Abstract
This paper presents various aspects of the use of chromatography to determine the biological activity of substances. On the one hand, the use of chromatography to determine the lipophilicity of a substance, a property that affects all LADME steps in various biomimetic systems, is presented, using various descriptors such as the retention factor in pure water (or buffer with physiological plasma pH), the CHI value, and Chrom logD. The use of chromatography in biomimetic systems to determine the interaction of substances with phospholipids (IAM stationary phases) and transport proteins (stationary phases with immobilised proteins) is also discussed. On the basis of the retention data obtained in these systems, the volume of distribution of the substance and the degree of binding of the substance with the proteins in question can be determined. Chromatography is also a method used to determine the interaction of substances with specific membrane receptors at their site of action using membrane chromatography (MCM). Thanks to biological detection, chromatography can also be used to determine the antimicrobial activity (bioautography) of substances and the effect of substances on biochemical reactions taking place in organisms, such as antioxidant properties and the inhibitory activity of various enzymes (biological assay).
1. Introduction
Biologically active substances can be identified among the many naturally occurring and synthesised substances. According to current pharmaceutical legislation, a biologically active substance (API) is a component of a medicinal product that is responsible for its pharmacological, metabolic, and immunological effects. The purpose of using such substances is to treat or prevent diseases and to restore the normal functioning of the organism. In nature, biologically active substances are usually secondary metabolites of plants. Biologically active substances can also be formed during the fermentation of foods [1,2].
The biological activity of a substance is related to its physicochemical and chemical properties [3,4,5,6,7,8,9,10]. A biologically active substance has an effect on the functioning of an organism if it is present in the right concentration in the vicinity of the corresponding receptor—its site of action. From administration to interaction with the receptor, the substance goes through a series of stages: release of the active substance from the preparation—in the case of non-intravenous administration, uptake into the bloodstream (absorption), and distribution to the individual tissues.
In order to reduce the number of in vivo and in vitro studies on the biological activity of substances, methods are being sought to determine biological activity on the basis of knowledge of the physicochemical properties of substances. Chromatographic methods are part of this research trend. The role of chromatographic studies in the biological activity of compounds includes the determination of:
- -
- The physicochemical parameters that determine the biological activity of a compound [11,12,13].
- -
- Pharmacokinetic parameters from chromatographic data [14,15,16,17,18,19,20].
- -
- The interaction with cellular or plasma proteins [18,20,21,22,23,24].
- -
- The effects of the substance on microorganisms and its enzyme-inhibiting properties and hormonal activity [25,26,27,28,29].
2. Biomimetic Chromatographic System
Biomimetic chromatographic systems are systems that mimic the biological environment of drug action. The chromatography in such systems is called biomimetic chromatography [17,26,27].
2.1. Stationary Phases in Biomimetic Chromatographic Systems
Substances that enter the body interact with biological membranes—cell membranes consisting of a phospholipid bilayer, with transport proteins in the blood and with membrane receptors. Until recently, the most popular biomimetic phases in chromatography were non-polar stationary RP phases with aliphatic ligands C-18 and C-8. Such phases mimic the non-polar interior of a biological membrane. These phases are used in reversed phase chromatography (RP) and micellar liquid chromatography (MLC) [30,31,32,33]. Other stationary phases that mimic cell membranes include IAM (Immobilised Artificial Membrane) and phases called IAM.PC DDC, which were developed by Pidgeon [34]. These phases are manufactured on a silicon dioxide base with phosphatidylcholine, the main component of cell membranes. The matrix of the IAM phase is silicon dioxide with chemically bound phosphatidylcholine molecules, which form a structure on their surface that is identical to the monolayer of the cell membrane (Figure 1). The retention values of substances obtained on such columns illustrate the interactions of the molecules of the substance with phospholipids—the affinity to phospholipids is essential for the permeation of substances through biological membranes [35,36]. High retention characterises molecules that have a high affinity for phospholipids—too high an affinity for phospholipids reduces the permeability of the compound, as it prefers to remain within the cell membrane. Such compounds are not only poorly absorbed and distributed in the body but can also disrupt the cell membrane or disrupt phospholipid metabolism in the cells (phospholipidosis). New stationary phases with phospholipids are those with immobilised sphingomyelin (SPH) [37] and phosphatylethanolamine. Stationary phases with phospholipid bilayers are immobilised—liposomes are stationary phases [38,39]. Stationary phases with active cell membranes—cell membrane stationary phases (CMSP)—are used to study the interactions of substances with membrane receptors. Fragments of cell membranes are deposited on the surface of silica or a polymer by physical adsorption, whereby the structure of the cell membranes is preserved and the spatial structure of the membrane receptors, their environment, and the enzyme activity remain unchanged [40,41]. Currently, CMSP phases are obtained from cell cultures with high expression of a specific membrane receptor. Cholesterol phases are another stationary phase that contains an important component of the cell membrane. Like the IAM phases, they are usually composed of a silica base with chemically bound cholesterol molecules (Figure 1). Chromatography with stationary membrane phases is known as biomimetic phospholipid membrane chromatography (BPMC) [42].
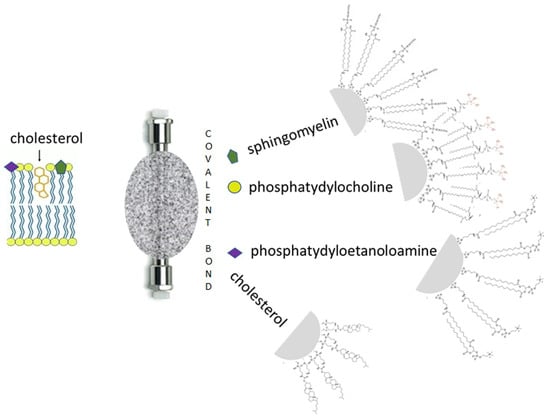
Figure 1.
Stationary phases composed of compounds that are part of cell membranes.
In stationary phases with immobilised proteins, interaction with proteins plays an important role in the pharmacological activity of a substance. The binding of a drug to proteins influences the volume of distribution of the drug, the absorption, and the duration of action. The most common stationary phases are those with immunised albumin and globulins [41,43,44]. Studies have shown that proteins in such stationary phases retain their most important properties, including sorption properties that determine their interaction with substances, including biologically active substances. When using columns with immobilised proteins, it is important to check that the proteins are not deactivated during the experiment. Many researchers recommend injecting a racemic warfarin mixture before each experiment to check the availability of the main HSA binding site—if the racemates separate, it means that the warfarin binding sites are active.
2.2. Mobile Phases in Biomimetic Chromatographic Systems
The composition of the mobile phases in biomimetic chromatographic systems depends on the type of stationary phase and the properties of the substances to be analysed, but the best possible representation of the operating environment of the substance should be taken into account.
Chromatographic systems with C-18, IAM, and cholesterol stationary phases are RP systems. The mobile phases used in these systems are aqueous–organic mixtures. Especially in studies on the biological activity of compounds, it is important that the ‘aqueous’ part of the mobile phase has properties as similar as possible to those of the drug. Therefore, mobile phases in which water is replaced by a buffer solution with a physiological plasma pH of 7.4 are often used to test the biological activity of compounds [45,46]. Instead of water, a solution that simulates the composition of blood plasma—SBF (Simulated Body Fluid)—can also be used [47].
For phases with immobilised proteins or phases with MCSP cell membranes, mobile phases should be used whenever possible, since they simulate the active environment of the substance but do not degrade the biomolecules.
A special mobile phase for biomimetic systems is micellar mobile phases, which are used in micellar liquid chromatography. These phases are aqueous solutions of surfactants above the critical micellization concentration. In addition to the surfactant monomers, such a solution also contains micelles that are similar in structure to cell membranes [48,49,50]. BRIJ 35 surfactant micelles are considered to be a very good imitation of cell membranes. Micellar chromatography with this surfactant is known as bio-partitioning micellar chromatography (BMC) [51,52,53].
3. Chromatography in the Determination of Lipophilicity of Compounds
In order to reach the site of action, a substance must overcome a number of biological barriers—cell membranes. In order to penetrate a cell membrane, a substance must overcome its lipophilic interior. According to the principle of ‘like dissolves like’, the ability of a substance to penetrate biological membranes depends on its lipophilic properties. Therefore, lipophilicity is one of the most important physicochemical properties of biologically active substances after interaction with the corresponding receptor. The ability of a substance to penetrate biological membranes has a direct influence on the processes that the substances undergo in the body—absorption, distribution and, consequently, the biological activity of the substance. For a substance to have a therapeutic effect, it must be present at the site of action in the correct concentration (the so-called therapeutic window). To summarise, lipophilicity is the property of a substance that allows us to predict the ability of that substance to penetrate cell membranes; its ADME profile, i.e., the individual processes that describe the fate of the drug in the body; and its ability to reach the site of action [10,54].
Quantitatively, lipophilicity describes the partition coefficient of a substance between n-octanol and water, P. It is one of the first parameters determined to describe the biological activity of a substance in testing different substances. Nowadays, many new substances that are potential drugs are developed every day. To be a drug, a substance must of course have pharmacokinetic properties, in addition to its effect on the body. Determining the biological activity of a substance, especially in the early stages of research, can be achieved outside living organisms, using biomimetic systems. Based on the knowledge of physiological fluid composition and cell membrane structure, a model system for n-octanol–water extraction was developed for the determination of P values. It was found that within the limits of logP 1–3, the penetration rate through biological membrane substances is directly proportional to the logP value.
The partition coefficient P was first used by Fujita et al. to determine the lipophilicity of substances [55]. It can be determined by the extraction method (shake flash)—until recently, this was the method recommended by IUPAC. Although this method is easy to perform, it has a number of limitations: the substances used must be very pure and large amounts of the substance and non-polar organic solvents are required. Therefore, alternative methods for the determination of P values are being sought out—one of these methods is chromatography in systems that mimic the environment of the drug. Collander observed a linear relationship between the partition coefficients of substances obtained in different aqueous extraction systems that differ in the organic solvents [56].
The retention factor of a substance (k) is the ratio between the amount of the substance in the stationary phase and the mobile phase and therefore logk values can be equated with logP values (Figure 2).
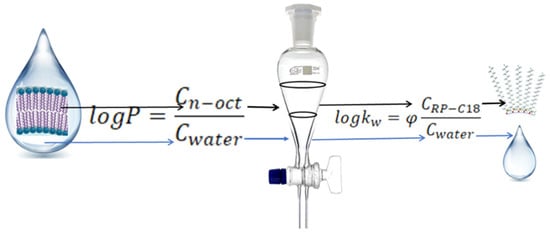
Figure 2.
Relationship between logP and logkw.
If the stationary phase mimics cell membranes and the mobile phase mimics physiological fluids, the retention factor in such a chromatographic system can be equated to the Po/w partition coefficient. For many substances, a linear relationship between logkw in a biomimetic system and log Po/w has been observed, which is a special case of Collander’s equation:
where logkw—logarithm of the retention factor, a, b—constants describing the rectilinear relationship (1).
logkw = alogPo/w + b
The simplest biomimetic system is the chromatographic system, which involves a stationary phase; a non-polar phase (usually C-18, which imitates the non-polar part of the cell membrane); and a mobile phase (usually water or a buffer solution with a pH of 7.4), which imitates physiological fluids. The determination of kw values directly in the RP system is not possible for most substances due to the hydrophobicity of the stationary phase and the zero elution power of water in RP systems. Therefore, the logkw values are determined by extrapolating the straight-line dependence of the logkw value on the content of organic modifiers in the aqueous–organic mobile phase. This is achieved using the Soczewiński–Wachtmeister equation [57] (Figure 3).
where logk is a retention factor of the substance obtained in a chromatographic system with a mobile phase with the concentration of the organic modifier ϕ; s represents the slope of the straight line; logkw is the log of the value of the retention factor of the substance in water when water is acting as the mobile phase.
logk = logkw − s ϕ
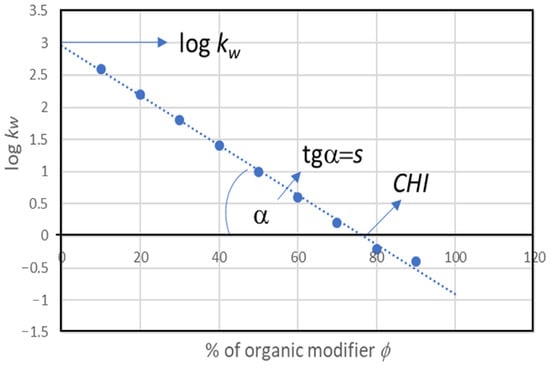
Figure 3.
Lipophilicity descriptors obtained from the Soczewiński–Wachtmeister equation (Equation (2)).
Three parameters that describe the lipophilicity of substances can be derived from the Soczewiński–Wachtmeister equation. The first is the retention factor kw, which can be identified based on the partition coefficient of the substance P. The second is the s value, which informs us about the non-polar part of the substance, and the last parameter (CHI (0)) is the amount of organic modifier in the mobile phase where the retention factor is equal to one [58,59].
The CHI values range from 0 (for hydrophilic) to 100 (for more lipophilic) substances. In extreme cases involving very hydrophilic or lipophilic substances, these values can be exceeded. However, it should be noted that substances with such extremely lipophilic properties have unfavourable pharmacokinetic parameters. To avoid a series of isocratic measurements, fast gradient chromatography was proposed to determine the chromatographic hydrophobicity index (CHI). In the RP system with fast gradient elution, the compounds hardly move in the column until the corresponding concentration of the mobile phase is reached. A straight line relationship was observed between the CHI values obtained during isocratic elution and the retention times of the compound during fast gradient elution [26,60,61].
It was found that although correlations between logkw and logP n-octanol–water are observed, the values of logkw and logP differ for some reasons:
- -
- Chromatography is a dynamic process, whereas the extraction process is a static equilibrium process;
- -
- The chromatographic process of substance distribution between the stationary and the mobile phases takes place on a much larger surface than in the extraction method (as in the body);
- -
- The C-18 phase has a more structured structure than liquid n-octanol (the structure of the stationary C-18 phase is more similar to the structure of the cell membrane than n-octanol).
When comparing the logP values with the logkw values, it was found that significant differences occur with proton donor compounds, since the -OH group of n-octanol is a proton acceptor, whereas the C-18 ligands of the stationary phase are not.
The values of the obtained lipophilicity descriptors depend on the type of organic modifier. Linear relationships were observed, with large fitting coefficients between CHI logP and logPo/w (n-octanol–water) values obtained in the chromatographic system in which the organic modifier was acetonitrile. When methanol was used as the organic modifier, the relationship was parabolic [60].
For large amphiphilic molecules such as peptides and macrolides, the n-octanol–water system is not used to determine the partition coefficient P value. In the extraction system, these molecules accumulate at the interface and, for this reason, their concentration in the volumetric phases in the extraction system is underestimated. To determine the acid–base properties of compounds with large molecules, which play a major role in the distribution of substances, pH gradient elution chromatography is used. Compounds that can be present in ionic form show different lipophilicity in different pH environments [62,63].
New descriptors, CHI logD and Chrom logD (D partition coefficient), were created to better describe the lipophilic properties of substances, taking into account their ionisation in the substance’s active medium. These can be derived from the CHI value using the following relationship [60]:
- -
- CHI logD = 0.054 ChI − 1.467—obtained on the basis of 98 compounds.
- -
- Chrom logD = 0.088 ChI − 2—obtained on the basis of 40,000 compounds.
- -
- The Chrom logD values are about two times higher than the logD values of octanol water.
Although the n-octanol–water partition coefficient characterises the lipophilic properties of substances, it is not suitable for estimating the uptake and distribution of molecules ionised at a physiological blood plasma pH (7.4).
Analysing the logkw values obtained in different biomimetic chromatographic systems can provide us with information on the interactions of substances with cell membranes. Kaliszan et al. compared the logP values obtained in the n-octanol–water system with the logkw values of substances obtained in systems with stationary IAM and C-18 phases for compounds with different acid–base properties from different pharmacological classes and showed that these values correlate differently, indicating different interactions of substances with non-polar phases and IAM phases [64,65].
The lipophilicity of substances can also be determined in micellar biomimetic systems [51,66,67,68,69,70]. Micellar liquid chromatography (MLC) is reversed-phase chromatography with C-18 stationary phase (most often used) or C-8 or RP-NH2 or RP-CN stationary phase and mobile phase—aqueous surfactant solutions above the critical micelle concentration (cmc). In such a system, there are two media that mimic biological membranes—the stationary phase (RP) and the micelles in the mobile phase. The retention of substances in micellar systems depends on the interaction between stationary phases, mobile phases, and micelles (Figure 4). The retention in MLC depends on the concentration of the micelles in the mobile phases and is described by Foley’s equation:
where [M]—concentration of the micellated surfactant, which corresponds to the difference between the total concentration of the surfactant (CS) and its critical micelle concentration (Ccmc), [M] = Cs—Ccmc, k—retention factor of the substance in the mobile phase with the concentration [M], km—retention factor of the substance at zero concentration of the surfactant Ccmc.
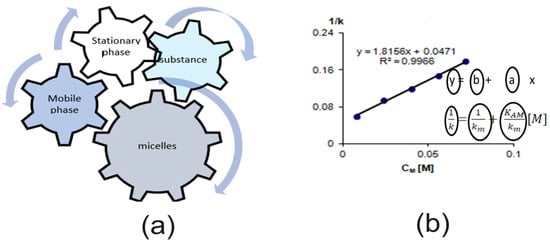
Figure 4.
(a) interaction in a MLC system; (b) parameters obtained from Foley’s equation (Equation (3)).
Based on this relationship, the interaction of the substance with the micelles (KAM value) and with the RP stationary phase (km value) can be determined.
In micellar systems, Foley’s equation provides us with information about the interaction of substances with two models of biological membranes, namely the stationary phase and the micelles. It was found that the logkm values obtained in micellar systems with C-18 mobile phase differ from the logkw values obtained in RP systems [71]. The reason for this is that the stationary phase is modified by surfactant molecules, and this modification changes its character. Linear correlations with a large fitting coefficient were detected between the logkm values obtained for the test substances and the logkw values obtained in systems with the IAM stationary phase, indicating the similarity of the interactions of these substances with the IAM phase and the BRIJ-35 monomer-modified C-18 phase. This is of great economic and environmental importance. IAM columns are expensive columns which do not have very long lifetimes, and the mobile phases used are aqueous–organic solutions with a high content of organic modifiers, whereas C-18 columns are many times cheaper, have a longer lifetime, and the mobile phases used in MLC systems contain small amounts of organic modifiers.
If the mobile phase in MLC is a surfactant buffer, the elution power of such a phase is often not sufficient to obtain the retention coefficients of substances in the analytical range. Therefore, an organic modifier is added to the mobile phase, and this is referred to as hybrid micellar chromatography. In addition, the presence of organic modifiers in the mobile phase affects the properties of the stationary phases in micellar systems, as it causes the desorption of surfactant monomers from their surface. Hence, the values resulting from the Foley relationship in hybrid micellar systems differ from each other.
4. Chromatography in the Determination of Pharmacokinetic Parameters of Substances
The pharmacokinetic parameters of a drug enable the determination of the drug dosage and the prediction of the expected drug efficacy. Compounds with optimal lipophilicity often have suitable pharmacokinetic parameters so that the determination of the lipophilic properties of compounds can be used to determine important pharmacokinetic parameters.
If the number of aromatic rings of a substance is combined with the Chrom logD value, the so-called PFI values (Property Forecast Index) are obtained [72,73,74]. This parameter indicates which properties of a compound influence the LADME process.
The PFI value is calculated on the basis of the following relationship:
where n is the number of aromatic rings in the molecule and Chrom logD is determined based on chromatographic measurements.
PFI = Chrom logD + n
Based on the analysis of PFI values for more than 40,000 substances, compounds with PFI values between three and six were found to have good pharmacokinetic parameters.
The distribution of a drug is another important process that influences the pharmacological effect of the preparation. The pharmacokinetic parameters that characterise the distribution of a drug in the body are the distribution constant, the volume of distribution, and the degree of binding of the drug to blood proteins. The volume of distribution can be defined as the ratio between the drug dose and the plasma concentration at steady state:
where Vd—the volume of distribution [dm3], X—the drug dose (total content of the drug in the body), C—the measured concentration of the drug in the blood (serum).
This quantity (volume of distribution), which helps to describe the quantitative distribution of the drug in the body, is proportional to the distribution of the drug between the plasma and the tissue compartment.
- -
- If Vd < 5 dm3, then the drug is in the blood and it is distributed only in the circulatory system.
- -
- If 10 dm3 < Vd < 20 dm3, this means that the drug penetrates the extracellular fluid;
- -
- If 25 dm3 < Vd < 30 dm3, the drug is in the intracellular fluid (ICFV);
- -
- If it is approx. 40 dm3, the drug is distributed in all the bodily fluids;
- -
- If it is approx. 100 dm3, this means that the drug accumulates in tissues and organs.
Drugs of lipophilic nature (e.g., diazepam) easily penetrate cell membranes and have a large volume of distribution, as their plasma concentration is low. Drugs that bind to plasma proteins have a small volume of distribution (up to 10 dm3), drugs that have an affinity for binding to peripheral tissue have a very high volume of distribution (e.g., digoxin—about 500 dm3), whereas azithromycin, which accumulates in intracellular lysosomes, has a volume of distribution of about 2000 dm3.
The volume of distribution depends on the affinity of a substance for phospholipids and plasma proteins (Figure 5). It has been observed that positively charged compounds bind more strongly to phospholipids and negatively charged cell membranes, indicating a high accumulation of drugs in tissues [75]. Although tissues contain large amounts of proteins (including albumin), they have many barriers, many biological membranes, and other lipid components, so the accumulation of drugs in tissues is mainly determined by the interactions of molecules with lipids. Negatively charged compounds bind more strongly to albumin and remain present in the blood plasma. In summary, compounds that bind more strongly to phospholipids show greater accumulation in tissues than compounds that bind strongly to plasma proteins, which mostly remain in the blood. Until recently, the value of the volume of distribution could only be determined by in vivo experiments. The determination of this volume is of great importance for determining the efficacy of a drug and should be carried out at an early stage of drug development. Therefore, the search for methods other than in vivo methods to estimate the volume of distribution is very important. The affinity of drugs for phospholipids and proteins can be determined by retention volumes in two biomimetic chromatographic systems with phospholipid (IAM) and protein (HSA) phases [20,76,77,78,79].
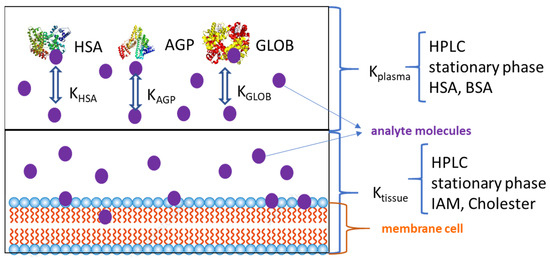
Figure 5.
Distribution of a substance in plasma and tissue.
4.1. Determination of the Distribution of Substances in Tissues
Chromatographic data obtained in IAM systems and with HSA phases can be used to estimate the distribution of substances in tissues. There are different proportions of phospholipids and proteins in the tissues, and the compositions of the cell membranes of the cells of different tissues differ from each other. Depending on the proportions of phospholipids and proteins in different tissues, the binding of drugs to tissues can be modelled by weighted sums of albumin and IAM binding. The binding of a substance in mucus can be modelled by determining the binding of the substance to a glycoprotein (AGP), since 90% of mucus is a glycoprotein. The permeability of a substance through the blood–brain barrier can be estimated by modelling the binding to brain tissue and the binding to plasma proteins. Of course, such studies only give us estimates, but with their help, it is possible to identify compounds that can penetrate the central nervous system. The distribution of drugs in the brain is determined by the properties of the molecule itself, such as its lipophilicity, its small size, its lack of binding to plasma proteins and its presence in a non-dissociated form.
Any drug that is absorbed can remain in the blood—in which case, its duration of action is relatively short—or bind to the so-called transport proteins (serum albumin, α- and β-globulins, and acidic α-1-glycoprotein). A drug that is bound to a protein is not pharmacologically active—it is not transported to the tissues, is not metabolised, and is not excreted from the body. The binding of a drug by plasma proteins influences the concentration of the free form of the drug, which in turn influences the drug dose, the duration of the drug effect, and many other pharmacokinetic parameters. Different disease states cause changes in the amount of transport proteins, meaning that the drug dose may not be correct in some cases. Therefore, estimating the degree of binding of a drug, in addition to determining the volume of distribution, is of great importance, especially for drugs with a narrow therapeutic index (the effect of the drug may be ineffective or side effects may occur).
The factors that influence the degree of binding of a drug to a protein are as follows:
- -
- The pH of the plasma—it can alter the ionisation of many compounds;
- -
- The drug concentration—different saturation of the binding sites;
- -
- The protein concentration—disease-related changes, age;
- -
- The affinity of the drug molecules for protein-binding sites (physicochemical properties);
- -
- The presence of other substances in the body that bind to proteins at the same active sites;
- -
- Exogenous (drugs, toxins), endogenous (urea, bilirubin, fatty acids, hormones).
The degree of binding of a drug to proteins can be determined in systems with stationary phase HSA or BSA—basic transport proteins. It has been found that the retention times of drugs with protein phases are related to the percentage of drug binding by the following equation [60,77]:
Chromatographic measurements with gradient elution are frequently carried out. In systems with HSA columns, a phosphate buffer with a pH value of 7.4 is used as the mobile phase and 2-propanol (up to 35% by volume, as the proteins of the stationary phase are denatured above this concentration) is used as the organic modifier. Since the retention time of the substance in gradient elution depends on many factors, e.g., the flow rate of the mobile phase and the size of the column, it is necessary to calibrate the retention times in gradient elution with compounds whose percentage binding is known and which have been obtained using other methods, e.g., equilibrium dialysis, organic modifiers, ultrafiltration, capillary electrophoresis, etc., in order to standardise the results obtained so that the data can be compared between different laboratories. It is also necessary to check the stationary phase of the protein to ensure that no denaturation or irreversible binding of the protein has taken place during the measurements. Since the binding of substances to proteins and phospholipids occurs naturally at physiological pH values, there is no need for mobile phases with different pH values [26,60].
The calibration measurements are carried out for 8–10 compounds. Based on the value of the percentage of the bound form of the drug and the chromatographic test data, a calibration curve is drawn up for the dependence of the retention time in the gradient elution and the percentage of the bound form of the drug.
Using chromatographic methods, it is possible to determine the interaction of a drug with a specific protein—the degree of binding of the drug to the protein. For this purpose, the drug–protein association constant (KD) is determined by chromatography with stationary phases in which proteins are immobilised. The investigation of the drug–protein interaction can be carried out in various ways.
4.1.1. Determination of KD Values Using Zonal Elution
Zonal elution is the most commonly used method for determining the association constant. During the zonal elution method, a substance which has a known binding site to a specific receptor is added to the mobile phase (in different concentrations). This is referred to as the competing agent. Meanwhile, the test substance is added to the chromatographic column to test its retention in such a system. The higher the concentration of the competing substance, the lower the retention of the test substance. This method assumes that the competing substance and the test substance are bound with some kind of bond. There is a linear relationship between the retention factor of the substance and the molar concentration of the competing substance in the mobile phase. This relationship can be represented by the following equation [80]:
where k is the retention coefficient of the analysed substance, V is the empty volume of the column, [I] is the concentration of the competing agent, mL represents the moles of binding sites in the column, and KA and K1 are the association constants of the competing agent or analyte.
4.1.2. Determination of KD Values Using Frontal Analysis
In the frontal analysis method, the analyte is continuously fed to the chromatographic column. Depending on the concentration of the analyte in the mobile phase, the chromatographic column is broken through at different times. The analyte is added to the column until a plateau is reached in the breakthrough curve (Figure 6). KD values can be obtained by the following equation:
where mLapp is the number of moles of the analyte required for column breakthrough (the mean position of the breakthrough curve)—it can be determined based on the breakthrough time, the volumetric migration rate of the mobile phase, and the concentration of the analyte in the mobile phase.
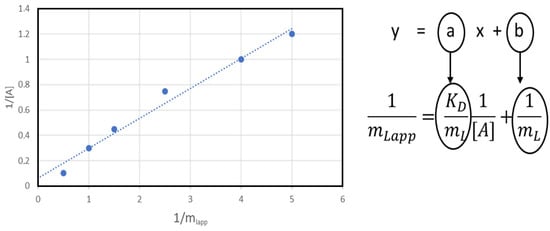
Figure 6.
Determination of KD values from Equation (8).
4.1.3. Determination of KD Values against a Standard
Another method for determining the KD value is to determine the receptor dosage constant based on the retention time of the substance. The method for determining the KD value in relation to a standard is to determine the value of the retention factor for an S standard with a known KDS:
where kx and ks represent the retention coefficient of the investigated substance and standard, respectively; KDX and KDS represent the KD value of the investigated substance and the standard, respectively.
This is a practical method that requires much smaller substitution amounts than the frontal analysis method so that KD values for several substances can be determined from a single standard. Zhang et al. investigated the affinity of different ligands for α1-AR and three subtypes of this receptor in stationary-phase CMC systems with cell membranes with high expression of α1A-adrenoreceptor (AR), α1B-AR, and α1D-AR. They ranked the test substances according to increasing affinity for the receptors studied and obtained results that were consistent with those found in vivo in the literature [81]. On the other hand, in stationary phase, Zeng et al. showed CMC systems based on guinea pig cardiac muscle and jejunum cells as well as cardiac muscle, jejunum, and rat brain, and the results obtained were in agreement with those of the frontal analysis.
4.1.4. Chromatography as an Analytical Method for the Determination of Interactions of Substances with Protein
Another application of chromatographic methods to determine the bound form of a drug is their use as methods of quantitative analysis. The percentage of the bound form is determined in a sample of buffer solution (pH = 7.4), test substance (at a known concentration), and test protein (at a known concentration). In such a solution, the test substance is adsorbed onto the protein molecules. The protein must then be removed from the solution, usually by adsorption onto suitable adsorbents. The deproteinised sample prepared in this way is subjected to quantitative analysis in a chromatographic system that is adapted to the type of drug.
Another way to determine the percentage of the bound form, which allows the tedious step of deproteinising the sample to be avoided, is to use micellar chromatography to quantify the percentage of the bound drug. In micelle chromatography, proteins can dissolve in micelles so that a protein-containing sample can be injected into the chromatography column [18,19,49,54,82].
In order to determine the free form of drugs with a micellar system, it is necessary to use a surfactant whose surface charge of the micelles is opposite to that of the protein—they then become binding analytes. In mimetic MLC systems, the pH value of the mobile phase is 7.4 (physiological plasma pH value). The pI value of albumin is 5.8. Under the conditions of the experiment, the albumin molecules are therefore negatively charged at pH = 7.4. Thus, it is important to use a cationic surfactant (e.g., CTAB), which forms positively charged micelles, and not an anionic one (e.g., SDS).
5. Cell Membrane Chromatography (CMC)
CMC is a biomimetic chromatographic method that was proposed by He et al. in the early 1990s and is based on the ability of substances to bind to cell membrane receptors [83,84]. Membrane receptors are integral proteins of the cell membranes which transfer information into the cell by interacting with substances that cannot penetrate the cell membranes (ligands). About 45% of small molecule drugs act by interacting with membrane receptors. Such studies are mostly conducted in vivo—they are important because they inform us about the interactions that directly produce the therapeutic effect of the drug. Therefore, it was hypothesised that chromatography with stationary phases containing membrane receptors could be used to study such interactions. The interaction of a specific ligand with a receptor leads to an increase in the retention of the substance.
Membrane cell chromatography is an HPLC method, in which the retention of a substance depends on its affinity to membrane receptors, and this influences its retention. The information obtained from CMC, combined with knowledge of cell biology and receptor pharmacology, enables the evaluation of the interaction of a specific ligand with a specific receptor and the simulation of the drug action process. With the development of molecular biology, methods for the cultivation of cell lines with high expression of certain receptor proteins have been developed, and such lines are now recommended for the preparation of the CMSP phase. In recent years, cell lines with high expression of certain receptor proteins have been cultivated with the help of modern molecular biology tools. Plants have always been factories for biologically active compounds. Their therapeutic effects are observed when plant-derived preparations are used. It is therefore important to identify the active substances in plants and herbal preparations that have a therapeutic effect in certain diseases. In the case of substances whose therapeutic effect is achieved through interactions with membrane receptors, the chromatography of cell membranes can provide answers. In order to identify substances that exhibit activity on the receptors in question in question and therefore exert a corresponding therapeutic effect, a sample of an extract of a particular plant is applied to a CMC column, usually with cell lines with high expression of the receptors in question. Substances that interact with receptors have longer retention times than non-receptors. The coupling of CMC with MS enables the identification of the substance. Due to the interaction of substances with receptors, KD values can be obtained by zone elution frontal analysis and the relative standard method described above [85]. Using cell membrane chromatography in combination with mass spectrometry, a number of substances have been identified that interact with specific membrane receptors from different plant materials. CMC is also a chromatography with EGFR receptor (which is strongly expressed in tumour cells and can be used to determine the interaction of biologically active substances with specific receptors. Zang and others have determined the KD value for nine substances with the receptors α1A-AR, β1B-AR [81,86], on the basis of guinea pig myocardium cell membrane/CMC interaction of some drugs with β-AR receptor [87,88] and M-AChR [89], L-Calcium channels [90], H1R receptor [91], and dopamine receptor [92].
CMC can also be applied to detect substances that act against a specific receptor in natural mixtures. Chromatography of cell membranes is an excellent tool with which to identify substances that interact with specific membrane receptors [93,94,95], as well as substances with specific biological activity: anti-allergic [96], anticancer [97,98,99,100,101,102,103,104,105,106], at anti-cardiovascular disease [107,108,109,110], antineoplastic [104,111,112,113], and allergenic substances [114,115].
6. Bioassay
Another way to test the biological activity of a substance in a single chromatographic step is biological detection. Biological detection methods are used to detect substances that exhibit biological activity. They can be divided into two groups: microbiological methods and microbiochemical methods. Microbiological detection methods test the activity of individual substances or mixtures of substances against microorganisms to determine whether they have the ability to inhibit the reproduction of certain microorganisms—specifically bacteria and fungi. Microbiochemical detection methods analyse the effects of substances or substance mixtures on biochemical reactions. Microbiochemical detection methods can be used to determine the inhibitory properties of enzymes and the antioxidant properties of substances or substance mixtures and to detect the hormonal effects of substances. The combination of thin-layer chromatography and biological detection is known as bioautography. Bioautography was first introduced as a detection method in chromatography by Gudall and Levy in 1946 [116], who used paper chromatography with biological detection to estimate the purity of penicillin, and in combination with planar chromatography in 1961 by Fischer and Lautner [117] and Nicolaus et al. for the detection of antibiotics [118]. Nowadays, due to the variety of biological detection methods in chromatographic systems, the term bioautography is mainly used in relation to microbial detection, and when referring to microbiochemical detection, the term bioassay is used.
Bioautography and biological tests are carried out in three stages:
- Stage I:
- Chromatography. This stage encompasses the preparation of the sample for analysis, application of the test samples to the chromatographic plate, and development of the chromatogram, resulting in a separation of the sample components.
- Stage II:
- Bioassays. These make it possible to determine which substances on the chromatographic plate exhibit a particular type of biological activity.
- Stage III:
- Identification of biologically active substances. This is based on a standard or on coupling of TLC with UV/VIS, MS, or NMR, IR detection.
Three different types of bioautography can be implemented (Figure 7):
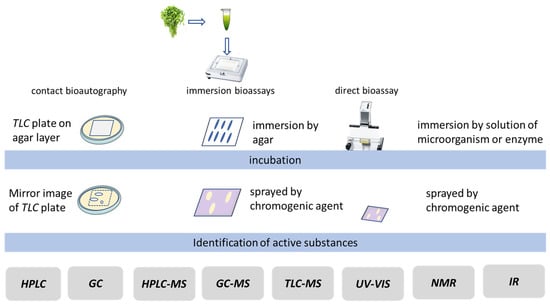
Figure 7.
Scheme of different modes of bioassays.
TLC-CB (contact bioautography). This involves direct contact of the chromatographic plate with the corresponding microorganisms in a Petri dish after the development of the chromatogram with an agar layer. Substances from the chromatographic plate diffuse into the agar layer (a mirror image of the plate is obtained on the agar layer). Substances that exhibit a certain type of biological activity appear in spots that are a different colour than the background colour [119,120,121].
TLC-IBs (immersion/overlay bioassays). In this method, the surface of the chromatographic plate is covered with an agar layer containing the corresponding microorganism or enzyme after the chromatogram has been developed and dried. The substances from the plate diffuse into the agar layer. The plate with the agar layer is then incubated according to the requirements of the respective analysis. The biologically active substances become visible in the form of spots that are a different colour than the plate (background)—[121,122].
In TLC-DBs (direct bioassays), after the chromatogram has been developed and dried, the chromatographic plate is sprayed or immersed in a solution containing microorganisms/enzymes and then subjected to an incubation process. If the microorganisms or enzymes do not show fluorescence or are not naturally coloured, the plate is sprayed with a suitable reagent to create a coloured background of the plate. The substances that exhibit a certain type of biological activity become visible on the chromatographic plate in the form of spots that are a different colour to the background. This variant of bioautography is most frequently used in research [29,121,122,123,124,125].
The effect of direct bioautography is influenced by many factors:
- -
- The composition of the bacterial suspension/enzyme concentration.
- -
- The incubation temperature.
- -
- The incubation time.
- -
- The composition of the mobile phase used to develop the chromatogram.
- -
- Additives to the mobile phase, such as acetic acid or formic acid.
- -
- The pH value of the mobile phase.
- -
- The type of stationary phase.
6.1. Microbial Detection—Determination of Antimicrobial Properties by Bioautography
The bactericidal and fungicidal properties of a substance can be determined by direct bioautography with microbial detection. In this method, suspensions of microorganisms are applied to the chromatographic plate after the chromatogram has been developed by immersion or spraying. The plate prepared in this way is then subjected to incubation for the purpose of multiplying the microorganisms. After incubation, substances that cause inhibition of microorganism multiplication are observed on the chromatographic plate in the form of spots that are a different colour than the background colour of the plate (zones of inhibition of microorganism growth are observed).
In the case of microorganisms that show luminescence (Allivibro fisheri or Photobacterium phosphoreum), under UV light, substances with antibacterial properties are visible on the plate as non-luminescent or luminescent zones of lower intensity on the luminescent substrate (only living bacteria show luminescence) [126]. Some of the bacteria, such as gramm (-) Serracia marcescens, have a natural red colour. After a certain period of inhibition, white or light-yellow zones of bacterial inhibition on a red background are observed on the chromatographic plate. Colourless microorganisms are visualised by adding exogenous dyes, usually tetrazolium salts. Tetrazolium salts penetrate the interior of the bacteria, where they are converted into coloured formazans. Such reactions only take place in living microorganisms. Therefore, the presence of substances that inactivate the microorganisms under investigation is represented by light-coloured spots on a dark background. The tetrazolium salts used in bioautography are 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide—MTT (the most popular tetrazolium salt in bioautography), p-iodinotetrazolium violet (INT) and 2,3,5-triphenyltetrazolium chloride. The microorganisms used in the bioautography investigation are presented in Table 1.

Table 1.
List of microorganisms used in bioautography to test the biological activity of substances.
6.2. Microbiochemical Detection
Thin-layer chromatography can also be a good tool for microbiological detection. As in microbiological detection, the first step involves separating the components of the mixture by thin-layer chromatography (TLC or HPTLC), followed by the determination of the effect of individual substances on the biochemical reactions under investigation, which is usually achieved by a direct bioassay. Another reagent used to determine the antioxidant properties of substances is 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS). It has the ability to react with potassium persulphate. Antioxidants inhibit this reaction and are visible as colourless or pink spots on a green background.
The antioxidant properties of a substance can also be tested with ß-carotene using two methods. In the first method, a chloroform solution of ß-carotene is used. As a result of the photochemical reaction of the antioxidant substance with ß-carotene under visible light, orange-coloured spots on a white or light-yellow background become visible on the chromatographic plate. A modification of this method involves the addition of a linoleic acid solution to the ß-carotene solution. Substances with antioxidant properties become visible on the chromatographic plate in the form of orange-coloured spots on a white or light-yellow background. As a result of this modification, substances that stabilise lipid oxidation can also be seen on the plate in addition to the substances with antioxidant properties.
The type of stationary phase influences the determination of the antioxidant properties of a substance. Non-polar stationary phases, such as C-18, have no influence on the reaction between radicals and antioxidants. If the investigation of antioxidant properties is carried out on a silica gel layer and the plate is sprayed with a methanolic DPPH solution, the results of the antioxidant activity of substances may be overestimated.
Detection of Enzyme Inhibitors by Bioassay
Enzyme reactions are very important in every living organism. They should be specifically catalysed by enzymes that provide optimal amounts of reaction products. Too many products of enzymatic reactions in the body lead to various diseases. In such cases, inhibition of reactions catalysed by a specific enzyme eliminates the causes of disease. The detection of enzyme inhibitors (i.e., the determination of which component of the analysed samples has inhibitory properties against a specific enzyme) can be performed directly on the chromatographic plate on which the components of the test mixtures are separated. The chromatographic plate is sprayed with a solution of the enzyme in question and the substance whose reactions the enzyme catalyses. After the incubation period, the plate is sprayed with a solution of the substance that produces a coloured compound when it reacts with the product of the enzymatic reaction. In the areas of the chromatographic plate containing substances that inhibit the action of the enzyme, the reactions do not take place and these substances (inhibitors of the enzyme in question) are visible on the chromatographic plate as spots that are a different colour to the background colour. The most commonly used bioassays are shown in Table 2.

Table 2.
Bioassays for determination of antioxidant properties and inhibitory properties of substances.
7. Summary
The biomimetic chromatographic systems discussed in this paper are systems that mimic the drug’s environment of action. Biomimetic stationary phases are non-polar phases with different hydrocarbon chain lengths, but also stationary phases that mimic biological membranes consisting of a monolayer of phospholipids forming the cell membrane, protein phases with immobilised proteins, and cholesterol phases. Biomimetic systems are not only systems with a stationary phase that mimics that of biological membranes, but also micellar liquid chromatography systems in which the cell membrane environment is mimicked by surfactant micelles (especially BRIJ 35 -BMC), but also by a stationary C-18 phase whose properties are modified by the sorption of surfactant molecules and mimic the cell membranes better than the C-18 phase.
Chromatography in non-mimetic systems provides much more information about the effect of a drug compared to studies based only on the n-octanol–water partition coefficient. The efficacy of a drug under development can be assessed using chromatographic data from different biomimetic systems early in the drug’s development, long before animal studies are conducted. Chromatographic data can be used to screen new compounds being produced as potential drugs. Chromatographic studies can be performed for compounds that do not meet the Lipinski Rule of Five, i.e., peptides, macrolides [171,172]. The databases already contain a lot of data obtained for different mimetic systems, and based on these data, semi-empirical models have been developed to predict different pharmacokinetic parameters, i.e., the volume of distribution, the degree of binding of the drug to proteins, toxicity, distribution in tissues, and absorption of the drug from different areas of the body. The study of compounds by biomimetic chromatography is limited to compounds with molar masses up to 2000.
The retention data obtained in different biomimetic chromatographic systems can be used for the prediction of different pharmacokinetic parameters. The IAM-HPLC system can be used for the investigation of drug–membrane interactions [173,174] and for predicting the permeability of substances through different biological membranes, e.g., blood–brain permeation [173,175,176]. Based on chromatographic data obtained in different chromatographic systems and theoretical studies, several models for the prediction of different pharmacokinetic parameters have been developed [18,19,54,71,174,177,178,179].
Using chromatographic methods, such as chromatography of cell membranes, it is also possible to study the efficacy of substances’ interaction with membrane receptors and through bioassays. Both chromatography of cell membranes and bioassays on a chromatographic plate are used for two purposes: to test the biological activity of newly synthesised substances with potential therapeutic properties and to determine which naturally occurring substances (most commonly those in plants) exhibit biological activity. In the latter aspect, the coupling of chromatography with various methods that allow the identification of substances, i.e., mass spectrometry, NMR, and many others, is invaluable. The use of chromatographic methods in the study of biological activity, supported by numerical and statistical methods, can significantly reduce the time and cost of testing and animal studies required for newly synthesised substances and can be used to detect biologically active substances occurring in nature.
Author Contributions
Conceptualization, I.M.; writing—original draft preparation, I.M.; writing—review and editing, I.M. and E.G.; visualization, I.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pai, S.; Hebbar, A.; Selvaraj, S. A Critical Look at Challenges and Future Scopes of Bioactive Compounds and Their Incorporations in the Food, Energy, and Pharmaceutical Sector. Environ. Sci. Pollut. Res. 2022, 29, 35518–35541. [Google Scholar] [CrossRef]
- Guaadaoui, A.; Benaicha, S.; Elmajdoub, N.; Bellaoui, M.; Hamal, A. What Is a Bioactive Compound? A Combined Definition for a Preliminary Consensus. Int. J. Nutr. Food Sci. 2014, 3, 174. [Google Scholar] [CrossRef]
- Cheng, Y.-Y.; Yuan, H. Quantitative Study of Electrostatic and Steric Effects on Physicochemical Property and Biological Activity. J. Mol. Graph. Model. 2006, 24, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Garner, C.W.; Behal, F.J. Effect of pH on Substrate and Inhibitor Kinetic Constants of Human Liver Alanine Aminopeptidase. Evidence for Two Ionizable Active Center Groups. Biochemistry 1975, 14, 5084–5088. [Google Scholar] [CrossRef] [PubMed]
- Bloom, B.M.; Laubach, G.D. The Relationship Between Chemical Structure and Pharmacological Activity. Annu. Rev. Pharmacol. 1962, 2, 67–108. [Google Scholar] [CrossRef]
- Sakai, M.; Nagayasu, K.; Shibui, N.; Andoh, C.; Takayama, K.; Shirakawa, H.; Kaneko, S. Prediction of Pharmacological Activities from Chemical Structures with Graph Convolutional Neural Networks. Sci. Rep. 2021, 11, 525. [Google Scholar] [CrossRef]
- Selassie, C.D.; Shusterman, A.J.; Kapur, S.; Verma, R.P.; Zhang, L.; Hansch, C. On the Toxicity of Phenols to Fast Growing Cells. A QSAR Model for a Radical-Based Toxicity. J. Chem. Soc. Perkin Trans. 1999, 2, 2729–2733. [Google Scholar] [CrossRef]
- Gehler, J.; Cantz, M.; O’Brien, J.F.; Tolksdorf, M.; Spranger, J. Mannosidosis: Clinical and Biochemical Findings. Birth Defects Orig. Artic. Ser. 1975, 11, 269–272. [Google Scholar]
- Robak, W.; Apostoluk, W.; Ochromowicz, K. Linear Solvation Energy Relationship (LSER) Analysis of Liquid–Liquid Distribution Constants of 8-Hydroxyquinoline and Its Derivatives. J. Chem. Eng. Data 2011, 56, 3971–3983. [Google Scholar] [CrossRef]
- Rudrapal, M.; Egbuna, C. Computer Aided Drug Design (CADD): From Ligand-Based Methods to Structure-Based Approaches; Elsevier: San Diego, CA, USA, 2022; ISBN 978-0-323-91433-8. [Google Scholar]
- Abraham, M.H.; Acree, W.E. Descriptors for the Prediction of Partition Coefficients of 8-Hydroxyquinoline and Its Derivatives. Sep. Sci. Technol. 2014, 49, 2135–2141. [Google Scholar] [CrossRef]
- Poole, C.F.; Atapattu, S.N. Determination of Physicochemical Properties of Small Molecules by Reversed-Phase Liquid Chromatography. J. Chromatogr. A 2020, 1626, 461427. [Google Scholar] [CrossRef] [PubMed]
- Smart, K.; Garcia, E.; Oloyede, B.; Fischer, R.; Golden, T.D.; Acree, W.E.; Abraham, M.H. The Partition of Organic Compounds from Water into the Methyl Isobutyl Ketone Extraction Solvent with Updated Abraham Model Equation. Phys. Chem. Liq. 2021, 59, 431–441. [Google Scholar] [CrossRef]
- Czaplicki, S. Chromatography in Bioactivity Analysis of Compounds. In Column Chromatography; Martin, D., Ed.; InTech: London, UK, 2013; ISBN 978-953-51-1074-3. [Google Scholar]
- Quinones-Torrelo, C.; Martin-Biosca, Y.; Martinez-pla, J.; Sagrado, S.; Villanueva-Camanas, R.; Medina-Hernandez, M. QRAR Models for Central Nervous System Drugs Using Biopartitioning Micellar Chromatography. Mini-Rev. Med. Chem. 2002, 2, 145–161. [Google Scholar] [CrossRef]
- Wang, S.; Yang, G.; Zhang, H.; Liu, H.; Li, Z. QRAR Models for Cardiovascular System Drugs Using Biopartitioning Micellar Chromatography. J. Chromatogr. B 2007, 846, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Stępnik, K. Biomimetic Chromatographic Studies Combined with the Computational Approach to Investigate the Ability of Triterpenoid Saponins of Plant Origin to Cross the Blood–Brain Barrier. Int. J. Mol. Sci. 2021, 22, 3573. [Google Scholar] [CrossRef] [PubMed]
- Ciura, K.; Kawczak, P.; Greber, K.E.; Kapica, H.; Nowakowska, J.; Bączek, T. Application of Reversed-Phase Thin Layer Chromatography and QSRR Modelling for Prediction of Protein Binding of Selected β-Blockers. J. Pharm. Biomed. Anal. 2019, 176, 112767. [Google Scholar] [CrossRef]
- Ciura, K.; Dziomba, S. Application of Separation Methods for in Vitro Prediction of Blood–Brain Barrier Permeability—The State of the Art. J. Pharm. Biomed. Anal. 2020, 177, 112891. [Google Scholar] [CrossRef] [PubMed]
- Valko, K.; Nunhuck, S.; Bevan, C.; Abraham, M.H.; Reynolds, D.P. Fast Gradient HPLC Method to Determine Compounds Binding to Human Serum Albumin. Relationships with Octanol/Water and Immobilized Artificial Membrane Lipophilicity. J. Pharm. Sci. 2003, 92, 2236–2248. [Google Scholar] [CrossRef] [PubMed]
- Hage, D.S.; Anguizola, J.A.; Jackson, A.J.; Matsuda, R.; Papastavros, E.; Pfaunmiller, E.; Tong, Z.; Vargas-Badilla, J.; Yoo, M.J.; Zheng, X. Chromatographic Analysis of Drug Interactions in the Serum Proteome. Anal. Methods 2011, 3, 1449. [Google Scholar] [CrossRef]
- Hage, D.S. Analysis of Biological Interactions by Affinity Chromatography: Clinical and Pharmaceutical Applications. Clin. Chem. 2017, 63, 1083–1093. [Google Scholar] [CrossRef]
- Matsuda, R.; Li, Z.; Zheng, X.; Hage, D.S. Analysis of Multi-Site Drug–Protein Interactions by High-Performance Affinity Chromatography: Binding by Glimepiride to Normal or Glycated Human Serum Albumin. J. Chromatogr. A 2015, 1408, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.; Beeram, S.; Li, Z.; Zheng, X.; Hage, D.S. Kinetic Analysis of Drug–Protein Interactions by Affinity Chromatography. Drug Discov. Today Technol. 2015, 17, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Mahran, E.; Keusgen, M.; Morlock, G.E. New Planar Assay for Streamlined Detection and Quantification of β-Glucuronidase Inhibitors Applied to Botanical Extracts. Anal. Chim. Acta X 2020, 4, 100039. [Google Scholar] [CrossRef] [PubMed]
- Valko, K.L. Biomimetic Chromatography—A Novel Application of the Chromatographic Principles. Anal. Sci. Adv. 2022, 3, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Ulenberg, S.; Ciura, K.; Georgiev, P.; Pastewska, M.; Ślifirski, G.; Król, M.; Herold, F.; Bączek, T. Use of Biomimetic Chromatography and in Vitro Assay to Develop Predictive GA-MLR Model for Use in Drug-Property Prediction among Anti-Depressant Drug Candidates. Microchem. J. 2022, 175, 107183. [Google Scholar] [CrossRef]
- Legerská, B.; Chmelová, D.; Ondrejovič, M.; Miertuš, S. The TLC-Bioautography as a Tool for Rapid Enzyme Inhibitors Detection—A Review. Crit. Rev. Anal. Chem. 2022, 52, 275–293. [Google Scholar] [CrossRef]
- Choma, I.M.; Grzelak, E.M. Bioautography Detection in Thin-Layer Chromatography. J. Chromatogr. A 2011, 1218, 2684–2691. [Google Scholar] [CrossRef] [PubMed]
- Andrić, F.; Bajusz, D.; Rácz, A.; Šegan, S.; Héberger, K. Multivariate Assessment of Lipophilicity Scales—Computational and Reversed Phase Thin-Layer Chromatographic Indices. J. Pharm. Biomed. Anal. 2016, 127, 81–93. [Google Scholar] [CrossRef]
- Chmiel, T.; Mieszkowska, A.; Kempińska-Kupczyk, D.; Kot-Wasik, A.; Namieśnik, J.; Mazerska, Z. The Impact of Lipophilicity on Environmental Processes, Drug Delivery and Bioavailability of Food Components. Microchem. J. 2019, 146, 393–406. [Google Scholar] [CrossRef]
- Ciura, K.; Dziomba, S.; Nowakowska, J.; Markuszewski, M.J. Thin Layer Chromatography in Drug Discovery Process. J. Chromatogr. A 2017, 1520, 9–22. [Google Scholar] [CrossRef]
- Sobańska, A.W. RP-18 TLC Retention Data and Calculated Physico-Chemical Parameters as Predictors of Soil-Water Partition and Bioconcentration of Organic Sunscreens. Chemosphere 2021, 279, 130527. [Google Scholar] [CrossRef] [PubMed]
- Pidgeon, C.; Venkataram, U.V. Immobilized Artificial Membrane Chromatography: Supports Composed of Membrane Lipids. Anal. Biochem. 1989, 176, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Ermondi, G.; Vallaro, M.; Caron, G. Learning How to Use IAM Chromatography for Predicting Permeability. Eur. J. Pharm. Sci. 2018, 114, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Grumetto, L.; Carpentiero, C.; Barbato, F. Lipophilic and Electrostatic Forces Encoded in IAM-HPLC Indexes of Basic Drugs: Their Role in Membrane Partition and Their Relationships with BBB Passage Data. Eur. J. Pharm. Sci. 2012, 45, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.; Ermondi, G.; Caron, G.; Verzele, D.; Lynen, F. Into the First Biomimetic Sphingomyelin Stationary Phase: Suitability in Drugs’ Biopharmaceutic Profiling and Block Relevance Analysis of Selectivity. Eur. J. Pharm. Sci. 2021, 156, 105585. [Google Scholar] [CrossRef] [PubMed]
- Beigi, F.; Qing, Y.; Lundahl, P. Immobilized-Liposome Chromatographic Analysis of Drug Partitioning into Lipid Bilayers. J. Chromatogr. A 1995, 704, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Lundahl, P.; Yang, Q. Liposome Chromatography: Liposomes Immobilized in Gel Beads as a Stationary Phase for Aqueous Column Chromatography. J. Chromatogr. A 1991, 544, 283–304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhao, X.; Xu, B.; Cheng, S.; Tang, C.; Duan, H.; Xiao, X.; Du, W.; Xu, L. Preparation and Characterization of Micro-Cell Membrane Chromatographic Column with Silica-Based Porous Layer Open Tubular Capillary as Cellular Membrane Carrier. Anal. Bioanal. Chem. 2016, 408, 2441–2448. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.S.; Habicht, K.-L.; Dossou, K.S.S.; Shimmo, R.; Wainer, I.W.; Moaddel, R. Multiple Protein Stationary Phases: A Review. J. Chromatogr. B 2014, 968, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, J.; Xu, D.; He, L.; Qu, J.-H.; Wang, Q.; Crommen, J.; Jiang, Z. Development of Biomimetic Phospholipid Membrane Chromatography for Drug Discovery: A Comprehensive Review. TrAC Trends Anal. Chem. 2024, 171, 117512. [Google Scholar] [CrossRef]
- Pabst, T.M.; Thai, J.; Hunter, A.K. Evaluation of Recent Protein A Stationary Phase Innovations for Capture of Biotherapeutics. J. Chromatogr. A 2018, 1554, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Sanghvi, M.; Moaddel, R.; Wainer, I.W. The Development and Characterization of Protein-Based Stationary Phases for Studying Drug–Protein and Protein–Protein Interactions. J. Chromatogr. A 2011, 1218, 8791–8798. [Google Scholar] [CrossRef] [PubMed]
- Subirats, X.; Bosch, E.; Rosés, M. Retention of Ionisable Compounds on High-Performance Liquid Chromatography XVII. J. Chromatogr. A 2007, 1138, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Subirats, X.; Bosch, E.; Rosés, M. Retention of Ionisable Compounds on High-Performance Liquid Chromatography. XV. Estimation of the pH Variation of Aqueous Buffers with the Change of the Acetonitrile Fraction of the Mobile Phase. J. Chromatogr. A 2004, 1059, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Pietrzyńska, M.; Voelkel, A. Stability of Simulated Body Fluids Such as Blood Plasma, Artificial Urine and Artificial Saliva. Microchem. J. 2017, 134, 197–201. [Google Scholar] [CrossRef]
- Shokry, D.S.; Waters, L.J.; Parkes, G.M.B.; Mitchell, J.C. Incorporating Physiologically Relevant Mobile Phases in Micellar Liquid Chromatography for the Prediction of Human Intestinal Absorption. Biomed. Chromatogr. 2018, 32, e4351. [Google Scholar] [CrossRef] [PubMed]
- Waters, L.J.; Shokry, D.S.; Parkes, G.M.B.; Mitchell, J.C. The Use of Bile Salt Micelles for the Prediction of Human Intestinal Absorption. J. Pharm. Sci. 2016, 105, 3611–3614. [Google Scholar] [CrossRef]
- Rambla-Alegre, M. Basic Principles of MLC. Chromatogr. Res. Int. 2012, 2012, 898520. [Google Scholar] [CrossRef]
- Rukhadze, M.D.; Bezarashvili, G.S.; Kutkhashvili, M.G.; Sigua, K.I. Investigation of Artificial Biomembrane Systems in Biopartitioning Micellar Chromatography by Method of Mathematical Design. Biomed. Chromatogr. 2005, 19, 169–177. [Google Scholar] [CrossRef]
- Tsopelas, F.; Danias, P.; Pappa, A.; Tsantili-Kakoulidou, A. Biopartitioning Micellar Chromatography under Different Conditions: Insight into the Retention Mechanism and the Potential to Model Biological Processes. J. Chromatogr. A 2020, 1621, 461027. [Google Scholar] [CrossRef]
- Escuder-Gilabert, L.; Martínez-Pla, J.J.; Sagrado, S.; Villanueva-Camañas, R.M.; Medina-Hernández, M.J. Biopartitioning Micellar Separation Methods: Modelling Drug Absorption. J. Chromatogr. B 2003, 797, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, B.; Abed, S.N.; Al-Attraqchi, O.; Kuche, K.; Tekade, R.K. Computer-Aided Prediction of Pharmacokinetic (ADMET) Properties. In Dosage Form Design Parameters; Elsevier: Amsterdam, The Netherlands, 2018; pp. 731–755. ISBN 978-0-12-814421-3. [Google Scholar]
- Fujita, T.; Iwasa, J.; Hansch, C. A New Substituent Constant, π, Derived from Partition Coefficients. J. Am. Chem. Soc. 1964, 86, 5175–5180. [Google Scholar] [CrossRef]
- Hawker, D. A Theoretical Basis for Collander Equations. Toxicol. Environ. Chem. 1994, 45, 87–95. [Google Scholar] [CrossRef]
- Soczewiński, E.; Wachtmeister, C.A. The Relation between the Composition of Certain Ternary Two-Phase Solvent Systems and RM Values. J. Chromatogr. A 1962, 7, 311–320. [Google Scholar] [CrossRef]
- Valkó, K.; Slégel, P. New Chromatographic Hydrophobicity Index (Φ0) Based on the Slope and the Intercept of the Log K′ versus Organic Phase Concentration Plot. J. Chromatogr. A 1993, 631, 49–61. [Google Scholar] [CrossRef]
- Valkó, K.; Bevan, C.; Reynolds, D. Chromatographic Hydrophobicity Index by Fast-Gradient RP-HPLC: A High-Throughput Alternative to Log P/Log D. Anal. Chem. 1997, 69, 2022–2029. [Google Scholar] [CrossRef] [PubMed]
- Valko, K.L. Application of Biomimetic HPLC to Estimate in Vivo Behavior of Early Drug Discovery Compounds. Future Drug Discov. 2019, 1, FDD11. [Google Scholar] [CrossRef]
- Valko, K.; Du, C.; Bevan, C.; Reynolds, D.; Abraham, M. Rapid Method for the Estimation of Octanol/Water Partition Coefficient (Log Poct) from Gradient RP-HPLC Retention and a Hydrogen Bond Acidity Term (Sigma alpha2H). Curr. Med. Chem. 2001, 8, 1137–1146. [Google Scholar] [CrossRef]
- Wiczling, P.; Kubik, Ł.; Kaliszan, R. PH Effects on Chromatographic Retention Modes. In Analytical Separation Science; Pino, V., Anderson, J.L., Berthod, A., Stalcup, A.M., Eds.; Wiley: Hoboken, NJ, USA, 2015; pp. 263–278. ISBN 978-3-527-33374-5. [Google Scholar]
- Subirats, X.; Rosés, M.; Bosch, E. On the Effect of Organic Solvent Composition on the pH of Buffered HPLC Mobile Phases and the p Ka of Analytes—A Review. Sep. Purif. Rev. 2007, 36, 231–255. [Google Scholar] [CrossRef]
- Kaliszan, R.; Haber, P.; Bączek, T.; Siluk, D.; Valko, K. Lipophilicity and pKa Estimates from Gradient High-Performance Liquid Chromatography. J. Chromatogr. A 2002, 965, 117–127. [Google Scholar] [CrossRef]
- Kawczak, P.; Vander Heyden, Y.; Nasal, A.; Bączek, T.; Drabczyñska, A.; Kieć-Kononowicz, K.; Kaliszan, R. Micellar Liquid Chromatography for Lipophilicity Determination of New Biologically Active 1,3-purinodiones. J. Sep. Sci. 2010, 33, 1546–1557. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, I.; Studziński, M.; Malinowski, H. Changes of 1,2,4-Triazole Retention and Lipophilicity Descriptor Values in RP-TLC and MLC—TLC Systems in the Presence of an External Magnetic Field. JPC-J. Planar Chromatogr.-Mod. TLC 2017, 30, 106–112. [Google Scholar] [CrossRef]
- Breyer, E.D.; Strasters, J.K.; Khaledi, M.G. Quantitative Retention-Biological Activity Relationship Study by Micellar Liquid Chromatography. Anal. Chem. 1991, 63, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Khaledi, M.G.; Breyer, E.D. Quantitation of Hydrophobicity with Micellar Liquid Chromatography. Anal. Chem. 1989, 61, 1040–1047. [Google Scholar] [CrossRef]
- González, V.; Rodríguez-Delgado, M.A.; Sánchez, M.J.; García-Montelongo, F. Solute-Micelle Association Constants and Correlation of Octanol-Water Coefficients with Hydrophobicity for Polycyclic Aromatic Hydrocarbons by Micellar Chromatography. Chromatographia 1992, 34, 627–635. [Google Scholar] [CrossRef]
- Stępnik, K.E. A Concise Review of Applications of Micellar Liquid Chromatography to Study Biologically Active Compounds. Biomed. Chromatogr. 2017, 31, e3741. [Google Scholar] [CrossRef] [PubMed]
- Stępnik, K.E.; Malinowska, I. The Use of Biopartitioning Micellar Chromatography and Immobilized Artificial Membrane Column for in Silico and in Vitro Determination of Blood–Brain Barrier Penetration of Phenols. J. Chromatogr. A 2013, 1286, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Young, R.J.; Green, D.V.S.; Luscombe, C.N.; Hill, A.P. Getting Physical in Drug Discovery II: The Impact of Chromatographic Hydrophobicity Measurements and Aromaticity. Drug Discov. Today 2011, 16, 822–830. [Google Scholar] [CrossRef]
- Ritchie, T.J.; Macdonald, S.J.F.; Young, R.J.; Pickett, S.D. The Impact of Aromatic Ring Count on Compound Developability: Further Insights by Examining Carbo- and Hetero-Aromatic and -Aliphatic Ring Types. Drug Discov. Today 2011, 16, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.P.; Young, R.J. Getting Physical in Drug Discovery: A Contemporary Perspective on Solubility and Hydrophobicity. Drug Discov. Today 2010, 15, 648–655. [Google Scholar] [CrossRef]
- Smith, D.A.; Beaumont, K.; Maurer, T.S.; Di, L. Volume of Distribution in Drug Design: Miniperspective. J. Med. Chem. 2015, 58, 5691–5698. [Google Scholar] [CrossRef] [PubMed]
- Hollósy, F.; Valkó, K.; Hersey, A.; Nunhuck, S.; Kéri, G.; Bevan, C. Estimation of Volume of Distribution in Humans from High Throughput HPLC-Based Measurements of Human Serum Albumin Binding and Immobilized Artificial Membrane Partitioning. J. Med. Chem. 2006, 49, 6958–6971. [Google Scholar] [CrossRef]
- Valko, K.L.; Ivanova-Berndt, G.; Beswick, P.; Kindey, M.; Ko, D. Application of Biomimetic HPLC to Estimate Lipophilicity, Protein and Phospholipid Binding of Potential Peptide Therapeutics. ADMET DMPK 2018, 6, 162–175. [Google Scholar] [CrossRef]
- Hage, D.S.; Anguizola, J.; Barnaby, O.; Jackson, A.; Yoo, M.J.; Papastavros, E.; Pfaunmiller, E.; Sobansky, M.; Tong, Z. Characterization of Drug Interactions with Serum Proteins by Using High-Performance Affinity Chromatography. Curr. Drug Metab. 2011, 12, 313–328. [Google Scholar] [CrossRef]
- Li, Z.; Beeram, S.R.; Bi, C.; Suresh, D.; Zheng, X.; Hage, D.S. High-Performance Affinity Chromatography: Applications in Drug-Protein Binding Studies and Personalized Medicine. Adv. Protein Chem. Struct. Biol. 2016, 102, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Hage, D.S.; Tweed, S.A. Recent Advances in Chromatographic and Electrophoretic Methods for the Study of Drug-Protein Interactions. J. Chromatogr. B Biomed. Sci. Appl. 1997, 699, 499–525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D. Chromatography Studies on Bio-Affinity of Nine Ligands of A1- Adrenoceptor to a1D Subtypes Overexpressed in Cell Membrane. Sci. China Ser. C 2004, 47, 376. [Google Scholar] [CrossRef]
- Esteve-Romero, J.; Albiol-Chiva, J.; Peris-Vicente, J. A Review on Development of Analytical Methods to Determine Monitorable Drugs in Serum and Urine by Micellar Liquid Chromatography Using Direct Injection. Anal. Chim. Acta 2016, 926, 1–16. [Google Scholar] [CrossRef]
- He, L.; Wang, S.; Geng, X. Coating and Fusing Cell Membranes onto a Silica Surface and Their Chromatographic Characteristics. Chromatographia 2001, 54, 71–76. [Google Scholar] [CrossRef]
- He, L.; Yang, G.; Geng, X. Enzymatic Activity and Chromatographic Characteristics of the Cell Membrane Immobilized on Silica Surface. Chin. Sci. Bull. 1999, 44, 826–831. [Google Scholar] [CrossRef]
- Ma, W.; Yang, L.; Lv, Y.; Fu, J.; Zhang, Y.; He, L. Determine Equilibrium Dissociation Constant of Drug-Membrane Receptor Affinity Using the Cell Membrane Chromatography Relative Standard Method. J. Chromatogr. A 2017, 1503, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Ren, J.; Wang, S.; He, L. Cell Membrane Chromatography Competitive Binding Analysis for Characterization of α1A Adrenoreceptor Binding Interactions. Anal. Bioanal. Chem. 2011, 400, 3625–3633. [Google Scholar] [CrossRef] [PubMed]
- Zeng, A.; Yuan, B.; Zhu, F.; Zhao, L.; He, L.; Yang, G. Cell Membrane Chromatography Correlated with Functional Assay for Ligand–β-Adrenergic Receptor Affinities. Chromatographia 2009, 69, 1373–1377. [Google Scholar] [CrossRef]
- Duan, L.; Guo, L.; Wang, L.; Yin, Q.; Zhang, C.-M.; Zheng, Y.-G.; Liu, E.-H. Application of Metabolomics in Toxicity Evaluation of Traditional Chinese Medicines. Chin. Med. 2018, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Hou, J.; He, L.; Yang, G. Evaluation of Drug-Muscarinic Receptor Affinities Using Cell Membrane Chromatography and Radioligand Binding Assay in Guinea Pig Jejunum Membrane. Acta Pharmacol. Sin. 2005, 26, 113–116. [Google Scholar] [CrossRef]
- Du, H.; He, J.; Wang, S.; He, L. Investigation of Calcium Antagonist–L-Type Calcium Channel Interactions by a Vascular Smooth Muscle Cell Membrane Chromatography Method. Anal. Bioanal. Chem. 2010, 397, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Zhang, D.; Li, J.; Che, D.; Liu, R.; Zhang, J.; Zhang, Y. Interactions between Histamine H1 Receptor and Its Antagonists by Using Cell Membrane Chromatography Method. J. Pharm. Pharmacol. 2015, 67, 1567–1574. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, Y.; Li, J.; Liu, R.; Che, D.; He, L. Analysis of Drug Interactions with Dopamine Receptor by Frontal Analysis and Cell Membrane Chromatography. Chromatographia 2015, 78, 649–654. [Google Scholar] [CrossRef]
- Hou, X.; Wang, S.; Zhang, T.; Ma, J.; Zhang, J.; Zhang, Y.; Lu, W.; He, H.; He, L. Recent Advances in Cell Membrane Chromatography for Traditional Chinese Medicines Analysis. J. Pharm. Biomed. Anal. 2014, 101, 141–150. [Google Scholar] [CrossRef]
- Bu, Y.; Hu, Q.; Bao, T.; Xie, X.; Wang, S. Recent Advances in Cell Membrane-Coated Technology for Drug Discovery from Natural Products. TrAC Trends Anal. Chem. 2022, 151, 116601. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, A.; Zhou, X.; Liu, Q.; Nan, Y.; Guan, Y.; Kong, L.; Han, Y.; Sun, H.; Yan, G. An Integrated Chinmedomics Strategy for Discovery of Effective Constituents from Traditional Herbal Medicine. Sci. Rep. 2016, 6, 18997. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Sun, Y.; Fu, J.; Kong, L.; Han, S. Screening Anti-allergic Components of Astragali Radix Using LAD2 Cell Membrane Chromatography Coupled Online with UHPLC-ESI-MS/MS Method. Biomed. Chromatogr. 2017, 31, e3806. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cao, Y.; Lv, D.; Zhu, Z.; Zhang, J.; Chai, Y. Comprehensive Two-Dimensional HepG2/Cell Membrane Chromatography/Monolithic Column/Time-of-Flight Mass Spectrometry System for Screening Anti-Tumor Components from Herbal Medicines. J. Chromatogr. A 2012, 1242, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Huang, J.; Hou, J.; Wang, S. Screening Epidermal Growth Factor Receptor Antagonists from Radix et Rhizoma Asari by Two-dimensional Liquid Chromatography. J. Sep. Sci. 2014, 37, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Han, S.; Zhang, T.; Zhang, J.; Wang, S.; Hou, J. Screening Active Compounds Acting on the Epidermal Growth Factor Receptor from Radix Scutellariae via Cell Membrane Chromatography Online Coupled with HPLC/MS. J. Pharm. Biomed. Anal. 2012, 62, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Guo, Y.; Dai, B.; Wang, C.; He, L. High-expression EGFR/Cell Membrane Chromatography-online-high-performance Liquid Chromatography/Mass Spectrometry: Rapid Screening of EGFR Antagonists from Semen Strychni. Rapid Commun. Mass Spectrom. 2012, 26, 2027–2032. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, M.; Zhang, Y.; Zhang, J.; He, L. EGFR/Cell Membrane Chromatography-Online-High Performance Liquid Chromatography/Mass Spectrometry Method for Screening EGFR Antagonists from Radix Angelicae Pubescentis. Sci. China Chem. 2010, 53, 2357–2362. [Google Scholar] [CrossRef]
- Han, S.-L.; Zhang, T.; Huang, J.; Hu, Z.-G.; Wang, S.-C. Screening Target Components from Radix Salviae Miltiorrhiae Using an EGFR/CMC-Online-HPLC/MS Method. Anal. Methods 2012, 4, 1078. [Google Scholar] [CrossRef]
- Sun, M.; Ma, W.; Guo, Y.; Hu, Z.; He, L. Simultaneous Screening of Four Epidermal Growth Factor Receptor Antagonists from Curcuma Longa via Cell Membrane Chromatography Online Coupled with HPLC–MS. J. Sep. Sci. 2013, 36, 2096–2103. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Hu, Q.; Huang, J.; Han, S.; Wang, S. Screening Active Compounds from Corydalis Yanhusuo by Combining High Expression VEGF Receptor HEK293 Cell Membrane Chromatography with HPLC–ESI–IT–TOF–MSn Method. J. Pharm. Biomed. Anal. 2017, 136, 134–139. [Google Scholar] [CrossRef]
- Zhang, T.; Han, S.; Huang, J.; Wang, S. Combined Fibroblast Growth Factor Receptor 4 Cell Membrane Chromatography Online with High Performance Liquid Chromatography/Mass Spectrometry to Screen Active Compounds in Brassica Albla. J. Chromatogr. B 2013, 912, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ding, Y.; An, H.; Feng, L.; Wang, S. Screening Anti-tumor Compounds from Ligusticum Wallichii Using Cell Membrane Chromatography Combined with High-performance Liquid Chromatography and Mass Spectrometry. J. Sep. Sci. 2015, 38, 3247–3253. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.-J.; He, L.-C.; Yang, G.-D. Screening, Analysis and in Vitro Vasodilatation of Effective Components from Ligusticum Chuanxiong. Life Sci. 2005, 78, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Dou, L.; Wang, X.; Xue, H.; Song, Y.; Li, X. Screening β1AR Inhibitors by Cell Membrane Chromatography and Offline UPLC/MS Method for Protecting Myocardial Ischemia. J. Pharm. Biomed. Anal. 2015, 115, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhou, M.; Jiang, Q.; Wang, S.; He, L. A Vascular Smooth Muscle/Cell Membrane Chromatography–Offline-Gas Chromatography/Mass Spectrometry Method for Recognition, Separation and Identification of Active Components from Traditional Chinese Medicines. J. Chromatogr. A 2009, 1216, 7081–7087. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, N.; Ma, J.; Zhu, Y.; Wang, M.; Wang, X.; Zhang, P. A Platelet/CMC Coupled with Offline UPLC-QTOF-MS/MS for Screening Antiplatelet Activity Components from Aqueous Extract of Danshen. J. Pharm. Biomed. Anal. 2016, 117, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, M.; Zhang, Y.; Du, H.; He, L. A New A431/Cell Membrane Chromatography and Online High Performance Liquid Chromatography/Mass Spectrometry Method for Screening Epidermal Growth Factor Receptor Antagonists from Radix Sophorae Flavescentis. J. Chromatogr. A 2010, 1217, 5246–5252. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Ren, J.; Wang, S.; He, L. Establishment of a High Expression of α1A Adrenergic Receptor Cell Membrane Chromatography-RPLC Method for Screening Target Components from Radix Caulophylli. Chromatographia 2010, 72, 635–640. [Google Scholar] [CrossRef]
- Wu, X.; Chen, X.; Jia, D.; Cao, Y.; Gao, S.; Guo, Z.; Zerbe, P.; Chai, Y.; Diao, Y.; Zhang, L. Characterization of Anti-Leukemia Components from Indigo Naturalis Using Comprehensive Two-Dimensional K562/Cell Membrane Chromatography and in Silico Target Identification. Sci. Rep. 2016, 6, 25491. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Che, D.; Zhang, T.; Liu, R.; Cao, J.; Wang, J.; Zhao, T.; Ma, P.; Dong, X.; He, L. Saikosaponin A Inhibits Compound 48/80-Induced Pseudo-Allergy via the Mrgprx2 Pathway in Vitro and in Vivo. Biochem. Pharmacol. 2018, 148, 147–154. [Google Scholar] [CrossRef]
- Han, S.; Lv, Y.; Xue, W.; Cao, J.; Cui, R.; Zhang, T. Screening Anaphylactic Components of MaiLuoNing Injection by Using Rat Basophilic leukemia-2H3 Cell Membrane Chromatography Coupled with HPLC–ESI-TOF-MS. J. Sep. Sci. 2016, 39, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Goodall, R.R.; Levi, A.A. A Microchromatographic Method for the Detection and Approximate Determination of the Different Penicillins in a Mixture. Nature 1946, 158, 675–676. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Lautner, H. Zum Papierchromatographischen Nachweis von Penicillinpräparaten. Arch. Pharm. 1961, 294, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nicolaus, B.J.R.; Coronelli, C.; Binaghi, A. Mikrobiologischer Nachweis von Antibiotika auf Dünnschichtchromatogrammen. Experientia 1961, 17, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Sherma, J. Planar Chromatography. Anal. Chem. 2008, 80, 4253–4267. [Google Scholar] [CrossRef] [PubMed]
- Morlock, G.; Schwack, W. Hyphenations in Planar Chromatography. J. Chromatogr. A 2010, 1217, 6600–6609. [Google Scholar] [CrossRef] [PubMed]
- Poole, C.F. Instrumental Thin-Layer Chromatography; Elsevier: Waltham, MA, USA, 2014; ISBN 978-0-12-417223-4. [Google Scholar]
- Rios, J.L.; Recio, M.C.; Villar, A. Screening Methods for Natural Products with Antimicrobial Activity: A Review of the Literature. J. Ethnopharmacol. 1988, 23, 127–149. [Google Scholar] [CrossRef]
- Meyers, E.; Smith, D.A. Bioautography of Antibiotic Spread-Layer Chromatograms. J. Chromatogr. A 1964, 14, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Choma, I.; Jesionek, W. TLC-Direct Bioautography as a High Throughput Method for Detection of Antimicrobials in Plants. Chromatography 2015, 2, 225–238. [Google Scholar] [CrossRef]
- Mehl, A.; Morlock, G.E. Strong Antibacterial Effects in Animal-Derived Food Detected via Non-Target Planar Bioassays. Food Chem. Adv. 2023, 2, 100283. [Google Scholar] [CrossRef]
- Cretu, G.C.; Morlock, G.E. Analysis of Anthocyanins in Powdered Berry Extracts by Planar Chromatography Linked with Bioassay and Mass Spectrometry. Food Chem. 2014, 146, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Ristivojević, P.; Dimkić, I.; Trifković, J.; Berić, T.; Vovk, I.; Milojković-Opsenica, D.; Stanković, S. Antimicrobial Activity of Serbian Propolis Evaluated by Means of MIC, HPTLC, Bioautography and Chemometrics. PLoS ONE 2016, 11, e0157097. [Google Scholar] [CrossRef] [PubMed]
- Jesionek, W.; Majer-Dziedzic, B.; Horváth, G.; Móricz, Á.M.; Choma, I.M. Screening of Antibacterial Compounds in Thymus Vulgaris L. Tincture Using Thin-Layer Chromatography—Direct Bioautography and Liquid Chromatography—Tandem Mass Spectrometry Techniques. JPC-J. Planar Chromatogr.-Mod. TLC 2017, 30, 131–135. [Google Scholar] [CrossRef]
- Jamshidi-Aidji, M.; Morlock, G.E. From Bioprofiling and Characterization to Bioquantification of Natural Antibiotics by Direct Bioautography Linked to High-Resolution Mass Spectrometry: Exemplarily Shown for Salvia Miltiorrhiza Root. Anal. Chem. 2016, 88, 10979–10986. [Google Scholar] [CrossRef] [PubMed]
- Orłowska, M.; Kowalska, T.; Sajewicz, M.; Jesionek, W.; Choma, I.M.; Majer-Dziedzic, B.; Szymczak, G.; Waksmundzka-Hajnos, M. A Comparison of Antibacterial Activity of Selected Thyme (Thymus) Species by Means of the Dot Blot Test with Direct Bioautographic Detection. J. AOAC Int. 2015, 98, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Krüzselyi, D.; Vetter, J.; Ott, P.G.; Móricz, Á.M. Investigation of Antibacterial Components of Button Mushroom (Agaricus Bisporus) by Direct Bioautography and HPLC–DAD–MS. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 298–302. [Google Scholar] [CrossRef]
- Móricz, Á.M.; Szeremeta, D.; Knaś, M.; Długosz, E.; Ott, P.G.; Kowalska, T.; Sajewicz, M. Antibacterial Potential of the Cistus Incanus L. Phenolics as Studied with Use of Thin-Layer Chromatography Combined with Direct Bioautography and in Situ Hydrolysis. J. Chromatogr. A 2018, 1534, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Móricz, Á.M.; Ott, P.G.; Böszörményi, A.; Lemberkovics, É.; Mincsovics, E.; Tyihák, E. Bioassay-Guided Isolation and Identification of Antimicrobial Compounds from Thyme Essential Oil by Means of Overpressured Layer Chromatography, Bioautography and GC–MS. Chromatographia 2012, 75, 991–999. [Google Scholar] [CrossRef]
- Grzelak, E.M.; Majer-Dziedzic, B.; Choma, I.M.; Pilorz, K.M. Development of a Novel Direct Bioautography-Thin-Layer Chromatography Test: Optimization of Growth Conditions for Gram-Positive Bacteria, Bacillus Subtilis. J. AOAC Int. 2013, 96, 386–391. [Google Scholar] [CrossRef]
- Jesionek, W.; Fornal, E.; Majer-Dziedzic, B.; Móricz, Á.M.; Nowicky, W.; Choma, I.M. Investigation of the Composition and Antibacterial Activity of UkrainTM Drug Using Liquid Chromatography Techniques. J. Chromatogr. A 2016, 1429, 340–347. [Google Scholar] [CrossRef]
- Kovács, J.K.; Horváth, G.; Kerényi, M.; Kocsis, B.; Emődy, L.; Schneider, G. A Modified Bioautographic Method for Antibacterial Component Screening against Anaerobic and Microaerophilic Bacteria. J. Microbiol. Methods 2016, 123, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.-L.; Liu, T.; Wang, S.-Y.; Ji, X.-Y.; Mu, Q. TLC-Bioautography Directed Isolation of Antibacterial Compounds from Active Fractionation of Ferula Ferulioides. Nat. Prod. Res. 2019, 33, 1761–1764. [Google Scholar] [CrossRef] [PubMed]
- Jesionek, W.; Choma, I.M.; Majer-Dziedzic, B.; Malinowska, I. Screening bacterial and radical scavenging properties of chosen plant extracts using thin-layer chromatography–direct bioautography. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 2882–2891. [Google Scholar] [CrossRef]
- Jesionek, W.; Grzelak, E.; Majer-Dziedzic, B.; Choma, I. Thin-Layer Chromatography—Direct Bioautography for the Screening of Antimicrobial Properties of Plant Extracts. J. Planar Chromatogr.-Mod. TLC 2013, 26, 109–113. [Google Scholar] [CrossRef]
- McGaw, L.J.; Bagla, V.P.; Steenkamp, P.A.; Fouche, G.; Olivier, J.; Eloff, J.N.; Myer, M.S. Antifungal and Antibacterial Activity and Chemical Composition of Polar and Non-Polar Extracts of Athrixia Phylicoides Determined Using Bioautography and HPLC. BMC Complement. Altern. Med. 2013, 13, 356. [Google Scholar] [CrossRef] [PubMed]
- Mincsovics, E.; Ott, P.; Alberti, Á.; Böszörményi, A.; Héthelyi, É.; Szőke, É.; Kéry, Á.; Lemberkovics, É.; Móricz, Á. In-Situ Clean-up and OPLC Fractionation of Chamomile Flower Extract to Search Active Components by Bioautography. J. Planar Chromatogr.-Mod. TLC 2013, 26, 172–179. [Google Scholar] [CrossRef]
- Móricz, Á.; Szarka, S.; Ott, P.; Kertesy, D.; Héthelyi, É.; Szőke, É.; Tyihák, E. Application of Direct Bioautography and SPME-GC-MS for the Study of Antibacterial Chamomile Ingredients. J. Planar Chromatogr.-Mod. TLC 2012, 25, 220–224. [Google Scholar] [CrossRef]
- Jesionek, W.; Móricz, Á.M.; Alberti, Á.; Ott, P.G.; Kocsis, B.; Horváth, G.; Choma, I.M. TLC-Direct Bioautography as a Bioassay Guided Method for Investigation of Antibacterial Compounds in Hypericum perforatum L. J. AOAC Int. 2015, 98, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Lomarat, P.; Phanthong, P.; Wongsariya, K.; Chomnawang, M.T.; Bunyapraphatsara, N. Bioautography-Guided Isolation of Antibacterial Compounds of Essential Oils from Thai Spices against Histamine-Producing Bacteria. Pak. J. Pharm. Sci. 2013, 26, 473–477. [Google Scholar]
- Grzelak, E.M.; Hwang, C.; Cai, G.; Nam, J.-W.; Choules, M.P.; Gao, W.; Lankin, D.C.; McAlpine, J.B.; Mulugeta, S.G.; Napolitano, J.G.; et al. Bioautography with TLC-MS/NMR for Rapid Discovery of Anti-Tuberculosis Lead Compounds from Natural Sources. ACS Infect. Dis. 2016, 2, 294–301. [Google Scholar] [CrossRef]
- Jesionek, W.; Móricz, Á.M.; Ott, P.G.; Kocsis, B.; Horváth, G.; Choma, I.M. TLC-Direct Bioautography and LC/MS as Complementary Methods in Identification of Antibacterial Agents in Plant Tinctures from the Asteraceae Family. J. AOAC Int. 2015, 98, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Móricz, A.M.; Szarka, S.; Ott, P.G.; Héthelyi, E.B.; Szoke, E.; Tyihák, E. Separation and Identification of Antibacterial Chamomile Components Using OPLC, Bioautography and GC-MS. Med. Chem. 2012, 8, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Móricz, A.M.; Ott, P.G.; Alberti, A.; Böszörményi, A.; Lemberkovics, E.; Szoke, E.; Kéry, A.; Mincsovics, E. Applicability of Preparative Overpressured Layer Chromatography and Direct Bioautography in Search of Antibacterial Chamomile Compounds. J. AOAC Int. 2013, 96, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Móricz, Á.M.; Häbe, T.T.; Böszörményi, A.; Ott, P.G.; Morlock, G.E. Tracking and Identification of Antibacterial Components in the Essential Oil of Tanacetum vulgare L. by the Combination of High-Performance Thin-Layer Chromatography with Direct Bioautography and Mass Spectrometry. J. Chromatogr. A 2015, 1422, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Cheng, Z.; Wu, T. TLC Bioautography on Screening of Bioactive Natural Products: An Update Review. Curr. Anal. Chem. 2020, 16, 545–556. [Google Scholar] [CrossRef]
- Choma, I.M.; Jesionek, W. Effect-Directed Detection in Chromatography. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2017; p. B9780124095472126794. ISBN 978-0-12-409547-2. [Google Scholar]
- Marston, A. Thin-Layer Chromatography with Biological Detection in Phytochemistry. J. Chromatogr. A 2011, 1218, 2676–2683. [Google Scholar] [CrossRef] [PubMed]
- Morlock, G.E. Chromatography Combined with Bioassays and Other Hyphenations—The Direct Link to the Compound Indicating the Effect. In ACS Symposium Series; Jayprakasha, G.K., Patil, B.S., Pellati, F., Eds.; American Chemical Society: Washington, DC, USA, 2014; Volume 1185, pp. 101–121. ISBN 978-0-8412-2976-1. [Google Scholar]
- Curry, A.S.; Mercier, M. Detection and Identification of Monoamine Oxidase Inhibitors in Biological Samples. Nature 1970, 228, 281–282. [Google Scholar] [CrossRef]
- Liang, J.-B.; Yang, Z.-D.; Shu, Z.-M.; Yu, C.-C. A Rapid Thin-Layer Chromatography Bioautographic Method for Detecting the Monoamine Oxidase Inhibitors in Plants. Nat. Prod. Res. 2014, 28, 1318–1321. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.; Bastidas, E.; Rodríguez, M.; Lucena, E.; Castillo, A.; Hasegawa, M. Aporphine Alkaloids from Guatteria Stenopetala (Annonaceae). Nat. Prod. Commun. 2008, 3, 1934578X0800300408. [Google Scholar] [CrossRef]
- Darwish, R.S.; Shawky, E.; Hammoda, H.M.; Harraz, F.M. A New Thin-Layer Chromatography–Direct Bioautography Assay for the Qualitative and Quantitative Determination of Peroxidase Inhibitors in Plant Extracts. JPC-J. Planar Chromatogr.-Mod. TLC 2020, 33, 3–9. [Google Scholar] [CrossRef]
- Ramallo, I.A.; Zacchino, S.A.; Furlan, R.L.E. A Rapid TLC Autographic Method for the Detection of Xanthine Oxidase Inhibitors and Superoxide Scavengers. Phytochem. Anal. 2006, 17, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Li, X.; Zhang, N.; Miao, Y.; Feng, H.; Wu, T.; Cheng, Z. Improved Bioautographic Assay on TLC Layers for Qualitative and Quantitative Estimation of Xanthine Oxidase Inhibitors and Superoxide Scavengers. J. Pharm. Biomed. Anal. 2018, 150, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Salazar, M.O.; Furlan, R.L.E. A Rapid TLC Autographic Method for the Detection of Glucosidase Inhibitors. Phytochem. Anal. 2007, 18, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Simões-Pires, C.A.; Hmicha, B.; Marston, A.; Hostettmann, K. A TLC Bioautographic Method for the Detection of A- and Β-glucosidase Inhibitors in Plant Extracts. Phytochem. Anal. 2009, 20, 511–515. [Google Scholar] [CrossRef]
- Marston, A.; Kissling, J.; Hostettmann, K. A Rapid TLC Bioautographic Method for the Detection of Acetylcholinesterase and Butyrylcholinesterase Inhibitors in Plants. Phytochem. Anal. PCA 2002, 13, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Mroczek, T.; Mazurek, J. Pressurized Liquid Extraction and Anticholinesterase Activity-Based Thin-Layer Chromatography with Bioautography of Amaryllidaceae Alkaloids. Anal. Chim. Acta 2009, 633, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Song, Z.; Ren, J.; Yang, M.; Li, S. Improved Thin-layer Chromatography Bioautographic Assay for the Detection of Actylcholinesterase Inhibitors in Plants. Phytochem. Anal. 2011, 22, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Taibon, J.; Ankli, A.; Schwaiger, S.; Magnenat, C.; Boka, V.-I.; Simões-Pires, C.; Aligiannis, N.; Cuendet, M.; Skaltsounis, A.-L.; Reich, E.; et al. Prevention of False-Positive Results: Development of an HPTLC Autographic Assay for the Detection of Natural Tyrosinase Inhibitors. Planta Med. 2015, 81, 1198–1204. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tang, Q.; Wu, T.; Cheng, Z. Improved TLC Bioautographic Assay for Qualitative and Quantitative Estimation of Tyrosinase Inhibitors in Natural Products. Phytochem. Anal. 2017, 28, 115–124. [Google Scholar] [CrossRef]
- Hassan, A.M.S. TLC Bioautographic Method for Detecting Lipase Inhibitors. Phytochem. Anal. PCA 2012, 23, 405–407. [Google Scholar] [CrossRef]
- Bayineni, V.K.; Suresh, S.; Singh, G.; Kadeppagari, R.-K. Development of a Bioautographic Method for the Detection of Lipase Inhibitors. Biochem. Biophys. Res. Commun. 2014, 453, 784–786. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhou, J.; Tang, Q.; Wu, T.; Cheng, Z. A New TLC Bioautographic Assay for Qualitative and Quantitative Estimation of Lipase Inhibitors. Phytochem. Anal. 2016, 27, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.H.; Liao, L.P.; Hu, H.J.; Annie Bligh, S.W.; Wang, C.H.; Chou, G.X.; Wang, Z.T. A Thin-Layer Chromatography-Bioautographic Method for Detecting Dipeptidyl Peptidase IV Inhibitors in Plants. J. Chromatogr. A 2015, 1411, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Stepanić, V.; Koštrun, S.; Malnar, I.; Hlevnjak, M.; Butković, K.; Ćaleta, I.; Dukši, M.; Kragol, G.; Makaruha-Stegić, O.; Mikac, L.; et al. Modeling Cellular Pharmacokinetics of 14- and 15-Membered Macrolides with Physicochemical Properties. J. Med. Chem. 2011, 54, 719–733. [Google Scholar] [CrossRef]
- Munić Kos, V.; Koštrun, S.; Fajdetić, A.; Bosnar, M.; Kelnerić, Ž.; Stepanić, V.; Eraković Haber, V. Structure-Property Relationship for Cellular Accumulation of Macrolones in Human Polymorphonuclear Leukocytes (PMNs). Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2013, 49, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.; Liu, H.; Pidgeon, C. Immobilized-Artificial-Membrane Chromatography: Measurements of Membrane Partition Coefficient and Predicting Drug Membrane Permeability. J. Chromatogr. A 1996, 728, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Barbato, F.; di Martino, G.; Grumetto, L.; La Rotonda, M.I. Prediction of Drug-Membrane Interactions by IAM-HPLC: Effects of Different Phospholipid Stationary Phases on the Partition of Bases. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2004, 22, 261–269. [Google Scholar] [CrossRef]
- Grumetto, L.; Carpentiero, C.; Di Vaio, P.; Frecentese, F.; Barbato, F. Lipophilic and Polar Interaction Forces between Acidic Drugs and Membrane Phospholipids Encoded in IAM-HPLC Indexes: Their Role in Membrane Partition and Relationships with BBB Permeation Data. J. Pharm. Biomed. Anal. 2013, 75, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Janicka, M.; Sztanke, M.; Sztanke, K. Modeling the Blood-Brain Barrier Permeability of Potential Heterocyclic Drugs via Biomimetic IAM Chromatography Technique Combined with QSAR Methodology. Molecules 2024, 29, 287. [Google Scholar] [CrossRef] [PubMed]
- Gilar, M.; Hill, J.; McDonald, T.S.; Gritti, F. Utility of Linear and Nonlinear Models for Retention Prediction in Liquid Chromatography. J. Chromatogr. A 2020, 1613, 460690. [Google Scholar] [CrossRef]
- Stępnik, K.E.; Malinowska, I.; Rój, E. In Vitro and in Silico Determination of Oral, Jejunum and Caco-2 Human Absorption of Fatty Acids and Polyphenols. Micellar Liquid Chromatography. Talanta 2014, 130, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Waters, L.J.; Shokry, D.S.; Parkes, G.M.B. Predicting Human Intestinal Absorption in the Presence of Bile Salt with Micellar Liquid Chromatography. Biomed. Chromatogr. 2016, 30, 1618–1624. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).