Abstract
Nerve-transfer surgery is the treatment of choice for traumatic brachial plexus injuries (tBPIs). Combined electromyography (EMG) follow-ups and results obtained by transcranial magnetic stimulation (TMS) may provide useful follow-up of rehabilitation outcomes of elbow flexion in C5-C7 tBPIs. A total of 11 patients with complex tBPIs, operated by the Oberlin surgical technique, were assessed clinically (British Medical Research Council’s score—MRC) and by EMG + TMS after undergoing neuromuscular electrical stimulation and proprioceptive neuromuscular facilitation. Dynamometer quantitative muscle strength (DQMS) was also assessed for overall grip strength evaluation. Six patients continued rehabilitation three times a week, whereas five patients did not follow recommendations for continuous physical therapy (PT). All patients were assessed after 6 months as planned. Following a 6-month PT protocol, clinical improvements correlated with decreases of the Motor Evoked Potential (MEP) latency recorded at the first dorsal interosseous muscle, biceps brachii, and cortical level in the sublot group with continuous PT protocol compliance. We obtained significant amelioration of MEP latency and needle EMG signs of amelioration in these six patients. These cases also correlated to the MRC improvement in elbow flexion, as well as DQMS parameters. TMS parameters also mildly and inconstantly improved in the other five patients who limited themselves just to PT after surgery; however, there was no correlation with the EMG findings or MRC scaling. PT influences the cortical representation within the motor area of the upper limb when performed continuously. The electrical signals within the motor cortex promote the utility adherence to long-term PT protocols.
1. Introduction
Traumatic brachial plexus injuries (tBPIs) can be characterized by different mechanisms, elongation, and avulsion amongst the most burdensome, which is very often invalidating for the patient, with a huge impact on the quality of life [1]. In addition to magnetic resonance imaging (MRI), the extent of the lesion can be accurately described within the neurapraxia, axonotmesis, or neurotmesis phenomena by the use of different clinical and electrophysiological instruments [2].
Electromyography (EMG) provides useful information about the early degeneration processes that occur, as well as the proper moment for surgical intervention. This depends on the spontaneous and uncoordinated muscle activity that can be recorded from the recent denervated muscle territories, as a result of the muscle fiber’s hyper sensibility to acetylcholine, but also during the rehabilitation period after surgery [2,3]. Needle EMG can, in a positive scenario, provide information about motor unit action potential (MUAP) recruitment [2]. The measurement of the sensory nerve action potential (SNAP) can also provide useful data about the possible reinnervation that is taking place at a specific moment [1]. Recording a low amplitude compound muscle action potential (CMAP) is a much better predictor of the reinnervation process [1,2].
In tBPIs, neurotization is the procedure of choice, when possible (dependent on the extent of atrophy of the muscle: less atrophy, the better the outcomes). The transfer of a healthy nerve to the damaged one facilitates reinnervation [2,3].
Functional MRI has already proven the remodeling of sensory-motor cortical areas in peripheral nerve impairment [2]. The phenomenon of neuroplasticity, especially after surgery, can be assessed by early electric activity measurable by transcranial magnetic stimulation (TMS) and recording of motor evoked potential (MEP), especially by evidence of its improved latencies following motor rehabilitation-induced facilitation. The modulation of cortical reorganization mechanisms correlates with the amelioration of the motor deficit, as appreciated by the use of the standardized British Medical Research Council’s score (MRC) [2,4,5].
Among the brachial plexus (BP), the nerves that originate from the C5-C6 roots tend to be less affected by avulsion and, therefore, especially in paraganglion lesions, they are better candidates for neurotization, with good results in restoring elbow flexion [2,6]. On the other hand, a lesion with a higher extent (up to multilevel C5-T1 origin, and possible avulsions) is more challenging, and multiple surgical procedures are often required with less spectacular results over time [7,8].
The Oberlin procedure, described by Oberlin in 1994, realizes motor nerve graphon transfer from the ulnar nerve to the biceps muscle (musculocutaneous nerve), with good recovery of elbow flexion [9]. It is useful in a variety of cases, from simple C5-C6 preganglionic lesions to many complex cases (along with other neurotization interventions over the years). As well as other surgical procedures, EMG can assess results in a period of 3–4 to 6 months, depending on the complexity of the case [2,4]. Sometimes, even in early stages, EMG can provide a favorable prognosis by evidence of a decrease in spontaneous pathological activity (such as fibrillation potentials), correlated with an increase in the number of MUAP [2,4]. Original techniques, such as direct coaptation in C5-C6-C7 roots in a spinal tangential traumatic lesion can result in good recovery of elbow flexion at 6 months after surgery [10].
Physical therapy (PT) neurorehabilitation treatment for tBPIs is focused on returning the limb to its previous level of function and preventing potential disability. The goals of PT rehabilitation are pain control through electrotherapy, increase muscle strength and endurance, restore coordination, maintain range of motion, and control edema [1].
Our study relies on the use of neuromuscular electrical stimulation (NEMS) and proprioceptive neuromuscular facilitation (PNF). The retrospective study we present aims to understand the opportunity of continuous rehabilitation programs according to improvement either in electrophysiological parameters, clinical assessment, or both.
2. Materials and Methods
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Institutional Review Board (or Ethics Committee) of the University of Medicine and Pharmacy “Grigore T. Popa” Iași with no. 13940/11.07.2019. All patients were informed about the study protocol and expressed written consent in accordance with ethical principles. The study was spread over a period of 4 years, the patients being investigated in ambulatory regime and at the Neurology Department within the Clinical Rehabilitation Hospital in Iași, Romania.
A total of 11 patients with complex tBPIs (Table 1), operated by Oberlin surgical technique, were assessed by clinical evaluation using the British Medical Research Council’s score (MRC), and EMG + TMS after 6 months of undergoing rehabilitation therapy, consisting of NEMS and PNF. The patients were enrolled in the study at variable amount of time after the specific Oberlin procedure, which was performed in various centers across Romania, related to a medical record summarized in Table 2.

Table 1.
Patients’ descriptions.

Table 2.
Moment of Oberlin surgical intervention.
The initial step was the 10-day period of initial medical rehabilitation according to the protocol. Patients underwent a 40 min NEMS program at a frequency of 20 Hz in continuous pulse trains. The muscle stimulated was the biceps brachii and bipolar two-channel stimulation was used with equal electrodes placed on the ends of the muscle. After NEMS, patients followed a 30 min PNF program starting with strengthening exercises on D1 diagonal flexion, extension, and D2 diagonal flexion; extension was added to the program. PNF with the slow-reversal-hold PNF strengthening technique was used to strengthen the biceps brachii. The technique was performed with ten repetitions per set with a break between sets. After the first 10 days of therapy, six patients underwent rehabilitation treatment for another 6 months three times a week. These sessions were realized at the Physiokinetic Therapy and Recovery Center within the treatment base of the University of Medicine and Pharmacy in Iași.
Although the goal was to observe improvement in elbow flexion, dynamometer quantitative muscle strength (DQMS) was also assessed for grip strength evaluation. DQMS was chosen on the consideration that most patients had complex, multilevel PB lesions or, in the case of less complex C5-C6 cases, their visit to the reconstructive surgeon occurred late, once the atrophy phenomena were already present.
All the described evaluations, both clinical and electrophysiological, were performed after the Oberlin intervention had already been practiced. Some of the complex cases described had already undergone other surgical procedures before Oberlin, such as for restoring elevation of the shoulder; nevertheless, all the cases described were selected to have gone through the Oberlin procedure last, in order to better assess the elbow flexion rehabilitation in relation to this intervention (Table 3).

Table 3.
Complexity of the surgical cases.
No other specific investigations were performed; however, one patient had a recent MRI of the shoulder with no imagistic results that would influence the results.
Most of the patients included are sure to get future interventions, according to the complexity of the case. From our experience, tBPIs patients may receive several surgical procedures over a variable course of time, which sometimes expands easily over a decade (especially in younger patients).
The aim of the study is to prove the utility of NEMS and PNF at a specific endpoint in the rehabilitation journey, in correlation with central reactivity to therapy. All these interdisciplinary cases have in common the surgical attempt of restoring elbow flexion, prior to enrolling in the current study protocol. Observing cortical activity that does not necessarily correlate to motor function improvement creates the premise of future results when associating further surgical procedures with ongoing rehabilitation.
None of the patients had any associated comorbidities that would in any way limit the protocol, especially related to the presence of pacemakers or a history of epileptic seizures. In order to assess proper neuroplasticity phenomena, an exclusion criterion was that all selected patients were right handed.
Statistical analyses were performed using STATISTICA 6.0 StatSoft (Europe). The comparison of data between the initial and final evaluation was performed using Student’s t test or variance analysis (ANOVA) for continuous data. The results were expressed as mean ± standard deviation. A p < 0.05 value was considered statistically significant.
3. Results
Although the protocol was to determine both clinical and electrophysiological modifications following a 6-month rehabilitation protocol for all of the 11 patients, 5 of them invoked personal subjective reasons for non-compliance to recommendations for further recovery in the specialized outpatient clinic and only showed up to the scheduled final evaluation.
At the 6-month re-evaluation endpoint, decreases of the Motor Evoked Potential (MEP) latency were recorded at the first dorsal interosseous muscle (FDI), biceps brachii, as well as the cortical level in the ongoing therapy group. Since the selected cases were complex, we included the FDI to correlate with the DQMS values. We considered normal dynamometric values as a minimum of 12 KgF for women and 14 KgF for men.
The electrophysiological data obtained revealed the amelioration of MEP latency in these six compliant patients that stuck to the program in territorial rehabilitation facilities. We also found the presence of signs of variable motor unit recruitment at the needle EMG exam. These cases also correlated to MRC improvement in elbow flexion (Figure 1): from an MRC of 2 to 4 for two of the patients, from 2 to 3 in the other two, and from an MRC of 1 to 3 and 3 to 4 respectively. DQMS also improved (Figure 2). Regardless of the discontinuity in the rehabilitation protocol, the TMS parameters were also ameliorated in all the other five patients, nevertheless, with a lack of correlation to EMG findings or MRC scaling.
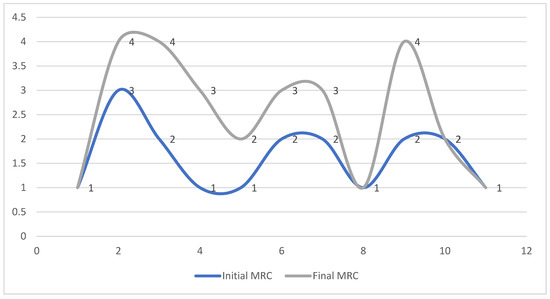
Figure 1.
MRC evolution of the 11 patients at the 6-month endpoint.
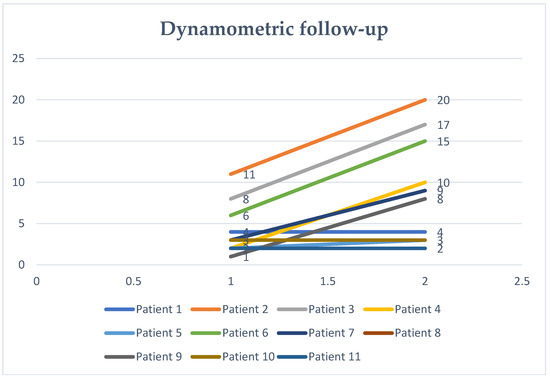
Figure 2.
Dynamometric testing.
The significant amelioration of MEP latency measured on the left cerebral hemisphere in the six compliant patients (p < 0.002), correlated with motor unit recruitment of MUAP and evidence of increased spontaneous activity, suggesting reinnervation at the needle EMG exam. Neurography parameters: CMAP in the radial nerve, as well as SNAP showed improvement (p < 0.0001), correlating with MRC amelioration (p < 0.001). DQMS was also relevant for these patients (p < 0.01) (Table 4).

Table 4.
Evolution of electrophysiological parameters at the 6-month endpoint for compliant patients.
In Table 5, patients that discontinued rehabilitation therapy showed mild TMS improvements, especially when measured at the bicipital (p < 0.04) and cervical level (p < 0.05). SNAP amplitude in the radial superficial nerve also showed improvement (p < 0.05).

Table 5.
Initial and final evaluation of the patients that discontinued rehabilitation.
4. Discussion
As TBPIs usually require several surgical techniques, the best results are usually obtained in non-complex, simple C5-C6 lesions by performing flexion of the forearm against the arm [2]. It is common that the patient would also need surgery for the other associated deficits, such as restoring opposition of the thumb [2,11].
From our experience, although this is a modifiable morbidity factor, in our region at least, some tBPI patients travel to different medical centers throughout the country, see different surgeons, and even receive interventions with very poor history documentation during long periods. It is not rare that a patient would not have the ability to give a correct history and, also, throughout the year, keeping track of some interventions is not always possible. On the other hand, compliance with rehabilitation protocols can also be reduced, related to long periods or, in our case, even to specific intervals. It is also difficult to gather a significant numerical group of patients to follow up at a given amount of time. Addressability issues, such as those during the SARS-CoV2 pandemic, also limited the follow-up of many patients with various types of brachial plexus lesions. Therefore, a single-center and relatively nonrandomized retrospective study acts as a major limitation. Needless to say, the number of tBPIs that were treated with surgical Oberlin procedures under these circumstances is relatively low to gather in our region, and quite challenging as an interdisciplinary approach. Accordingly, the small sample size is a mentionable limitation. The small group also required all patients to be right-handed.
Our preoccupations related to neuroplasticity in brachial plexus lesions started from Lundborg’s concept of the hand as an extension of the brain, as synaptic reorganization in the sensory-motor cortex enormously relies on the tactical experiences of the hand [5]. In this context, defective reinnervation and even the phantom-limb syndrome actually suggest a profound cortical reorganization, thus successful surgical procedures in the upper limb highly depend on neuroplasticity [4,5]. Earlier, Wall J et al. proved the brain’s reorganization patterns in 12 monkeys with transection of the ulnar and radial nerves. Cortical reorganization by analysis of the motor response in only one nerve-innervated limb (the median nerve) was assessed up to 1 and a half years, showing neuronal aggregates activated by inputs from each nerve [12].
Mano Y et al. assessed four patients with cervical root avulsions after intercostal to musculocutaneous nerve anastomosis. Six months after the surgery, motor unit action potentials were recorded from the operated biceps while the patient was doing a profound inspiration [13]. These MUAP became independent from breathing throughout the following 2 to 3 years and, at that point, the TMS suggested a transition of the representation of the reinnervated biceps brachii from the intercostal muscle towards the arm’s cortical area, as a post-surgical reorganization behavior [13].
PT neurorehabilitation plays a major role in the patient’s outcomes [1,14]. Neuromuscular electrical stimulation (NEMS) and proprioceptive neuromuscular facilitation (PNF) can not only improve peripherally, but we also consider that the rehabilitation intervention can sustain cortical reorganization patterns that can allow better amelioration of active voluntary elbow flexion [1,14]. Therefore, we align our research with the correspondent available body of data and sustain that EMG and TMS may provide a useful assessment of the rehabilitation outcomes in tBPIs [4].
Zinon Kokkalis et al., in a case study with bilateral tBPIs, showed that an intensive PT rehabilitation program that was composed of electrostimulation and finger movement amplitude exercises achieved promising results [15]. At 3 months, the patient’s muscle movement against gravity without any resistance in both upper limbs was 3/5 on the MRC scale; at 4 months the score was 4/5 and at 12 months, unrestricted range of motion was present with a score of 5/5 on the MRC scale [15].
Roger M. Enoka, Ioannis G. Amiridis, and Jacques Duchateau in a review published in 2019 have shown that the rehabilitative potential of electrical stimulation can range from modulating motor function to reducing symptom severity in clinical cohorts [16]. NEMS currents can increase muscle strength in targeted muscles, but spectacularly these currents have the ability to increase strength in muscles that are not directly targeted as well as in contralateral muscles [16].
NMES works by creating a strong electric field near the motor axons of peripheral nerves, which manages to depolarize the axonal membranes causing action potentials, which in turn will cause muscle contractions. However, for NMES to be effective, the peripheral nerves to the target muscles must be intact and the muscles must be normally vascularized, which is why they are recommended in central nervous system disorders. [17].
Proprioceptive neuromuscular facilitation (PNF) is a PT technique for the development of muscle strength that is based on the application of mechanical resistance to muscle contraction, thereby facilitating the contractile strength of the muscle. PNF is suitable for patients with partial peripheral nerve damage involving significant muscle weakness [18].
Chagas et al. tested a protocol developed specifically for tBPIs in which PNF exercises were chosen according to the deficits presented by the patients aiming to recover limb function with minimal compensation from increased blood flow [19]. The selected PNF exercises represent different graded combinations of resistance application on diagonal movement patterns in the three planes to achieve high neural recruitment. Agonistic techniques, among which rhythmic-initiation and combination of isotones are considered to be the first choice in rehabilitation programs, aim to give the patient a first explanation of the movement. Antagonistic techniques, including stabilizing inversion and dynamic inversion, have been used to promote functional and task-oriented movement aimed at improving motor learning [19].
In our case, in the five non-compliant patients, the amelioration of TMS parameters in bicipital and cervical measurements can be attributed directly to nerve regeneration following the surgical procedure alone (more or less related to initial rehabilitation), especially in terms of restoring nervous continuity of the plexus. These results correlate less with signs of ongoing brain plasticity, as compared to the other six patients, when related to MEP latency measured at the cerebral magnetic stimulation. Also, SNAP amplitude alone does not provide information other than a favorable background to continue physical treatment or, if necessary, complementary surgical procedures.
The main idea is that prolonged rehabilitation protocols maintain and improve segmental representation among cortical motor areas with favorable outcomes. Maintaining activity by the use of rehabilitation protocols, prolongs the brain’s reactivity and adaptability to future situations. This would reiterate the need for finding a more suitable invasive restoration technique adapted to each patient’s current situation. This includes associations of direct neurotization, complex neuro-neuronal neurotization, or muscle transfers.
One limitation of this study is represented by the low number of participants which may decrease the statistical power. Further studies with larger groups are needed to confirm these results. Taking into consideration left-handed patients might be important as well.
Nevertheless, each case is different when it comes to long-term compliance and evolution; therefore, electrophysiological combined techniques may provide a background for long-term therapeutic plans.
5. Conclusions
While the chosen surgical procedure depends on the ingenuity of the surgeon faced with the complexity of the case, NEMS and PNF may contribute to both peripheral amelioration and enhancement of the cortical representation within the motor area of the upper limb. Even in patients without visible motor amelioration at the 6-month endpoint, there are slightly better electrical signals measured, especially at the cervical level, which is an encouraging fact if further surgical procedures are to be performed. These can signal mild ongoing cortical reorganization and reinforce the need for long-term continuous rehabilitation protocols, as well as the need for further larger studies to identify these patterns.
Author Contributions
Conceptualization, D.T., I.O., D.-V.M. and T.S.; methodology, D.T., D.M.T. and T.S.; software, I.O. and D.A.I.; validation, I.O., D.-V.M., D.T., D.A.I. and T.S.; formal analysis, D.-V.M.; investigation, D.T. and D.M.T.; resources, D.T., I.O. and D.A.I.; data curation, D.M.T. and T.S.; writing—original draft preparation, D.T., D.M.T. and T.S.; writing—review and editing, D.T., I.O., D.-V.M. and T.S.; visualization, D.T., D.M.T., I.O., D.-V.M. and T.S.; supervision, T.S. and D.-V.M.; project administration, D.T., D.M.T. and T.S.; funding acquisition, I.O. and D.A.I. All authors have read and agreed to the published version of the manuscript.
Funding
The work of Daniel Andrei Iordan was supported by the project “PROINVENT”, contract no. 62487/03.06.2022—POCU/993/6/13—code 153299, financed by The Human Capital Operational Programme 2014–2020 (POCU), Romania.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the University of Medicine and Pharmacy “Grigore T. Popa” Iasi with no. 13940/11.07.2019.
Informed Consent Statement
Informed consent was obtained from all subjects that were involved in the study.
Data Availability Statement
Data are contained within the main text of the article.
Acknowledgments
All authors have equally contributed to this article as senior authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huang, X.; Jiang, Z.; Sun, H.; Xie, B.; Lu, F.; Huang, W.; Wang, T.; Xiong, H. The effect of a high-quality nursing model employing low-frequency pulse electrical stimulation combined with early systemic functional exercises on the function of the affected limb in brachial plexus injury patients. Am. J. Transl. Res. 2021, 13, 4939–4948. [Google Scholar] [PubMed]
- Chung, K.; Yan, L.; McGillicuddy, J. Practical Management of Pediatric and Adult Brachial Plexus Palsies; Elsevier Saunders: Philadelphia, PA, USA, 2012; pp. 1–32, 173–211, 220–233. [Google Scholar]
- Narakas, A.O.; Hentz, V.R. Neurotization in brachial plexus injuries. Indication and results. Clin. Orthop. Relat. Res. 1988, 237, 43–56. [Google Scholar] [CrossRef]
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.G.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.B.; George, M.S. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef] [PubMed]
- Lundborg, G. Brain plasticity and hand surgery: An overview. J. Hand. Surg. Br. 2000, 25, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, P.S.; Sadhotra, L.P.; Bhargava, P.; Bath, A.S.; Mukherjee, M.K.; Bhatti, T.S.; Maurya, S. Multiple nerve transfers for the reanimation of shoulder and elbow functions in irreparable C5, C6 and upper truncal lesions of the brachial plexus. Indian J. Neurotrauma 2008, 5, 95–104. [Google Scholar] [CrossRef]
- Dawson, S.E.; Gross, J.N.; Berns, J.M.; Weinzerl, T.; Adkinson, J.M.; Borschel, G.H. Supraclavicular Approach to the Brachial Plexus. Plast. Reconstr. Surg. Glob. Open 2023, 11, e4771. [Google Scholar] [PubMed]
- Rohde, R.S.; Wolfe, S.W. Nerve transfers for adult traumatic brachial plexus palsy (brachial plexus nerve transfer). HSS J. 2007, 3, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Oberlin, C.; Béal, D.; Leechavengvongs, S.; Salon, A.; Dauge, M.C.; Sarcy, J.J. Nerve transfer to biceps muscle using a part of ulnar nerve for C5-C6 avulsion of the brachial plexus: Anatomical study and report of four cases. J. Hand. Surg. Am. 1994, 19, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Stamate, T.; Mazilu, G.; Stamate, M.; Stamate, G.; Topa, I. Direct Coaptation of the C5-C6-C7 Brachial Plexus Roots in Traumatic Tangential Spine Lesions. Personal Technique. J. Reconstr. Microsurg. 2014, 30, A073. [Google Scholar] [CrossRef]
- Sakellariou, V.I.; Badilas, N.K.; Stavropoulos, N.A.; Mazis, G.; Kotoulas, H.K.; Kyriakopoulos, S.; Tagkalegkas, I.; Sofianos, I.P. Treatment options for brachial plexus injuries. ISRN Orthop. 2014, 2014, 314137. [Google Scholar] [CrossRef]
- Wall, J.T.; Huerta, M.F.; Kaas, J.H. Changes in the cortical map of the hand following postnatal ulnar and radial nerve injury in monkeys: Organization and modification of nerve dominance aggregates. J. Neurosci. 1992, 12, 3456–3465. [Google Scholar] [CrossRef]
- Mano, Y.; Nakamuro, T.; Tamura, R.; Takayanagi, T.; Kawanishi, K.; Tamai, S.; Mayer, R.F. Central motor reorganization after anastomosis of the musculocutaneous and intercostal nerves following cervical root avulsion. Ann. Neurol. 1995, 38, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Belviso, I.; Palermi, S.; Sacco, A.M.; Romano, V.; Corrado, B.; Zappia, M.; Sirico, F. Brachial Plexus Injuries in Sport Medicine: Clinical Evaluation, Diagnostic Approaches, Treatment Options, and Rehabilitative Interventions. J. Funct. Morphol. Kinesiol. 2020, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Kokkalis, Z.; Papagiannis, S.; Kouzelis, A.; Diamantakis, G.; Panagopoulos, A. Traumatic Bilateral Brachial Plexus Injury. Cureus 2022, 14, e24626. [Google Scholar] [CrossRef] [PubMed]
- Enoka, R.M.; Amiridis, I.G.; Duchateau, J. Electrical Stimulation of Muscle: Electrophysiology and Rehabilitation. Physiology 2020, 35, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Knutson, J.S.; Makowski, N.S.; Kilgore, K.L.; Chae, J. 43—Neuromuscular Electrical Stimulation Applications. In Atlas of Orthoses and Assistive Devices, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 432–439.e3. ISBN 9780323483230. [Google Scholar] [CrossRef]
- Fritz, S.; Chaitow, L.; Hymel, G.M. Chapter 15—Medical Treatment for Illness and Injury. In Clinical Massage in the Healthcare Setting; Mosby: Maryland, MI, USA, 2008; pp. 514–533. ISBN 9780323039963. [Google Scholar] [CrossRef]
- Teodor, C.I.; Claudiu, M. The role of motivation in education through sport. Procedia-Soc. Behav. Sci. 2013, 83, 1054–1058. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).