The Influence of Substrate and Strain on Protein Quality of Pleurotus ostreatus
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Raw Materials
2.3. Preparation of P. ostreatus Protein Concentrates
2.4. Protein Solubility
2.5. Protein Solubility Index
2.6. Amino Acid Composition
2.6.1. Acidic Hydrolysis of P. ostreatus Flour for Amino Acid Analysis
2.6.2. Amino Acid Derivatization and HPLC Analysis
2.7. Crude Protein Content
2.8. FTIR Spectroscopy
2.9. Functional Properties
2.9.1. Water and Oil Absorption Capacity
2.9.2. Foam Properties
2.9.3. Emulsion Properties
2.10. Statistical Analysis
3. Results and Discussion
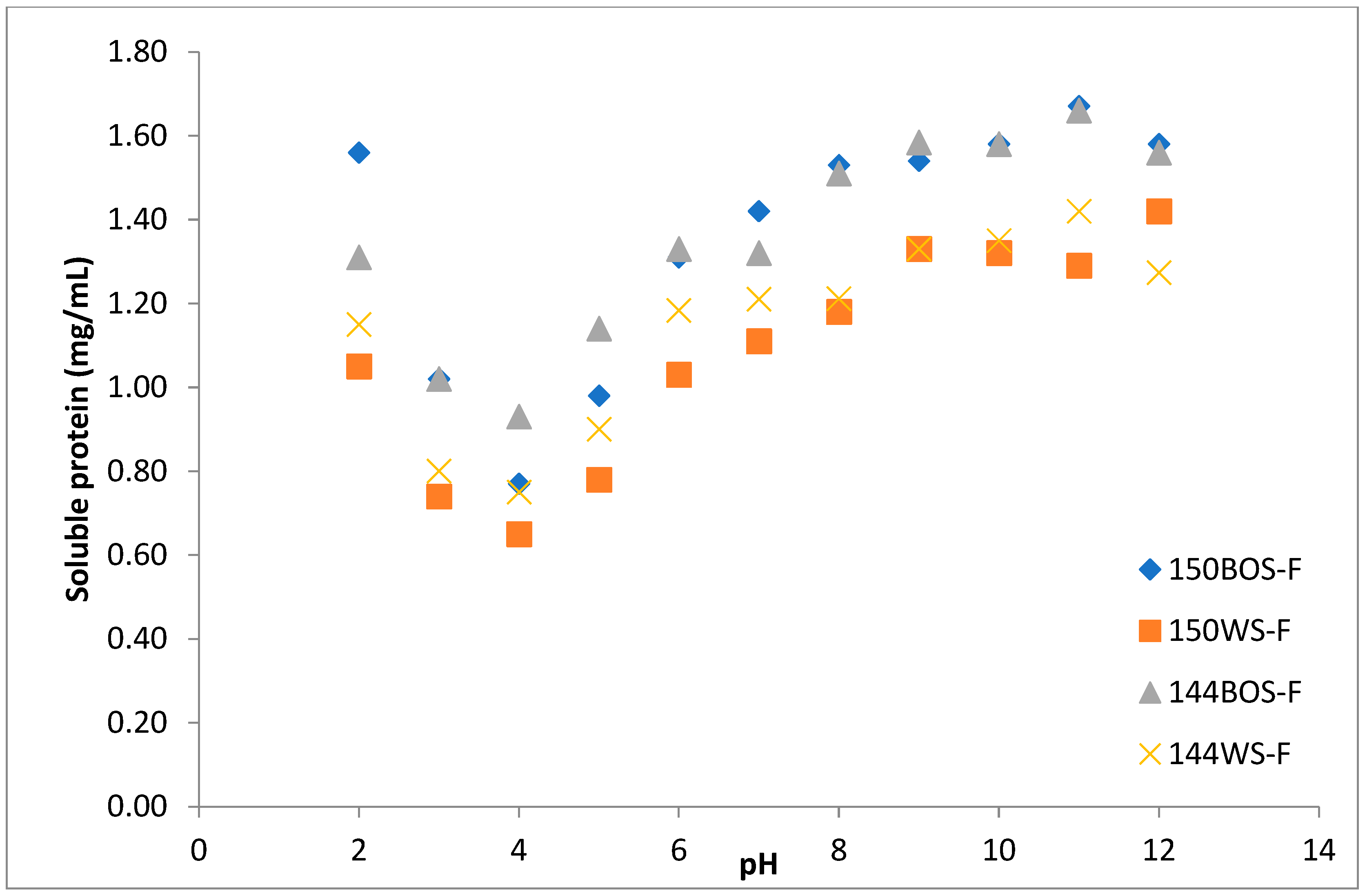
3.1. Protein Solubility
3.2. Protein Content and Extraction Yield
3.3. Protein Solubility Index (PSI)
3.4. Amino Acid Composition
3.5. Spectroscopic Analysis
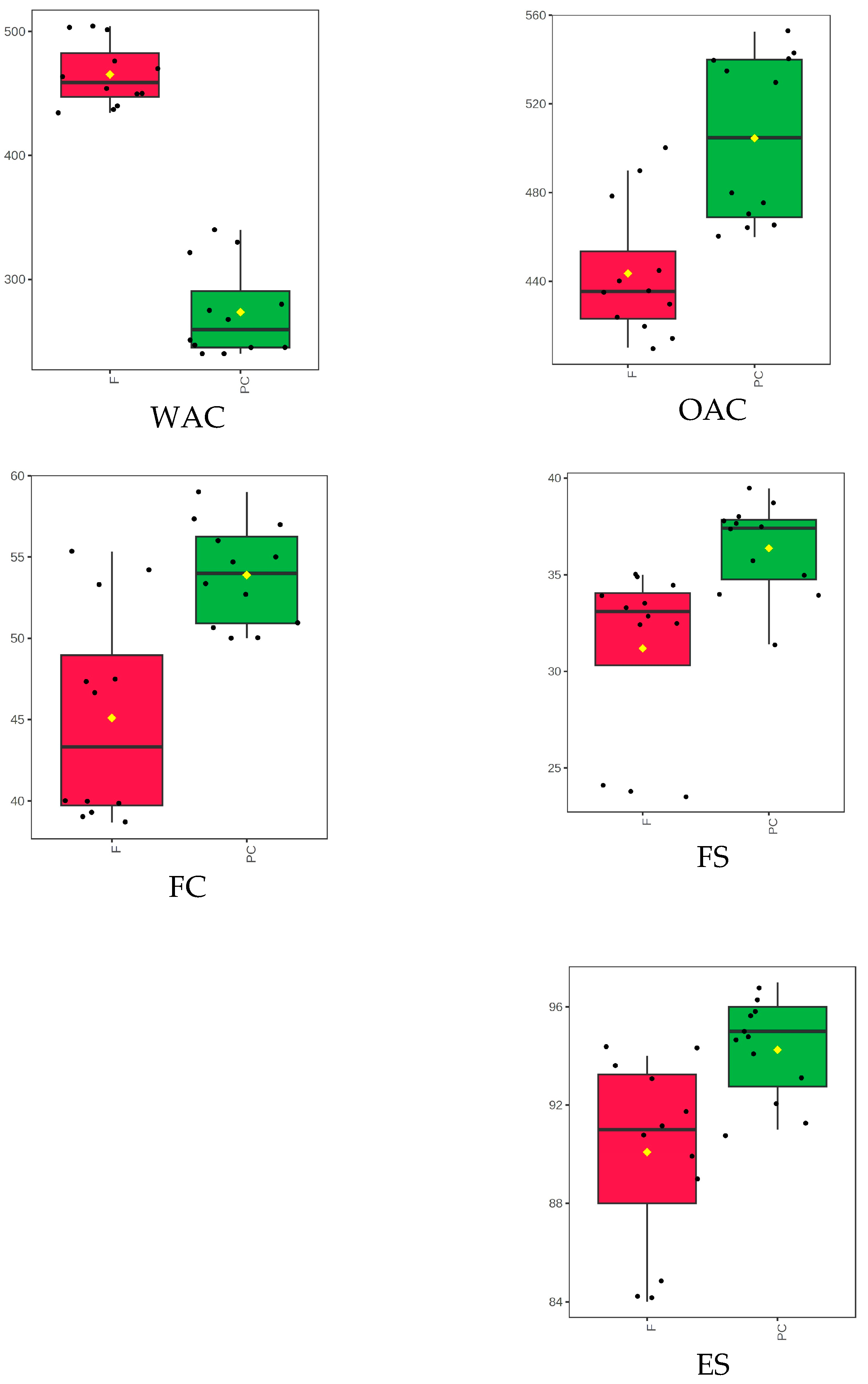
3.6. Functional Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Royse, D.J.; Baars, J.; Tan, Q. Current Overview of Mushroom Production in the World. In Edible and Medicinal Mushrooms; Diego, C.Z., Pardo-Giménez, A., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 5–13. ISBN 978-1-119-14941-5. [Google Scholar]
- Törős, G.; El-Ramady, H.; Prokisch, J. Edible Mushroom of Pleurotus spp.: A Case Study of Oyster Mushroom (Pleurotus ostreatus L.). Environ. Biodivers. Soil Secur. 2022, 6, 51–59. [Google Scholar] [CrossRef]
- Aida, F.M.N.A.; Shuhaimi, M.; Yazid, M.; Maaruf, A.G. Mushroom as a Potential Source of Prebiotics: A Review. Trends Food Sci. Technol. 2009, 20, 567–575. [Google Scholar] [CrossRef]
- Ulziijargal, E.; Mau, J.-L. Nutrient Compositions of Culinary-Medicinal Mushroom Fruiting Bodies and Mycelia. Int. J. Med. Mushrooms 2011, 13, 343–349. [Google Scholar] [CrossRef]
- Economou, C.N.; Philippoussis, A.N.; Diamantopoulou, P.A. Spent Mushroom Substrate for a Second Cultivation Cycle of Pleurotus Mushrooms and Dephenolization of Agro-Industrial Wastewaters. FEMS Microbiol. Lett. 2020, 367, fnaa060. [Google Scholar] [CrossRef]
- Singdevsachan, S.K.; Auroshree, P.; Mishra, J.; Baliyarsingh, B.; Tayung, K.; Thatoi, H. Mushroom Polysaccharides as Potential Prebiotics with Their Antitumor and Immunomodulating Properties: A Review. Bioact. Carbohydr. Diet. Fibre 2016, 7, 1–14. [Google Scholar] [CrossRef]
- Murphy, E.J.; Rezoagli, E.; Major, I.; Rowan, N.J.; Laffey, J.G. β-Glucan Metabolic and Immunomodulatory Properties and Potential for Clinical Application. J. Fungi 2020, 6, 356. [Google Scholar] [CrossRef] [PubMed]
- Tagkouli, D.; Kaliora, A.; Bekiaris, G.; Koutrotsios, G.; Christea, M.; Zervakis, G.I.; Kalogeropoulos, N. Free Amino Acids in Three Pleurotus Species Cultivated on Agricultural and Agro-Industrial By-Products. Molecules 2020, 25, 4015. [Google Scholar] [CrossRef]
- El Enshasy, H.; Maftoun, P.; Johari, H.J.; Soltani, M.; Malek, R.A.; Othman, N.Z. The Edible Mushroom Pleurotus spp.: I. Biodiversity and Nutritional Values. Int. J. Biotechnol. Wellness Ind. 2015, 4, 67–83. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Development of a Novel Methodology for the Analysis of Ergosterol in Mushrooms. Food Anal. Methods 2014, 7, 217–223. [Google Scholar] [CrossRef]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of Phenolic Acids: Metabolites versus Parent Compounds: A Review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Muszyńska, B.; Piotrowska, J.; Krakowska, A.; Gruba, A.; Kała, K.; Sułkowska-Ziaja, K.; Kryczyk, A.; Opoka, W. Study of Physiologically Active Components in Different Parts of Fruiting Bodies of Varieties of Agaricus bisporus (White Mushroom). Eur. Food Res. Technol. 2017, 243, 2135–2145. [Google Scholar] [CrossRef]
- Melanouri, E.-M.; Dedousi, M.; Diamantopoulou, P. Cultivating Pleurotus ostreatus and Pleurotus eryngii Mushroom Strains on Agro-Industrial Residues in Solid-State Fermentation. Part I: Screening for Growth, Endoglucanase, Laccase and Biomass Production in the Colonization Phase. Carbon Resour. Convers. 2022, 5, 61–70. [Google Scholar] [CrossRef]
- Dedousi, M.; Melanouri, E.-M.; Karayannis, D.; Kaminarides, E.-I.; Diamantopoulou, P. Utilization of Spent Substrates and Waste Products of Mushroom Cultivation to Produce New Crops of Pleurotus ostreatus, Pleurotus eryngii and Agaricus bisporus. Carbon Resour. Convers. 2024, 7, 100196. [Google Scholar] [CrossRef]
- Dedousi, M.; Melanouri, E.-M.; Diamantopoulou, P. Carposome Productivity of Pleurotus ostreatus and Pleurotus eryngii Growing on Agro-Industrial Residues Enriched with Nitrogen, Calcium Salts and Oils. Carbon Resour. Convers. 2023, 6, 150–165. [Google Scholar] [CrossRef]
- González, A.; Cruz, M.; Losoya, C.; Nobre, C.; Loredo, A.; Rodríguez, R.; Contreras, J.; Belmares, R. Edible Mushrooms as a Novel Protein Source for Functional Foods. Food Funct. 2020, 11, 7400–7414. [Google Scholar] [CrossRef]
- Bach, F.; Helm, C.V.; Bellettini, M.B.; Maciel, G.M.; Haminiuk, C.W.I. Edible Mushrooms: A Potential Source of Essential Amino Acids, Glucans and Minerals. Int. J. Food Sci. Technol. 2017, 52, 2382–2392. [Google Scholar] [CrossRef]
- González, A.; Nobre, C.; Simões, L.S.; Cruz, M.; Loredo, A.; Rodríguez-Jasso, R.M.; Contreras, J.; Texeira, J.; Belmares, R. Evaluation of Functional and Nutritional Potential of a Protein Concentrate from Pleurotus ostreatus Mushroom. Food Chem. 2021, 346, 128884. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.C.Y.; Warkentin, T.D.; Tyler, R.T.; Nickerson, M.T. Physicochemical and Functional Properties of Protein Isolates Obtained from Several Pea Cultivars. Cereal Chem. J. 2017, 94, 89–97. [Google Scholar] [CrossRef]
- Lavelli, V.; Proserpio, C.; Gallotti, F.; Laureati, M.; Pagliarini, E. Circular Reuse of Bio-Resources: The Role of Pleurotus spp. in the Development of Functional Foods. Food Funct. 2018, 9, 1353–1372. [Google Scholar] [CrossRef] [PubMed]
- You, S.W.; Hoskin, R.T.; Komarnytsky, S.; Moncada, M. Mushrooms as Functional and Nutritious Food Ingredients for Multiple Applications. ACS Food Sci. Technol. 2022, 2, 1184–1195. [Google Scholar] [CrossRef]
- Zhang, Y.; Sharan, S.; Rinnan, Å.; Orlien, V. Survey on Methods for Investigating Protein Functionality and Related Molecular Characteristics. Foods 2021, 10, 2848. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, M.; Khaksar, F.B.; Pagan, J.; Ibarz, A. Application of Ultrasound-Ultrafiltration-Assisted Alkaline Isoelectric Precipitation (UUAAIP) Technique for Producing Alfalfa Protein Isolate for Human Consumption: Optimization, Comparison, Physicochemical, and Functional Properties. Food Res. Int. 2020, 130, 108907. [Google Scholar] [CrossRef] [PubMed]
- Kheto, A.; Sehrawat, R.; Gul, K.; Kumar, L. Effect of Extraction pH on Amino Acids, Nutritional, In-Vitro Protein Digestibility, Intermolecular Interactions, and Functional Properties of Guar Germ Proteins. Food Chem. 2024, 444, 138628. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zheng, Q.; Chen, X.; Ye, Z.; Wei, T.; Guo, L.; Lin, J. Physicochemical and Emulsifying Properties of Protein Isolated from Phlebopus portentosus. LWT 2021, 142, 111042. [Google Scholar] [CrossRef]
- Gao, K.; Rao, J.; Chen, B. Plant Protein Solubility: A Challenge or Insurmountable Obstacle. Adv. Colloid Interface Sci. 2024, 324, 103074. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of the Association of Official Analytical Chemists, 16th ed.; Association of Official Analytical Chemists: Arlington, TX, USA, 2005. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein meaurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Liu, K. A New Method for Determining Protein Solubility Index (PSI) Based on Extraction with 5 mM Alkali Hydroxide and Its Correlation with Trypsin Inhibitor Activity in Soybean Products. J. Am. Oil Chem. Soc. 2022, 99, 855–871. [Google Scholar] [CrossRef]
- Dai, Z.; Wu, Z.; Jia, S.; Wu, G. Analysis of Amino Acid Composition in Proteins of Animal Tissues and Foods as Pre-Column o-Phthaldialdehyde Derivatives by HPLC with Fluorescence Detection. J. Chromatogr. B 2014, 964, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Rutherfurd, S.M.; Gilani, G.S. Amino Acid Analysis. Curr. Protoc. Protein Sci. 2009, 58, 6. [Google Scholar] [CrossRef] [PubMed]
- Agilent Application #5591-5571EN: Automated Amino Acid Analysis Using an Agilent Poroshell HPH-C18 Column. Available online: https://www.agilent.com/cs/library/applications/5991-5571EN.pdf (accessed on 17 March 2024).
- Kalač, P. Chemical Composition and Nutritional Value of European Species of Wild Growing Mushrooms: A Review. Food Chem. 2009, 113, 9–16. [Google Scholar] [CrossRef]
- Cruz-Solorio, A.; Villanueva-Arce, R.; Garín-Aguilar, M.E.; Leal-Lara, H.; Valencia-del Toro, G. Functional Properties of Flours and Protein Concentrates of 3 Strains of the Edible Mushroom Pleurotus ostreatus. J. Food Sci. Technol. 2018, 55, 3892–3901. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S. MetaboAnalyst: A Web Server for Metabolomic Data Analysis and Interpretation. Nucleic Acids Res. 2009, 37, W652–W660. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.J.; von Elbe, J.H.; Giusti, M.M. Chapter 5—Amino acids, Peptides, and Proteins. In Fennema’s Food Chemistry; Damodaran, S., Parkin, K.L., Fennema, O.R., Eds.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Diamantopoulou, P.; Fourtaka, K.; Melanouri, E.M.; Dedousi, M.; Diamantis, I.; Gardeli, C.; Papanikolaou, S. Examining the Impact of Substrate Composition on the Biochemical Properties and Antioxidant Activity of Pleurotus and Agaricus Mushrooms. Fermentation 2023, 9, 689. [Google Scholar] [CrossRef]
- Akharume, F.U.; Aluko, R.E.; Adedeji, A.A. Modification of Plant Proteins for Improved Functionality: A Review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 198–224. [Google Scholar] [CrossRef] [PubMed]
- Mendez, L.A.; Sandoval Castro, C.A.; Belmar Casso, R.; Capetillo Leal, C.M. Effect of Substrate and Harvest on the Amino Acid Profile of Oyster Mushroom (Pleurotus ostreatus). J. Food Compos. Anal. 2005, 18, 447–450. [Google Scholar] [CrossRef]
- Li, Z.; Hong, T.; Shen, G.; Gu, Y.; Guo, Y.; Han, J. Amino Acid Profiles and Nutritional Evaluation of Fresh Sweet–Waxy Corn from Three Different Regions of China. Nutrients 2022, 14, 3887. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.F.; Soares, C.; Machado, S.; Correia, M.; Ramalhosa, M.J.; Oliva-teles, M.T.; Paula Carvalho, A.; Domingues, V.F.; Antunes, F.; Oliveira, T.A.C.; et al. Seaweeds from the Portuguese Coast as a Source of Proteinaceous Material: Total and Free Amino Acid Composition Profile. Food Chem. 2018, 269, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Weltgesundheitsorganisation; FAO; Vereinte Nationen (Eds.) Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint WHO/FAO/UNU Expert Consultation; [Geneva, 9–16 April 2002]; WHO Technical Report Series; WHO: Geneva, Switzerland, 2007; ISBN 978-92-4-120935-9. [Google Scholar]
- Machado, M.; Machado, S.; Pimentel, F.B.; Freitas, V.; Alves, R.C.; Oliveira, M.B.P.P. Amino Acid Profile and Protein Quality Assessment of Macroalgae Produced in an Integrated Multi-Trophic Aquaculture System. Foods 2020, 9, 1382. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, F.A.; Chuang, L.T.; Torun, H.; Colak, A.; Sesli˙, E.; Presley, J.; Smith, B.R.; Glew, R.H. Fatty Acid and Amino Acid Compositions of Selected Wild-Edible Mushrooms Consumed in Turkey. Int. J. Food Sci. Nutr. 2011, 62, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, J.; Fan, S.; Mi, S.; Zhang, Y. Comparison of the Nutritional and Taste Characteristics of 5 Edible Fungus Powders Based on the Composition of Hydrolyzed Amino Acids and Free Amino Acids. J. Food Qual. 2022, 2022, 1–10. [Google Scholar] [CrossRef]
- Ayimbila, F.; Keawsompong, S. Nutritional Quality and Biological Application of Mushroom Protein as a Novel Protein Alternative. Curr. Nutr. Rep. 2023, 12, 290–307. [Google Scholar] [CrossRef] [PubMed]
- Koutrotsios, G.; Tagkouli, D.; Bekiaris, G.; Kaliora, A.; Tsiaka, T.; Tsiantas, K.; Chatzipavlidis, I.; Zoumpoulakis, P.; Kalogeropoulos, N.; Zervakis, G.I. Enhancing the Nutritional and Functional Properties of Pleurotus citrinopileatus Mushrooms through the Exploitation of Winery and Olive Mill Wastes. Food Chem. 2022, 370, 131022. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, M.A.; Nadeeshani, H.; Sewwandi, S.M.; Rathnayake, I.; Kananke, T.C.; Liyanage, R. Comparison of Nutritional Composition, Bioactivities, and FTIR- ATR Microstructural Properties of Commercially Grown Four Mushroom Species in Sri Lanka; Agaricus bisporus, Pleurotus ostreatus, Calocybe sp. (MK-White), Ganoderma lucidum. Food Prod. Process. Nutr. 2023, 5, 43. [Google Scholar] [CrossRef]
- Pilafidis, S.; Tsouko, E.; Sougleri, G.; Diamantopoulou, P.; Gkatzionis, K.; Ioannou, Z.; Sarris, D. Submerged Cultivation of Selected Macro-Fungi to Produce Mycelia Rich in β-Glucans and Other Bioactive Compounds, Valorizing Side Streams of the Food Industry. Carbon Resour. Convers. 2024, 7, 100198. [Google Scholar] [CrossRef]
- Duan, Z.; Zhang, Y.; Zhu, C.; Wu, Y.; Du, B.; Ji, H. Structural Characterization of Phosphorylated Pleurotus ostreatus Polysaccharide and Its Hepatoprotective Effect on Carbon Tetrachloride-Induced Liver Injury in Mice. Int. J. Biol. Macromol. 2020, 162, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Limberger-Bayer, V.M.; De Francisco, A.; Chan, A.; Oro, T.; Ogliari, P.J.; Barreto, P.L.M. Barley β-Glucans Extraction and Partial Characterization. Food Chem. 2014, 154, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Zervakis, G.I.; Bekiaris, G.; Tarantilis, P.A.; Pappas, C.S. Rapid Strain Classification and Taxa Delimitation within the Edible Mushroom Genus Pleurotus through the Use of Diffuse Reflectance Infrared Fourier Transform (DRIFT) Spectroscopy. Fungal Biol. 2012, 116, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Fang, X.; Feng, Z.; Chen, M.; Qiu, X.; Sun, J.; Wu, M.; He, J. Protein from Rapeseed for Food Applications: Extraction, Sensory Quality, Functional and Nutritional Properties. Food Chem. 2024, 439, 138109. [Google Scholar] [CrossRef] [PubMed]
- Boureghda, Y.; Satha, H.; Bendebane, F. Chitin–Glucan Complex from Pleurotus ostreatus Mushroom: Physicochemical Characterization and Comparison of Extraction Methods. Waste Biomass Valorization 2021, 12, 6139–6153. [Google Scholar] [CrossRef]
- Cernescu, A.; Szuwarzyński, M.; Kwolek, U.; Wydro, P.; Kepczynski, M.; Zapotoczny, S.; Nowakowska, M.; Quaroni, L. Label-Free Infrared Spectroscopy and Imaging of Single Phospholipid Bilayers with Nanoscale Resolution. Anal. Chem. 2018, 90, 10179–10186. [Google Scholar] [CrossRef]
- Pellegrino, R.M.; Ianni, F.; Blasi, F.; Angelini, P.; Emiliani, C.; Venanzoni, R.; Cossignani, L. Lipidomic Profiling of Pleurotus ostreatus by LC/MS Q-TOF Analysis. Food Res. Int. 2022, 156, 111335. [Google Scholar] [CrossRef] [PubMed]
- Effiong, M.E.; Umeokwochi, C.P.; Afolabi, I.S.; Chinedu, S.N. Assessing the Nutritional Quality of Pleurotus ostreatus (Oyster Mushroom). Front. Nutr. 2024, 10, 1279208. [Google Scholar] [CrossRef] [PubMed]
- Alobo, A.P. Proximate Composition and Functional Properties of Pleurotus tuberregium Sclerotia Flour and Protein Concentrate. Plant Foods Hum. Nutr. 2003, 58, 1–9. [Google Scholar] [CrossRef]
- Jarpa-Parra, M.; Bamdad, F.; Wang, Y.; Tian, Z.; Temelli, F.; Han, J.; Chen, L. Optimization of Lentil Protein Extraction and the Influence of Process pH on Protein Structure and Functionality. LWT-Food Sci. Technol. 2014, 57, 461–469. [Google Scholar] [CrossRef]
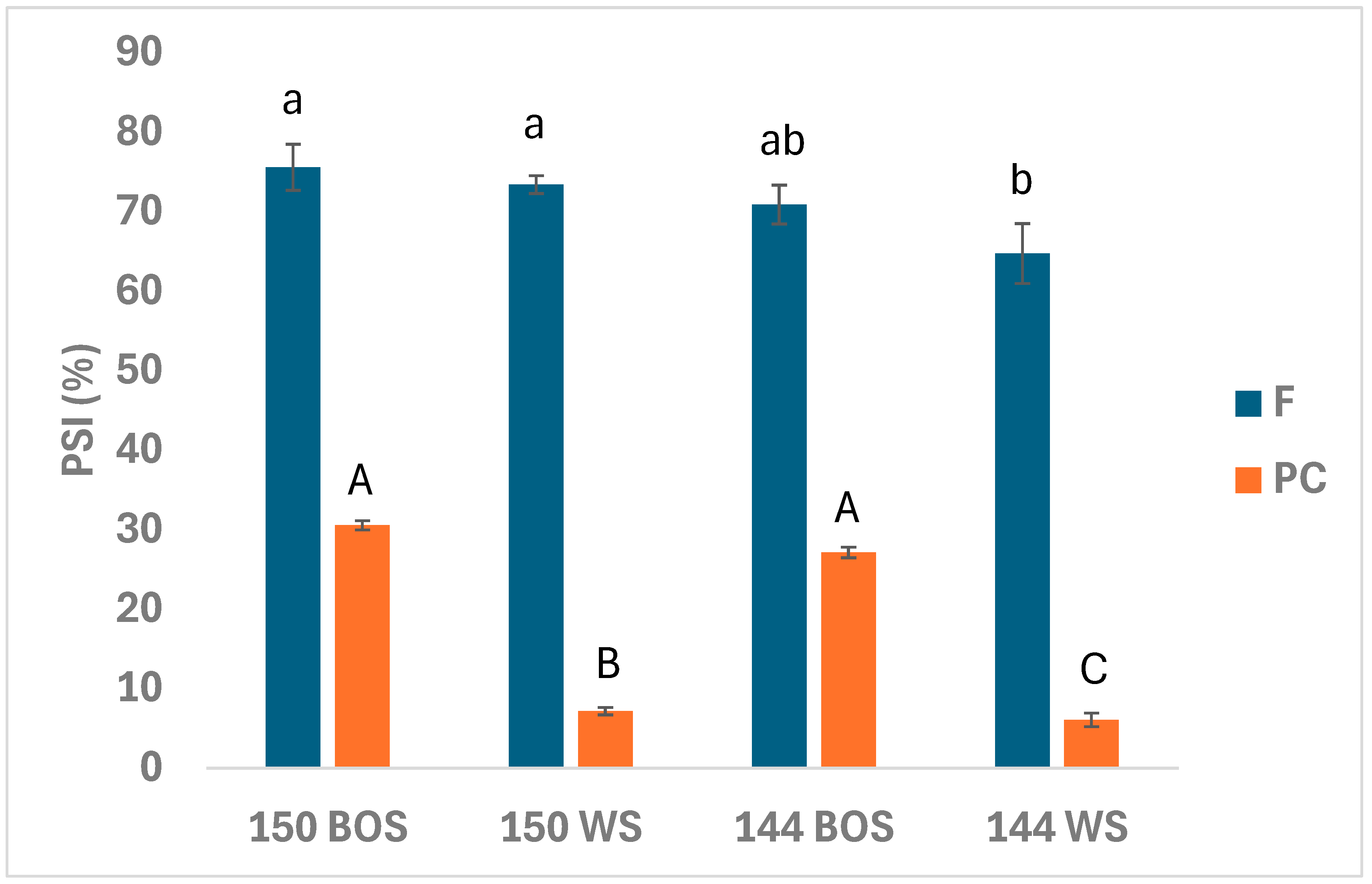


| Sample | Protein Content (% m/m on a Dry Mass Basis) | |
|---|---|---|
| Mushroom Flour (F) | Protein Concentrate (PC) | |
| 150WS | 17.2 ± 0.1 a | 37.2 ± 0.0 a |
| 150BOS | 24.6 ± 0.4 b | 43.1 ± 0.4 b |
| 144WS | 18.7 ± 0.5 c | 39.8 ± 0.6 c |
| 144BOS | 22.7 ± 0.2 d | 43.5 ± 0.1 b |
| Amino Acid (AA) | P. ostreatus Strains and Substrates 1 | |||
|---|---|---|---|---|
| 150WS | 150BOS | 144WS | 144BOS | |
| Asp + Asn | 18.82 ± 0.85 a | 25.34 ± 6.74 a | 22.81 ± 1.86 a | 24.85 ± 0.86 a |
| Glu + Gln | 28.80 ± 0.69 a | 39.92 ± 6.32 b | 33.00 ± 0.92 a | 39.60 ± 3.05 b |
| Ser | 10.61 ± 0.71 a | 13.60 ± 1.39 b | 11.68 ± 0.79 a | 13.56 ± 1.30 b |
| His | 6.28 ± 0.34 a | 8.66 ± 0.17 b | 6.43 ± 0.20 a | 8.28 ± 0.80 b |
| Gly | 9.89 ± 3.48 a | 13.59 ± 4.30 a | 10.10 ± 4.14 a | 12.01 ± 3.30 a |
| Thr | 9.82 ± 0.44 a | 13.04 ± 1.93 b | 10.97 ± 0.32 a | 12.45 ± 1.22 b |
| Arg | 20.89 ± 0.80 a | 30.58 ± 1.94 b | 21.60 ± 2.27 a | 27.70 ± 1.37 b |
| Ala | 12.93 ± 0.52 a | 18.28 ± 1.85 b | 14.35 ± 1.43 a | 17.72 ± 1.52 b |
| Tyr | 5.74 ± 0.25 a | 8.33 ± 1.85 b | 6.81 ± 1.10 a | 8.97 ± 0.67 b |
| Val | 9.98 ± 0.26 a | 13.48 ± 1.88 b | 11.78 ± 0.22 a | 13.54 ± 0.48 b |
| Met | 3.55 ± 0.32 a | 5.01 ± 0.22 b | 3.97 ± 0.17 a | 4.14 ± 0.56 b |
| Cys-Cys | 8.90 ± 0.98 a | 4.68 ± 1.70 a | 3.61 ± 1.74 a | 6.54 ± 2.53 a |
| Phe | 8.43 ± 0.24 a | 10.74 ± 1.84 b | 9.64 ± 0.51 a | 11.09 ± 0.45 b |
| Ile | 8.34 ± 0.56 a | 10.61 ± 1.08 a | 9.64 ± 0.88 a | 10.15 ± 1.30 a |
| Leu | 14.71 ± 1.81 a | 17.96 ± 0.94 a | 17.11 ± 3.35 a | 18.18 ± 2.80 a |
| Lys | 24.77 ± 6.67 a | 32.98 ± 2.48 a | 30.82 ± 12.51 a | 31.50 ± 5.55 a |
| Sum | 202.45 ± 4.46 a | 266.20 ± 11.50 b | 224.32 ± 6.78 a | 259.98 ± 6.79 b |
| Essential AA | 106.77 ± 11.44 a | 143.06 ± 12.48 b | 121.96 ± 20.43 a | 137.03 ± 14.53 b |
| Essential AA (%) | 52.70 ± 0.11 a | 53.80 ± 0.09 a | 54.30 ± 2.40 a | 52.70 ± 0.10 a |
| SAA 2 (Met + Cys-Cys) | 12.45 ± 1.3 a | 9.69 ± 1.92 a | 7.58 ± 1.91 a | 10.68 ± 3.09 a |
| AAA 3 (Phe + Tyr) | 14.17 ± 0.49 a | 19.07 ± 3.69 b | 16.45 ± 1.61 a | 20.06 ± 1.12 b |
| Sum of acidic amino acids (Asp + Glu) | 47.62 ± 1.54 a | 65.26 ± 13.06 b | 5 5.81 ± 2.78 a | 64.45 ± 3.91 b |
| Sum of basic amino acids (Lys + Arg + His) | 51.94 ± 7.81 a | 72.22 ± 5.49 b | 58.85 ± 14.98 a | 67.48 ± 7.72 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gardeli, C.; Mela, N.; Dedousi, M.; Kandyliari, A.; Kaparakou, E.; Diamantopoulou, P.; Pappas, C.; Mallouchos, A. The Influence of Substrate and Strain on Protein Quality of Pleurotus ostreatus. Appl. Sci. 2024, 14, 4040. https://doi.org/10.3390/app14104040
Gardeli C, Mela N, Dedousi M, Kandyliari A, Kaparakou E, Diamantopoulou P, Pappas C, Mallouchos A. The Influence of Substrate and Strain on Protein Quality of Pleurotus ostreatus. Applied Sciences. 2024; 14(10):4040. https://doi.org/10.3390/app14104040
Chicago/Turabian StyleGardeli, Chrysavgi, Nektaria Mela, Marianna Dedousi, Aikaterini Kandyliari, Eleftheria Kaparakou, Panagiota Diamantopoulou, Christos Pappas, and Athanasios Mallouchos. 2024. "The Influence of Substrate and Strain on Protein Quality of Pleurotus ostreatus" Applied Sciences 14, no. 10: 4040. https://doi.org/10.3390/app14104040
APA StyleGardeli, C., Mela, N., Dedousi, M., Kandyliari, A., Kaparakou, E., Diamantopoulou, P., Pappas, C., & Mallouchos, A. (2024). The Influence of Substrate and Strain on Protein Quality of Pleurotus ostreatus. Applied Sciences, 14(10), 4040. https://doi.org/10.3390/app14104040








