Abstract
This study examined age-related changes in unimanual and bimanual hand-grip force control capabilities by focusing on absolute and relative outcome measures. Thirty-two older adults and thirty-two younger adults performed isometric hand-grip force control tasks across three hand conditions (unimanual dominant, unimanual non-dominant, and bimanual) and two submaximal targeted levels (10% and 40% of maximal voluntary contraction). Force control performances were evaluated by calculating absolute and relative variables on force accuracy and variability. Furthermore, to determine which force control variables and experimental conditions effectively indicate age-related sensorimotor control deficits, we conducted receiver operating characteristic curve analyses. Older adults demonstrated impaired force control capabilities at 10% of maximal voluntary contraction collapse across the three hand conditions compared with younger adults, and these deficits were identified by both relative force accuracy and relative force variability. Moreover, relative force accuracy showed a good diagnostic quality at 10% of maximal voluntary contraction. These findings suggested that aging may induce unimanual and bimanual hand-grip force control deficits at a lower targeted level, and these motor impairments were sensitively estimated by quantifying relative force control outcome measures that may reflect age-related muscle weakness as compared with absolute measurements.
1. Introduction
Aging generally results in neuromuscular deficits including fewer numbers of motor units and muscle atrophy [1]. Furthermore, these age-related motor impairments interfere with activities of daily living, such as holding a cup, buttoning clothes, or driving vehicles [2,3,4]. Specifically, older adults frequently experience a loss of strength and fine motor control capabilities during hand-grip movements [5], associated with the incidence of dementia and depression [6,7]. Thus, estimating the ability to produce and modulate hand-grip force outputs may be an effective way to predict aging progression [8].
The isometric force control paradigm has been frequently used to estimate sensorimotor processing capabilities [9,10]. Participants were required to match their isometric forces to the submaximal targeted force level using online visual feedback (e.g., simultaneous visual information on their force outputs and a targeted level). Thus, successful isometric force control performances suggest advanced visuomotor correction functions necessary for independent living in older adults [11,12]. For unimanual hand-grip force control tasks, older women produced greater force errors using the dominant hand than younger women [13], and other studies reported age-induced hand-grip force control deficits for unimanual dominant and non-dominant hands, respectively [14,15]. Furthermore, force control impairments in older adults increased at lower targeted force levels (e.g., 2.5–10% maximal voluntary contraction; MVC) because of potential changes in motor unit activation patterns such as more variable inter-spike intervals [16,17,18].
Moreover, these sensorimotor impairments may increase in bimanual force control tasks because of altered neural resources in the brains of older adults while executing interlimb motor actions [19,20,21]. During bimanual visuomotor tasks, older adults showed higher brain connectivity between the interhemispheric dorsal and ventral premotor areas [20]. Furthermore, hyperactivations across the supplementary motor area, dorsolateral prefrontal cortex, and secondary somatosensory cortex were observed in older adults because additional cognitive and sensorimotor processes may be required for interacting and coordinating motor actions between hands [19,22]. In fact, older adults showed impaired bimanual hand-grip force control and interlimb coordination patterns [23,24]. Although isometric hand-grip force control capabilities may be related to motor impairments caused by aging progression, whether age-related force control deficits predominantly appear in either unimanual or bimanual hands (i.e., using both hands simultaneously) is still unclear.
Typically, force control capabilities are estimated by quantifying the accuracy and variability of force outputs [10]. Two perspectives on force accuracy and variability assessments include (a) absolute variables (e.g., absolute force error = root mean square error, RMSE, and force variability = standard deviation, SD) and (b) relative variables (i.e., RMSE relative to a targeted force level and SD relative to a mean force). In comparison to absolute force error and variability increased with greater force production [25,26,27], relative force control variables may effectively indicate fine motor control functions because of the minimal effects of altered targeted levels on force control performances [28]. Considering aging-induced muscle weakness [29,30], relative variables may be more appropriate for comparing changes in force control capabilities between younger and older adults [10,28]. Previous studies have reported no significant differences in force accuracy and variability estimated by absolute variables for older groups compared with younger groups [24,31]. Although several studies have revealed lower hand-grip force variability as assessed by relative variables in older adults at relatively low targeted force levels [13,24,32], there is a lack of studies that have used relative variables to examine force accuracy [14]. Considering reduced muscle strength in older adults, it is necessary to investigate age-related force control capabilities using both absolute and relative force accuracy and variability. Therefore, this study aimed to examine unimanual and bimanual hand-grip force control performances between older and younger adult groups by quantifying absolute and relative variables. On the basis of previous findings [19,23,28], we hypothesized that the older group would show greater relative force error and variability at the lower target force level, and these impairments would be increased in the bimanual condition.
2. Materials and Methods
2.1. Participants
Thirty-two older adults (age: M ± SD = 64.6 ± 3.4 years; 26 females and 6 males) and 32 younger adults (age: M ± SD = 23.3 ± 2.4 years; 26 females and 6 males) participated in this study consistent with priori power analysis using G*Power (power = 0.99; alpha = 0.05; version 3.1.9.4). Using social media announcements and offline flyers from November 2019 to November 2021, participants who met the inclusion criteria were recruited: younger adults aged 19–29 years and older adults over 60 years who had no cognitive impairments (i.e., Korean Mini-Mental State Exam score ≥24) [33,34], vision disorders, and neuromuscular deficits in the upper extremities by self-reports. Additionally, we advised participants to refrain from vigorous physical activities and alcohol consumption for 24 h, and to abstain from consuming caffeine, as well as any medications (e.g., painkillers or sedatives), for 12 h before the experiment [35]. All participants were right-handed, as estimated by the Edinburgh Handedness Questionnaire [36], and specific demographic details are shown in Table 1. Before starting the experiments, all participants read and signed the study protocols and an informed consent form approved by the University’s Institutional Review Board.

Table 1.
Demographic information for participants.
2.2. Experimental Setup
Before beginning the force control tasks, height and body composition were measured for each participant using an aluminum stadiometer (Sam-Hwa Machinery, Gyeongsan-si, Republic of Korea) and a bioelectrical impedance device (Inbody 720, Biospace, Seoul, Republic of Korea). For the isometric hand-grip force tasks, a customized isometric force control device (SEED TECH Co., Ltd., Bucheon, Republic of Korea; Figure 1a) was used. Based on previous studies [24,37,38], participants sat 80 cm away from a 54.6 cm LCD monitor and grabbed handles embedded with a force transducer (Micro Load Cell-CZL635-3135, range = 220 lbs., Phidgets Inc., Calgary, AB, Canada) in a comfortable position (i.e., 15°–20° of shoulder flexion and 20°–45° of elbow flexion; Figure 1b). While performing force control tasks, all force signals were collected at a 200 Hz sampling rate using a 16-bit analog-to-digital converter (A/D; ADS1148 16-bit 2kSPS and a minimum detectable force = 0.0192 N) and amplified using an INA122 with a 5 V excitation voltage (Texas Instruments Inc., Dallas, TX, USA). All force control tasks were administered using a custom Microsoft Visual C++ program (Microsoft Corp., Redmond, WA, USA).
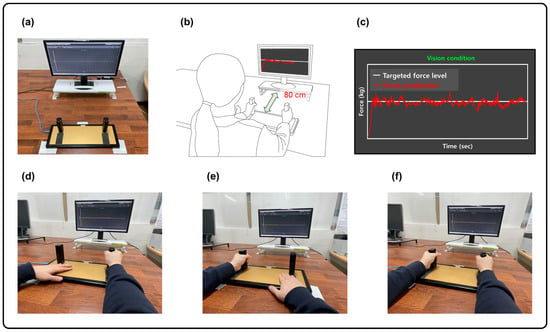
Figure 1.
Experimental setup. (a) Isometric hand-grip force measurement system. (b) Hand-grip force control position. (c) Two types of visual information: force production (red trajectory line) and targeted force level (white horizontal line). (d) Unimanual dominant hand-grip force control task. (e) Unimanual non-dominant hand-grip force control task. (f) Bimanual hand-grip force control task.
In this study, isometric force control tasks comprised the following three hand conditions: unimanual dominant hand, unimanual non-dominant hand, and bimanual hands. Initially, participants completed two MVC trials (trial duration = 3 s, and 60 s of rest between trials) producing isometric hand-grip force for each hand condition. The mean value of two maximum forces from each trial was used to set the targeted force levels for each individual. We randomly assigned three hand conditions for the MVC tasks and provided 180 s of rest between hand conditions to minimize potential fatigue effects. Finally, we selected 10% and 40% MVC as the targeted force levels consistent with previous studies [24,39].
The goal of the isometric force control task was to generate and maintain force outputs produced by unimanual or bimanual hands (the sum of forces from each hand) around a targeted force level for 20 s. While performing the task, participants received two pieces of visual information (Figure 1c): (1) force production (i.e., red trajectory line) and (2) a targeted force level (i.e., white horizontal line). We randomly administered six experimental blocks across three hand conditions and two targeted force levels. For each block, two practice trials were administered so that we confirmed that participants understood and were familiar with the overall procedures for force control tasks. Participants completed three consecutive trials for each experimental block. To minimize potential fatigue effects, we provided 30 s of rest between trials and 60 s of rest between experimental blocks.
2.3. Data Analysis
We conducted the following offline analyses using the Matlab program (R2022a Update 3 (9.12.0.1975300) 64-bit (win64), Math Works TM Inc., Natick, MA, USA). For each trial, we eliminated the first 3 s and last 1 s to prevent initial adjustment and termination effects so that the middle 16 s of force signals could be analyzed. Further, we used a low-pass filter on the force data using a bidirectional fourth-order Butterworth filter at a 30 Hz cut-off frequency.
To evaluate force control performances, we focused on force accuracy and variability. Force accuracy can be estimated by the absolute force error and relative force error. We used RMSE to quantify absolute force error, and lower values of RMSE denote higher force accuracy and lower absolute force error. Also, we used relative RMSE (rRMSE = RMSE/targeted force) to minimize the potential effects of different MVC levels between participants. Force variability can be estimated by calculating absolute and relative force variabilities. We used the SD of force output to compute absolute force variability and quantified relative force variability using the coefficient of variation (%CV = SD/mean force). Higher values of SD and %CV indicated greater force variability.
2.4. Statistical Analysis
We confirmed the normality of distribution for all dependent variables using Shapiro–Wilk’s test. For all of the force control variables, we performed a three-way mixed ANOVA (Group × Hand Condition × Force Level; 2 × 3 × 2) with repeated measures on the last two factors. When assumptions of sphericity were violated, we reported Greenhouse–Geisser’s degrees of freedom adjustment. For the post hoc analysis, we used Bonferroni’s pairwise comparisons. Moreover, we performed ROC curve analyses on all dependent variables across different hand and targeted force level conditions. Diagnostic accuracy was evaluated by Area Under the Curve (AUC) from the ROC curve, and the levels of AUC were normally classified into: (a) 1.00 − 0.90 = excellent, (b) 0.89 − 0.80 = good, (c) 0.79 − 0.70 = fair, (d) 0.69 − 0.60 = poor, and (e) 0.59 − 0.00 = fail [40,41]. Finally, our secondary analysis included Pearson’s correlation analysis to investigate the potential association between body composition and relative force control variables for the older group. All statistical analyses were performed using IBM SPSS Statistics 28 (SPSS Inc., Chicago, IL, USA). The alpha level was set at 0.05.
3. Results
3.1. Force Accuracy
A three-way mixed ANOVA on the RMSE revealed the following two interactions: (a) Group × Force Level interaction [F(1, 62) = 6.313; p = 0.015; partial η2 = 0.092] and (b) Hand Condition × Force Level interaction [F(1.399, 86.747) = 39.625; p < 0.001; partial η2 = 0.390]. Specifically, RMSE values significantly increased at a higher targeted force level with no group differences (Figure 2a), and RMSE values in the bimanual hand condition were greater than those in the unimanual dominant and non-dominant hand conditions (Supplementary Figure S1). Analysis of the rRMSE showed the following significant main effect and interaction: (a) Hand Condition main effect [F(1.795, 111.272) = 21.237; p < 0.001; partial η2 = 0.255] and (b) Group × Force Level interaction [F(1, 62) = 10.719; p = 0.002; partial η2 = 0.147]. Specifically, the older group produced greater rRMSE values than the younger group at 10% MVC (Figure 2b). The rRMSE values in both unimanual dominant and non-dominant hand conditions were greater than those in the bimanual hand condition collapsed across group and force level conditions (Supplementary Figure S2). Taken together, these findings indicate that older adults produced greater relative force errors as compared with younger adults at a lower targeted force level.
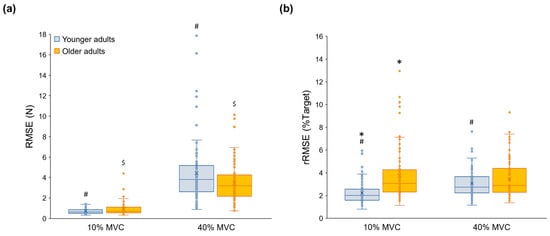
Figure 2.
Force accuracy variables for the younger and older groups. (a) RMSE showing a significant Group × Force Level interaction, (b) rRMSE showing a significant Group × Force Level interaction. Asterisk (*) indicates a significant difference between groups. Number sign (#) denotes a significant difference between Force Levels in the younger group. Dollar sign ($) means a significant difference between Force Levels in the older group. (p < 0.05).
3.2. Force Variability
A three-way mixed ANOVA on the SD reported a Group × Hand Condition × Force Level interaction [F(1.402, 86.906) = 4.577; p = 0.023; partial η2 = 0.069;]. Specifically, the younger group produced a greater SD value than the older group at 40% MVC for the three hand conditions (Figure 3a). SD values in the bimanual hands were greater than those in the unimanual dominant and non-dominant hands, and the SD values significantly increased at a higher targeted force level for all groups and force level conditions. Analysis of the %CV showed a significant main effect and interaction: (a) Hand Condition main effect [F(1.765, 109.434) = 31.795; p < 0.001; partial η2 = 0.339] and (b) Group × Force Level interaction [F(1, 62) = 16.122; p < 0.001; partial η2 = 0.206]. Specifically, the older group produced greater %CV values than the younger group at 10% MVC (Figure 3b). Values of %CV in both unimanual dominant and non-dominant hand conditions were greater than those in bimanual hands collapsed across group and force level conditions (Supplementary Figure S3). Overall, these results suggest that older adults produced greater relative force variability as compared with younger adults at a lower targeted force level.

Figure 3.
Force variability variables for the younger and older groups. (a) SD showing a significant Group × Hand Condition × Force Level interaction. (b) %CV showing a significant Group × Force Level interaction. Asterisk (*) indicates a significant difference between groups. Ampersand sign (&) means a significant difference between bimanual and unimanual (both dominant and non-dominant) hands in the younger group. Section sign (§) indicates a significant difference between bimanual and unimanual (both dominant and non-dominant) hands in the older group. Number sign (#) denotes a significant difference between Force Levels in the younger group. Dollar sign ($) means a significant difference between Force Levels in the older group (p < 0.05).
3.3. ROC Curve Findings
The ROC curve analysis showed that the rRMSE significantly differentiated aging at 10% MVC (Table 2): (a) unimanual dominant hand at 10% MVC (AUC = 0.813), (b) unimanual non-dominant hand at 10% MVC (AUC = 0.785), and (c) bimanual hands at 10% MVC (AUC = 0.808). The analysis on %CV at 10% MVC significantly distinguished between younger and older adults: (a) unimanual dominant hand at 10% MVC (AUC = 0.789), (b) unimanual non-dominant hand at 10% MVC (AUC = 0.756), and (c) bimanual hands at 10% MVC (AUC = 0.713). Given that an AUC above 0.7 was considered indicative of a fair level of diagnostic quality [40,41], the AUC of rRMSE in unimanual dominant and bimanual hand conditions at a 10% MVC level showed good quality. The specific details of the ROC curve analysis results are shown in Figure 4 and Supplementary Table S1.

Table 2.
ROC curve analysis on variables that had a significant group effect in ANOVA.
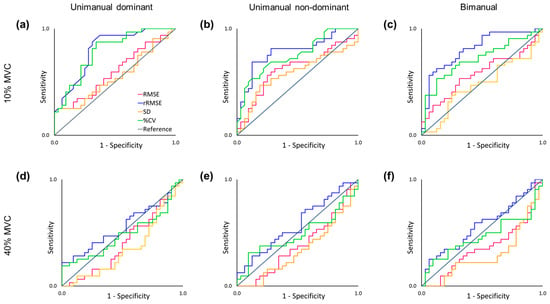
Figure 4.
Receiver Operating Characteristic curve analysis on force control variables. (a) The unimanual dominant hand-grip task at 10% of MVC. (b) The unimanual non-dominant hand-grip task at 10% of MVC. (c) The bimanual hand-grip task at 10% MVC. (d) The unimanual dominant hand-grip task at 40% MVC. (e) The unimanual non-dominant hand-grip task at 40% MVC. (f) The bimanual hand-grip task at 40% MVC.
3.4. Correlation Finding: Between Body Composition and Force Control in Older Adults
Pearson’s correlation analyses showed significant correlations between body mass index (BMI) and unimanual non-dominant hand force control at 10% MVC (Supplementary Table S2): (a) greater BMI versus lower rRMSE (r = −0.370; p = 0.037; Figure 5a) and (b) greater BMI versus lower %CV (r = −0.384; p = 0.030; Figure 5b). These findings suggest that decreased BMI values in older adults are associated with more impairments in non-dominant hand force control capabilities at a lower targeted level.
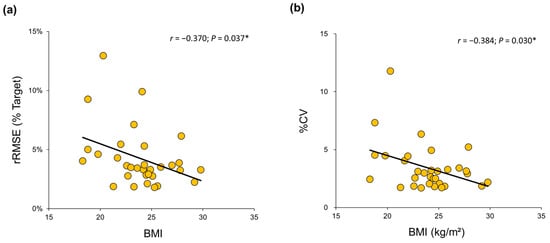
Figure 5.
Correlation between BMI for the older group and relative force variables at 10% of MVC in their non-dominant hands. (a) Lower values of BMI were significantly related to higher values of rRMSE. (b) Lower values of BMI were significantly related to higher values of %CV. * indicates p < 0.05.
4. Discussion
We examined unimanual and bimanual hand-grip force control performances between older and younger groups by estimating absolute and relative variables. The absolute force accuracy was not significantly different between the younger and older adult groups, and further, younger adults revealed greater absolute force variability for unimanual and bimanual hand conditions than older adults at 40% of MVC. However, the older adult group showed lower relative force accuracy and higher relative force variability than the younger adult group at 10% MVC for all hand conditions. The ROC curve analysis identified that the values of relative force accuracy in unimanual dominant and bimanual hand conditions at 10% MVC were favorable for distinguishing force control deficits in older adults. Moreover, secondary analysis revealed that increased body mass index values in older adults were positively correlated with higher relative force accuracy and lower relative force variability at 10% MVC in the unimanual non-dominant hand condition.
Absolute force control variables found no significant age-related differences, whereas younger adults produced greater absolute force variability at 40% MVC. These findings can account for the relationship between increased force control deficits estimated by absolute force accuracy and variability and greater force productions [21,25,26,27]. For example, younger and older adults showed greater SDs of forces as targeted force levels increased, and younger adult groups produced greater absolute force variability than older adults at 80% MVC [42,43]. Presumably, the application of absolute force control variables may be insufficient for differentiating age-related motor control changes from those in younger adults because of decreased maximal and submaximal force production in older adults.
Relative variables on force accuracy and variability identified significant impaired force control capabilities in older adults at 10% MVC. These results support the proposition that age-related unimanual force control deficits appear in tasks requiring lower force modulation (e.g., 2–10% of MVC) [13,44,45]. The relative measurement of force variability is frequently used to identify potential force control deficits observed in older adults [10,16,28,46]. In contrast, previous studies on force accuracy frequently used absolute outcome measures (e.g., RMSE), and they failed to identify altered force control capabilities in older adults [14,24,31,47]. Considering the dependence of absolute force control error on the levels of force outputs [26,48], age-related muscle weakness may account for underestimating the ability to precisely sustain forces. For example, a significant reduction in relative force accuracy appeared in older adults across all hand (dominant vs. non-dominant) and task (stationary vs. forward-reaching vs. backward-reaching) conditions, whereas absolute force accuracy was only reduced in the stationary task with the non-dominant hand [14]. Presumably, the level of relative force accuracy may be an effective indicator of age-induced changes in force control capabilities across various experimental conditions [47].
The ROC analysis additionally confirmed that relative outcome variables on force accuracy and variability produced by unimanual and bimanual hands at 10% MVC successfully identified age-related force control deficits. Moreover, we observed that relative force accuracy showed a relatively good diagnostic quality (i.e., AUC ≥ 0.8). Force control deficits in older adults at lower targeted levels may be related to greater variability in neural activation in motor neuron pools [17,18]. Older adults showed higher inter-spike interval variability across multiple motor units during index finger force control at 10% MVC that significantly correlated with increased force variability [18]. Perhaps, aging-induced loss of motor units alternatively increases the number of muscle fibers innervated by the remaining motor units, presumably resulting in increased motor unit discharge variability and higher force fluctuations. In contrast, a force control training program for older adults advanced force accuracy and increased the motor unit discharge rate [49]. These findings suggest that force control capabilities as indicated by relative force control variables are effective for representing altered neuromuscular systems in the aging population.
Increased BMI in older adults was related to lower relative force accuracy and greater relative force variability at 10% MVC in the unimanual non-dominant hand. The obesity paradox indicated that being overweight in the older adult population could decrease mortality by increasing strength and bone mineral density, protecting against falls, and acting as a metabolic reserve during disease [50,51,52]. For older adults, a decrease in BMI was associated with low hand-grip strength [53,54], and some studies suggested that older adults with either lower (<25 kg/m2) or higher BMI (>35 kg/m2) values experience motor deficits [55,56]. Considering the range of BMI (i.e., 18.3–29.8 kg/m2) for older adults in our study, the current findings suggest that decreased BMI is related to fine motor impairments in the aging population.
Potential mechanisms underlying age-induced hand-grip force control deficits for unimanual and bimanual conditions may include structural and functional changes in key brain regions. Previous studies reported decreased gray and white matter volumes across the prefrontal cortex, primary motor cortex, and cerebellum in older adults, related to hand motor impairments [57,58]. Despite the greater importance of interhemispheric neural communications for successful bimanual force control in older adults [59], age-induced atrophy of the corpus callosum additionally interfered with their bilateral motor control capabilities [60,61]. Moreover, older adults may require more neural resources to successfully perform unimanual and bimanual force control by compensating for degenerative changes in neuromuscular systems (e.g., reduced brain volume, decreased neurotransmitter interactions, and impaired sensorimotor functions) [62,63,64,65,66]. For example, while showing lower accuracy during unimanual hand-grip force control tasks, older adults revealed hyperactivation patterns across bilateral sides of premotor and sensorimotor areas compared with younger adults [67]. However, older adults who revealed fewer neural activations related to sensorimotor processing showed more impairments in unimanual and bimanual motor functions [63,68]. Thus, future studies may use exercise protocols combined with non-invasive brain stimulation techniques (e.g., transcranial direct current stimulation) that may modulate cortical activation patterns for improving force control capabilities in older adults.
Despite the finding that relative outcome variables effectively identified age-related force control deficits, several study limitations remain. First, the proportion of female participants in this study was relatively high (i.e., female ratio = 81.3%) so that the current findings could be affected by sexual bias. Therefore, future studies should determine whether gender differences influence age-related force control capabilities identified by relative outcome measures. Second, given that we focused on isometric hand-grip force control tasks, whether these findings are observed in other force control tasks (e.g., lower limb force control) should be investigated. Moreover, future studies should examine how relative force control variables are associated with structural and functional changes in key brain regions and motor neuron pools.
5. Conclusions
This study revealed that older adults showed more impairments in bimanual hand-grip force control performances than younger adults, and these deficits were identified by quantifying relative force accuracy and variability variables at a lower targeted force level across all hand conditions. Furthermore, these patterns were effective indicators that could discriminate age-related motor dysfunctions from younger adults. These findings suggested that force control capabilities normalized via an individual’s muscle strength can accurately demonstrate specific fine motor control changes caused by aging and neurological diseases. Potentially, aging researchers or rehabilitation specialists may use relative hand-grip force control outcome measures for effectively estimating age-induced fine motor control impairments and accurately quantifying the progress of fine motor control improvements after applying exercise programs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app14114408/s1, Table S1: Non-significant result of ROC curve analysis; Table S2: Pearson’s correlation analyses between body composition and relative force control variables that significant group effect in ANOVA for the older group; Figure S1: RMSE showing a significant Hand Condition × Force Level interaction. Ampersand sign (&) means a significant difference between bimanual and unimanual (both dominant and non-dominant) hands. Number sign (#) denotes a significant difference between Force Levels. (p < 0.05); Figure S2: rRMSE showing a significant Hand Condition main effect. (p < 0.05); Figure S3: %CV showing a significant Hand Condition main effect. (p < 0.05).
Author Contributions
Conceptualization, D.-K.K.; data curation, D.-K.K.; formal analysis, D.-K.K.; funding acquisition, N.K.; investigation, D.-K.K. and N.K.; methodology, N.K.; project administration, N.K.; resources, N.K.; software, D.-K.K. and N.K.; supervision, N.K.; visualization, D.-K.K.; writing - original draft, D.-K.K.; writing—review and editing, N.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science and ICT (MSIT) of the Korean government (NRF-2018R1C1B5084455).
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Incheon National University’s Institutional Review Board (7007971-201810-002A).
Informed Consent Statement
All participants read and signed the study protocols and an informed consent before starting the experiments.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Booth, F.W.; Weeden, S.H.; Tseng, B.S. Effect of aging on human skeletal muscle and motor function. Med. Sci. Sports Exerc. 1994, 26, 556–560. [Google Scholar] [CrossRef]
- Hayase, D.; Mosenteen, D.; Thimmaiah, D.; Zemke, S.; Atler, K.; Fisher, A.G. Age-related changes in activities of daily living ability. Aust. Occup. Ther. J. 2004, 51, 192–198. [Google Scholar] [CrossRef]
- Gulde, P.; Schmidle, S.; Aumüller, A.; Hermsdörfer, J. The effects of speed of execution on upper-limb kinematics in activities of daily living with respect to age. Exp. Brain Res. 2019, 237, 1383–1395. [Google Scholar] [CrossRef] [PubMed]
- Hortobágyi, T.; Mizelle, C.; Beam, S.; DeVita, P. Old adults perform activities of daily living near their maximal capabilities. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, M453–M460. [Google Scholar] [CrossRef] [PubMed]
- Carmeli, E.; Patish, H.; Coleman, R. The aging hand. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, M146–M152. [Google Scholar] [CrossRef] [PubMed]
- Zasadzka, E.; Pieczyńska, A.; Trzmiel, T.; Kleka, P.; Pawlaczyk, M. Correlation between handgrip strength and depression in older adults—A systematic review and a meta-analysis. Int. J. Environ. Res. Public Health 2021, 18, 4823. [Google Scholar] [CrossRef] [PubMed]
- Duchowny, K.A.; Ackley, S.F.; Brenowitz, W.D.; Wang, J.; Zimmerman, S.C.; Caunca, M.R.; Glymour, M.M. Associations between handgrip strength and dementia risk, cognition, and neuroimaging outcomes in the UK Biobank cohort study. JAMA Netw. Open 2022, 5, e2218314. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W.; Bear-Lehman, J.; Desrosiers, J.; Massy-Westropp, N.; Mathiowetz, V. Average grip strength: A meta-analysis of data obtained with a Jamar dynamometer from individuals 75 years or more of age. J. Geriatr. Phys. Ther. 2007, 30, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Pethick, J.; Clark, N.C.; Liew, B. Alterations in peripheral joint muscle force control in adults with musculoskeletal disease, injury, surgery, or arthroplasty: A systematic review and meta-analysis. J. Electromyogr. Kinesiol. 2022, 66, 102696. [Google Scholar] [CrossRef]
- Pethick, J.; Taylor, M.J.; Harridge, S.D. Aging and skeletal muscle force control: Current perspectives and future directions. Scand. J. Med. Sci. Sports 2022, 32, 1430–1443. [Google Scholar] [CrossRef]
- Fiogbé, E.; Carnavale, B.F.; de Medeiros Takahashi, A.C. Exercise training in older adults, what effects on muscle force control? A systematic review of randomized clinical trials. Arch. Gerontol. Geriatr. 2019, 83, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Marshall, M.M.; Armstrong, T.J. Observational assessment of forceful exertion and the perceived force demands of daily activities. J. Occup. Rehabil. 2004, 14, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, Y.-M.; Kang, N. Unilateral hand force control impairments in older women. EXCLI J. 2022, 21, 1231–1244. [Google Scholar] [PubMed]
- Lin, B.-S.; Kuo, S.-F.; Lee, I.; Lu, L.-H.; Chen, P.-Y.; Wang, P.-C.; Lai, C.-H.; Wang, X.-M.; Lin, C.-H. The impact of aging and reaching movements on grip stability control during manual precision tasks. BMC Geriatr. 2021, 21, 703. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Sung, W.-H.; Chiang, S.-L.; Lee, S.-C.; Lu, L.-H.; Wang, P.-C.; Wang, X.-M. Influence of aging and visual feedback on the stability of hand grip control in elderly adults. Exp. Gerontol. 2019, 119, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Enoka, R.M.; Christou, E.A.; Hunter, S.K.; Kornatz, K.W.; Semmler, J.G.; Taylor, A.M.; Tracy, B.L. Mechanisms that contribute to differences in motor performance between young and old adults. J. Electromyogr. Kinesiol. 2003, 13, 1–12. [Google Scholar] [CrossRef]
- Laidlaw, D.H.; Bilodeau, M.; Enoka, R.M. Steadiness is reduced and motor unit discharge is more variable in old adults. Muscle Nerve 2000, 23, 600–612. [Google Scholar] [CrossRef]
- Tracy, B.L.; Maluf, K.S.; Stephenson, J.L.; Hunter, S.K.; Enoka, R.M. Variability of motor unit discharge and force fluctuations across a range of muscle forces in older adults. Muscle Nerve 2005, 32, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Maes, C.; Gooijers, J.; de Xivry, J.-J.O.; Swinnen, S.P.; Boisgontier, M.P. Two hands, one brain, and aging. Neurosci. Biobehav. Rev. 2017, 75, 234–256. [Google Scholar] [CrossRef]
- Solesio-Jofre, E.; Serbruyns, L.; Woolley, D.G.; Mantini, D.; Beets, I.A.; Swinnen, S.P. Aging effects on the resting state motor network and interlimb coordination. Hum. Brain Mapp. 2014, 35, 3945–3961. [Google Scholar] [CrossRef]
- Smits-Engelsman, B.C.; Van Galen, G.P.; Duysens, J. Force levels in uni-and bimanual isometric tasks affect variability measures differently throughout lifespan. Motor Control 2004, 8, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Goble, D.J.; Coxon, J.P.; Van Impe, A.; De Vos, J.; Wenderoth, N.; Swinnen, S.P. The neural control of bimanual movements in the elderly: Brain regions exhibiting age-related increases in activity, frequency-induced neural modulation, and task-specific compensatory recruitment. Hum. Brain Mapp. 2010, 31, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Chou, L.-W.; Wei, S.-H.; Lieu, F.-K.; Chiang, S.-L.; Sung, W.-H. Influence of aging on bimanual coordination control. Exp. Gerontol. 2014, 53, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kang, N. Altered Bimanual Kinetic and Kinematic Motor Control Capabilities in Older Women. Int. J. Environ. Res. Public Health 2023, 20, 2153. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.E.; Hamilton, A.F.d.C.; Wolpert, D.M. Sources of signal-dependent noise during isometric force production. J. Neurophysiol. 2002, 88, 1533–1544. [Google Scholar] [CrossRef] [PubMed]
- Novak, T.; Newell, K.M. Physiological tremor (8–12 Hz component) in isometric force control. Neurosci. Lett. 2017, 641, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.A.; Zelaznik, H.; Hawkins, B.; Frank, J.S.; Quinn, J.T., Jr. Motor-output variability: A theory for the accuracy of rapid motor acts. Psychol. Rev. 1979, 86, 415. [Google Scholar] [CrossRef]
- Hunter, S.K.; Pereira, H.M.; Keenan, K.G. The aging neuromuscular system and motor performance. J. Appl. Physiol. 2016, 121, 982–995. [Google Scholar] [CrossRef]
- Aagaard, P.; Suetta, C.; Caserotti, P.; Magnusson, S.P.; Kjær, M. Role of the nervous system in sarcopenia and muscle atrophy with aging: Strength training as a countermeasure. Scand. J. Med. Sci. Sports 2010, 20, 49–64. [Google Scholar] [CrossRef]
- Rice, C.L.; Cunningham, D.A.; Paterson, D.H.; Rechnitzer, P.A. Strength in an elderly population. Arch. Phys. Med. Rehabil. 1989, 70, 391–397. [Google Scholar]
- Tillman, M.; Ambike, S. Expectation of movement generates contrasting changes in multifinger synergies in young and older adults. Exp. Brain Res. 2018, 236, 2765–2780. [Google Scholar] [CrossRef] [PubMed]
- Galganski, M.E.; Fuglevand, A.J.; Enoka, R.M. Reduced control of motor output in a human hand muscle of elderly subjects during submaximal contractions. J. Neurophysiol. 1993, 69, 2108–2115. [Google Scholar] [CrossRef]
- Kang, Y.; NA, D.L.; Hahn, S. A validity study on the Korean Mini-Mental State Examination (K-MMSE) in dementia patients. J. Korean Neurol. Assoc. 1997, 15, 300–308. [Google Scholar]
- Tombaugh, T.N.; McIntyre, N.J. The mini-mental state examination: A comprehensive review. J. Am. Geriatr. Soc. 1992, 40, 922–935. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, H.; Kim, H.; Kim, R.K.; Lee, T.L.; Ko, D.K.; Lee, H.J.; Kang, N. Resistance band training with functional electrical stimulation improves force control capabilities in older adults: A preliminary study. EXCLI J. 2024, 23, 130. [Google Scholar] [PubMed]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Kang, N. Low-frequency oscillations and force control capabilities as a function of force level in older women. Appl. Sci. 2022, 12, 1812. [Google Scholar] [CrossRef]
- Kim, J.S.; Hwang, M.H.; Kang, N. Bilateral deficits during maximal grip force production in late postmenopausal women. Appl. Sci. 2021, 11, 8426. [Google Scholar] [CrossRef]
- Hu, X.; Loncharich, M.; Newell, K.M. Visual information interacts with neuromuscular factors in the coordination of bimanual isometric force. Exp. Brain Res. 2011, 209, 129–138. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef]
- Lasko, T.A.; Bhagwat, J.G.; Zou, K.H.; Ohno-Machado, L. The use of receiver operating characteristic curves in biomedical informatics. J. Biomed. Inform. 2005, 38, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, J.M.; Luchies, C.W.; Richards, L.G.; Zebas, C.J. The effects of age and feedback on isometric knee extensor force control abilities. Clin. Biomech. 2002, 17, 486–493. [Google Scholar] [CrossRef]
- Tracy, B.L. Force control is impaired in the ankle plantarflexors of elderly adults. Eur. J. Appl. Physiol. 2007, 101, 629–636. [Google Scholar] [CrossRef]
- Sosnoff, J.J.; Newell, K.M. Aging, visual intermittency, and variability in isometric force output. J. Gerontol. B Psychol. Sci. Soc. Sci. 2006, 61, P117–P124. [Google Scholar] [CrossRef] [PubMed]
- Tracy, B.L.; Hitchcock, L.N.; Welsh, S.J.; Paxton, R.J.; Feldman-Kothe, C.E. Visuomotor correction is a robust contributor to force variability during index finger abduction by older adults. Front. Aging Neurosci. 2015, 7, 229. [Google Scholar] [CrossRef] [PubMed]
- Oomen, N.M.; van Dieen, J.H. Effects of age on force steadiness: A literature review and meta-analysis. Ageing Res. Rev. 2017, 35, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.N.; Shinohara, M. Corticomuscular coherence with and without additional task in the elderly. J. Appl. Physiol. 2012, 112, 970–981. [Google Scholar] [CrossRef]
- Baweja, H.S.; Patel, B.K.; Martinkewiz, J.D.; Vu, J.; Christou, E.A. Removal of visual feedback alters muscle activity and reduces force variability during constant isometric contractions. Exp. Brain Res. 2009, 197, 35–47. [Google Scholar] [CrossRef]
- Patten, C.; Kamen, G. Adaptations in motor unit discharge activity with force control training in young and older human adults. Eur. J. Appl. Physiol. 2000, 83, 128–143. [Google Scholar] [CrossRef]
- Cheng, F.W.; Gao, X.; Mitchell, D.C.; Wood, C.; Still, C.D.; Rolston, D.; Jensen, G.L. Body mass index and all-cause mortality among older adults. Obesity 2016, 24, 2232–2239. [Google Scholar] [CrossRef]
- Oreopoulos, A.; Kalantar-Zadeh, K.; Sharma, A.M.; Fonarow, G.C. The obesity paradox in the elderly: Potential mechanisms and clinical implications. Clin. Geriatr. Med. 2009, 25, 643–659. [Google Scholar] [CrossRef] [PubMed]
- Bales, C.W.; Buhr, G.T. Body mass trajectory, energy balance, and weight loss as determinants of health and mortality in older adults. Obes. Facts 2009, 2, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Soraya, N.; Parwanto, E. The Controversial Relationship between Body Mass Index and Handgrip Strength in the Elderly: An Overview. Malays. J. Med. Sci. 2023, 30, 73. [Google Scholar] [CrossRef] [PubMed]
- Lenardt, M.H.; Grden, C.R.B.; Sousa, J.A.V.d.; Reche, P.M.; Betiolli, S.E.; Ribeiro, D.K.M.N. Factors associated with loss of handgrip strength in long-lived elderly. Rev. Esc. Enferm. USP 2014, 48, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Sergi, G.; Perissinotto, E.; Toffanello, E.D.; Maggi, S.; Manzato, E.; Buja, A.; Coin, A.; Frigo, A.C.; Inelmen, E.M.; Enzi, G. Lower extremity motor performance and body mass index in elderly people: The Italian Longitudinal Study on Aging. J. Am. Geriatr. Soc. 2007, 55, 2023–2029. [Google Scholar] [CrossRef] [PubMed]
- Kıskaç, M.; Soysal, P.; Smith, L.; Capar, E.; Zorlu, M. What is the optimal body mass index range for older adults? Ann. Geriatr. Med. Res. 2022, 26, 49. [Google Scholar] [CrossRef]
- Kennedy, K.M.; Raz, N. Age, sex and regional brain volumes predict perceptual-motor skill acquisition. Cortex 2005, 41, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Koppelmans, V.; Hirsiger, S.; Mérillat, S.; Jäncke, L.; Seidler, R.D. Cerebellar gray and white matter volume and their relation with age and manual motor performance in healthy older adults. Hum. Brain Mapp. 2015, 36, 2352–2363. [Google Scholar] [CrossRef] [PubMed]
- Fling, B.; Seidler, R. Fundamental differences in callosal structure, neurophysiologic function, and bimanual control in young and older adults. Cereb. Cortex 2012, 22, 2643–2652. [Google Scholar] [CrossRef]
- Ryberg, C.; Rostrup, E.; Paulson, O.; Barkhof, F.; Scheltens, P.; Van Straaten, E.; Van Der Flier, W.; Fazekas, F.; Schmidt, R.; Ferro, J. Corpus callosum atrophy as a predictor of age-related cognitive and motor impairment: A 3-year follow-up of the LADIS study cohort. J. Neurol. Sci. 2011, 307, 100–105. [Google Scholar] [CrossRef]
- Fling, B.W.; Walsh, C.M.; Bangert, A.S.; Reuter-Lorenz, P.A.; Welsh, R.C.; Seidler, R.D. Differential callosal contributions to bimanual control in young and older adults. J. Cogn. Neurosci. 2011, 23, 2171–2185. [Google Scholar] [CrossRef] [PubMed]
- Larivière, S.; Xifra-Porxas, A.; Kassinopoulos, M.; Niso, G.; Baillet, S.; Mitsis, G.D.; Boudrias, M.H. Functional and effective reorganization of the aging brain during unimanual and bimanual hand movements. Hum. Brain Mapp. 2019, 40, 3027–3040. [Google Scholar] [CrossRef] [PubMed]
- Heuninckx, S.; Wenderoth, N.; Swinnen, S.P. Systems neuroplasticity in the aging brain: Recruiting additional neural resources for successful motor performance in elderly persons. J. Neurosci. 2008, 28, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.; Frackowiak, R. Age-related changes in the neural correlates of motor performance. Brain 2003, 126, 873–888. [Google Scholar] [CrossRef] [PubMed]
- Mora, F.; Segovia, G.; Del Arco, A. Glutamate–dopamine–GABA interactions in the aging basal ganglia. Brain Res. Rev. 2008, 58, 340–353. [Google Scholar] [CrossRef]
- Seidler, R.D.; Bernard, J.A.; Burutolu, T.B.; Fling, B.W.; Gordon, M.T.; Gwin, J.T.; Kwak, Y.; Lipps, D.B. Motor control and aging: Links to age-related brain structural, functional, and biochemical effects. Neurosci. Biobehav. Rev. 2010, 34, 721–733. [Google Scholar] [CrossRef]
- Berger, A.; Steinberg, F.; Thomas, F.; Doppelmayr, M. Neural correlates of age-related changes in precise grip force regulation: A combined EEG-fNIRS study. Front. Aging Neurosci. 2020, 12, 594810. [Google Scholar] [CrossRef]
- Mattay, V.S.; Fera, F.; Tessitore, A.; Hariri, A.; Das, S.; Callicott, J.; Weinberger, D. Neurophysiological correlates of age-related changes in human motor function. Neurology 2002, 58, 630–635. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).