Solvent Bar Microextraction Method Based on a Natural Deep Eutectic Solvent and Multivariate Optimization for Determination of Steroid Hormones in Urine and Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagent and Materials
2.2. Synthesis of HDEs
2.3. Preparation of Standard Solutions
2.4. Urine and Water Samples
2.5. HDE-SBME Procedure
2.6. Instrumentation
2.7. Method Validation
2.8. Computational Methods
2.9. Experimental Design
3. Results and Discussion
3.1. Selection of HDEs
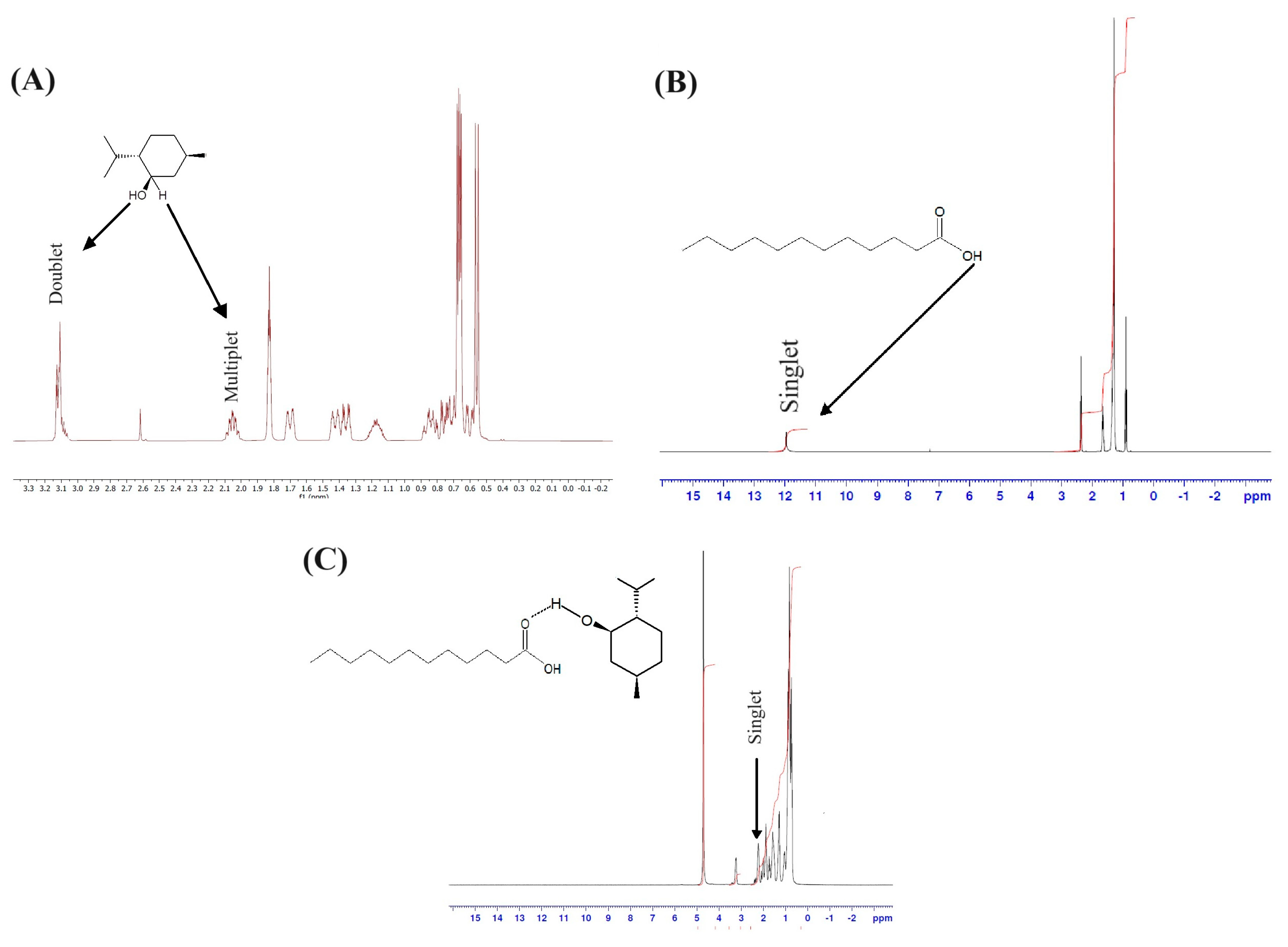
3.1.1. HDE Characterization
3.1.2. Interaction of the 4:1 HDEs and Selected Steroids Based on Computational Methods
3.2. Optimize the Sample pH
3.3. Optimize Parameters Using a Chemometric Approaches
2.0862AC + 1.2078AD + 0.79794BC + 3.7513BD − 3.9001CD − 21.147A2 −
16.705B2 − 4.8185C2 − 1.5980D2 (R2 = 0.9239)
2.7738AC + 1.9213AD + 0.77775BC + 5.0723BD − 3.9554CD − 25.545A2 −
19.826B2 − 2.4962C2 − 6.2847D2 (R2 = 0.9108)
5.21419AB + 2.79081AC + 1.93831AD + 0.52206BC + 4.91131BD − 3.83681CD
− 21.93324 A2 − 18.87824B2 − 4.70274C2 − 6.13174D2 (R2 = 0.9177)
3.4. Method Performance and Application
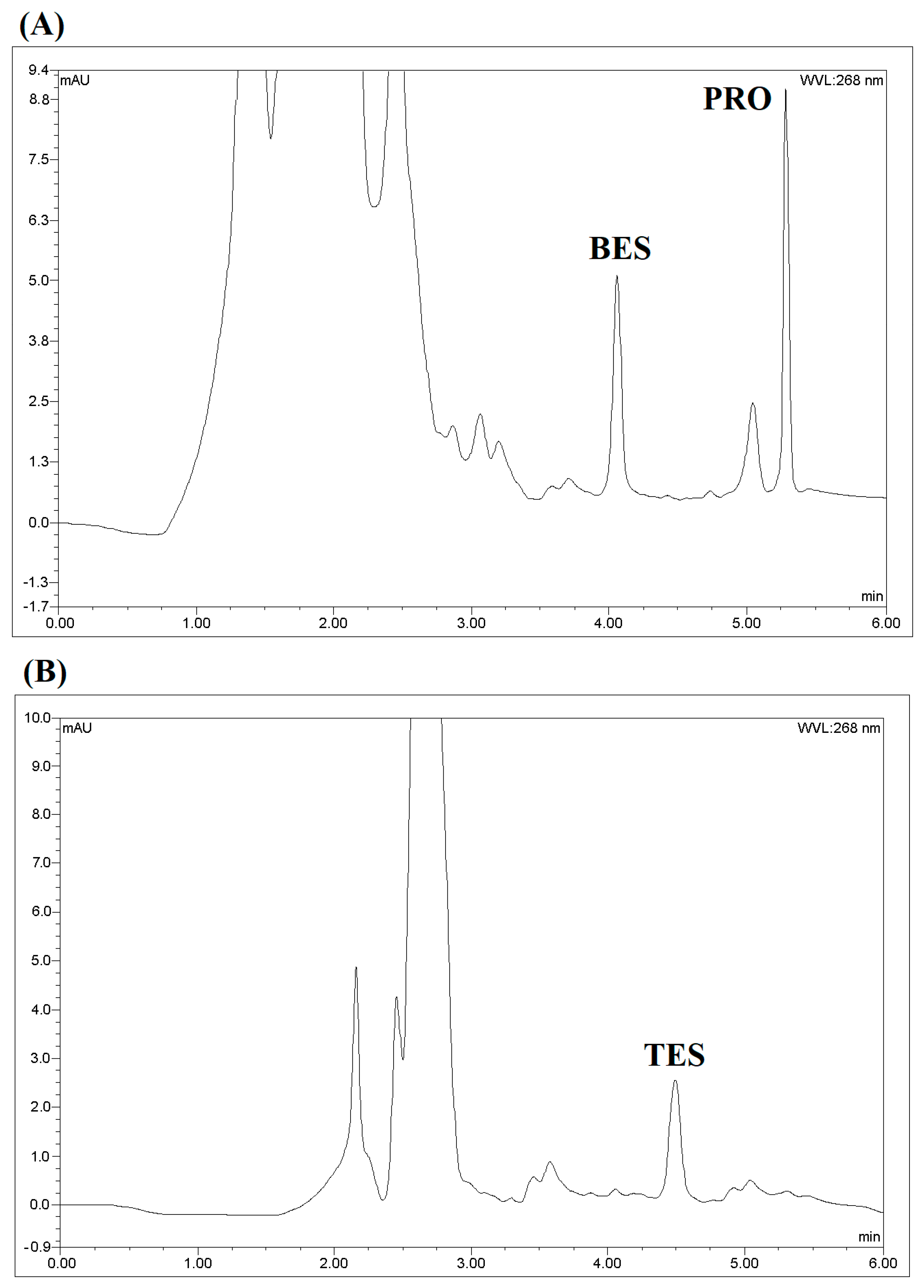
3.5. Method Comparison
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walker, W.H.; Cooke, P.S. Functions of steroid hormones in the male reproductive tract as revealed by mouse models. Int. J. Mol. Sci. 2023, 24, 2748. [Google Scholar] [CrossRef] [PubMed]
- McManus, J.M.; Bohn, K.; Alyamani, M.; Chung, Y.-M.; Klein, E.A.; Sharifi, N. Rapid and structure-specific cellular uptake of selected steroids. PLoS ONE 2019, 14, e0224081. [Google Scholar] [CrossRef] [PubMed]
- Zubeldia-Brenner, L.; Roselli, C.E.; Recabarren, S.E.; Gonzalez Deniselle, M.C.; Lara, H.E. Developmental and functional effects of steroid hormones on neuroendocrine axis and spinal cord. J. Neuroendocrinol. 2016, 28, 7. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Bourguignon, J.-P.; Giudice, L.C.; Hauser, R.; Prins, C.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An endocrine society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef]
- Ojoghoro, J.O.; Scrimshaw, M.D.; Sumpter, J.P. Steroid hormones in the aquatic environment. Sci. Total Environ. 2021, 792, 148306. [Google Scholar] [CrossRef] [PubMed]
- Luque-Córdoba, D.; Priego-Capote, F. Fully automated method for quantitative determination of steroids in serum: An approach to evaluate steroidogenesis. Talanta 2021, 224, 121923. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Fent, K. Determination of two progestin metabolites (17α-hydroxypregnanolone and pregnanediol) and different classes of steroids (androgens, estrogens, corticosteroids, progestins) in rivers and wastewaters by high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). Sci. Total Environ. 2018, 610–611, 1164–1172. [Google Scholar]
- Liu, W.; Yuan, D.; Han, M.; Huang, J.; Xie, Y. Development and validation of a sensitive LC-MS/MS method for simultaneous quantification of thirteen steroid hormones in human serum and its application to the study of type 2 diabetes mellitus. J. Pharma. Biomed. Anal. 2021, 199, 114059. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cai, Z. Determination of hormones in human urine by ultra-high performance liquid chromatography/triple-quadrupole mass spectrometry. Rapid Commun. Mass Spectrom. 2019, 34, e8583. [Google Scholar] [CrossRef]
- McDonald, J.G.; Matthew, S.; Auchus, R.J. Steroid profiling by gas chromatography-mass spectrometry and high performance liquid chromatography-mass spectrometry for adrenal diseases. Horm. Ther. Cancer 2011, 2, 324–332. [Google Scholar] [CrossRef]
- Krone, N.; Hughes, B.A.; Lavery, G.G.; Stewart, P.M.; Arlt, W.; Shackleton, C.H. Gas chromatography/mass spectrometry (GC/MS) remains a pre-eminent discovery tool in clinical steroid investigations even in the era of fast liquid chromatography tandem mass spectrometry (LC/MS/MS). J. Steroid Biochem. Mol. Biol. 2010, 121, 496–504. [Google Scholar] [CrossRef]
- Appa, R.; Mhaisalker, V.A.; Bafana, A.; Devi, S.S.; Krishnamurthi, K.K.; Chakrabarti, T.; Naoghare, P. Simultaneous quantitative monitoring of four indicator contaminants of emerging concern (CEC) in different water sources of central India using, SPE/LC-(ESI)MS-MS. Environ. Monit. Assess 2018, 190, 489. [Google Scholar] [CrossRef]
- Luque-Córdoba, D.; López-Bascón, M.A.; Priego-Capote, F. Development of a quantitative method for determination of steroids in human plasma by gas chromatography-negative chemical ionization-tandem mass spectrometry. Talanta 2020, 220, 121415. [Google Scholar] [CrossRef]
- Li, Z.-M.; Kannan, K. Determination of 19 steroid hormones in human serum and urine using liquid chromatography-tandem mass spectrometry. Toxics 2022, 10, 687. [Google Scholar] [CrossRef]
- Temerdashev, A.; Nesterenko, P.; Dmitrieva, E.; Zhurkina, K.; Feng, Y.-Q. GC-MS/MS determination of steroid hormones in urine using solid-phase derivatization as an alternative to conventional methods. Molecules 2022, 27, 5796. [Google Scholar] [CrossRef]
- Lee, J.; Lee, H.K.; Rasmussen, K.E.; Pedersen-Bjergaard, S. Environmental and bioanalytical application of hollow fiber membrane liquid-phase microextraction: A review. Anal. Chim. Acta 2008, 624, 254–268. [Google Scholar] [CrossRef]
- Jiang, X.; Lee, H.K. Solvent bar microextraction. Anal. Chem. 2004, 76, 5591–5596. [Google Scholar] [CrossRef]
- Prosen, H. Application of hollow-fiber and related microextraction techniques for the determination of pesticides in environmental and food samples-a mini review. Separation 2019, 6, 57. [Google Scholar] [CrossRef]
- Van Osh, D.J.; Dietz, C.H.; Warrag, S.E.; Kroon, M.C. The curious case of hydrophobic deep eutectic solvents: A story on the discovery, design and application. ACS Sustain. Chem. Eng. 2020, 8, 10591–10612. [Google Scholar] [CrossRef]
- Florindo, C.; Romero, L.; Rintoul, I.; Branco, L.; Marrucho, I.M. From phase change materials to green solvent: Hydrophobic low viscous fatty acid-based deep eutectic solvents. ACS Sustain. Chem. Eng. 2018, 6, 3888–3895. [Google Scholar] [CrossRef]
- Hansen, B.; Horton, A.; Chen, B.; Poe, D.; Zhang, Y.; Spittle, S.; Klein, J.; Adhikari, L.; Zelovich, T.; Doherty, B.; et al. Deep eutectic solvents: A review of fundamentals and applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Naik, P.K.; Kundu, D.; Bairagva, P.; Banerjee, T. Phase behavior of water-menthol based deep eutectic solvent-dodecane system. Chem. Therm. Therm. Anal. 2021, 3–4, 100011. [Google Scholar] [CrossRef]
- Křížek, T.; Bursová, M.; Horsley, R.; Kuchař, M.; Tůma, P. Menthol-based hydrophobic deep eutectic solvents: Towards greener and efficient extraction of phytocannabinoids. J. Clean. Prod. 2018, 193, 391–396. [Google Scholar] [CrossRef]
- Fan, T.; Yan, Z.; Yan, C.; Qiu, S.; Peng, X.; Zhang, J.; Hu, L.; Chen, L. Preparation of menthol-based hydrophobic deep eutectic solvents for the extraction of triphenylmethane dyes: Quantitative properties and extraction mechanism. Analyst 2012, 146, 1996–2008. [Google Scholar] [CrossRef]
- Verma, R.; Banerjee, T. Liquid-liquid extraction of lower alcohols using menthol-based hydrophobic deep eutectic solvent: Experiments and COSMO-SAC prediction. Ind. Eng. Chem. Res. 2018, 57, 3371–3381. [Google Scholar] [CrossRef]
- Silva, J.; Pereira, C.; Mano, F.; Silva, E.; Castro, V.; Sá-Nogueira, I.; Reis, R.; Paiva, A.; Matias, A.; Duarte, A. Therapeutic role of deep eutectic solvents based on menthol and saturated fatty acids on wound healing. ACS Appl. Bio Mater. 2019, 2, 4346–4355. [Google Scholar] [CrossRef]
- Gonzalo-Lumbreras, R.; Pimentel-Trapero, D.; Izquierdo-Hornillos, R. Development and method validation for testosterone and epitestosterone in human urine samples by liquid chromatography applications. J. Chromatogr. Sci. 2003, 41, 261–266. [Google Scholar] [CrossRef]
- Naldi, A.; Fayad, P.; Prévost, M.; Sauvé, S. Analysis of steroid hormones and their conjugated forms in water and urine by on-line solid-phase extraction coupled to liquid chromatography tandem mass spectrometry. Chem. Cent. J. 2016, 10, 349–360. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V. Method for the determination of limit of detection and limit of quantification of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Parrish, D.; Zhurova, E.; Kirschbaum, K.; Pinkerton, A. Experimental charge density study of estrogens: 17β-estradiol urea. J. Phys. Chem. B. 2006, 110, 26442–26447. [Google Scholar] [CrossRef]
- Roberts, P.; Pettersen, R.; Sheldrick, G.; Isaacs, N.; Kennard, O. Crystal and molecular structure of 17β-hydroxyandrost-4-en-3-one (testosterone). J. Chem. Soc. Perkin Trans. 1999, 2, 2655–2657. [Google Scholar] [CrossRef]
- Lancaster, R.; Karamertzanis, P.; Hulme, A.; Tocher, D.; Lewis, T.; Price, S. The polymorphism of progesterone: Stabilization of a ‘disappearing’ polymorph by co-crystallization. J. Pharm. Sci. 2007, 96, 3419–3431. [Google Scholar] [CrossRef]
- Corvis, Y.; Négrier, P.; Massip, S.; Leger, J.-M.; Espeau, P. Insights into the crystal structure, polymorphism and thermal behavior of menthol optical isomers and racemates. CrystEngComm 2012, 14, 7055–7064. [Google Scholar] [CrossRef]
- Lipkowski, J.; Komarov, V.; Radionova, T.; Aladko, L. X-ray investigation of compounds crystallized in aqueous solution of tetrabutylammonium laurate. The structure of (C4H9)4N(C11H23COO)3C11H23COOH.4H2O. J. Struct. Chem. 2005, 46, S51–S57. [Google Scholar] [CrossRef]
- AL-Hashimi, N.; AL-Degs, Y.; Al Momany, E.; El-Sheikh, A.; Alqudah, A.; Oqal, M.; Abdelghani, J. Solvent bar microextraction combined with HPLC-DAD and multivariate optimization for simultaneous determination of three antiarrhythmic drugs in human urine and plasma samples. Talanta Open 2022, 6, 100140. [Google Scholar] [CrossRef]
- Neale, P.A.; Escher, B.I.; Schäfer, A.I. PH depesndence of steroid hormone-organic matter interactions at environmental concentrations. Sci. Total Environ. 2009, 407, 1164–1173. [Google Scholar] [CrossRef]
- Selahle, S.; Nqombolo, A.; Nomngongo, P. From polyethylene waste bottles to UIO-66 (Zr) for preconcentration of steroid hormones from river water. Sci. Rep. 2023, 13, 6808. [Google Scholar] [CrossRef]
- Zhao, L.; Lee, H. Liquid-phase microextraction combined with hollow fiber as a sample preparation technique prior to gas chromatography/mass spectrometry. Anal. Chem. 2002, 74, 2486–2492. [Google Scholar] [CrossRef]
- Suslick, K.S. Sonochemistry. Science 1990, 247, 1439–1445. [Google Scholar] [CrossRef]
- Sampaio, N.; Castilhos, N.; Silva, B.; Riegel-Vidotti, I.; Silva, B. Evaluation of polyvinyl alcohol/pectin-based hydrogel disks as extraction phase for determination of steroidal hormones in aqueous sample by GC-MS/MS. Molecules 2019, 24, 40. [Google Scholar] [CrossRef]
- Lia, K.; Mei, M.; Li, H.; Huang, X.; Wu, C. Multiple monolithic fiber solid-phase microextraction based on a polymeric ionic liquid with high-performance liquid chromatography for the determination of steroid sex hormones in water and urine. J. Sep. Sci. 2016, 39, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Czarny, K.; Szczukocki, D.; Krawczyk, B.; Juszczak, R.; Skrzypek, S.; Gadzała-Kopciuch, R. Molecularly imprinted polymer film grafted from porous silica for efficient enrichment of steroid hormones in water samples. J. Sep. Sci. 2019, 42, 2858–2866. [Google Scholar] [CrossRef] [PubMed]
- Ričanyová, J.; Gadzała-Kopciuch, R.; Reiffova, K.; Bazel, Y.; Buszewski, B. Molecularly imprinted adsorbents for preconcentration and isolation of progesterone and testosterone by solid phase extraction combined with HPLC. Adsorption 2010, 16, 473–483. [Google Scholar] [CrossRef]
- Studzińska, S.; Buszewski, B. Fast method for the resolution and determination of sex steroids in urine. J. Chromatogr. B 2013, 927, 158–163. [Google Scholar] [CrossRef] [PubMed]




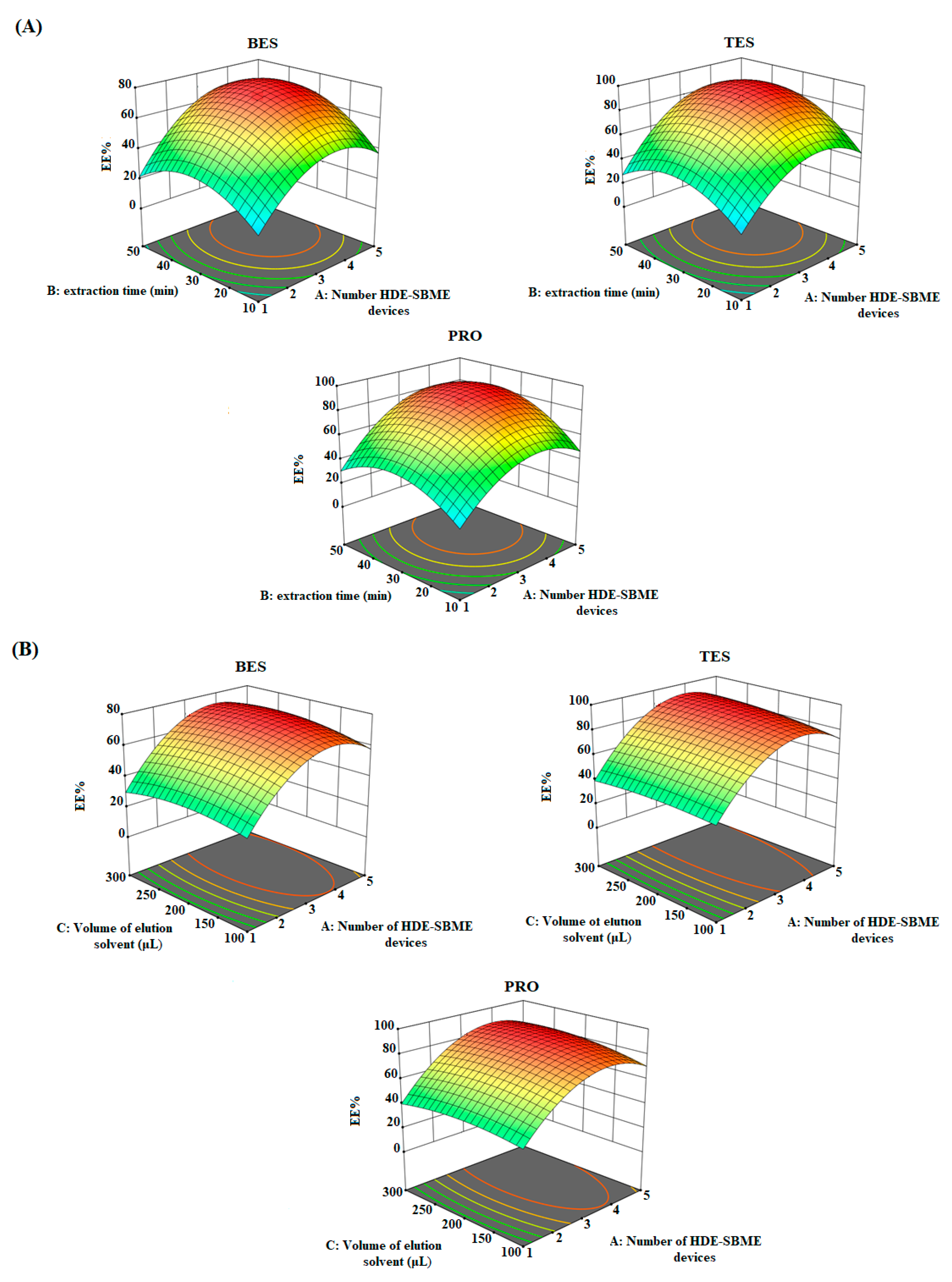
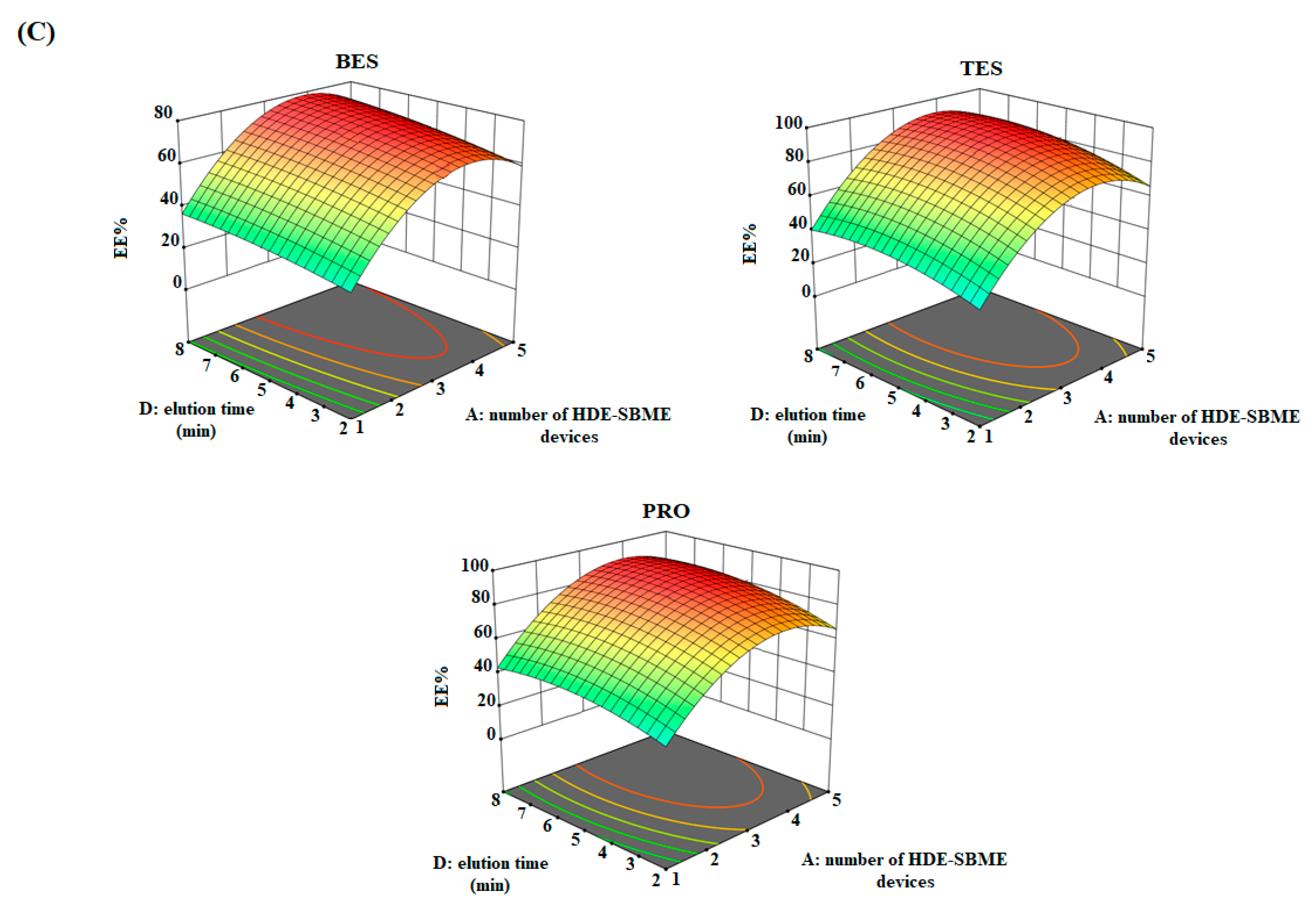
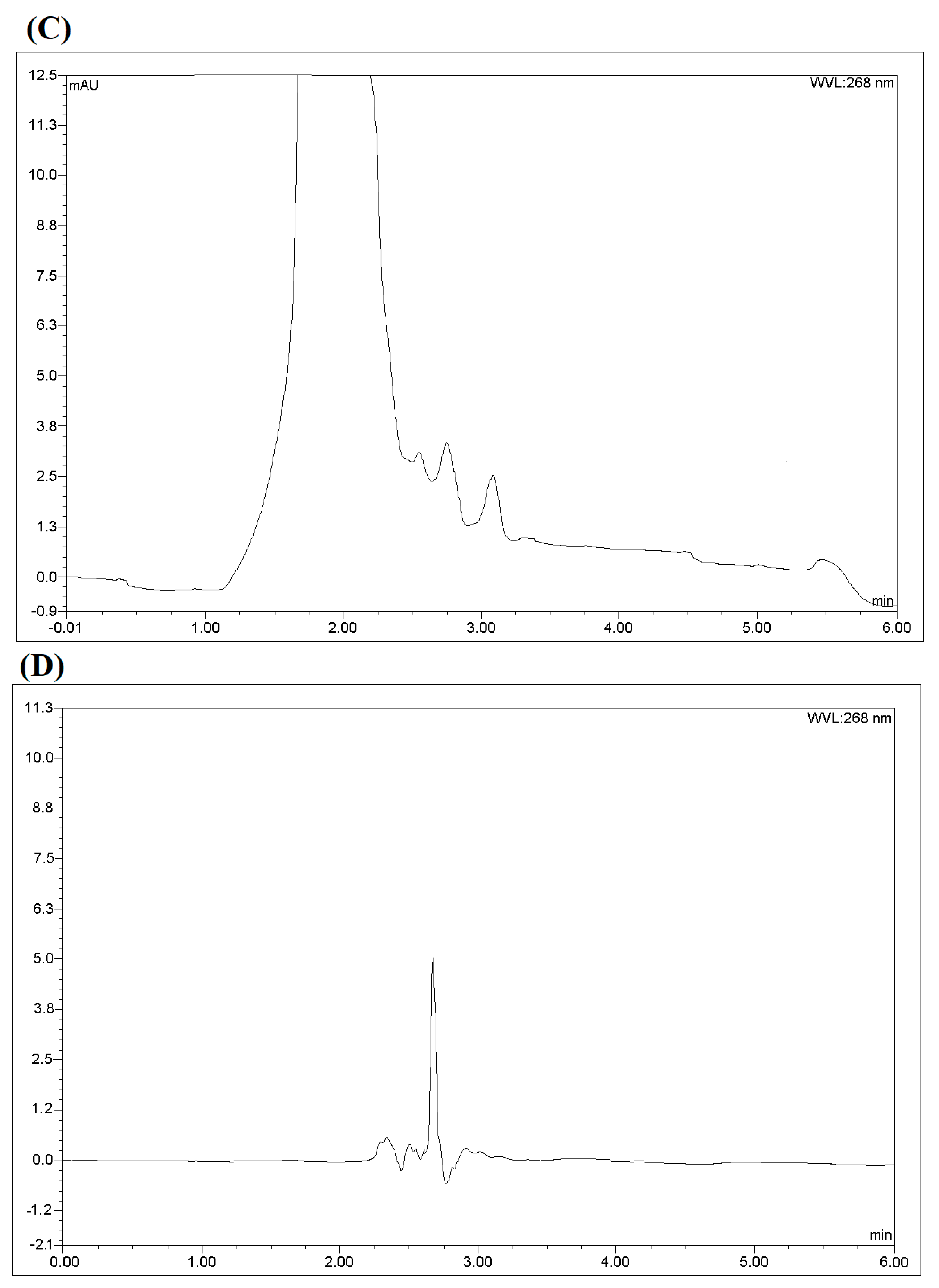
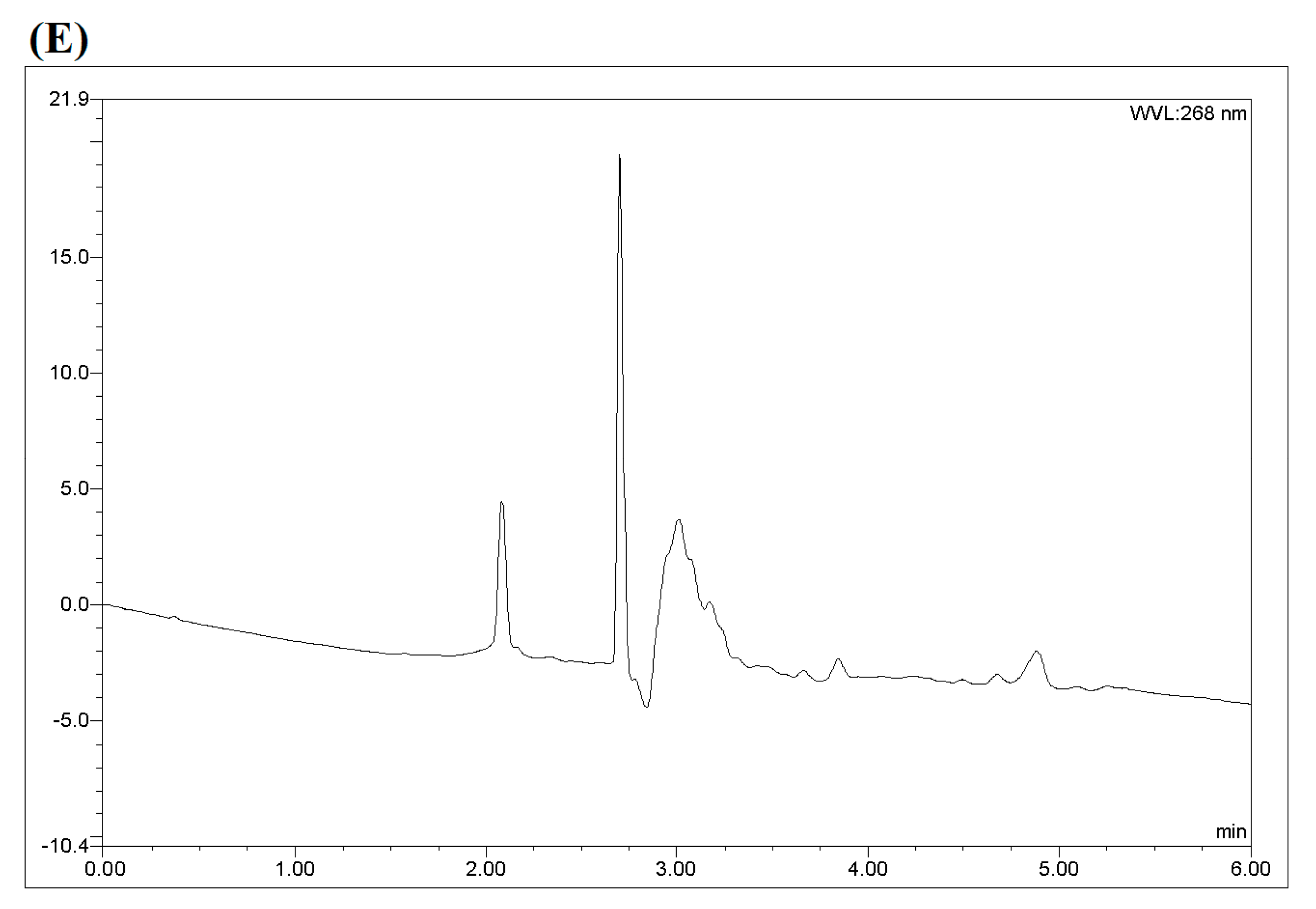
| Test | Influence Factors | Analyte Response (EE%) | |||||
|---|---|---|---|---|---|---|---|
| A: Number of HDE-SBME Devices | B: Extraction Time (min) | C: Volume of Elution (μL) | D: Elution Time (min) | BES | TES | PRO | |
| 1 | 5 | 10 | 300 | 2 | 27.33 | 32.50 | 32.51 |
| 2 | 1 | 10 | 300 | 2 | 17.11 | 19.18 | 22.35 |
| 3 | 3 | 30 | 200 | 8 | 70.21 | 82.92 | 81.36 |
| 4 | 3 | 50 | 200 | 5 | 70.63 | 86.65 | 83.21 |
| 5 | 1 | 10 | 100 | 8 | 11.28 | 13.41 | 14.23 |
| 6 | 3 | 30 | 200 | 5 | 73.26 | 90.39 | 87.92 |
| 7 | 5 | 50 | 100 | 8 | 61.89 | 75.45 | 71.70 |
| 8 | 3 | 30 | 200 | 5 | 73.26 | 90.39 | 87.92 |
| 9 | 3 | 30 | 200 | 5 | 73.26 | 90.39 | 87.92 |
| 10 | 5 | 10 | 300 | 8 | 32.43 | 39.44 | 40.01 |
| 11 | 1 | 50 | 300 | 8 | 13.42 | 16.02 | 17.01 |
| 12 | 5 | 50 | 300 | 2 | 59.42 | 67.45 | 65.67 |
| 13 | 5 | 50 | 300 | 8 | 67.11 | 82.63 | 81.47 |
| 14 | 3 | 30 | 200 | 5 | 73.26 | 90.39 | 87.92 |
| 15 | 1 | 50 | 100 | 8 | 32.43 | 39.21 | 40.23 |
| 16 | 1 | 10 | 300 | 8 | 9.67 | 11.44 | 12.83 |
| 17 | 3 | 30 | 200 | 2 | 68.38 | 73.41 | 70.73 |
| 18 | 1 | 50 | 300 | 2 | 8.77 | 10.52 | 11.74 |
| 19 | 3 | 30 | 300 | 5 | 70.26 | 88.45 | 83.25 |
| 20 | 1 | 50 | 100 | 2 | 6.11 | 8.47 | 9.35 |
| 21 | 3 | 10 | 200 | 5 | 37.74 | 42.59 | 43.38 |
| 22 | 5 | 30 | 200 | 5 | 72.26 | 88.63 | 85.65 |
| 23 | 1 | 10 | 100 | 2 | 3.26 | 5.74 | 6.623 |
| 24 | 5 | 10 | 100 | 8 | 35.66 | 40.59 | 40.03 |
| 25 | 5 | 10 | 100 | 2 | 30.14 | 36.51 | 35.16 |
| 26 | 3 | 30 | 200 | 5 | 73.26 | 90.39 | 87.92 |
| 27 | 5 | 50 | 100 | 2 | 29.33 | 34.76 | 34.61 |
| 28 | 3 | 30 | 100 | 5 | 61.89 | 75.45 | 71.70 |
| 29 | 1 | 30 | 200 | 5 | 27.23 | 29.18 | 34.84 |
| HNDE Ratio of Menthol–Lauric Acid | Peak Area | ||
|---|---|---|---|
| BES | TES | PRO | |
| 2:1 | 1.081 | 5.309 | 3.864 |
| 3:1 | 1.757 | 4.467 | 5.236 |
| 4:1 | 1.760 | 4.608 | 5.523 |
| 5:1 | 1.741 | 4.184 | 5.308 |
| BES | TES | PRO | |
|---|---|---|---|
| Binding Mode 1 | −11.5 | −13.9 | −13.3 |
| Binding Mode 2 | −11.4 | −15.8 | −15.8 |
| Parameters | Water | Urine | ||||
|---|---|---|---|---|---|---|
| BES | TES | PRO | BES | TES | PRO | |
| Calibration curve | PA = 0.5917x − 0.1226 | PA = 1.2458x + 0.0538 | PA = 1.7164x − 0.3684 | PA = 0.5779x − 0.1481 | PA = 1.2149x + 0.0359 | PA = 1.6629x − 0.3086 |
| Correlation coefficient (R2) | 0.999 | 0.997 | 0.998 | 0.995 | 0.996 | 0.994 |
| Standard deviation, n = 3 | 1.07–3.44 | 2.13–3.42 | 1.54–4.08 | 1.52–3.81 | 2.76–3.90 | 1.82–5.13 |
| Inter precision (RSD%, n = 5) | 2.71–3.04 | 1.90–3.67 | 1.43–4.21 | 3.51–4.32 | 2.22–4.46 | 2.13–5.09 |
| Intra precision (RSD%, n = 5) | 2.43–5.32 | 2.71–5.83 | 1.98–5.11 | 2.71–7.11 | 3.06–6.21 | 2.78–6.53 |
| LOD (µg L−1) | 0.269 | 0.337 | 0.171 | 0.407 | 0.401 | 0.278 |
| LOQ (µg L−1) | 0.899 | 1.124 | 0.573 | 1.357 | 1.337 | 0.929 |
| Linear range (µg L−1) | 0.899–104 | 1.124–104 | 0.573–104 | 1.357–104 | 1.337–104 | 0.929–104 |
| Matrix | Level | Spiked Concentration (μg L−1, n = 3) | Steroids | Mean Extracted Concentration (μg L−1, n = 3) | Accuracy RE % | Recovery % |
|---|---|---|---|---|---|---|
| Water | 1 | 10 | BES | 698.2 | −4.68 | 95.32 |
| TES | 880.3 | −2.58 | 97.41 | |||
| PRO | 852.9 | −2.98 | 97.01 | |||
| 2 | 10 | BES | 704.3 | −3.84 | 96.16 | |
| TES | 890.9 | −1.43 | 98.58 | |||
| PRO | 862.2 | −1.93 | 98.06 | |||
| 3 | 10 | BES | 687.8 | −6.09 | 93.91 | |
| TES | 876.6 | −2.99 | 97.00 | |||
| PRO | 839.0 | −4.57 | 95.43 | |||
| Steroids-free urine | 1 | 10 | BES | 675.6 | −7.75 | 92.24 |
| TES | 852.3 | −5.68 | 94.31 | |||
| PRO | 814.2 | −7.38 | 92.61 | |||
| 2 | 10 | BES | 688.8 | −5.95 | 94.04 | |
| TES | 862.4 | −4.57 | 95.42 | |||
| PRO | 828.7 | −5.73 | 94.26 | |||
| 3 | 10 | BES | 673.1 | −8.09 | 91.90 | |
| TES | 845.1 | −6.48 | 93.51 | |||
| PRO | 806.2 | −8.29 | 91.70 |
| Method | Matrix | Steroid | Linear Range (μg L−1) | LOD (μg L−1) | Recovery (%) | Refs. |
|---|---|---|---|---|---|---|
| HDE-SBME-HPLC-DAD | Water | BES | 0.89–104 | 0.269 | 93.9–96.1 | This work |
| TES | 1.12–104 | 0.337 | 97.0–98.5 | |||
| PRO | 0.57–104 | 0.171 | 95.4–98.0 | |||
| Urine | BES | 1.357–104 | 0.407 | 91.9–94 | ||
| TES | 1.337–104 | 0.401 | 93.5–95.4 | |||
| PRO | 0.929–104 | 0.278 | 91.7–94.2 | |||
| a PVOHD-GC-MS/MS | Water | BES | 0.5 | 0.5–100 | - | [40] |
| BES | 1 | 1–100 | - | |||
| PRO | 1 | 1–100 | - | |||
| b AMED-MMF-SPME-HPLC-DAD | Water and urine | BES | 0.10–200 | 0.027 | 78.1–115 | [41] |
| TES | 0.50–200 | 0.085 | 77.3–113 | |||
| PRO | 0.50–200 | 0.12 | 77.0–116 | |||
| c MISPE-HPLC-DAD | Water | BES | 5 × 103−105 | 39.6–76.1 | >90 | [42] |
| TES | 5 × 103−105 | 24.0–89.8 | >90 | |||
| PRO | 5 × 103−105 | 59.4–77.7 | >90 | |||
| d MISPE-LC-DAD | Urine | BES | 0.05–0.5 | 0.003 | 97–107 | [43] |
| TES | 0.005–0.5 | 0.002 | 93–107 | |||
| PRO | 0.025–0.75 | 0.008 | 87–107 | |||
| e UHPLC-DAD | Urine | BES | 0.116–10.447 | 0.034 | - | [44] |
| TES | 0.089–4.456 | 0.026 | - | |||
| PRO | 0.102–9.029 | 0.030 | - | |||
| f LLE-UHPLC-MS/MS | Urine | BES | 0.2–200 | 0.40 | 103 | [9] |
| TES | 0.2–200 | 0.20 | 109 | |||
| PRO | 0.2–200 | 0.33 | 94 | |||
| g LLE-LC-MS/MS | Serum and urine | BES | 0.04 | 0.14–200 | 93.3–120 | [14] |
| TES | 0.14 | 0.46–200 | 113–119 | |||
| PRO | 0.15 | 0.5–200 | 117–141 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AL-Hashimi, N.N.; Abed Alfattah, H.; El-Barghouthi, M.I.; El-Sheikh, A.H.; Ale-nezi, H.M.; Sunjuk, M.S.; Fahelelbom, K.M. Solvent Bar Microextraction Method Based on a Natural Deep Eutectic Solvent and Multivariate Optimization for Determination of Steroid Hormones in Urine and Water. Appl. Sci. 2024, 14, 4438. https://doi.org/10.3390/app14114438
AL-Hashimi NN, Abed Alfattah H, El-Barghouthi MI, El-Sheikh AH, Ale-nezi HM, Sunjuk MS, Fahelelbom KM. Solvent Bar Microextraction Method Based on a Natural Deep Eutectic Solvent and Multivariate Optimization for Determination of Steroid Hormones in Urine and Water. Applied Sciences. 2024; 14(11):4438. https://doi.org/10.3390/app14114438
Chicago/Turabian StyleAL-Hashimi, Nabil N., Husam Abed Alfattah, Musa I. El-Barghouthi, Amjad H. El-Sheikh, Hanan M. Ale-nezi, Mahmoud S. Sunjuk, and Khairi M. Fahelelbom. 2024. "Solvent Bar Microextraction Method Based on a Natural Deep Eutectic Solvent and Multivariate Optimization for Determination of Steroid Hormones in Urine and Water" Applied Sciences 14, no. 11: 4438. https://doi.org/10.3390/app14114438
APA StyleAL-Hashimi, N. N., Abed Alfattah, H., El-Barghouthi, M. I., El-Sheikh, A. H., Ale-nezi, H. M., Sunjuk, M. S., & Fahelelbom, K. M. (2024). Solvent Bar Microextraction Method Based on a Natural Deep Eutectic Solvent and Multivariate Optimization for Determination of Steroid Hormones in Urine and Water. Applied Sciences, 14(11), 4438. https://doi.org/10.3390/app14114438







