Can Hediste diversicolor Speed Up the Breakdown of Cigarette Butts in Marine Sediments?
Abstract
1. Introduction
2. Material and Methods
2.1. Study Area and Sample Collection
2.2. Experimental Design
2.3. Sediment Contamination
2.4. Biological Parameters
2.5. Physical Degradation of CBs
2.6. Cigarette Filter Characterization and Degradation Tracking
2.7. Statistical Analysis
3. Results
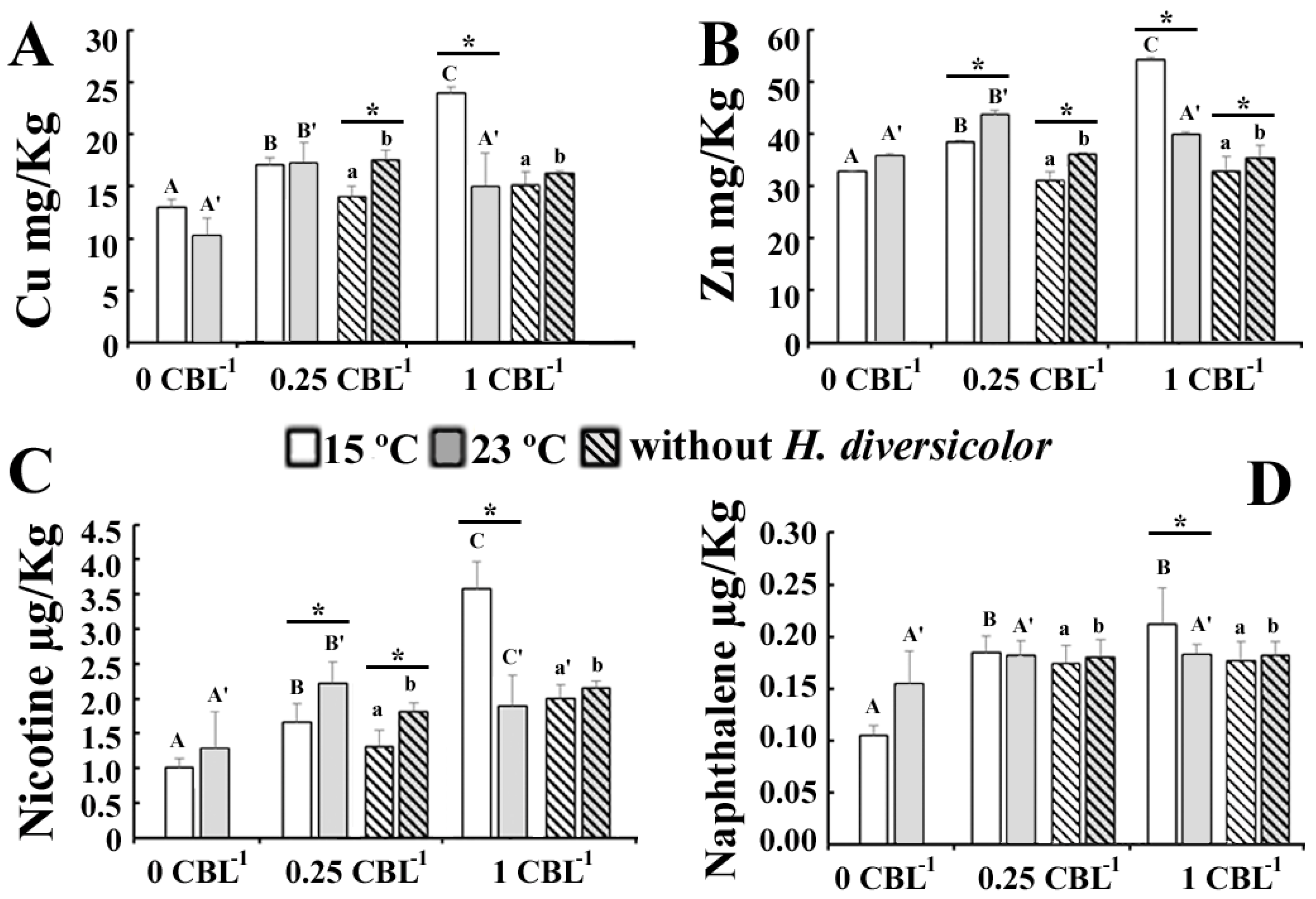
3.1. Physicochemical Parameters
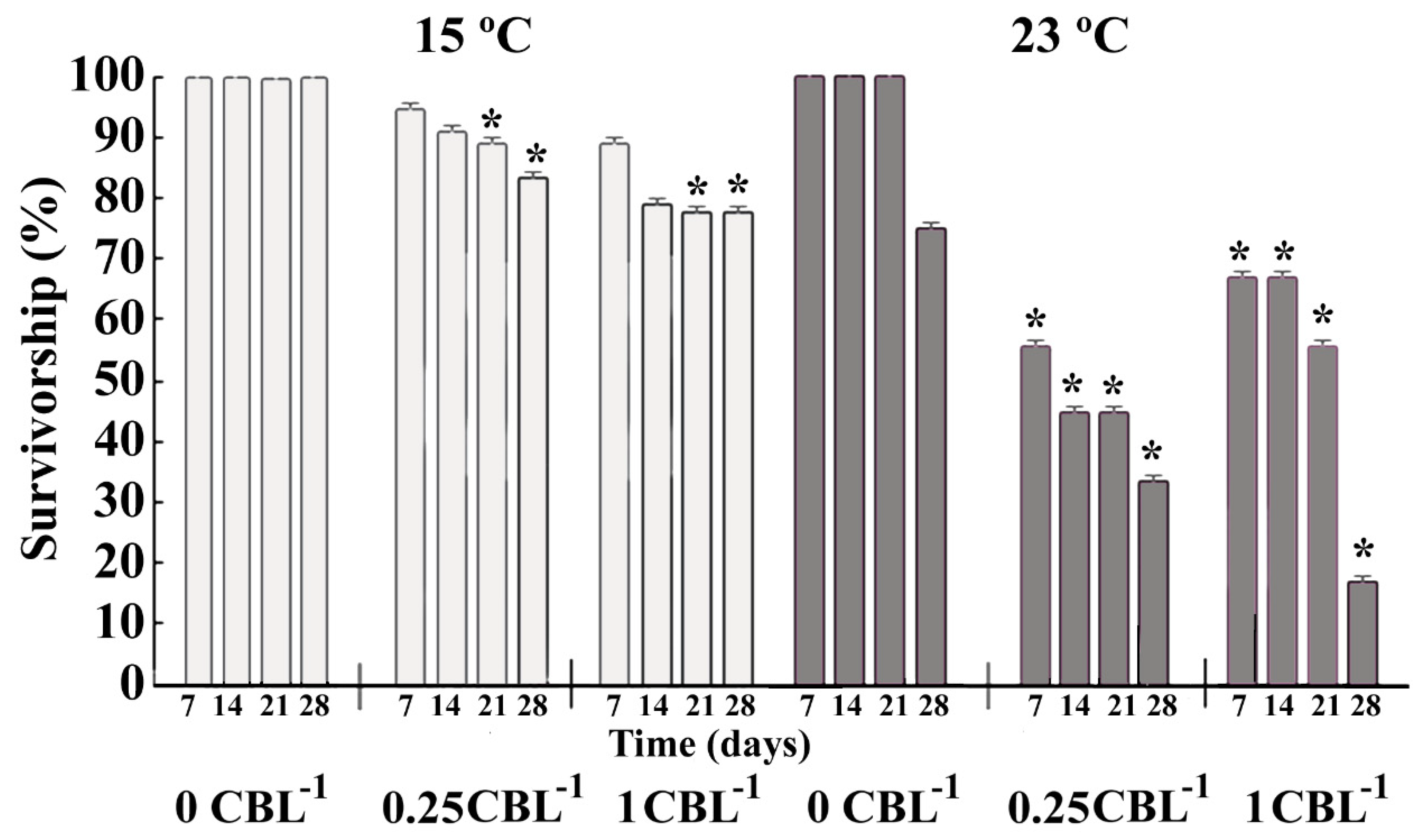
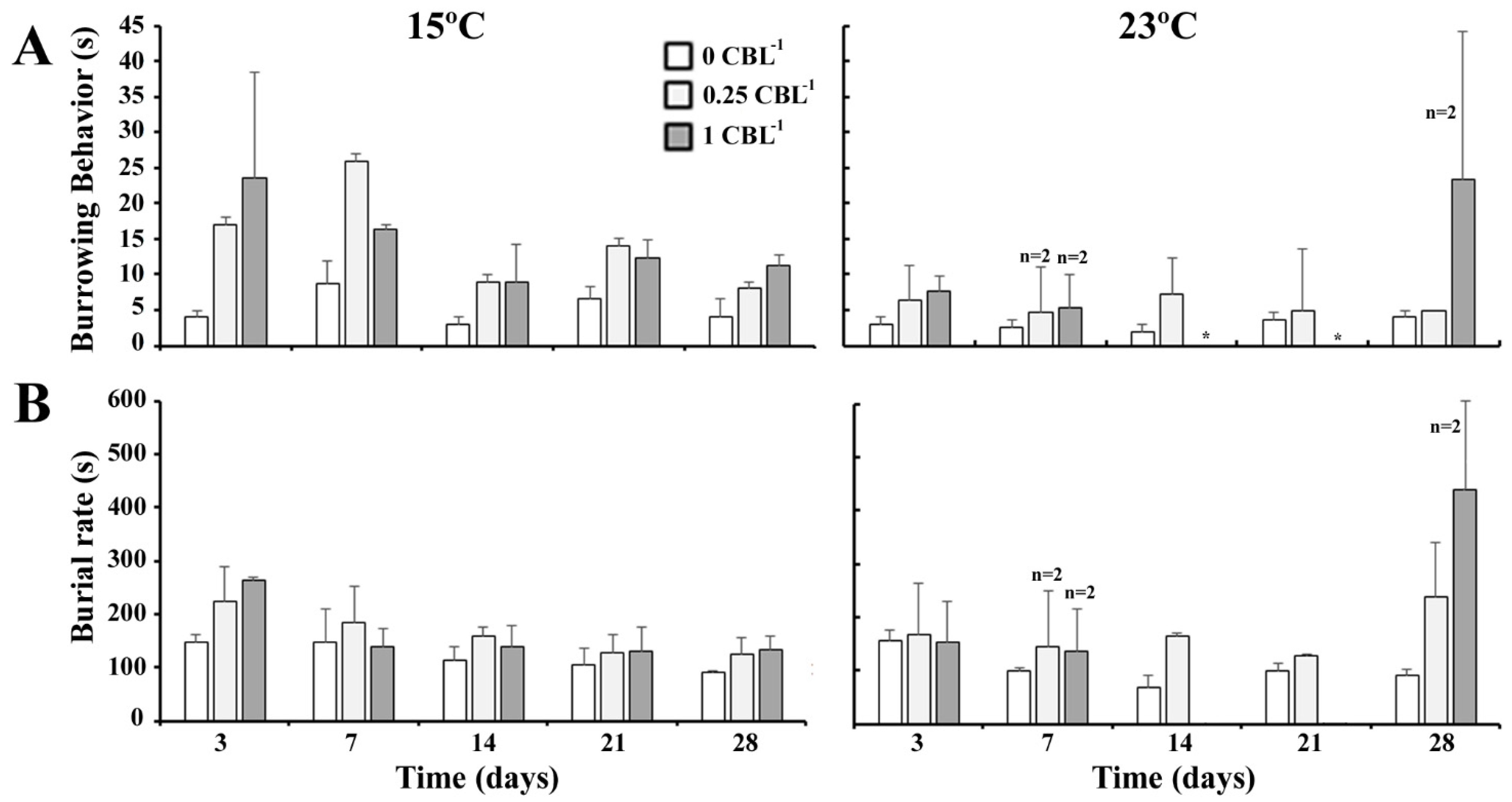
3.2. Biological Parameters
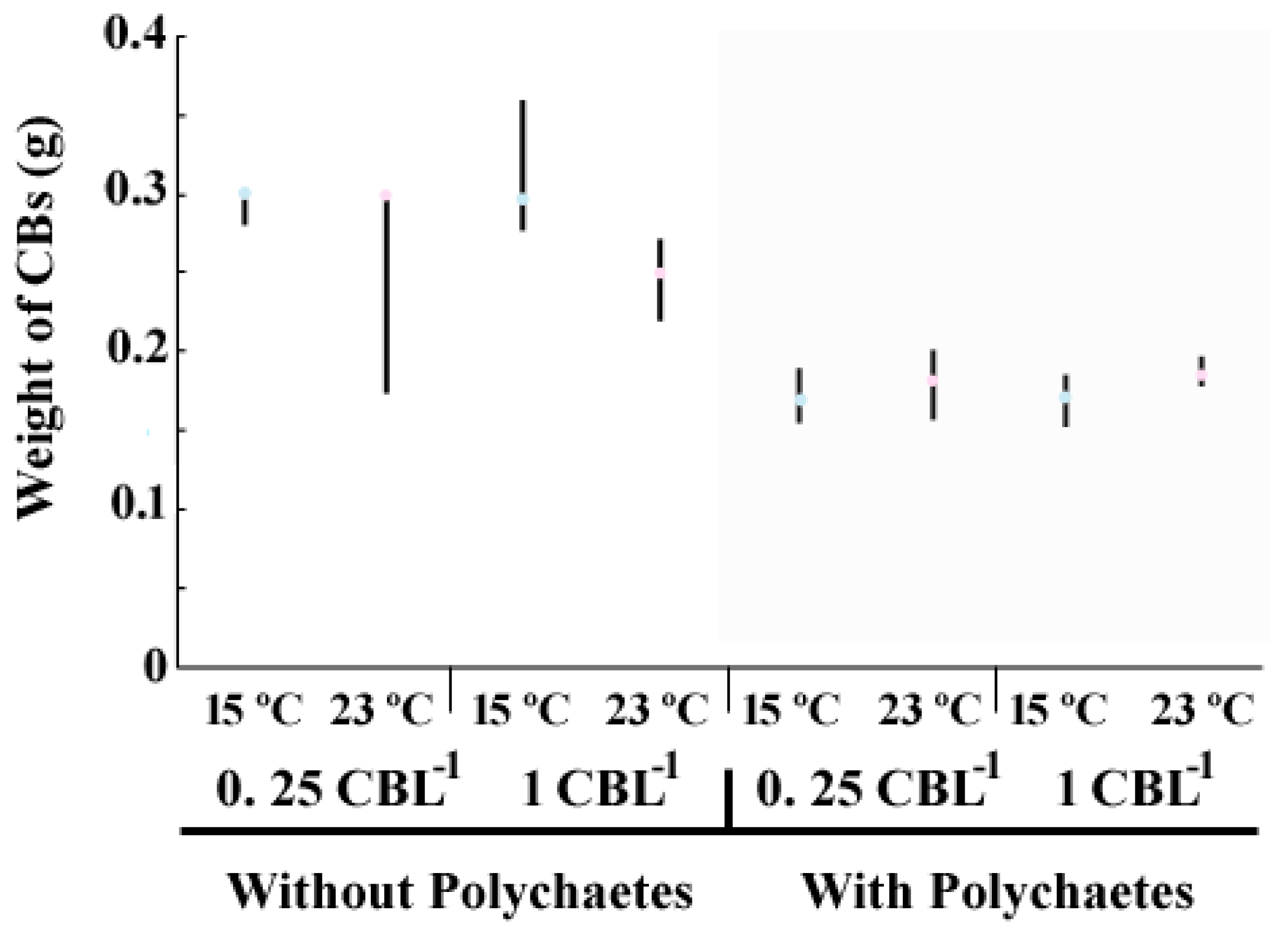
3.3. Physical Degradation of the CBs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Marinello, S.; Lolli, F.; Gamberini, R.; Rimini, B. A second life for cigarette butts? A review of recycling solutions. J. Hazard. Mater. 2020, 384, 121245. [Google Scholar] [CrossRef] [PubMed]
- Blickley, L.C.; Currie, J.J.; Kaufman, G.D. Trends and drivers of debris accumulation on maui shorelines: Implications for local mitigation strategies. Mar. Pollut. Bull. 2016, 105, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Ruz, V.; Honorato-Zimmer, D.; Gatta-Rosemary, M.; Nuñez, P.; Hinojosa, I.A.; Thiel, M. Spatio-temporal variation of anthropogenic marine debris on Chilean beaches. Mar. Pollut. Bull. 2018, 126, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Ocean Conservancy. Pandemic Pollution: The Rising Tide of Plastic PPE. 2021. Available online: https://oceanconservancy.org/wp-content/uploads/2021/03/FINAL-Ocean-Conservancy-PPE-Report-March-2021.pdf (accessed on 10 January 2024).
- Soares, J.; Miguel, I.; Venâncio, C.; Lopes, I.; Oliveira, M. Public views on plastic pollution: Knowledge, perceived impacts, and pro-environmental behaviours. J. Hazard. Mater. 2021, 412, 125227. [Google Scholar] [CrossRef] [PubMed]
- Ballance, A.; Ryan, P.G.; Turpie, J.K. How much is a clean beach worth? The impact of litter on beach users in the Cape Peninsula, South Africa. S. Afr. J. Sci. 2000, 96, 210–230. [Google Scholar]
- Krelling, A.P.; Williams, A.T.; Turra, A. Differences in perception and reaction of tourist groups to beach marine debris that can influence a loss of tourism revenue in coastal areas. Mar. Policy 2017, 85, 87–99. [Google Scholar] [CrossRef]
- Ogi, H.; Fukumoto, Y. A sorting method for small plastic debris floating on the sea Surface and stranded on sandy beaches. Bull. Fac. Fish. Hokkaido Univ. 2000, 51, 71–93. [Google Scholar]
- Cruz, C.J.; Muñoz-Perez, J.J.; Carrasco-Braganza, M.I.; Poullet, P.; Lopez-Garcia, P.; Contreras, A.; Silva, R. Beach cleaning costs. Ocean Coast. Manag. 2020, 188, 105118. [Google Scholar] [CrossRef]
- Piccardo, M.; Provenza, F.; Anselmi, S.; Broccoli, A.; Terlizzi, A.; Renzi, M. Use of Sediqualsoft® to determine the toxicity of cigarette butts to marine species: A weather simulation test. J. Mar. Sci. Eng. 2021, 9, 734. [Google Scholar] [CrossRef]
- Loizidou, X.I.; Loizides, M.I.; Orthodoxou, D.L. Persistent marine litter: Small plastics and cigarette butts remain on beaches after organized beach cleanups. Environ. Monit. Assess. 2018, 190, 414. [Google Scholar] [CrossRef]
- Dobaradaran, S.; Schmidt, T.C.; Kaziur-Cegla, W.; Jochmann, M.A. BTEX compounds leachates from cigarette butts into water environment: A primary study. Environ. Pollut. 2021, 269, 116185. [Google Scholar] [CrossRef] [PubMed]
- Belzagui, F.; Buscio, V.; Gutiérrez-Bouzán, C.; Vilaseca, M. Cigarette butts as a microfiber source with a microplastic level of concern. Sci. Total Environ. 2021, 762, 144165. [Google Scholar] [CrossRef] [PubMed]
- Green, D.S.; Kregting, L.; Boots, B. Effects of cigarette butts on marine keystone species (Ulva lactuca L. and Mytilus edulis L.) and sediment microphytobenthos. Mar. Pollut. Bull. 2021, 165, 112152. [Google Scholar] [CrossRef] [PubMed]
- Green, D.S.; Tongue, A.D.; Boots, B. The ecological impacts of discarded cigarette butts. Trends Ecol. Evol. 2022, 37, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Fagiano, V.; Compa, M.; Alomar, C.; Ríos-Fuster, B.; Morato, M.; Capó, X.; Deudero, S. Breaking the paradigm: Marine sediments hold two-fold microplastics than sea surface waters and are dominated by fibers. Sci. Total Environ. 2023, 858, 159722. [Google Scholar] [CrossRef] [PubMed]
- Dobaradaran, S.; Schmidt, T.C.; Lorenzo-Parodi, N.; Jochmann, M.A.; Nabipour, I.; Raeisi, A.; Mahmoodi, M. Cigarette butts: An overlooked source of PAHs in the environment? Environ. Pollut. 2019, 249, 932–939. [Google Scholar] [CrossRef]
- Dobaradaran, S.; Schmidt, T.C.; Lorenzo-Parodi, N.; Kaziur-Cegla, W.; Jochmann, M.A.; Nabipour, I.; Telgheder, U. Polycyclic aromatic hydrocarbons (PAHs) leachates from cigarette butts into water. Environ. Pollut. 2020, 259, 113916. [Google Scholar] [CrossRef] [PubMed]
- Koutela, N.; Fernández, E.; Saru, M.L.; Psillakis, E. A comprehensive study on the leaching of metals from heated tobacco sticks and cigarettes in water and natural waters. Sci. Total Environ. 2020, 714, 136700. [Google Scholar] [CrossRef]
- Soleimani, F.; Dobaradaran, S.; De la Torre, G.E.; Schmidt, T.C.; Saeedi, R. Content of toxic components of cigarette, cigarette smoke vs cigarette butts: A comprehensive systematic review. Sci. Total Environ. 2022, 813, 152667. [Google Scholar] [CrossRef]
- da Silva, N.F.; de Araújo, M.C.B.; Silva-Cavalcanti, J.S. Cigarette butts in the environment: A growing global threat? Environ. Rev. 2023, 31, 229–242. [Google Scholar] [CrossRef]
- Santos-Echeandía, J.; Rivera-Hernández, J.R.; Rodrigues, J.P.; Molto, V. Interaction of mercury with beached plastics with special attention to zonation, degradation status, and polymer type. Mar. Chem. 2020, 222, 103788. [Google Scholar] [CrossRef]
- Yadav, N.; Hakkarainen, M. Degradation of Cellulose Acetate in Simulated Aqueous Environments: One-Year Study. Macromol. Mater. Eng. 2022, 307, 2100951. [Google Scholar] [CrossRef]
- Novotny, T.E.; Lum, K.; Smith, E.; Wang, V.; Barnes, R. Cigarette butts and the case for an environmental policy on hazardous cigarette waste. Int. J. Environ. Res. Public Health 2009, 6, 1691–1705. [Google Scholar] [CrossRef] [PubMed]
- Pauly, J.L.; Stegmeier, S.J.; Mayer, A.G.; Lesses, J.D.; Streck, R.J. Release of carbon granules from cigarettes with charcoal filters. Tob. Control 1997, 6, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Conradi, M.; Sánchez-Moyano, J.E. Toward a sustainable circular economy for cigarette butts, the most common waste worldwide on the coast. Sci. Total Environ. 2022, 847, 157634. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Choi, H.K.; Kwon, J.S.I. LSTM-based control of cellulose degree of polymerization in a batch pulp digester. In Proceedings of the 2023 American Control Conference (ACC), San Diego, CA, USA, 31 May–2 June 2023; pp. 4659–4664. [Google Scholar]
- Ali, S.S.; Elsamahy, T.; Al-Tohamy, R.; Zhu, D.; Mahmoud, Y.A.G.; Koutra, E.; Metwally, M.A.; Kornaros, M.; Sun, J. Plastic wastes biodegradation: Mechanisms, challenges and future prospects. Sci. Total Environ. 2021, 780, 146590. [Google Scholar] [CrossRef] [PubMed]
- Rummel, C.D.; Jahnke, A.; Gorokhova, E.; Kühnel, D.; Schmitt-Jansen, M. Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environ. Sci. Technol. Lett. 2017, 4, 258–267. [Google Scholar] [CrossRef]
- Helmberger, M.S.; Tiemann, L.K.; Grieshop, M.J. Towards an ecology of soil microplastics. Funct. Ecol. 2020, 34, 550–560. [Google Scholar] [CrossRef]
- Davidson, T.M. Boring crustaceans damage polystyrene floats under docks polluting marine waters with microplastic. Mar. Pollut. Bull. 2012, 64, 1821–1828. [Google Scholar] [CrossRef]
- Jang, M.; Shim, W.J.; Han, G.M.; Song, Y.K.; Hong, S.H. Formation of microplastics by polychaetes (Marphysa sanguinea) inhabiting expanded polystyrene marine debris. Mar. Pollut. Bull. 2018, 131, 365–369. [Google Scholar] [CrossRef]
- Brandon, A.M.; Gao, S.H.; Tian, R.; Ning, D.; Yang, S.S.; Zhou, J.; Wu, W.M.; Criddle, C.S. Biodegradation of polyethylene and plastic mixtures in mealworms (larvae of Tenebrio molitor) and effects on the gut microbiome. Environ. Sci. Technol. 2018, 52, 6526–6533. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, D.J.; Bréchon, A.L.; Thompson, R.C. Ingestion and fragmentation of plastic carrier bags by the amphipod Orchestia gammarellus: Effects of plastic type and fouling load. Mar. Pollut. Bull. 2018, 127, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Qiu, R.; Hu, J.; Li, X.; Zhang, X.; Chen, Y.; Wu, W.M.; He, D. Biodegradation and disintegration of expanded polystyrene by land snails Achatina fulica. Sci. Total Environ. 2020, 746, 141289. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Cárdenas, A.; O’Halloran, J.; van Pelt, F.N.; Jansen, M. Rapid fragmentation of microplastics by the freshwater amphipod Gammarus duebeni (Lillj.). Sci. Rep. 2020, 10, 12799. [Google Scholar] [CrossRef]
- Huerta Lwanga, E.; Mendoza Vega, J.; Ku Quej, V.; Chi, J.D.L.A.; Sanchez del Cid, L.; Chi, C.; Escalona Segura, G.; Gertsen, H.; Salánki, T.; van der Ploeg, M.; et al. Field evidence for transfer of plastic debris along a terrestrial food chain. Sci. Rep. 2017, 7, 14071. [Google Scholar]
- Dawson, A.L.; Kawaguchi, S.; King, C.K.; Townsend, K.A.; King, R.; Huston, W.M.; Bengtson Nash, S.M. Turning microplastics into nanoplastics through digestive fragmentation by Antarctic krill. Nat. Commun. 2018, 9, 1001. [Google Scholar] [CrossRef]
- Kwak, J.I.; An, Y.J. Microplastic digestion generates fragmented nanoplastics in soils and damages earthworm spermatogenesis and coelomocyte viability. J. Hazard. Mater. 2021, 402, 124034. [Google Scholar] [CrossRef]
- Bhuiyan, K.A.; Rodríguez, B.M.; Pires, A.; Riba, I.; Dellvals, Á.; Freitas, R.; Conradi, M. Experimental evidence of uncertain future of the keystone ragworm Hediste diversicolor (OF Müller, 1776) under climate change conditions. Sci. Total Environ. 2021, 750, 142031. [Google Scholar] [CrossRef] [PubMed]
- Müggler Zumstein, I.R.; Weber Marin, A. Textiles & Biodegradability: Challenges and Opportunities of sustainable textile Futures. In Proceedings of the International Association of Societies of Design Research Conference (IASDR), Manchester, UK, 2–5 September 2019; pp. 1–12. [Google Scholar]
- Basallote, M.D.; De Orte, M.R.; DelValls, T.A.; Riba, I. Studying the effect of CO2-induced acidification on sediment toxicity using acute amphipod toxicity test. Environ. Sci. Technol. 2014, 48, 8864–8872. [Google Scholar] [CrossRef]
- Passarelli, M.C.; Riba, I.; Cesar, A.; Serrano-Bernando, F.; DelValls, T.A. Assessing the influence of ocean acidification to marine amphipods: A comparative study. Sci. Total Environ. 2017, 595, 759–768. [Google Scholar] [CrossRef]
- Arias, A.M.; Drake, P. Distribution and production of the polychaete Nereis diversicolor in a shallow coastal lagoon in the Bay of Cádiz (SW Spain). Cah. Biol. Mar. 1995, 36, 201–210. [Google Scholar]
- Santos-Echeandía, J.; Zéler, A.; Gago, J.; Lacroix, C. The role of cigarette butts as vectors of metals in the marine environment: Could it cause bioaccumulation in oysters? J. Hazard. Mater. 2021, 416, 125816. [Google Scholar] [CrossRef] [PubMed]
- Lucia, G.; Giuliani, M.E.; d’Errico, G.; Booms, E.; Benedetti, M.; Di Carlo, M.; Fattorini, D.; Gorbi, S.; Regoli, F. Toxicological effects of cigarette butts for marine organisms. Environ. Int. 2023, 171, 107733. [Google Scholar] [CrossRef] [PubMed]
- Quéméneur, M.; Bel Hassen, M.; Armougom, F.; Khammeri, Y.; Lajnef, R.; Bellaaj-Zouari, A. Prokaryotic diversity and distribution along physical and nutrient gradients in the Tunisian coastal waters (South Mediterranean Sea). Front. Microbiol. 2020, 11, 2904. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Sanahuja, A.; Casado-Coy, N.; Simó-Cabrera, L.; Sanz-Lázaro, C. Monitoring polymer degradation under different conditions in the marine environment. Environ. Pollut. 2020, 259, 113836. [Google Scholar] [CrossRef]
- Araújo, M.C.B.; Costa, M.F.; Silva-Cavalcanti, J.S.; Duarte, A.C.; Reis, V.; Rocha-Santos, T.A.; da Costa, J.P.; Girão, V. Different faces of cigarette butts, the most abundant beach litter worldwide. Environ. Sci. Pollut. Res. 2022, 29, 48926–48936. [Google Scholar] [CrossRef] [PubMed]
- Hummel, D.O. Elemental Analysis. In Atlas of Plastics Additives: Analysis by Spectrometric Methods; Springer: Berlin/Heidelberg, Germany, 2002; pp. 113–116. [Google Scholar]
- Frias, J.P.; Gago, J.; Otero, V.; Sobral, P. Microplastics in coastal sediments from Southern Portuguese shelf waters. Mar. Environ. Res. 2016, 114, 24–30. [Google Scholar] [CrossRef]
- Cardoso, L.S.; Estrela, F.N.; Chagas, T.Q.; da Silva, W.A.M.; Costa, D.R.D.O.; Pereira, I.; Vaz, B.G.; Rodrigues, A.S.L.; Malafaia, G. Exposure to water with cigarette residue changes the antipredator response in female Swiss albino mice. Environ. Sci. Pollut. Res. 2018, 25, 8592–8607. [Google Scholar] [CrossRef]
- Chevalier, Q.; El Hadri, H.; Petitjean, P.; Bouhnik-Le Coz, M.; Reynaud, S.; Grassl, B.; Gigault, J. Nano-litter from cigarette butts: Environmental implications and urgent consideration. Chemosphere 2018, 194, 125–130. [Google Scholar] [CrossRef]
- Dobaradaran, S.; Nabipour, I.; Saeedi, R.; Ostovar, A.; Khorsand, M.; Khajeahmadi, N.; Keshtkar, M. Association of metals (Cd, Fe, As, Ni, Cu, Zn, and Mn) with cigarette butts in northern part of the Persian Gulf. Tob. Control 2017, 26, 461–463. [Google Scholar] [CrossRef]
- Dobaradaran, S.; Soleimani, F.; Akhbarizadeh, R.; Schmidt, T.C.; Marzban, M.; BasirianJahromi, R. Environmental fate of cigarette butts and their toxicity in aquatic organisms: A comprehensive systematic review. Environ. Res. 2021, 195, 110881. [Google Scholar] [CrossRef] [PubMed]
- Dobaradaran, S.; Mutke, X.A.M.; Schmidt, T.C.; Swiderski, P.; De-la-Torre, G.E.; Jochmann, M.A. Aromatic amines contents of cigarette butts: Fresh and aged cigarette butts vs unsmoked cigarette. Chemosphere 2022, 301, 134735. [Google Scholar] [CrossRef] [PubMed]
- Poppendieck, D.; Gong, M.; Pham, V. Influence of temperature, relative humidity, and water saturation on airborne emissions from cigarette butts. Sci. Total Environ. 2020, 12, 136422. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Osako, M. Leaching characteristics of polycyclic aromatic hydrocarbons (PAHs) from spiked sandy soil. Chemosphere 2003, 51, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Rowe, D.; Reid, M.J.; Thomas, K.V.; Galloway, T.S. Bioaccumulation and biological effects of cigarette litter in marine worms. Sci. Rep. 2015, 5, 14119. [Google Scholar] [CrossRef] [PubMed]
- Boukadida, K.; Banni, M.; Gourves, P.Y.; Cachot, J. High sensitivity of embryo-larval stage of the Mediterranean mussel, Mytilus galloprovincialis to metal pollution in combination with temperature increase. Mar. Environ. Res. 2016, 122, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Delnat, V.; Tran, T.T.; Janssens, L.; Stoks, R. Daily temperature variation magnifies the toxicity of a mixture consisting of a chemical pesticide and a biopesticide in a vector mosquito. Sci. Total Environ. 2019, 659, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, I.M.; Lannig, G. Interactive effects of metal pollution and temperature on metabolism in aquatic ectotherms: Implications of global climate change. Clim. Res. 2008, 37, 181–201. [Google Scholar] [CrossRef]
- Verlecar, X.N.; Jena, K.B.; Chainy, G.B.N. Biochemical markers of oxidative stress in Perna viridis exposed to mercury and temperature. Chem. Biol. Interact. 2007, 167, 219–226. [Google Scholar] [CrossRef]
- Lima, C.F.; dos Santos Pinto, M.A.; Choueri, R.B.; Moreira, L.B.; Castro, Í.B. Occurrence, characterization, partition, and toxicity of cigarette butts in a highly urbanized coastal area. Waste Manag. 2021, 131, 10–19. [Google Scholar] [CrossRef]
- Xi, Y.; Diao, L.; Wang, Z.; Jin, Z.; Wang, Y.; Liu, W.; Wen, D.; Li, H.; Sun, C.; Lu, J. Toxicity of leachate from smoked cigarette butts to terrestrial animals: A case of study on the earthworm Eisenia fetida. Sci. Total Environ. 2023, 898, 165531. [Google Scholar] [CrossRef]
- Noisette, F.; Bordeyne, F.; Davoult, D.; Martin, S. Assessing the physiological responses of the gastropod Crepidula fornicata to predicted ocean acidification and warming. Limnol. Oceanogr. 2016, 61, 430–444. [Google Scholar] [CrossRef]
- Galasso, H.L.; Richard, M.; Lefebvre, S.; Aliaume, C.; Callier, M.D. Body size and temperature effects on standard metabolic rate for determining metabolic scope for activity of the polychaete Hediste (Nereis) diversicolor. PeerJ 2018, 6, 5675. [Google Scholar] [CrossRef]
- Clements, J.C.; Darrow, E.S. Eating in an acidifying ocean: A quantitative review of elevated CO2 effects on the feeding rates of calcifying marine invertebrates. Hydrobiologia 2018, 820, 1–21. [Google Scholar] [CrossRef]
- Fonseca, T.G.; Morais, M.B.; Rocha, T.; Abessa, D.M.S.; Aureliano, M.; Bebianno, M.J. Ecotoxicological assessment of the anticancer drug cisplatin in the polychaete Nereis diversicolor. Sci. Total Environ. 2018, 575, 162–172. [Google Scholar] [CrossRef]
- Murray, F.; Solan, M.; Douglas, A. Effects of algal enrichment and salinity on sediment particle reworking activity and associated nutrient generation mediated by the intertidal polychaete Hediste diversicolor. J. Exp. Mar. Biol. Ecol. 2017, 495, 75–82. [Google Scholar] [CrossRef]
- Dale, H.; Taylor, J.D.; Solan, M.; Lam, P.; Cunliffe, M. Polychaete mucopolysaccharide alters sediment microbial diversity and stimulates ammonia-oxidising functional groups. FEMS Microbiol. Ecol. 2019, 95, 234. [Google Scholar] [CrossRef]
- Bonanomi, G.; Maisto, G.; De Marco, A.; Cesarano, G.; Zotti, M.; Mazzei, P.; Incerti, G. The fate of cigarette butts in different environments: Decay rate, chemical changes, and ecotoxicity revealed by a 5-year decomposition experiment. Environ. Pollut. 2020, 261, 114108. [Google Scholar] [CrossRef]

| pH | Temperature | Salinity | DO | ||
|---|---|---|---|---|---|
| 15 °C | Control | 8.13 ± 0.09 | 14.99 ± 0.13 | 35.38 ± 0.09 | 131.79 ± 7.6 |
| 0.25 CBL−1 | 8.13 ± 0.08 | 14.97 ± 0.13 | 35.39 ± 0.11 | 131.64 ± 9.3 | |
| 1 CBL−1 | 8.12 ± 0.09 | 14.96 ± 0.13 | 35.39 ± 0.12 | 134.10 ±6.7 | |
| D-0.25 CBL−1 | 8.14 ± 0.08 | 14.94± 0.16 | 35.47 ± 0.11 | 129.75 ± 6.6 | |
| D-1 CBL−1 | 8.15 ± 0.08 | 14.96 ± 0.16 | 35.44 ± 0.11 | 130.64 ± 6.5 | |
| 23 °C | Control | 8.11 ± 0.08 | 23.15 ± 0.31 | 35.42 ± 0.12 | 126.62 ± 11.4 |
| 0.25 CBL−1 | 8.12 ± 0.10 | 23.15 ± 0.36 | 35.45 ± 0.08 | 125.60 ± 10.1 | |
| 1 CBL−1 | 8.11 ± 0.10 | 23.15 ± 0.36 | 35.44 ± 0.09 | 127.29 ± 10.6 | |
| D-0.25 CBL−1 | 8.11 ± 0.09 | 23.17 ± 0.34 | 35.50 ± 0.11 | 128.36 ± 10.4 | |
| D-1 CBL−1 | 8.13 ± 0.09 | 23.15 ± 0.36 | 35.49 ± 0.10 | 129.40 ± 9.8 |
| df | SS | MS | Pseudo-F | p | ||
|---|---|---|---|---|---|---|
| Cu | CB | 4 | 30.41 | 7.62 | 31.86 | 0.001 |
| Temperature | 1 | 0.30 | 0.30 | 1.26 | ns | |
| CB × Temperature | 4 | 23.77 | 5.94 | 24.81 | 0.001 | |
| Res | 62 | 14.82 | 0.24 | |||
| Total | 71 | 71 | ||||
| Zn | CB | 4 | 38.78 | 9.69 | 253.2 | 0.001 |
| Temperature | 1 | 6.88 | 6.88 | 179.7 | 0.001 | |
| CB × Temperature | 4 | 25.41 | 6.35 | 165.91 | 0.001 | |
| Res | 62 | 2.37 | 3.828 × 10−2 | |||
| Total | 71 | 71 | ||||
| Naftalene | CB | 4 | 37.23 | 9.31 | 26.56 | 0.001 |
| Temperature | 1 | 0.45 | 0.45 | 1.28 | ns | |
| CB × Temperature | 4 | 11.44 | 2.86 | 8.16 | 0.001 | |
| Res | 62 | 21.73 | 0.35 | |||
| Total | 71 | 71 | ||||
| Nicotine | CB | 3 | 29.15 | 9.72 | 38.45 | 0.001 |
| Temperature | 1 | 6.32 × 10−2 | 6.32 × 10−2 | 0.25 | ns | |
| CB × Temperature | 3 | 26.45 | 8.82 | 34.89 | 0.001 | |
| Res | 52 | 13.14 | 0.25 | |||
| Total | 59 | 69.14 |
| df | SS | MS | F | p | ||
|---|---|---|---|---|---|---|
| Mortality rate | Time | 1.32 | 8496.72 | 8496.70 | 21.91 | 0.0001 |
| Time × CBs | 2.63 | 1324.99 | 1324.99 | 3.42 | 0.0440 | |
| Time × Temperature | 1.32 | 6205.38 | 6205.38 | 16.00 | 0.0001 | |
| Error (Time) | 42 | 387.77 | 170.20 | - | - | |
| CBs | 2.00 | 7430.71 | 3715.36 | 3.500 | ns | |
| Temperature | 1.00 | 19,181.87 | 19,181.87 | 18.10 | 0.0010 | |
| Error | 14 | 14,843.52 | 1070.25 | - | - | |
| Burrowing behavior | Time | 2.45 | 24,140 | 24,140.29 | 1.65 | ns |
| Time × CBs | 4.40 | 67,741.38 | 13,010.80 | 2.30 | ns | |
| Time × Temperature | 2.40 | 28,048.55 | 11,436.80 | 1.90 | ns | |
| Error (Time) | 56 | 205,169.38 | 5975.56 | - | - | |
| CBs | 2.00 | 58,063.40 | 29,031.70 | 4.70 | 0.0270 | |
| Temperature | 1.00 | 36,200.28 | 36,200.28 | 5.90 | 0.0290 | |
| Error | 14 | 85,485.62 | 6106.18 | - | - | |
| Burial rate | Time | 2.00 | 119,836.15 | 57,548.40 | 3.60 | 0.0370 |
| Time × CBs | 4.20 | 54,932.31 | 54,932.31 | 0.84 | ns | |
| Time × Temperature | 2.10 | 42,614.38 | 20,464.52 | 1.30 | ns | |
| Error (Time) | 56 | 458,695.55 | 8190.99 | - | - | |
| CBs | 2.00 | 23,835.47 | 11,917.73 | 0.82 | ns | |
| Temperature | 1.00 | 77,088.40 | 77,088.40 | 5.32 | 0.0370 | |
| Error | 14 | 20,2705.33 | 14,478.95 | - | - |
| df | SS | MS | F | p | ||
|---|---|---|---|---|---|---|
| Weigth loss | CB | 3 | 16,426.65 | 5475.55 | 6.26 | 0.0001 |
| Temperature | 1 | 450.76 | 450.76 | 1.137 | 0.248 | |
| CB × Temperature | 3 | 597.90 | 199.30 | 1.137 | 0.298 | |
| Error | 22 | 8719.28 | 396.33 | - | ||
| Total | 30 | 26,412.62 | - | - | ||
| Degradation level | CB | 3 | 24.53 | 8.18 | 38.55 | 0.0001 |
| Temperature | 1 | 0.19 | 0.19 | 0.89 | 0.345 | |
| CB × Temperature | 3 | 0.53 | 0.17 | 0.38 | 0.487 | |
| Error | 22 | 4.67 | 0.21 | |||
| Total | 30 | 350 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conradi, M.; Sánchez-Moyano, J.E.; Rodríguez-Martín, F.J.; Bayo, J. Can Hediste diversicolor Speed Up the Breakdown of Cigarette Butts in Marine Sediments? Appl. Sci. 2024, 14, 4409. https://doi.org/10.3390/app14114409
Conradi M, Sánchez-Moyano JE, Rodríguez-Martín FJ, Bayo J. Can Hediste diversicolor Speed Up the Breakdown of Cigarette Butts in Marine Sediments? Applied Sciences. 2024; 14(11):4409. https://doi.org/10.3390/app14114409
Chicago/Turabian StyleConradi, Mercedes, J. Emilio Sánchez-Moyano, Francisco J. Rodríguez-Martín, and Javier Bayo. 2024. "Can Hediste diversicolor Speed Up the Breakdown of Cigarette Butts in Marine Sediments?" Applied Sciences 14, no. 11: 4409. https://doi.org/10.3390/app14114409
APA StyleConradi, M., Sánchez-Moyano, J. E., Rodríguez-Martín, F. J., & Bayo, J. (2024). Can Hediste diversicolor Speed Up the Breakdown of Cigarette Butts in Marine Sediments? Applied Sciences, 14(11), 4409. https://doi.org/10.3390/app14114409







