Abstract
The study aims to assess the myotonometer parameters of major pectoralis, biceps brachialis, femoral biceps and anterior tibialis in patients with Parkinson’s disease before and after medical treatment using comparisons with healthy controls. A total of 49 patients with Parkinson’s disease (69.76 ± 6.39 years) and 42 healthy controls (60.48 ± 7.62 years) were tested using MyotonPRO before and one hour after drug administration. Five parameters were recorded (frequency [Hz], stiffness [N/m], decrement, relaxation [ms] and creep). At pre-treatment assessment, significantly increased values of myotonometer parameters were recorded for major pectoralis and biceps brachialis, with the exception of decrement. Frequency and decrement were significantly higher in patients’ femoral biceps and anterior tibialis. For all assessed muscles, frequency was significantly higher in Parkinson’s disease patients one hour after medication intake. Stiffness, relaxation and creep had increased values in major pectoralis. For the lower limb muscles, decrement had greater values. We concluded that there were no significant differences of major pectoralis and biceps brachialis elasticity between patients with Parkinson’s disease and healthy controls pre and post drug administration, with improved viscoelastic properties of biceps brachialis after medication. After drug administration, no significant differences of femoral biceps and anterior tibialis stiffness were noted between patients and controls.
1. Introduction
Parkinson’s disease is a progressive neurodegenerative disorder and is considered one of the most frequent neurological disorders []. It is responsible for the destruction of the brain nerve cells that produce the neurotransmitter dopamine. The loss of these specific nerve cells results in major symptoms; those typical of Parkinson’s disease are tremor, muscular rigidity and bradykinesia []. As the disease progresses, the changes in posture and muscular action become more obvious: the shoulders and body are bent forward, and the difficulty of movement degenerates into a general muscular imbalance. It is known from the literature that muscle rigidity and progressive loss of muscle control are the most common symptoms of Parkinson’s disease. Approximately 90% of patients are confronted with these symptoms in the course of disease progression []. Alterations in the structure and functionality of the musculature in Parkinson’s disease determine unfavorable changes of lifestyle in these patients. Patients can encounter difficulties in simple activities, such as changing positions in bed, lifting from a chair or fine motor activities []. Muscle rigidity determines the absence of normal balance of the upper limbs while walking, which leads to falls [,]. Starting from the early stages of Parkinson’s disease, patients have decreased muscle activity and lesser ability to modulate their muscle activities; this is manifested especially during the gait initiation phase []. MyotonPRO technology is a unique, reliable, precise, fast and easy-to-use research tool. The role of this tool is important for the clinical and objective evaluation of the treatment efficiency, both in terms of medical treatment and physical therapy [,]. This device was conceived for research, evaluation and supervision [,,]. MyotonPRO records the natural oscillation cushion of the soft tissue as an acceleration signal, with a simultaneous calculus of an exterior mechanical impulse, with a reduced force, fast release and constant pre-load []. The technology describes a tissue using five features and through different characteristics []. The five specific parameters of the device are represented by the following: state of tension through oscillation frequency (Hz), biomechanical properties with the help of dynamic stiffness (N/m), elasticity represented by logarithmic decrement, viscous-elastic properties through mechanical stress relaxation time (ms) and ratio of deformation and relaxation time (creep) [,]. The testing allows the determination of the muscle status [,,]. Moreover, the myotonometry shows excellent reliability properties for test and retest [].
The systematic review of Ferreira-Sánchez MdR et al. compiled the objective quantitative methods (myotonometry, electromyography and elastography) for rigidity assessment in Parkinson’s disease patients []. These assessment tools have been used only for certain muscle groups of the upper or lower limbs; no analysis was performed for the trunk muscles. Previous studies showed that myotonometric measurements of the extensor digitorum [] and biceps and triceps brachialis muscles [,] had an increased rigidity-related resting muscle stiffness in Parkinson’s disease patients compared with healthy controls.
Patients suffering from Parkinson’s disease have difficulties in performing complex fast arm movements involving also the activity of major pectoralis []. The study of Corcos DM et al. showed that the decline in elbow flexor peak torque is due to a decrease in the activity of the biceps brachialis []. A shorter duration of anterior tibialis muscle activity occurs prematurely in patients with Parkinson’s disease prior to freezing []. A reduced anterior tibialis activity alters foot contact patterns, increasing the fall risk []. Rodriguez KL et al. identified a lower modular complexity of ankle plantar flexor (anterior tibialis) and knee flexors (femoral biceps) in Parkinson’s disease patients, which may contribute to gait deficits [].
The aim of our study is to identify the muscle mechanical properties in patients with Parkinson’s disease, before and after medical treatment (drug administration), and applying comparisons with healthy controls. We chose for testing four muscle groups that are important in daily activities in patients suffering from Parkinson’s disease: major pectoralis, biceps brachialis, femoral biceps and anterior tibialis of the dominant part. The secondary objective of our research was to analyze a possible correlation between myotonometric parameters of the assessed muscles and patients’ characteristics.
2. Materials and Methods
2.1. Participants
The participants were patients diagnosed with Parkinson’s disease (patients’ group) and healthy controls. For the patients’ group, the inclusion criteria were: people diagnosed with Parkinson’s disease who followed a specific medical treatment (different types of oral medication: Levodopa in combination with dopa-decarboxylase inhibitors, dopamine agonists, monoamine oxidase B (MAO-B) inhibitors, Catechol-O-methyl transferase (COMT) inhibitors, or amantadine. The exclusion criteria were: patients who do not take the recommended medical treatment on an ongoing basis, patients with psychiatric disorders (dementia or other disorders that affect rationality), recent surgical interventions (in the last 6 months), and other neurological disorders that lead to muscle structural changes (stroke, amyotrophic lateral sclerosis, muscular dystrophy, etc.). The control group consisted of gender and age-matched healthy controls.
The study was conducted between 15 September 2022 and 15 January 2023. Participation in the study was voluntary. Written informed consent was obtained from all the participants. The study was approved by the Ethics Committee of “Victor Babes” University of Medicine and Pharmacy, Timisoara, Romania, and was in accordance with the Helsinki Declaration (No. 49/15.09.2022).
Clinical characteristics of participants (age, weight, height and body mass index) were recorded. Disease duration and medication were also documented in Parkinson’s disease patients.
The study group consisted of 49 patients (51% women and 49% men) diagnosed with Parkinson’s disease who follow medical treatment. A total 42 healthy subjects (50% women and 50% men) comprised the control group. Table 1 presents the characteristics of Parkinson’s disease patients and controls. The two groups were homogenous, as we found no statistically significant differences in age, height, weight and body mass index. The patients included in the study were under neurological follow-up from 2 to 15 years from their diagnosis. The age of appearance of the disease in patients with Parkinson’s disease varied from 43 to 72 years old. The duration of the disease ranged from 2 to 15 years. According to the Hoehn and Yahr scale, the patients were in stages 2, 3 or 4 of the disease.

Table 1.
Characteristics of Parkinson’s disease patients and controls.
2.2. Assessments
All the participants at the study were tested at the dominant hemicorpus using a MyotonPRO device (Myoton AS, Tallinn, Estonia) (Figure 1).

Figure 1.
MyotonPRO device.
The MyotonPRO system measures the following five parameters: state of tension (1. natural oscillation frequency characterizing tone or state of tension); biomechanical properties (2. dynamic stiffness, and 3. logarithmic decrement, characterizing elasticity or dissipation of natural oscillation); viscoelastic properties (4. mechanical stress relaxation time (ms), and 5. ratio of relaxation time to deformation time, characterizing creep). The data provided by the device let us analyze the oscillation frequency (Hz), which characterizes the tonus (the intrinsic tension at a cellular level). An increased muscular tonus compared to normal levels alters the conditions of blood feeding of the muscle. The increased tonus is associated with muscular pain, fatigue, overburden, the decrease of physical performance and a late recovery []. Dynamic stiffness (N/m) is represented by the capacity of the muscle to endure a force that alters the shape of the muscle. A rigid musculature determines an increased effort for muscular elongation in that area, producing asymmetry between the parts of the body, which disturbs the functional movement rhythm [,]. Elasticity represents the capacity of the muscle to regain its initial shape. The functionality of a muscle can be quantified as efficient only if the muscle regains its shape quickly in the time between two contractions. Lack of elasticity of the muscle produces fatigue more rapidly during effort; the velocity of the movement is limited, and the structure is less elastic [,].
Parkinson’s disease patients have changes in the center of gravity with the forward position of the body, a slightly anterior bend of the trunk with the shoulders bent forward, and semi-flexed elbows and knees. The tested musculature was chosen after the examination of the specific posture in these patients. We also considered the muscles that are most affected in usual self-care activities. We chose to test the muscles involved in the activities of daily living [] at general level, and we included four muscle areas. The first tested muscular group is situated at the antero-superior trunk and is represented by the major pectoralis muscle; the second muscular group was tested at the dominant superior limb on the biceps brachialis muscle. The next two muscular tests were done at the lower limb on the posterior face of the thigh musculature at the level of the femoral biceps muscle, and the last test was done on the anterior tibialis muscle.
The chosen positions allowed patients to be fully relaxed. The measurements were performed at the level of the muscle belly; the device was always perpendicular to the skin surface. First determination was done on the major pectoralis muscle. The test was done with the patient relaxed, in a sitting position; the same position was kept for the upper limb test at the level of the biceps brachialis muscle (Figure 2). The next two measurements included the inferior limb. The test at the thigh level was done in the posterior area at the level of the femoral biceps muscle; the test at this level was done with the patient in a prone position because the device should be perpendicular to the surface of the skin. For the last determination, the patient was in the sitting position; the anterior tibialis muscle was tested (Figure 3). The same muscles were tested in the healthy controls. All muscles were measured in the same order for all participants (both groups).
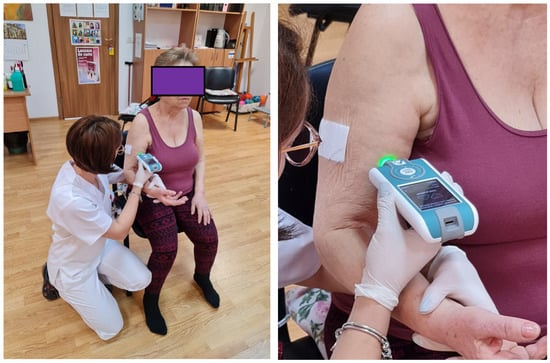
Figure 2.
Measurement of myotonometric parameters on biceps brachialis muscle.
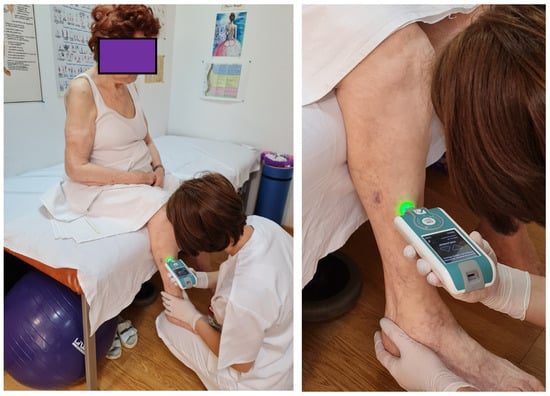
Figure 3.
Measurement of myotonometric parameters on anterior tibialis muscle.
In the patients’ group, the above-mentioned muscles were tested twice. The first determination was done before the medical treatment administration for the study group, and the second determination an hour after the medical treatment administration. In the control group, the two measurements were done one hour apart. All the measurements were performed in the morning. The myotonometric measurements were done by the same physical therapist. The four muscles were tested consecutively; the overall assessment for one subject lasted approximately 90 s.
We used the MDS-UPDRS (Movement Disorder Society–Unified Parkinson’s Disease Rating Scale) (patient section) to identify the patients’ physical status in the last weeks. The following motor activities were assessed: dressing, hygiene, hobbies, walking and balance, and freezing. Each item had a score ranging from 0 (normal) to 4 (severely affected) [].
2.3. Statistical Analysis
The sample size was calculated on the basis of results of Min-Jae and Dae-Sung using G*Power 3.1.9.7 (Universitat Kiel, Kiel, Germany) with the Wilcoxon signed-rank test. The effect size was 0.5, the type I error was α = 0.05, and power was 0.8. A total sample size of 38 patients was required. The data were processed in SPSS Statistics 23.0. The t-test was used for the couple samples. For these correlations, we applied regression with a single predictor, and for testing the relationship between variables, we used Pearson correlation. A p value less than 0.05 was considered statistically significant.
3. Results
The comparison between the myotonometer parameters of the Parkinson’s disease patients (before medical treatment, pre drug administration) and the control group (first measurement) are included in Table 2 (data of the all four muscle groups). We noticed a statistically significant difference of frequency, stiffness and decrement, with higher values for the patients’ group.

Table 2.
Myotonometer parameters (all muscle data) of the Parkinson’s disease patients (pre drug administration) and the control group (first measurement).
When performing the intergroup comparison of each muscle, significantly increased values of the myotonometer parameters were recorded for major pectoralis and biceps brachialis, with the exception of the decrement (Table 3 and Table 4). Frequency and decrement were significantly higher when assessing the patients’ femoral biceps and anterior tibialis (Table 5 and Table 6). For all the Parkinson’s disease patients, the five myotonometer parameters were increased in comparison to healthy controls.

Table 3.
Myotonometer parameters of major pectoralis in Parkinson’s disease patients (pre drug administration) and control group (first measurement).

Table 4.
Myotonometer parameters of biceps brachialis in Parkinson’s disease patients (pre drug administration) and the control group (first measurement).

Table 5.
Myotonometer parameters of femoral biceps in Parkinson’s disease patients (pre drug administration) and the control group (first measurement).

Table 6.
Myotonometer parameters of anterior tibialis in Parkinson’s disease patients (pre drug administration) and the control group (first measurement).
3.1. The Post-Treatment Assessment and Comparison to Healthy Controls
The comparison between the myotonometer parameters of the Parkinson’s disease patients (post medical treatment, drug administration) and the control group (second measurement) are presented in Table 7 (data of the all four muscle groups). Frequency, stiffness and decrement had significantly increased values for the patients’ group. For all the four assessed muscles, frequency was significantly higher in Parkinson’s disease patients one hour after medication intake. Moreover, stiffness, relaxation and creep had increased values in the major pectoralis. Stiffness was also significantly higher in biceps brachialis, while for the lower limb muscle groups, decrement had greater values (Table 8, Table 9, Table 10 and Table 11).

Table 7.
Myotonometer parameters (all muscle data) of the Parkinson’s disease patients (post medical treatment) and the control group (second measurement).

Table 8.
Myotonometer parameters of major pectoralis in Parkinson’s disease patients (post medical treatment) and the control group (second measurement).

Table 9.
Myotonometer parameters of biceps brachialis in Parkinson’s disease patients (post medical treatment) and the control group (second measurement).

Table 10.
Myotonometer parameters of femoral biceps in Parkinson’s disease patients (post medical treatment) and the control group (second measurement).

Table 11.
Myotonometer parameters of anterior tibialis in Parkinson’s disease patients (post medical treatment) and the control group (second measurement).
3.2. Correlations between Myotonometric Parameters and Patients’ Characteristics
Table 12 includes the correlations between myotonometric parameters (pre drug administration) and patients’ characteristics. The relationship between frequency and functional status shows a significant correlation; the more the frequency increases (muscular tension state), the better the functional status. There are also significant correlations between decrement and age of disease onset, and between decrement and functional status. The higher the decrement (elasticity), the lower the age of onset and the better the functional status.

Table 12.
Correlations between myotonometric parameters and patients’ characteristics.
The MDS-UPDRS scores were as follows: dressing 2.69 ± 0.93, hygiene 2.96 ± 0.82, hobbies 2.93 ± 0.85, walking and balance 2.63 ± 1.14, and freezing 2.63 ± 1.2.
4. Discussion
Our research showed that in patients suffering from Parkinson’s disease, the myotonometric parameters (frequency, stiffness and decrement) of the major pectoralis, biceps brachialis, femoral biceps and anterior tibialis, assessed in the morning before drug administration, had significantly higher values in comparison to age- and gender-matched healthy controls. We chose the above-mentioned muscle groups as they are involved in postural stability, thus affecting the everyday activities in Parkinson’s disease patients.
When analyzing the within-group myotonometric parameters (before medical treatment and an hour after taking the medication), the patients’ group reported a state of muscular tension (frequency), a muscular rigidity (stiffness) and a value of elasticity (decrement) significantly higher than before treatment. When considering the decrement, the bigger the value, the more the muscle cannot come back to the relative shape rapidly between two contractions. Thus, fatigue and movement limitation occurs due to the lack of elasticity. These elements change the grade of the patient’s functionality in daily activities, because the patient must make a more considerable effort in doing an activity due to muscular tension (frequency) and increased rigidity (stiffness). There were no statistically significant differences between pre and post treatment for all the myotonometric parameters.
To our knowledge, this is the first study to use the myotomometric assessment of four different muscle groups in Parkinson’s disease patients.
Rätsep and Asser assessed the extensor digitorum muscle in six patients with advanced-stage Parkinson’s disease. They concluded that increased rigidity is associated with increased values of viscoelastic stiffness in patients with Parkinson’s disease [].
The scientific literature includes the use MyotonPRO technology to determine the specificity of the parameters, such as a study done by Liang H et al. []. This study on 21 patients followed the negative effects of different postures involuntarily adopted by the patients at the level of trapezius muscles, provoking muscle rigidity. The results showed the grade of rigidity, depending on the maintained antalgic position. After the obtained results by means of MyotonPRO technology, it was identified in what measure the muscle rigidity triggers discomfort, pain and fatigue and how maintaining the correct position of the neck and back would decrease the level of rigidity [].
Another study on 18 patients (Marusiak J et al., 2010) analyzed the passive rigidity of the biceps brachialis muscle in a group of women with Parkinson’s disease in comparison with a group of healthy women. Myotonometry, electromyography and mechanomyography were used for assessment. Results showed that myotonometry provides valuable evaluation due to its sensitivity, as it detects muscle rigidity [].
Marusiak J et al. studied the relation between the dopaminergic dose treatment and the mechanical properties (myotonometry) of elbow flexor muscles. The results of the clinical evaluation of parkinsonian rigidity showed that the rigidity decreased during the outset phase in comparison with the ending phase. The study of 10 patients concluded that the anti-parkinsonian dopaminergic agents improved the mobility of the elbow joint by increasing its angle and decreased the parkinsonian rigidity of the superior limb []. In our study on 49 patients with Parkinson’s disease, we assessed by myotonometry the assigned muscle groups (major pectoralis, biceps brachialis, femoral biceps and anterior tibialis) one hour after oral medication intake. Frequency (state of tension), stiffness and viscoelastic properties (mechanical stress relaxation time and creep ratio of relaxation time to deformation time) of major pectoralis and biceps brachialis were significantly greater before the pharmacological medication in comparison to healthy controls. For the lower limb muscles (femoral biceps and anterior tibialis), frequency (state of tension) and decrement (elasticity) were significantly higher.
The patients in the current research followed the medical treatment recommended by the neurologist. The substance and dose were determined according to symptoms, age, associated pathologies, and therapeutic response. The treatments of the patients from the study group are Levodopa in combination with dopa-decarboxylase inhibitors, dopamine agonists, monoamine oxidase B inhibitors, Catechol-O-methyl transferase inhibitors or amantadine.
Levodopa reduces the progression of disability. However, long-term treatment is frequently affected by a series of motor fluctuations, such as oscillations of the motor response and the appearance of dyskinesia []. The side effects of levodopa can limit its use in patients under 70 years with mild symptoms of Parkinson’s disease. The dopamine agonists are considered a safe and efficient alternative, especially in younger patients. They are associated with a decrease of motor complications in the first 5 years of disease progression []. MAO-B (monoamine oxidase B) inhibitors are efficient in the control of motor symptoms, both as a monotherapy and in combination with levodopa or a dopa-decarboxylase inhibitor. MAO-B inhibitors are well tolerated; they are recommended in the treatment of akinesia and motor fluctuations []. Amantadine showed efficacy in the treatment of dyskinesia induced by levodopa in Parkinson’s disease patients. Studies have also proven its potential in reducing motor fluctuations []. Entacapone is a reversible peripheric inhibitor of Catechol-O-methyl transferase that increases the half time effect of levodopa on the motor symptoms. Studies have shown that combination therapy with levodopa/carbidopa/entacapone has a superior efficacy and a safer profile in the treatment of Parkinson’s disease patients [].
The study of Rätsep and Asser analyzed the changes of viscoelastic stiffness induced by deep brain stimulation. The extensor digitorum muscle was assessed by MyotonPRO in 15 patients in an advanced stage of Parkinson’s disease (evaluation in the off-medication conditions). The authors support the use of myotonometry for objective quantification of parkinsonian rigidity at rest []. In our study we also observed an increased rigidity in the patients’ group in the four tested muscles at rest.
Marusiak J et al. tested 12 patients with Parkinson’s disease and 12 healthy matched controls. Patients with Parkinson’s disease had higher stiffness in the biceps brachialis. In contrast, there was no significant intergroup difference in the triceps brachialis stiffness []. In our research we recorded an increased muscular tension and lack of elasticity in all the four tested muscles in Parkinson’s disease patients in comparison to healthy controls.
Recent research published in 2022 by Christine Lo and Siddharth A chose to combine MDS-UPDRS (motor examination score) with the Purdue Pegboard Test and Timed Up and Go to offer a greater sensitivity in detecting the motor changes in Parkinson’s disease []. The assessment of motor symptoms using MDS-UPDRS (motor examination score), Timed Up and Go, Berg balance Scale and Purdue Pegboard Test is an endorsed combination of scores used in Parkinson’s disease patients [,]. In our study we combined the MDS-UPDRS with an objective muscle assessment tool (MyotonPRO). As a future research direction, we have in view the comparison of MDS-UPDRS score and myotonometric parameters in patients with Parkinson’s disease before and after a 3-month rehabilitation program.
The patients’ follow-up is a limitation of the study; it was done between the treatment administration periods and involved functional status in consecutive hours and days. Another element that limited the research is that the time and means of data collection were dependent on the physical and emotional status of the patients. In the future, we aim to include in the analysis a larger group of patients for a longer period, as well as the assessment of postural stability in relation to myotonometric parameters. As the postural stability and physical activity was already studied in healthy subjects [,], it can be extended to patients suffering from Parkinson’s disease. The correlations among dynamic balance, physical activity and quality of life have been investigated [], as well as the efficacy of particular trunk exercises in the balance training in patients with Parkinson’s disease []. We envisage a future study aiming to assess postural stability in relation to myotonometry in Parkinson’s disease patients who follow a physical exercise program for postural correction as well as improved static and dynamic balance. We also intend to extend the muscle groups assessed by MyotonPRO to muscles involved in posture (ventral trunk muscles rectus and obliquus externus abdominis, and dorsal trunk muscles longissimus cervicis and lumbar erector spinae).
5. Conclusions
Our study analyzing the myotonometric parameters showed that before oral drug administration, there were significantly higher values of frequency, stiffness, relaxation and creep for the major pectoralis and biceps brachialis in patients with Parkinson’s disease in comparison to healthy controls. One hour after medication intake, these differences remained significant for frequency and stiffness for both muscle groups; for biceps brachialis, there remained increased values for relaxation and creep. For the pre–drug administration assessment, frequency and decrement were significantly higher in Parkinson’s disease patients for the femoral biceps and anterior tibialis, with only the decrement values being greater at post drug assessment. We concluded that there were no significant differences in the major pectoralis and biceps brachialis elasticity between patients with Parkinson’s disease and healthy controls pre and post drug administration. Improved viscoelastic properties of biceps brachialis were recorded after medication. After drug administration, no significant differences of femoral biceps and anterior tibialis stiffness were noted between patients and controls.
Author Contributions
Conceptualization, H.A.Z., E.A. and M.S.; methodology, H.A.Z. and E.A.; validation, H.A.Z. and E.A.; formal analysis, H.A.Z., M.M. and M.L.M.; data curation, H.A.Z.; writing—original draft preparation, H.A.Z.; writing—review and editing, H.A.Z., E.A., M.M. and M.L.M.; visualization, E.A., M.M., M.L.M. and M.S.; supervision, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
The publishing fee has been partially funded by “Victor Babes” University of Medicine and Pharmacy Timisoara, Romania.
Institutional Review Board Statement
In conformity with the ethical standards, participation in the study was voluntary, and participants were informed about the testing procedure. All participants in the study consented, in written form, to data processing and the scientific use of data. The study was approved by the Ethical Committee of Victor Babes University of Medicine and Pharmacy, Timisoara, Romania, Ethical Notification No. 49/15.09.2022, and is in conformity with the Helsinki Declaration.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent was obtained from the patients to publish this paper.
Data Availability Statement
Supplementary data available at request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- National Institute on Aging—Parkinson‘s Disease: Causes, Symptoms, and Treatments. Available online: https://www.nia.nih.gov/health/parkinsons-disease (accessed on 14 April 2022).
- Sigurlaug, S. The clinical symptoms of Parkinson’s disease. J. Neurochem. 2016, 139, 318–324. [Google Scholar]
- David Phinney Foundation of Parkinson Disease. Rigidity and Parkinson’s: What It Is and How to Treat It; David Phinney Foundation for Parkinson’s: Louisville, CO, USA, 2020; Available online: https://davisphinneyfoundation.org/ (accessed on 14 April 2022).
- European Parkinson’s Disease Association (EPDA). Guide-Life with Parkinson’s; European Parkinson’s Disease Association (EPDA): Munich, Germany, 2011. [Google Scholar]
- Grimes, D.; Fitzpatrick, M.; Gordon, J.; Miyasaki, J.; Fon, E.A.; Schlossmacher, M.; Suchowersky, O.; Rajput, A.; Lafontaine, A.L.; Mestre, T.; et al. Canadian guideline for Parkinson disease. Cmaj 2019, 36, E989–E1004. [Google Scholar] [CrossRef] [PubMed]
- Zippenfening, H.A.; Alexoi, I. Kinetoterapia și Nutriția Destinată Bolii Parkinson GHID; Editor Mirton: Timișoara, Romania, 2021; pp. 8–18. ISBN 978-973-52-1970-3. [Google Scholar]
- Keloth, S.M.; Arjunan, S.P.; Raghav, S.; Kumar, D.K. Muscle activation strategies of people with early-stage Parkinson’s during walking. J. Neuroeng. Rehabil. 2021, 18, 133. [Google Scholar] [CrossRef] [PubMed]
- Peipsi, A.; Kerpe, R.; Jäger, H.; Soeder, S.; Gordon, C.; Schleip, R. Myoton Pro: A Novel Tool for the Assessment of Mechanical Properties of Fascial Tissues. J. Bodyw. Mov. Ther. 2012, 16, 527. [Google Scholar] [CrossRef]
- MyotonTechnology. Available online: www.myoton.com (accessed on 3 December 2022).
- World Health Organization. Parkinson Disease. Available online: https://www.who.int/news-room/fact-sheets/detail/parkinson-disease (accessed on 15 June 2022).
- Bizzini, M.; Mannion, A.F. Reliability of a new, hand-held device for assessing skeletal muscle stiffness. Clin. Biomech. 2003, 18, 459–461. [Google Scholar] [CrossRef]
- Ferreira-Sánchez, M.d.R.; Moreno-Verdú, M.; Cano-de-la-Cuerda, R. Quantitative Measurement of Rigidity in Parkinson’s Disease: A Systematic Review. Sensors 2020, 20, 880. [Google Scholar] [CrossRef]
- Rätsep, T.; Asser, T. Changes in viscoelastic properties of skeletal muscles induced by subthalamic stimulation in patients with Parkinson’s disease. Clin. Biomech. 2011, 26, 213–217. [Google Scholar] [CrossRef]
- Marusiak, J.; Kisiel-Sajewicz, K.; Jaskólska, A.; Jaskólski, A. Higher muscle passive stiffness in Parkinson’s disease patients than in controls measured by myotonometry. Arch. Phys. Med. Rehabil. 2010, 91, 802–815. [Google Scholar] [CrossRef]
- Marusiak, J.; Jaskólska, A.; Budrewicz, S.; Koszewicz, M.; Jaskólski, A. Increased muscle belly and tendon stiffness in patients with Parkinson’s disease, as measured by myotonometry. Mov. Disord. 2011, 26, 2119–2122. [Google Scholar] [CrossRef]
- Berardelli, A.; Accornero, N.; Argenta, M.; Meco, G.; Manfredi, M. Fast complex arm movements in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1986, 49, 1146–1149. [Google Scholar] [CrossRef]
- Corcos, D.M.; Chen, C.M.; Quinn, N.P.; McAuley, J.; Rothwell, J.C. Strength in Parkinson’s disease: Relationship to rate of force generation and clinical status. Ann. Neurol. 1996, 39, 79–88. [Google Scholar] [CrossRef]
- Nieuwboer, A.; Dom, R.; De Weerdt, W.; Desloovere, K.; Janssens, L.; Stijn, V. Electromyographic profiles of gait prior to onset of freezing episodes in patients with Parkinson’s disease. Brain 2004, 127, 1650–1660. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wu, B.; Lou, X.; Zhao, T.; Li, J.; Xu, Z.; Hu, X.; Zheng, X. A self-adaptive foot-drop corrector using functional electrical stimulation (FES) modulated by tibialis anterior electromyography (EMG) dataset. Med. Eng. Phys. 2013, 35, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, K.L.; Roemmich, R.T.; Cam, B.; Fregly, B.J.; Hass, C.J. Persons with Parkinson’s disease exhibit decreased neuromuscular complexity during gait. Clin. Neurophysiol. 2013, 124, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Yu, S.; Hao, M.; Deng, W.; Lin, M.; Zhang, Z.; Liu, C. Effect of cervicothoracic postures on the stiffness of trapezius muscles. Med. Biol. Eng. Comput. 2022, 60, 3009–3301. [Google Scholar] [CrossRef]
- Balsalobre-Fernández, C.; Torres-Ronda, L. The Implementation of Velocity-Based Training Paradigm for Team Sports: Framework, Technologies, Practical Recommendations and Challenges. Sports 2021, 9, 47. [Google Scholar] [CrossRef]
- MDS-UPDRS. The MDS-sponsored Revision of the Unified Parkinson’s Disease Rating Scale. Official MDS Romanian Translation. 2018 International Parkinson and Movement Disorder Society. Available online: https://www.movementdisorders.org/MDS-Files1/PDFs/MDS-UPDRS-Rating-Scales/MDS_UPDRS_Romanian_OfficialTranslation_FINAL.pdf (accessed on 6 June 2022).
- Marusiak, J.; Jaskólska, A. Influence of dopaminergic treatment on resting elbow joint angle control mechanisms in patients with Parkinson’s disease—A preliminary report. Acta Bioeng. Biomech. 2018, 20, 75–82. [Google Scholar]
- Poewe, W.; Antonini, A.; Zijlmans, J.C.; Burkhard, P.R.; Vingerhoets, F. Levodopa in the treatment of Parkinson’s disease: An old drug still going strong. Clin. Interv. Aging 2010, 5, 229–238. [Google Scholar]
- Cánovas, A.A.; Luquin Piudo, R.; García Ruiz-Espiga, P.; Burguera, J.A.; Campos Arillo, V.; Castro, A.; Linazasoro, G.; López Del Val, J.; Vela, L.; Martínez Castrillo, J.C. Dopaminergic agonists in Parkinson’s disease. Neurologia 2014, 29, 230–241. [Google Scholar]
- Mutti, C.; Sarnataro, R.B.; Beretta, J.; Enzo, P.; Negrotti, A.; Rausa, F.; Pizzarotti, S.; Parrino, L. Rasagiline, sleep quality and well-being in Parkinson’s disease: A pilot study. Neurol. Sci. 2022, 43, 4791–4796. [Google Scholar] [CrossRef]
- Rascol, O.; Fabbri, M.; Poewe, W. Amantadine in the treatment of Parkinson’s disease and other movement disorders. Lancet Neurol. 2021, 20, 1048–1056. [Google Scholar] [CrossRef]
- Yi, Z.M.; Qiu, T.T.; Zhang, Y.; Liu, N.; Zha, S.D. Levodopa/carbidopa/entacapone versus levodopa/dopa-decarboxyiase inhibitor for the treatment of Parkinson’s disease: Systematic review, meta-analysis, and economic evaluation. Ther. Clin. Risk Manag. 2018, 14, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Rätsep, T.; Asser, T. The effect of subthalamic stimulation on viscoelastic stiffness of skeletal muscles in patients with Parkinson’s disease. Clin. Biomech. 2017, 44, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Christine, L.; Siddharth, A. A composite clinical motor score as a comprehensions and sensitive outcome measure for Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2022, 93, 617–624. [Google Scholar]
- Tomlinson, C.L.; Patel, S.; Meek, C.; Herd, C.P.; Clarke, C.E.; Stowe, R.; Shah, L.; Sackley, C.; Deane, K.H.O.; Wheatley, K.; et al. Physiotherapy intervention in Parkinson’s disease: Systematic review and meta-analysis. BMJ 2012, 345, e5004. [Google Scholar] [CrossRef] [PubMed]
- Johansson, C.; Lindström, B.; Forsgren, L.; Johansson, G.M. Balance and mobility in patients with newly diagnosed Parkinson’s disease: A five-year follow-up of a cohort in northern Sweden. Disabil. Rehabil. 2018, 42, 770–778. [Google Scholar] [CrossRef]
- Petroman, R.; Rata, A.L. Balance performance in sedentary and active healthy young individuals—A cross-sectional study. Phys. Educ. Stud. 2020, 24, 115–119. [Google Scholar] [CrossRef]
- Jo, S.H.; Choi, H.J.; Cho, H.S.; Yoon, J.H.; Lee, W.Y. Effect of Core Balance Training on Muscle Tone and Balance Ability in Adult Men and Women. Int. J. Environ. Res. Public Health 2022, 19, 12190. [Google Scholar] [CrossRef]
- Wu, S.Y.; Lin, T.K.; Pan, C.Y.; Tsai, C.L. The predictive relationships between advanced dynamic balance and physical activity/quality of life in Parkinson’s disease. Hum. Mov. Sci. 2023, 89, 103076. [Google Scholar] [CrossRef]
- López-Liria, R.; Vega-Tirado, S.; Valverde-Martínez, M.Á.; Calvache-Mateo, A.; Martínez-Martínez, A.M.; Rocamora-Pérez, P. Efficacy of Specific Trunk Exercises in the Balance Dysfunction of Patients with Parkinson’s Disease: A Systematic Review and Meta-Analysis. Sensors 2023, 23, 1817. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).