The Influence of the Used Bleaching Earth on the Content of Natural Dyes in Hemp (Cannabis sativa L.) Oils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hemp Oils Pressing
2.2. Low-Temperature Bleaching
2.3. Measurement Methodology
2.3.1. Measurements of Oil Color Using the CIE-Lab Method
- ∆L*—change of photometric brightness
- ∆a*—change of parameter (greenness for negative or redness for positive values)
- ∆b*—change of parameter (blueness for negative or yellowness for positive values)
2.3.2. Determination of Carotenoids and Chlorophylls in Hemp Oils
- A—absorbance at λ = 652.4 nm
- B—absorbance at λ = 665.2 nm
- C—absorbance at λ = 470.0 nm
2.4. Statistical Analysis
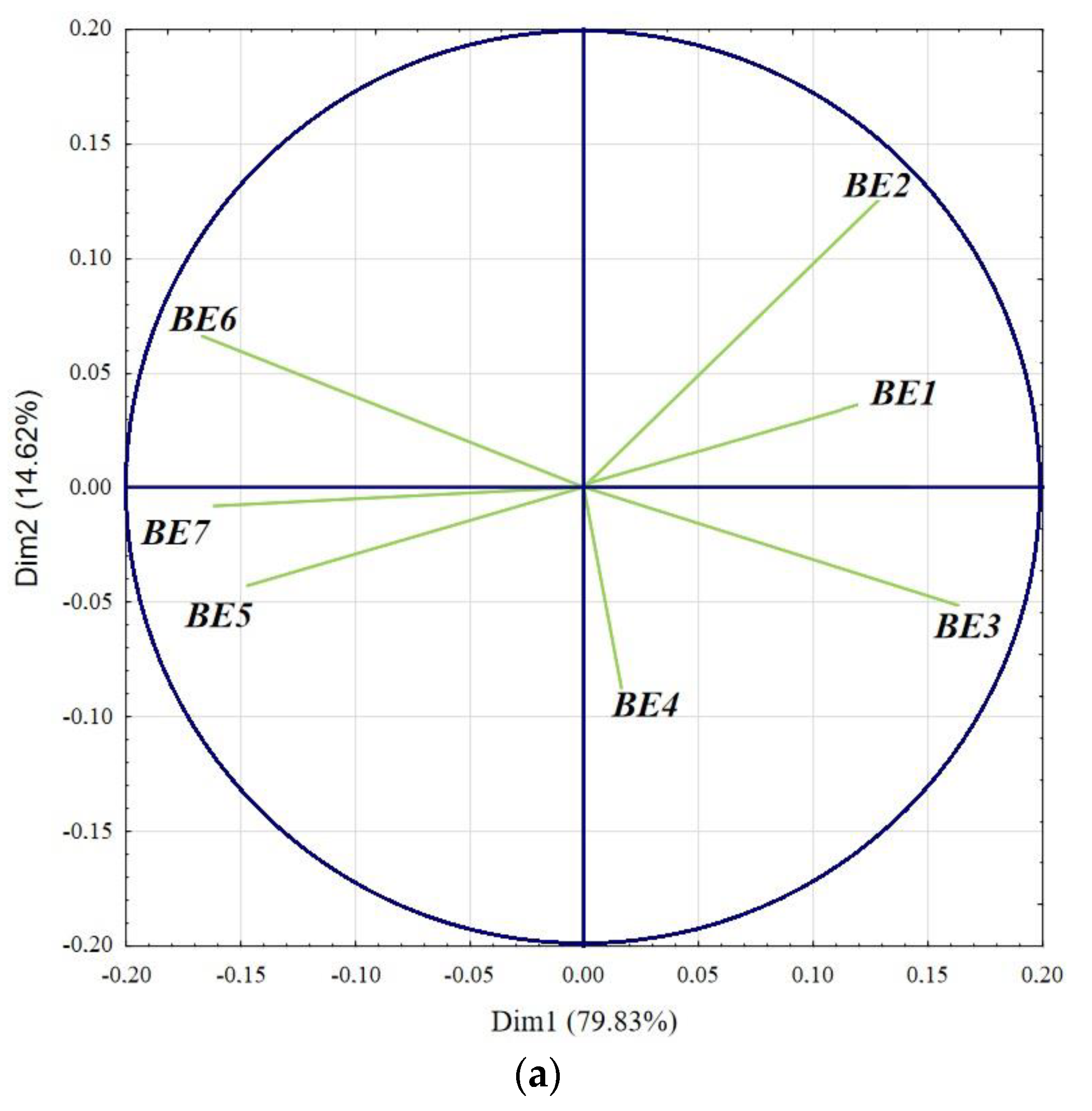
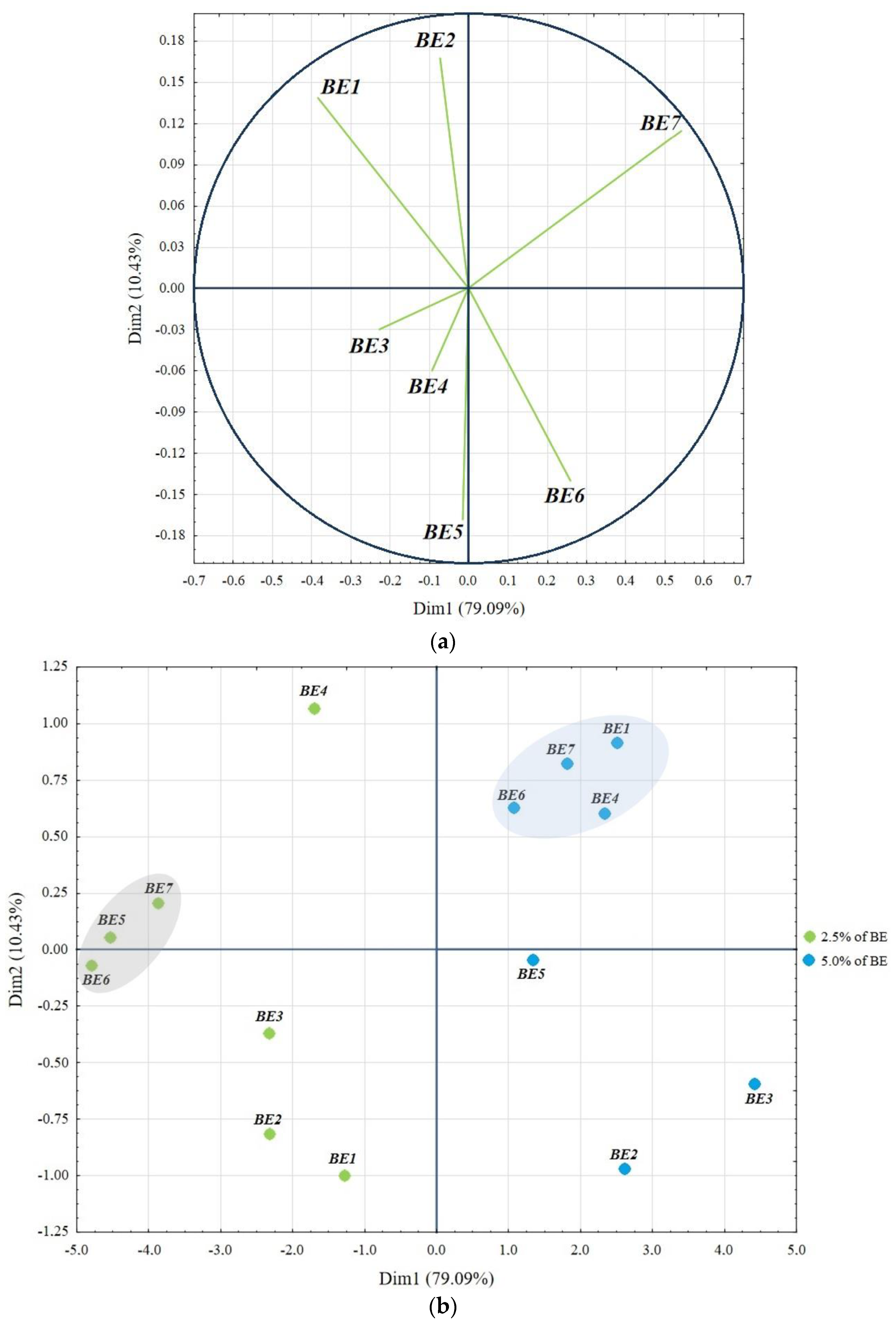
3. Results and Discussion
4. Conclusions
- Two doses of selected adsorbents were used during bleaching, and it was found that, in most cases, the use of more BE is associated with an increased reduction in dyes. This is not a favorable phenomenon for carotenoids, which are desirable compounds that have a beneficial effect on human health.
- Taking into account hemp varieties and pressing temperature, it was noted that for Finola CP and Earlina 8FC CP oils, the adsorbent with the most favorable adsorption ratio of chlorophylls to carotenoids was modified BEs 2.5%, especially kerolite-hydrated magnesium silicate. They remove sufficient chlorophylls while causing slight changes in carotenoid content. When bleaching HP oils from these varieties, using unmodified BEs 2.5% based on magnesian bentonite is recommended.
- Oil obtained from the Secuieni Jubileu CP and HP hemp variety should be bleached with unmodified magnesian bentonite at 2.5%. Unmodified attapulgite clay is not recommended for this variety, as it strongly adsorbs carotenoids from the oil.
- Tests conducted showed that an increase in the L* parameter (i.e., brightness) is caused by BEs based on modified magnesian bentonite and kerolite-hydrated magnesium silicate, although they do not show major changes in oil color (i.e., a* and b*), especially at doses below 5%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Symoniuk, E.; Ratusz, K.; Ostrowska-Ligęza, E.; Krygier, K. Impact of Selected Chemical Characteristics of Cold-Pressed Oils on Their Oxidative Stability Determined Using the Rancimat and Pressure Differential Scanning Calorimetry Method. Food Anal. Methods 2018, 11, 1095–1104. [Google Scholar] [CrossRef]
- Symoniuk, E.; Wroniak, M.; Napiórkowska, K.; Brzezińska, R.; Ratusz, K. Oxidative Stability and Antioxidant Activity of Selected Cold-Pressed Oils and Oils Mixtures. Foods 2022, 11, 1597. [Google Scholar] [CrossRef] [PubMed]
- Callaway, J.C. Hempseed as a Nutritional Resource: An Overview; Kluwer Academic Publishers: Norwell, MA, USA, 2004; Volume 140. [Google Scholar]
- Czechlowski, M.; Marcinkowski, D.; Golimowska, R.; Berger, W.A.; Golimowski, W. Spectroscopy Approach to Methanol Detection in Waste Fat Methyl Esters. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 210, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Weronika, G.; Damian, M.; Czechlowski, M.; Wojciech, G. Determination of optimal share of methyl esters from waste fats in biofuels with quality consistent with the standards EN 14214 and EN 590. J. Res. Appl. Agric. Eng. 2017, 62, 41–45. [Google Scholar]
- Gaglieri, C.; Alarcon, R.T.; de Moura, A.; Bannach, G. Vegetable Oils as Monomeric and Polymeric Materials: A Graphical Review. Curr. Res. Green Sustain. Chem. 2022, 5, 100343. [Google Scholar] [CrossRef]
- Hu, K.; Huyan, Z.; Ding, S.; Dong, Y.; Yu, X. Investigation on Food Packaging Polymers: Effects on Vegetable Oil Oxidation. Food Chem. 2020, 315, 126299. [Google Scholar] [CrossRef] [PubMed]
- Fridrihsone, A.; Romagnoli, F.; Cabulis, U. Environmental Life Cycle Assessment of Rapeseed and Rapeseed Oil Produced in Northern Europe: A Latvian Case Study. Sustainability 2020, 12, 5699. [Google Scholar] [CrossRef]
- Kwaśnica, A.; Teleszko, M.; Marcinkowski, D.; Kmiecik, D.; Grygier, A.; Golimowski, W. Analysis of Changes in the Amount of Phytosterols after the Bleaching Process of Hemp Oils. Molecules 2022, 27, 7196. [Google Scholar] [CrossRef]
- Czwartkowski, K.; Wierzbic, A.; Golimowski, W. Awareness and Expectations of Polish Consumers Regarding Edible Niche Oils as a Food Product. Sustainability 2022, 14, 14239. [Google Scholar] [CrossRef]
- Cavallo, P.; Dini, I.; Sepe, I.; Galasso, G.; Fedele, F.L.; Sicari, A.; Censi, S.B.; Gaspari, A.; Ritieni, A.; Lorito, M.; et al. An Innovative Olive Pâté with Nutraceutical Properties. Antioxidants 2020, 9, 581. [Google Scholar] [CrossRef]
- Matthäus, B.; Brühl, L. Virgin Hemp Seed Oil: An Interesting Niche Product. Eur. J. Lipid Sci. Technol. 2008, 110, 655–661. [Google Scholar] [CrossRef]
- Ramos-Escudero, F.; González-Miret, M.L.; Viñas-Ospino, A.; Ramos Escudero, M. Quality, Stability, Carotenoids and Chromatic Parameters of Commercial Sacha Inchi Oil Originating from Peruvian Cultivars. J. Food Sci. Technol. 2019, 56, 4901–4910. [Google Scholar] [CrossRef] [PubMed]
- Siano, F.; Moccia, S.; Picariello, G.; Russo, G.L.; Sorrentino, G.; Di Stasio, M.; La Cara, F.; Volpe, M.G. Comparative Study of Chemical, Biochemical Characteristic and ATR-FTIR Analysis of Seeds, Oil and Flour of the Edible Fedora Cultivar Hemp (Cannabis Sativa L.). Molecules 2019, 24, 83. [Google Scholar] [CrossRef] [PubMed]
- Plat, J.; Baumgartner, S.; Vreugdenhil, A.C.E.; Konings, M.C.J.M.; Calkins, K.L.; Mensink, R.P. Modifying Serum Plant Sterol Concentrations: Effects on Markers for Whole Body Cholesterol Metabolism in Children Receiving Parenteral Nutrition and Intravenous Lipids. Nutrients 2019, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Narayanankutty, A.; Illam, S.P.; Raghavamenon, A.C. Health Impacts of Different Edible Oils Prepared from Coconut (Cocos Nucifera): A Comprehensive Review. Trends Food Sci. Technol. 2018, 80, 1–7. [Google Scholar] [CrossRef]
- Rapa, M.; Ciano, S.; Rocchi, A.; D’Ascenzo, F.; Ruggieri, R.; Vinci, G. Hempseed Oil Quality Parameters: Optimization of Sustainable Methods by Miniaturization. Sustainability 2019, 11, 3104. [Google Scholar] [CrossRef]
- Łaska-Zieja, B.; Golimowski, W.; Marcinkowski, D.; Niedbała, G.; Wojciechowska, E. Low-Cost Investment with High Quality Performance. Bleaching Earths for Phosphorus Reduction in the Low-Temperature Bleaching Process of Rapeseed Oil. Foods 2020, 9, 603. [Google Scholar] [CrossRef] [PubMed]
- Marcinkowski, D.; Czwartkowski, K.; Bochniak, M.; Wereńska, M.; Krzaczek, P. Reuse of Bleaching Earth: The Green Solution for Rapeseed Oil Producers. Sustainability 2022, 14, 13071. [Google Scholar] [CrossRef]
- Sabah, E.; Majdan, M. Removal of Phosphorus from Vegetable Oil by Acid-Activated Sepiolite. J. Food Eng. 2009, 91, 423–427. [Google Scholar] [CrossRef]
- Yener, N.; Biçer, C.; Pekdemir, A.D.; Sarıkaya, Y.; Önal, M. Preparation and Characterization of Nanoporous Powders from Bentonite by Hydrochloric Acid Leaching and Using as Bleaching Earth. SN Appl. Sci. 2020, 2, 717. [Google Scholar] [CrossRef]
- Liu, W.; Yuan, K.; Yin, K.; Zuo, S.; Yao, C. Clay-Activated Carbon Adsorbent Obtained by Activation of Spent Bleaching Earth and Its Application for Removing Pb(II) Ion. Environ. Sci. Pollut. Res. 2021, 28, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Chakawa, D.P.; Nkala, M.; Hlabangana, N.; Muzenda, E. The Use of Calcium Sulphate Dihydrate (CaSO4·2H2O) as a Bleaching Agent for Crude Soya Bean Vegetable Oil. Procedia Manuf. 2019, 35, 802–807. [Google Scholar] [CrossRef]
- Silva, S.M.; Sampaio, K.A.; Ceriani, R.; Verhé, R.; Stevens, C.; De Greyt, W.; Meirelles, A.J.A. Effect of Type of Bleaching Earth on the Final Color of Refined Palm Oil. LWT 2014, 59, 1258–1264. [Google Scholar] [CrossRef]
- Didi, M.A.; Makhoukhi, B.; Azzouz, A.; Villemin, D. Colza Oil Bleaching through Optimized Acid Activation of Bentonite. A Comparative Study. Appl. Clay Sci. 2009, 42, 336–344. [Google Scholar] [CrossRef]
- Ferfuia, C.; Zuliani, F.; Piani, B.; Costa, L.D.; Corazzin, M.; Turi, M.; Baldini, M. Bleaching Techniques Impact on Some Quality Parameters in Two Different Cold-Pressed Oils Obtained at Farm Scale. J. Food Process Eng. 2023, 46, e14357. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis Sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 1–17. [Google Scholar] [CrossRef]
- Golimowski, W.; Łaska, B.; Pasyniuk, P.; Adamczyk, F.; Trawiński, A. Institute of Technology and Life Sciences. Patent PL 232781, 17 February 2016. [Google Scholar]
- Nkhata, S.G. Total Color Change (ΔE∗) Is a Poor Estimator of Total Carotenoids Lost during Post-Harvest Storage of Biofortified Maize Grains. Heliyon 2020, 6, e05173. [Google Scholar] [CrossRef]
- PN-A-86934:1995; Oils and Fats Vegetable and Animal—Determination of the General Spectrophotometric Color. Polish Committee for Standardization: Warszawa, Poland, 1995.
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Islam, M.; Rajagukguk, Y.V.; Siger, A.; Tomaszewska-Gras, J. Assessment of Hemp Seed Oil Quality Pressed from Fresh and Stored Seeds of Henola Cultivar Using Differential Scanning Calorimetry. Foods 2023, 12, 135. [Google Scholar] [CrossRef]
- Sacilik, K.; Öztürk, R.; Keskin, R. Some Physical Properties of Hemp Seed. Biosyst. Eng. 2003, 86, 191–198. [Google Scholar] [CrossRef]
- Santoso, H.; Iryanto; Inggrid, M. Effects of Temperature, Pressure, Preheating Time and Pressing Time on Rubber Seed Oil Extraction Using Hydraulic Press. Procedia. Chem. 2014, 9, 248–256. [Google Scholar] [CrossRef]
- Wroniak, M.; Krygier, K.; Kaczmarzyk, M. Comparison of the Quality of Cold Pressed and Virgin Rapeseed Oils with Industrially Obtained Oils. Pol. J. Food Nutr. Sci. 2008, 58, 85–89. [Google Scholar]
- Seçilmiş, Ş.S.; Koçak Yanık, D.; Fadiloğlu, S.; Göğüş, F. A Comparative Study on Performance of Industrial and Microwave Techniques for Sunflower Oil Bleaching Process. Food Chem. 2021, 365, 130488. [Google Scholar] [CrossRef] [PubMed]
- Dordevic, D.; Gablo, N.; Dordevic Janickova, S.; Tremlova, B. Effects of Centrifugation on the Oxidative Stability and Antioxidant Profile of Cold-Pressed Rapeseed Oil during Storage. Processes 2023, 11, 2224. [Google Scholar] [CrossRef]
- Suri, K.; Singh, B.; Kaur, A.; Yadav, M.P. Physicochemical Characteristics, Oxidative Stability, Pigments, Fatty Acid Profile and Antioxidant Properties of Co-Pressed Oil from Blends of Peanuts, Flaxseed and Black Cumin Seeds. Food Chem. Adv. 2023, 2, 100231. [Google Scholar] [CrossRef]
- Rossi, M.; Gianazza, M.; Alamprese, C.; Stanga, F. The Effect of Bleaching and Physical Refining on Color and Minor Components of Palm Oil. JAOCS J. Am. Oil Chem. Soc. 2001, 78, 1051–1055. [Google Scholar] [CrossRef]
- Marrakchi, F.; Kriaa, K.; Hadrich, B.; Kechaou, N. Experimental Investigation of Processing Parameters and Effects of Degumming, Neutralization and Bleaching on Lampante Virgin Olive Oil’s Quality. Food Bioprod. Process. 2015, 94, 124–135. [Google Scholar] [CrossRef]
- Kong, S.; Keang, T.; Bunthan, M.; Say, M.; Nat, Y.; Tan, C.P.; Tan, R. Hydraulic Cold-Pressed Extraction of Sacha Inchi Seeds: Oil Yield and Its Physicochemical Properties. ChemEngineering 2023, 7, 69. [Google Scholar] [CrossRef]
- Spano, M.; Di Matteo, G.; Rapa, M.; Ciano, S.; Ingallina, C.; Cesa, S.; Menghini, L.; Carradori, S.; Giusti, A.M.; Di Sotto, A.; et al. Commercial Hemp Seed Oils: A Multimethodological Characterization. Appl. Sci. 2020, 10, 6933. [Google Scholar] [CrossRef]
- Mansouri, F.; Allay, A.; Ben Moumen, A.; Benkirane, C.; Taaifi, Y.; Belhaj, K.; Addi, M.; Hano, C.; Fauconnier, M.L.; Caid, H.S.; et al. Laboratory-Scale Optimization of Hemp Seed Roasting Temperature and Time for Producing a High-Quality Pressed Oil. J. Food Process Preserv. 2023, 2023, 8261279. [Google Scholar] [CrossRef]
- Tura, M.; Mandrioli, M.; Valli, E.; Gallina Toschi, T. Quality Indexes and Composition of 13 Commercial Hemp Seed Oils. J. Food Compos. Anal. 2023, 117, 105112. [Google Scholar] [CrossRef]
- Lo Turco, V.; Litrenta, F.; Nava, V.; Albergamo, A.; Rando, R.; Bartolomeo, G.; Potortì, A.G.; Di Bella, G. Effect of Filtration Process on Oxidative Stability and Minor Compounds of the Cold-Pressed Hempseed Oil during Storage. Antioxidants 2023, 12, 1231. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.C.; Ali, M.A. Recent Advances in Ultrasound Technology Applications of Vegetable Oil Refining. Trends Food Sci. Technol. 2021, 116, 468–479. [Google Scholar] [CrossRef]
- Chew, S.C.; Tan, C.P.; Nyam, K.L. Optimization of Bleaching Parameters in Refining Process of Kenaf Seed Oil with a Central Composite Design Model. J. Food Sci. 2017, 82, 1622–1630. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Aachary, A.A.; Hydamaka, A.; Eskin, N.A.M.; Eck, P.; Thiyam-Holländer, U. Reduction of Chlorophyll in Cold-Pressed Hemp (Cannabis Sativa) Seed Oil by Ultrasonic Bleaching and Enhancement of Oxidative Stability. Eur. J. Lipid Sci. Technol. 2018, 120, 1700349. [Google Scholar] [CrossRef]
- Ramli, M.R.; Siew, W.L.; Ibrahim, N.A.; Hussein, R.; Kuntom, A.; Razak, R.A.A.; Nesaretnam, K. Effects of Degumming and Bleaching on 3-MCPD Esters Formation during Physical Refining. JAOCS J. Am. Oil Chem. Soc. 2011, 88, 1839–1844. [Google Scholar] [CrossRef]

| Hemp Variety | ||||
|---|---|---|---|---|
| Finola | Earlina 8FC | Secuieni Jubileu | ||
| Parameter | Oil content [%] | 29.97 | 31.04 | 29.47 |
| Seed size [mm] | 1.152 | 1.096 | 1.213 | |
| Average moisture content [%] | 8.389 | 8.319 | 8.312 | |
| Average bulk density [kg/m3] | 516.06 | 521.00 | 506.73 | |
| CP efficiency [%] | 66.26 | 69.26 | 62.66 | |
| HP efficiency [%] | 75.19 | 79.40 | 76.12 | |
| Oil | Parameters | ||||
|---|---|---|---|---|---|
| L* | a* | b* | Chlorophylls [μg/mL] | Carotenoids [μg/mL] | |
| Finola CP | 42.13 | 1.51 | 24.66 | 90.1 | 11.5 |
| Finola HP | 50.42 | 5.17 | 40.38 | 117.9 | 8.7 |
| Earlina 8FC CP | 37.89 | 0.99 | 20.14 | 81.2 | 17.3 |
| Earlina 8FC HP | 47.91 | 3.92 | 36.67 | 102.1 | 14.8 |
| Secuieni Jubileu CP | 45.01 | 2.12 | 26.81 | 106.7 | 12.9 |
| Secuieni Jubileu HP | 54.03 | 6.84 | 43.11 | 131.4 | 10.6 |
| Oil Variety | Parameter | BE1 (2.5%) | BE1 (5.0%) | BE2 (2.5%) | BE2 (5.0%) | BE3 (2.5%) | BE3 (5.0%) | BE4 (2.5%) | BE4 (5.0%) | BE5 (2.5%) | BE5 (5.0%) | BE6 (2.5%) | BE6 (5.0%) | BE7 (2.5%) | BE7 (5.0%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Finola CP | L* | 11.37 | 20.08 | 9.78 | 18.32 | 17.42 | 25.44 | 12.18 | 23.51 | 6.18 | 17.67 | 9.14 | 16.04 | 11.03 | 21.19 |
| a* | −124.81 | −202.26 | −95.49 | −172.93 | −188.72 | −210.53 | −145.11 | −215.04 | −90.23 | −209.02 | −53.38 | −189.47 | −81.95 | −222.56 | |
| b* | 55.03 | 31.26 | 53.56 | 41.50 | 106.03 | 23.22 | 82.45 | 110.24 | 34.73 | 105.30 | 26.33 | 90.86 | 46.80 | 110.97 | |
| Chlorophylls | −65.41 | −91.16 | −60.93 | −89.37 | −92.31 | −99.36 | −85.65 | −98.46 | −47.87 | −90.26 | −41.46 | −80.15 | −65.03 | −98.08 | |
| Carotenoids | −26.30 | −82.51 | −33.75 | −62.03 | −34.12 | −79.90 | −27.79 | −71.34 | −75.31 | −47.15 | −32.26 | −55.71 | −0.62 | −66.50 | |
| Finola HP | L* | 7.11 | 17.79 | 5.38 | 15.03 | 8.01 | 22.65 | 8.57 | 18.39 | 1.29 | 12.40 | 3.10 | 12.14 | 3.23 | 14.99 |
| a* | −89.42 | −184.13 | −53.44 | −137.04 | −89.95 | −187.83 | −79.37 | −147.62 | −39.68 | −110.58 | −35.98 | −111.64 | −42.86 | −157.67 | |
| b* | 131.10 | 179.60 | 104.35 | 180.60 | 158.19 | 234.78 | 142.14 | 269.90 | 66.22 | 179.93 | 62.21 | 187.63 | 67.22 | 231.77 | |
| Chlorophylls | −52.70 | −77.25 | −53.74 | −76.21 | −66.88 | −94.48 | −58.60 | −93.24 | −36.90 | −72.30 | −32.14 | −77.35 | −41.47 | −88.67 | |
| Carotenoids | −21.31 | −69.65 | −15.13 | −63.21 | −32.32 | −79.05 | −5.19 | −58.64 | −50.94 | −7.07 | −26.23 | −40.64 | −14.95 | −50.04 | |
| Earlina 8FC CP | L* | 13.62 | 21.65 | 11.14 | 20.11 | 16.31 | 24.55 | 12.51 | 21.56 | 7.73 | 18.62 | 6.45 | 20.96 | 7.98 | 20.03 |
| a* | −188.11 | −248.95 | −146.85 | −167.83 | −206.29 | −253.85 | −179.02 | −251.75 | −103.50 | −232.17 | −100.00 | −243.36 | −122.38 | −275.52 | |
| b* | 69.03 | 61.94 | 70.65 | 48.99 | 103.44 | 88.87 | 96.76 | 129.15 | 45.95 | 121.66 | 44.33 | 107.49 | 51.82 | 131.38 | |
| Chlorophylls | −67.88 | −87.17 | −67.10 | −87.40 | −84.72 | −95.43 | −78.70 | −94.87 | −50.71 | −88.85 | −54.28 | −90.30 | −58.62 | −93.64 | |
| Carotenoids | −43.98 | −78.63 | −5.87 | −65.06 | −38.79 | −74.59 | −22.04 | −69.68 | −10.30 | −50.34 | −13.76 | −60.73 | −3.66 | −67.37 | |
| Earlina 8FC HP | L* | 15.64 | 22.26 | 14.84 | 21.53 | 15.52 | 24.97 | 15.77 | 22.09 | 11.91 | 19.54 | 8.73 | 18.44 | 8.18 | 20.43 |
| a* | −220.54 | −264.29 | −240.18 | −226.79 | −278.57 | −280.36 | −191.96 | −276.79 | −150.00 | −264.29 | −158.04 | −303.57 | −150.00 | −321.43 | |
| b* | 96.66 | 52.12 | 110.02 | 55.46 | 134.97 | 85.52 | 124.50 | 122.05 | 86.41 | 162.81 | 88.86 | 140.09 | 94.43 | 144.54 | |
| Chlorophylls | −76.25 | −90.90 | −81.45 | −91.97 | −85.11 | −94.09 | −84.52 | −95.98 | −67.15 | −91.49 | −59.94 | −86.18 | −67.03 | −94.09 | |
| Carotenoids | −65.77 | −31.54 | −59.73 | −79.58 | −57.72 | −79.87 | −40.75 | −76.41 | −15.15 | −68.07 | −18.60 | −50.81 | −36.05 | −71.81 | |
| Secuieni Jubileu CP | L* | 10.35 | 25.15 | 5.36 | 15.12 | 12.71 | 28.42 | 9.94 | 24.83 | 6.22 | 17.75 | 3.50 | 18.02 | 4.95 | 18.75 |
| a* | −80.39 | −184.80 | −29.90 | −95.59 | −108.82 | −213.24 | −74.02 | −187.25 | −42.65 | −146.08 | −31.86 | −127.94 | −37.75 | −166.67 | |
| b* | 109.86 | 192.96 | 64.08 | 149.65 | 166.90 | 261.62 | 109.86 | 275.35 | 72.89 | 222.54 | 55.99 | 182.75 | 59.15 | 246.83 | |
| Chlorophylls | −52.01 | −81.69 | −38.15 | −73.93 | −68.51 | −94.27 | −57.06 | −92.39 | −36.19 | −81.54 | −22.70 | −76.87 | −36.49 | −84.40 | |
| Carotenoids | −26.98 | −66.72 | −0.56 | −53.55 | −28.09 | −75.34 | −3.19 | −54.27 | −23.54 | −30.09 | −30.49 | −41.58 | −19.71 | −44.45 | |
| Secuieni Jubileu HP | L* | 9.33 | 17.56 | 7.88 | 20.01 | 13.08 | 26.66 | 8.47 | 21.68 | 4.93 | 14.89 | 4.75 | 16.66 | 6.93 | 19.83 |
| a* | −79.17 | −171.88 | −43.75 | −119.27 | −105.73 | −208.85 | −50.52 | −183.85 | −32.29 | −132.29 | −31.77 | −106.25 | −38.54 | −142.19 | |
| b* | 91.28 | 126.17 | 67.60 | 136.14 | 138.01 | 212.15 | 56.70 | 238.63 | 52.96 | 179.75 | 42.68 | 125.55 | 34.89 | 167.91 | |
| Chlorophylls | −69.49 | −78.44 | −67.15 | −80.32 | −67.74 | −93.38 | −44.79 | −89.79 | −54.33 | −77.21 | −29.02 | −66.02 | −32.04 | −80.40 | |
| Carotenoids | −52.34 | −69.23 | −42.38 | −78.43 | −56.56 | −86.12 | −26.24 | −50.83 | −25.94 | −64.71 | −19.61 | −47.36 | −16.89 | −62.75 |
| Oil Variety | ΔE | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BE1 (2.5%) | BE1 (5.0%) | BE2 (2.5%) | BE2 (5.0%) | BE3 (2.5%) | BE3 (5.0%) | BE4 (2.5%) | BE4 (5.0%) | BE5 (2.5%) | BE5 (5.0%) | BE6 (2.5%) | BE6 (5.0%) | BE7 (2.5%) | BE7 (5.0%) | |
| Finola CP | 136.88 | 205.64 | 109.92 | 178.78 | 217.17 | 213.33 | 167.34 | 242.79 | 96.88 | 234.72 | 60.22 | 210.74 | 95.02 | 249.59 |
| Finola HP | 158.85 | 257.83 | 117.36 | 227.21 | 182.15 | 301.52 | 163.02 | 308.18 | 77.21 | 211.56 | 71.93 | 218.66 | 79.79 | 280.72 |
| Earlina 8FC CP | 200.84 | 257.45 | 163.34 | 175.99 | 231.35 | 270.07 | 203.88 | 283.76 | 113.50 | 262.77 | 109.58 | 266.86 | 133.14 | 305.90 |
| Earlina 8FC HP | 241.30 | 270.29 | 264.60 | 234.46 | 309.93 | 294.17 | 229.34 | 303.31 | 173.52 | 311.02 | 181.52 | 334.84 | 177.44 | 353.02 |
| Secuieni Jubileu CP | 136.52 | 268.36 | 70.92 | 178.21 | 199.65 | 338.71 | 132.84 | 333.92 | 84.68 | 266.79 | 64.51 | 223.81 | 70.35 | 298.42 |
| Secuieni Jubileu HP | 121.19 | 213.93 | 80.91 | 182.10 | 174.34 | 298.90 | 76.41 | 302.02 | 62.22 | 223.68 | 53.42 | 165.31 | 52.45 | 220.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcinkowski, D.; Nizio, E.; Golimowski, W.; Czwartkowski, K. The Influence of the Used Bleaching Earth on the Content of Natural Dyes in Hemp (Cannabis sativa L.) Oils. Appl. Sci. 2024, 14, 390. https://doi.org/10.3390/app14010390
Marcinkowski D, Nizio E, Golimowski W, Czwartkowski K. The Influence of the Used Bleaching Earth on the Content of Natural Dyes in Hemp (Cannabis sativa L.) Oils. Applied Sciences. 2024; 14(1):390. https://doi.org/10.3390/app14010390
Chicago/Turabian StyleMarcinkowski, Damian, Edyta Nizio, Wojciech Golimowski, and Kamil Czwartkowski. 2024. "The Influence of the Used Bleaching Earth on the Content of Natural Dyes in Hemp (Cannabis sativa L.) Oils" Applied Sciences 14, no. 1: 390. https://doi.org/10.3390/app14010390
APA StyleMarcinkowski, D., Nizio, E., Golimowski, W., & Czwartkowski, K. (2024). The Influence of the Used Bleaching Earth on the Content of Natural Dyes in Hemp (Cannabis sativa L.) Oils. Applied Sciences, 14(1), 390. https://doi.org/10.3390/app14010390










