni, H.; Forootanfar, H.; Khazaeli, P.; Mehrabani, M.; Rahimi, H.R.; Shakibaie, M.; Jafari, E.; Doostmohammadi, M.; Bami, M.S.; Shaghooei, P.M.; et al. Evaluation of antioxidant potential of Heliotropium bacciferum Forssk extract and wound healing activity of its topical formulation in rat. Ann. Pharm. Françaises 2022, 80, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.; Zang, H.; Yin, Z.; Guan, P.; Yu, C.; Shan, A.; Feng, X. Dietary pterostilbene exerts potential protective effects by regulating lipid metabolism and enhancing antioxidant capacity on liver in broilers. J. Anim. Physiol. Anim. Nutr. 2024, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Guan, P.; Yu, H.; Wang, S.; Sun, J.; Chai, X.; Sun, X.; Qi, X.; Zhang, R.; Jiao, Y.; Li, Z.; et al. Dietary rutin alleviated the damage by cold stress on inflammation reaction, tight junction protein and intestinal microbial flora in the mice intestine. J. Nutr. Biochem. 2024, 130, 109658. [Google Scholar] [CrossRef] [PubMed]
- Al-Subhi, L.; Ibrahim Waly, M. Two Cultivars of Ocimum basilicum leaves extracts attenuate streptozotocin-mediated oxidative stress in diabetic rats. Pak. J. Biol. Sci. 2020, 23, 1010–1017. [Google Scholar] [CrossRef]
- Shokrzadeh, M.; Chabra, A.; Ahmadi, A.; Naghshvar, F.; Habibi, E.; Salehi, F.; Assadpour, S. Hepatoprotective effects of Zataria multiflora ethanolic extract on liver toxicity induced by cyclophosphamide in mice. Drug Res. 2015, 65, 169–175. [Google Scholar] [CrossRef]




| Name of the Plant | Family | Accession Number | AFB1 Detoxification (%) |
|---|---|---|---|
| Aerva javanica (Burm.f.) Juss. ex Schult. | Amaranthaceae | 201800506 | 33 |
| Searsia aucheri (Boiss.) Moffett | Anacardiaceae | 201800777 | 0 |
| Desmidorchis adenensis (Deflers) Meve & Liede | Apocynaceae | 200604436 | 6 |
| Desmidorchis arabica (N.E.Br.) Meve & Liede | Apocynaceae | 201800303 | 0 |
| Desmidorchis flava (N.E.Br.) Meve & Liede | Apocynaceae | 201300308 | 32 |
| Desmidorchis penicillata (Deflers) Plowes | Apocynaceae | 201800596 | 0 |
| Monolluma quadrangular (Forssk.) Plowes | Apocynaceae | 201800011 | 55 |
| Aloe dhufarensis Lavranos | Asphodelaceae | 201000386 | 32 |
| Aloe praetermissa T.A.McCoy & Lavranos | Asphodelaceae | 201000250 | 0 |
| Aloe vera (L.) Burm.f. | Asphodelaceae | 201800270 | 10 |
| Kleinia odora (Forssk.) DC. | Asteraceae | 201000077 | 85 |
| Pluchea arabica (Boiss.) Qaiser & Lack | Asteraceae | 201000388 | 58 |
| Pulicaria glutinosa (Boiss.) Jaub. & Spach | Asteraceae | 201200418 | 40 |
| Tecomella undulata (Sm.) Seem. | Bignoniaceae | 200817492 | 85 |
| Heliotropium bacciferum Forssk. | Boraginaceae | 201600290 | 95 |
| Boswellia sacra Flück. | Burseraceae | 201300064 | 52 |
| Commiphora gileadensis (L.) C. Christ. | Burseraceae | 201000695 | 18 |
| Commiphora wightii (Arnott) Bhandari | Burseraceae | 200616808 | 16 |
| Capparis cartilaginea Decne. | Capparaceae | 201001415 | 42 |
| Capparis spinosa L. | Capparaceae | 201800536 | 22 |
| Maerua crassifolia Forssk. | Capparaceae | 200602732 | 24 |
| Ephedra ciliata Fisch. & C.A.Mey. | Ephedraceae | 201600189 | 88 |
| Euphorbia aff. Uzmuk S.Carter & J.R.I.Wood | Euphorbiaceae | 200604709 | 21 |
| Euphorbia larica Boiss. | Euphorbiaceae | 201800015 | 34 |
| Euphorbia balsamifera subsp. adenensis (Deflers) P.R.O.Bally | Euphorbiaceae | 200717381 | 7 |
| Euphorbia cactus Ehrenb. ex Boiss. | Euphorbiaceae | 201600143 | 0 |
| Euphorbia smithii S.Carter | Euphorbiaceae | 201200704 | 32 |
| Jatropha dhofarica Radcl.-Sm. | Euphorbiaceae | 201000400 | 10 |
| Vachellia flava (Forssk.) Kyal. & Boatwr. | Fabaceae | 201900813 | 50 |
| Prosopis cineraria (L.) Druce | Fabaceae | 200918235 | 8 |
| Tephrosia purpurea subsp. apollinea (Delile) Hosni & El-Karemy | Fabaceae | 201900065 | 0 |
| Tephrosia nubica (Bioss.) Baker | Fabaceae | 201900067 | 0 |
| Lavandula dhofarensis subsp. ayunensis A.G. Mill. | Lamiaceae | 201800010 | 86 |
| Lavandula subnuda Benth. | Lamiaceae | 201900964 | 81 |
| Ocimum dhofarense (Sebald) A.J.Paton | Lamiaceae | 202000071 | 93 |
| Coleus barbatus (Andrews) Benth. ex G.Don | Lamiaceae | 201600466 | 70 |
| Coleus cylindraceus (Hochst. ex Benth.) A.J.Paton | Lamiaceae | 201800554 | 9 |
| Salvia hillcoatiae Hedge | Lamiaceae | 201900818 | 84 |
| Teucrium nummularifolium Baker | Lamiaceae | 201800146 | 62 |
| Teucrium stocksianum subsp. stenophyllum R.A.King | Lamiaceae | 201900997 | 22 |
| Zataria multiflora Boiss. | Lamiaceae | 201100114 | 92 |
| Indigofera articulata Gouan | Leguminosae | 201000765 | 60 |
| Lawsonia inermis L. | Lythraceae | 200917997 | 35 |
| Abutilon fruticosum Guill. & Perr. | Malvaceae | 201901182 | 0 |
| Senra incana Cav. | Malvaceae | 201000211 | 0 |
| Acridocarpus orientalis A.Juss. | Malpighiaceae | 201500463 | 0 |
| Moringa peregrina (Forssk.) Fiori | Moringaceae | 200609782 | 8 |
| Myrtus communis L. | Myrtaceae | 201500311 | 0 |
| Olea europaea subsp. cuspidate (Wall. & G.Don) Cif. | Oleaceae | 200717412 | 0 |
| Schweinfurthia spinosa A.G.Mill., M.Short & D.A.Sutton | Plantaginaceae | 201800698 | 34 |
| Dionysia mira (Jaub. & Spach.) Wendelbo | Primulaceae | 201800663 | 33 |
| Ochradenus arabicus Chaudhary, Hillc. & A.G. Mill. | Resedaceae | 201900726 | 35 |
| Ziziphus spina-christi (L.) Desf. | Rhamnaceae | 200817484 | 71 |
| Hyoscyamus gallagheri A.G.Mill. & Biagi | Solanaceae | 201900407 | 35 |
| Hermannia paniculata Franch. | Sterculiaceae | 201900552 | 15 |
| Zygophyllum luntii (Baker) Christenh. & Byng | Zygophylaceae | 201901077 | 0 |
| Zygophyllum paulayanum (J.Wagner & Vierth.) Christenh. & Byng | Zygophylaceae | 201800475 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velazhahan, R.; Al-Sadi, A.M.; Waly, M.I.; Soundra Pandian, S.B.; Al-Sabahi, J.; Al-Farsi, K. Aflatoxin B1 Detoxification and Antioxidant Effect of Selected Omani Medicinal Plants against Aflatoxin B1-Induced Oxidative Stress Pathogenesis in the Mouse Liver. Appl. Sci. 2024, 14, 5378. https://doi.org/10.3390/app14135378
Velazhahan R, Al-Sadi AM, Waly MI, Soundra Pandian SB, Al-Sabahi J, Al-Farsi K. Aflatoxin B1 Detoxification and Antioxidant Effect of Selected Omani Medicinal Plants against Aflatoxin B1-Induced Oxidative Stress Pathogenesis in the Mouse Liver. Applied Sciences. 2024; 14(13):5378. https://doi.org/10.3390/app14135378
Chicago/Turabian StyleVelazhahan, Rethinasamy, Abdullah Mohammed Al-Sadi, Mostafa I. Waly, Sathish Babu Soundra Pandian, Jamal Al-Sabahi, and Khalid Al-Farsi. 2024. "Aflatoxin B1 Detoxification and Antioxidant Effect of Selected Omani Medicinal Plants against Aflatoxin B1-Induced Oxidative Stress Pathogenesis in the Mouse Liver" Applied Sciences 14, no. 13: 5378. https://doi.org/10.3390/app14135378
APA StyleVelazhahan, R., Al-Sadi, A. M., Waly, M. I., Soundra Pandian, S. B., Al-Sabahi, J., & Al-Farsi, K. (2024). Aflatoxin B1 Detoxification and Antioxidant Effect of Selected Omani Medicinal Plants against Aflatoxin B1-Induced Oxidative Stress Pathogenesis in the Mouse Liver. Applied Sciences, 14(13), 5378. https://doi.org/10.3390/app14135378








