Acoustic Characterization for The Feeding Activities of Haliotis discus Hannai
Abstract
1. Introduction
2. Materials and Methods
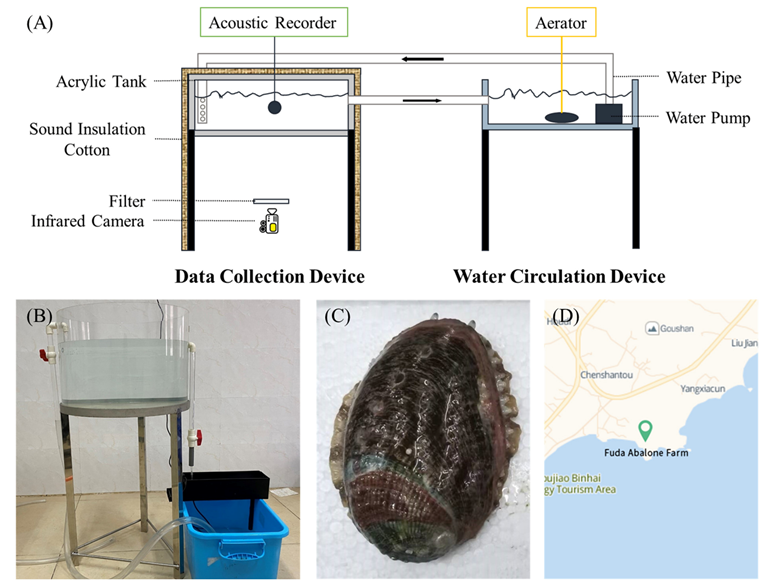
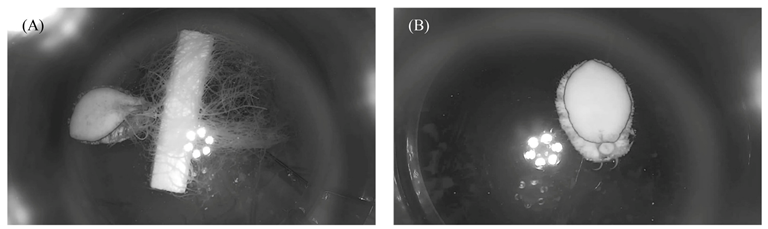
2.1. Experimental Design
2.2. Acoustic Signals Preprocessing
2.3. Analysis of Acoustic Variables
3. Results
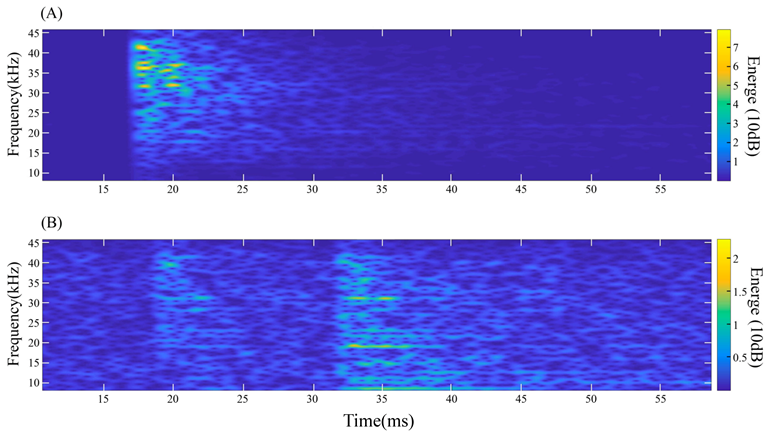
3.1. Eating-Based Sound Production
3.2. Signals of Eating Different Substances
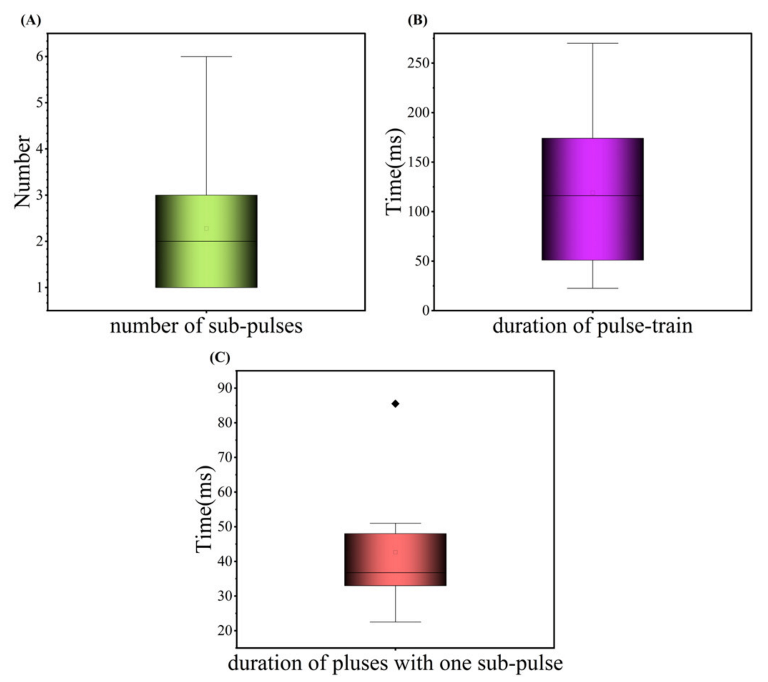
3.3. Analysis of Eating Acoustic Signals
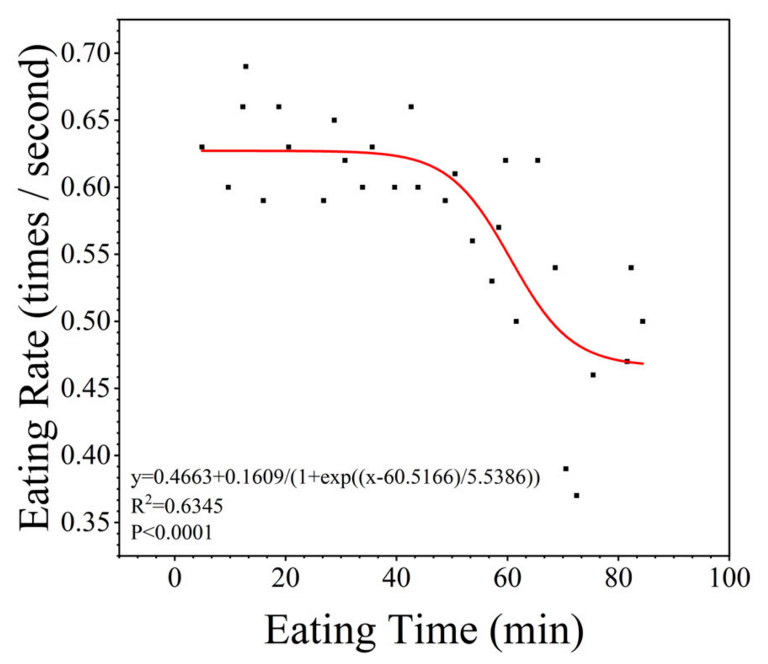
3.4. Relationship between the Eating Rate and the Hunger Level
4. Discussions
4.1. Eating-Based Sound Production Mechanism
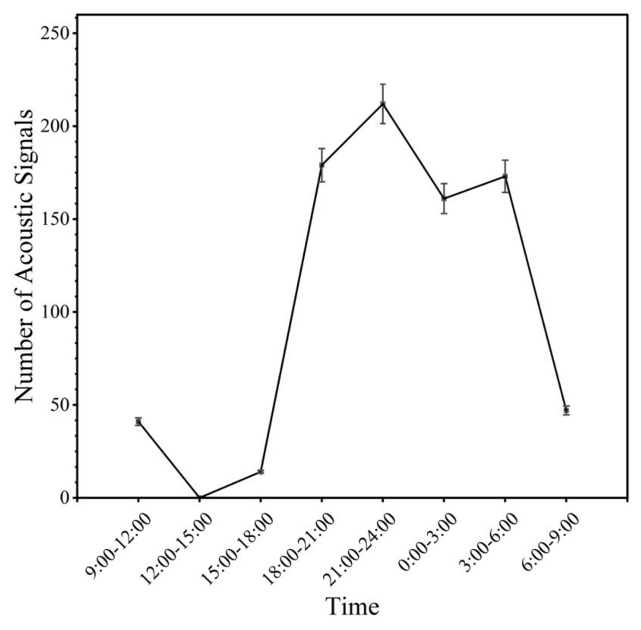
4.2. The Relationship between Acoustic Signals and Abalone Circadian Rhythm
4.3. Acoustic Signal Processing Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vermeij, G.J. Sound Reasons for Silence: Why Do Molluscs Not Communicate Acoustically? Biol. J. Linn. Soc. 2010, 100, 485–493. [Google Scholar] [CrossRef]
- Bergert, B.A.; Wainwright, P.C. Morphology and Kinematics of Prey Capture in the Syngnathid Fishes Hippocampus erectus and Syngnathus floridae. Mar. Biol. 1997, 127, 563–570. [Google Scholar] [CrossRef]
- Ladich, F. Comparative Analysis of Swimbladder (Drumming) and Pectoral (Stridulation) Sounds in Three Families of Catfishes. Bioacoustics 1997, 8, 185–208. [Google Scholar] [CrossRef]
- Crawford, J.D.; Huang, X. Communication Signals and Sound Production Mechanisms of Mormyrid Electric Fish. J. Exp. Biol. 1999, 202, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Kasumyan, A.O. Sounds and Sound Production in Fishes. J. Ichthyol. 2008, 48, 981–1030. [Google Scholar] [CrossRef]
- Juanes, F. Listening to Fish: An International Workshop on the Application of Passive Acoustics in Fisheries. Rev. Fish Biol. Fish. 2002, 12, 105–106. [Google Scholar] [CrossRef]
- Lagardère, J.P.; Mallekh, R.; Mariani, A. Acoustic Characteristics of Two Feeding Modes Used by Brown Trout (Salmo trutta), Rainbow Trout (Oncorhynchus mykiss) and Turbot (Scophthalmus maximus). Aquaculture 2004, 240, 607–616. [Google Scholar] [CrossRef]
- Lagardère, J.P.; Mallekh, R. Feeding Sounds of Turbot (Scophthalmus maximus) and Their Potential Use in the Control of Food Supply in Aquaculture: I. Spectrum Analysis of the Feeding Sounds. Aquaculture 2000, 189, 251–258. [Google Scholar] [CrossRef]
- Lammers, M.O.; Brainard, R.E.; Au, W.W.L.; Mooney, T.A.; Wong, K.B. An Ecological Acoustic Recorder (Ear) for Long-Term Monitoring of Biological and Anthropogenic Sounds on Coral Reefs and Other Marine Habitats. J. Acoust. Soc. Am. 2008, 123, 1720–1728. [Google Scholar] [CrossRef]
- Guinot-Dumortier, D.; Dumortier, B. La Stridulation Chez Les Crabes. Crustaceana 1960, 1, 117–155. [Google Scholar] [CrossRef]
- Knowlton, R.E.; Moulton, J.M. Sound Production in the Snapping Shrimps Alpheus (Crangon) and Synalpheus. Biol. Bull. 1963, 125, 311–331. [Google Scholar] [CrossRef]
- Jocelyn, C. Combat, Display and Ritualization in Fiddler Crabs (Ocypodidae, Genus uca). Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1966, 251, 459–472. [Google Scholar]
- Ikeda, H.; Imafuku, M. Sound Production in the Land Hermit Crab Coenobita Purpureus Stimpson, 1858 (Decapoda, Coenobitidae). Crustaceana 1990, 58, 168–174. [Google Scholar]
- Henninger, H.P.; Watson, W.H. Mechanisms Underlying the Production of Carapace Vibrations and Associated Waterborne Sounds in the American Lobster, Homarus americanus. J. Exp. Biol. 2005, 208, 3421–3429. [Google Scholar] [CrossRef]
- Patek, S.N.; Baio, J.E. The Acoustic Mechanics of Stick-Slip Friction in the California Spiny Lobster (Panulirus Interruptus). J. Exp. Biol. 2007, 210, 3538–3546. [Google Scholar] [CrossRef]
- Boon, P.Y.; Yeo, D.C.J.; Todd, P.A. Sound Production and Reception in Mangrove Crabs Perisesarma spp. (Brachyura: Sesarmidae). Aquat. Biol. 2009, 5, 107–116. [Google Scholar] [CrossRef]
- Patek, S.N.; Shipp, L.E.; Staaterman, E.R. The Acoustics and Acoustic Behavior of the California Spiny Lobster (Panulirus interruptus). J. Acoust. Soc. Am. 2009, 125, 3434–3443. [Google Scholar] [CrossRef]
- Au, W.; Banks, K. The Acoustics of the Snapping Shrimp Synalpheus Parneomeris in Kaneohe Bay. J. Acoust. Soc. Am. 1998, 103, 41–47. [Google Scholar] [CrossRef]
- Hamilton, S.; Silva, J.F.; Pereira-Neves, A.; Travassos, P.; Peixoto, S. Sound Production Mechanism in the Brazilian Spiny Lobsters (Family palinuridae). Zoomorphology 2019, 138, 475–482. [Google Scholar] [CrossRef]
- Smith, D.V.; Tabrett, S. The Use of Passive Acoustics to Measure Feed Consumption by Penaeus monodon (Giant Tiger Prawn) in Cultured Systems. Aquac. Eng. 2013, 57, 38–47. [Google Scholar] [CrossRef]
- Gao, X.S. Abalone; Liaoning Science and Technology Press: Liaoning, China, 2000. [Google Scholar]
- Kitting, C.L. The Use of Feeding Noises to Determine the Algal Foods Being Consumed by Individual Intertidal Molluscs. Oecologia 1979, 40, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Konzewitsch, N.; Evans, S.N. Examining the Movement of the Common Spider Conch Lambis Lambis in Shallow Water of a Northeastern Indian Ocean Atoll Using Passive Acoustic Tracking. J. Shellfish Res. 2020, 39, 389–397. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020; The State of World Fisheries and Aquaculture (Sofia); Food & Agriculture Org: Rome, Italy, 2020. [Google Scholar]
- Babcock, R.; Keesing, J. Fertilization Biology of the Abalone Haliotis Laevigata: Laboratory and Field Studies. Can. J. Fish. Aquat. Sci. 1999, 56, 1668–1678. [Google Scholar] [CrossRef]
- Wang, X.; Tang, B.; Luo, X.; Ke, C.; Huang, M.; You, W.; Wang, Y. Effects of Temperature, Diet and Genotype-Induced Variations on the Gut Microbiota of Abalone. Aquaculture 2020, 524, 735269. [Google Scholar] [CrossRef]
- Gao, X.L.; Pang, G.W.; Luo, X.; You, W.W.; Ke, C.H. Effects of Light Cycle on Motion Behaviour and Melatonin Secretion in Haliotis discus Hannai. Aquaculture 2021, 532, 735981. [Google Scholar] [CrossRef]
- Duong, D.N.; Stone, D.A.J.; Qin, J.G.; Bansemer, M.S.; Harris, J.O. Energy Budgets for Greenlip Abalone (Haliotis laevigata Donovan) Fed Graded Dietary Crude Protein Levels at Seasonal Water Temperatures. Aquaculture 2021, 536, 736499. [Google Scholar] [CrossRef]
- Li, X.; Chang, W.-C.; Chao, Y.J.; Wang, R.; Chang, M. Nanoscale Structural and Mechanical Characterization of a Natural Nanocomposite Material: The Shell of Red Abalone. Nano Lett. 2004, 4, 613–617. [Google Scholar] [CrossRef]
- Oedekoven, C.S.; Marques, T.A.; Harris, D.; Thomas, L.; Thode, A.M.; Blackwell, S.B.; Conrad, A.S.; Kim, K.H. A Comparison of Three Methods for Estimating Call Densities of Migrating Bowhead Whales Using Passive Acoustic Monitoring. Environ. Ecol. Stat. 2022, 29, 101–125. [Google Scholar] [CrossRef]
- Mann, D.A.; Hawkins, A.D.; Jech, J.M. Active and Passive Acoustics to Locate and Study Fish. In Fish Bioacoustics: With 81 Illustrations; Webb, J.F., Fay, R.R., Popper, A.N., Eds.; Springer: New York, NY, USA, 2008; pp. 279–309. [Google Scholar]
- Jonsson, P.; Sillitoe, I.; Dushaw, B.; Nystuen, J.; Heltne, J. Observing Using Sound and Light—A Short Review of Underwater Acoustic and Video-Based Methods. Ocean Sci. Discuss. 2009, 6, 819–870. [Google Scholar]
- Derby, C.D.; Elsayed, F.H.; Williams, S.A.; González, C.; Choe, M.; Bharadwaj, A.S.; Chamberlain, G.W. Krill Meal Enhances Performance of Feed Pellets through Concentration-Dependent Prolongation of Consumption by Pacific White Shrimp, Litopenaeus vannamei. Aquaculture 2016, 458, 13–20. [Google Scholar] [CrossRef]
- Bardera, G.; Owen, M.A.G.; Pountney, D.; Alexander, M.E.; Sloman, K.A. The Effect of Short-Term Feed-Deprivation and Moult Status on Feeding Behaviour of the Pacific White Shrimp (Litopenaeus vannamei). Aquaculture 2019, 511, 734222. [Google Scholar] [CrossRef]
- Smith, D.V.; Shahriar, M.S. A Context Aware Sound Classifier Applied to Prawn Feed Monitoring and Energy Disaggregation. Knowl.-Based Syst. 2013, 52, 21–31. [Google Scholar] [CrossRef]
- Peixoto, S.; Soares, R.; Davis, D.A. An Acoustic Based Approach to Evaluate the Effect of Different Diet Lengths on Feeding Behavior of Litopenaeus vannamei. Aquac. Eng. 2020, 91, 102114. [Google Scholar] [CrossRef]
- Qi, R.; Liu, H.; Liu, S. Effects of Different Culture Densities on the Acoustic Characteristics of Micropterus Salmoide Feeding. Fishes 2023, 8, 126. [Google Scholar] [CrossRef]
- Qu, R.; Liu, H.; Liu, J.W.; Zhang, Y.L. Acoustic Signal Characteristics of Largemouth Bass (Micropterus salmoides) in Feeding Process and the Effects of Breeding Density. Fish. Mod 2021, 48, 55–63. [Google Scholar]
- Reis, J.; Peixoto, S.; Soares, R.; Rhodes, M.; Ching, C.; Davis, D.A. Passive Acoustic Monitoring as a Tool to Assess Feed Response and Growth of Shrimp in Ponds and Research Systems. Aquaculture 2022, 546, 737326. [Google Scholar] [CrossRef]
- da Silva, J.F.; Hamilton, S.; Rocha, J.V.; Borie, A.; Travassos, P.; Soares, R.; Peixoto, S. Acoustic Characterization of Feeding Activity of Litopenaeus vannamei in Captivity. Aquaculture 2018, 501, 76–81. [Google Scholar] [CrossRef]
- Barber, A.H.; Lu, D.; Pugno, N.M. Extreme Strength Observed in Limpet Teeth. J. R. Soc. Interface 2015, 12, 20141326. [Google Scholar] [CrossRef]
- Hunt, M.J.; Winsor, H.; Alexander, C.G. Feeding by Penaeid Prawns: The Role of the Anterior Mouthparts. J. Exp. Mar. Biol. Ecol. 1992, 160, 33–46. [Google Scholar] [CrossRef]
- Colson, D.J.; Patek, S.N.; Brainerd, E.L.; Lewis, S.M. Sound Production During Feeding in Hippocampus seahorses (Syngnathidae). Environ. Biol. Fishes 1998, 51, 221–229. [Google Scholar] [CrossRef]
- Oliveira, T.P.R.; Ladich, F.; Abed-Navandi, D.; Souto, A.S.; Rosa, I.L. Sounds Produced by the Longsnout seahorse: A Study of Their Structure and Functions. J. Zool. 2014, 294, 114–121. [Google Scholar] [CrossRef]
- Parmentier, E.; Colleye, O.; Fine, M.L.; Frédérich, B.; Vandewalle, P.; Herrel, A. Sound Production in the Clownfish Amphiprion clarkii. Science 2007, 316, 1006. [Google Scholar] [CrossRef] [PubMed]
- Olivier, D.; Frédérich, B.; Herrel, A.; Parmentier, E. A Morphological Novelty for Feeding and Sound Production in the Yellowtail Clownfish. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2015, 323, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Bertucci, F.; Ruppé, L.; Van Wassenbergh, S.; Compère, P.; Parmentier, E. New Insights into the Role of the Pharyngeal Jaw Apparatus in the Sound-Producing Mechanism of Haemulon flavolineatum (Haemulidae). J. Exp. Biol. 2014, 217, 3862–3869. [Google Scholar] [CrossRef]
- Ripley, J.L.; Foran, C.M. Influence of Estuarine Hypoxia on Feeding and Sound Production by Two Sympatric Pipefish Species (Syngnathidae). Mar. Environ. Res. 2007, 63, 350–367. [Google Scholar] [CrossRef]
- Olivier, D.; Frédérich, B.; Spanopoulos-Zarco, M.; Balart, E.F.; Parmentier, E. The Cerato-Mandibular Ligament: A Key Functional Trait for Grazing in Damselfishes (Pomacentridae). Front. Zool. 2014, 11, 1–14. [Google Scholar] [CrossRef]
- Panda, S.; Hogenesch, J.B.; Kay, S.A. Circadian Rhythms from Flies to Human. Nature 2002, 417, 329–335. [Google Scholar] [CrossRef]
- Challet, E. Minireview: Entrainment of the Suprachiasmatic Clockwork in Diurnal and Nocturnal Mammals. Endocrinology 2007, 148, 5648–5655. [Google Scholar] [CrossRef]
- Gao, X.L.; Luo, X.; You, W.W.; Ke, C.H. Circadian Movement Behaviours and Metabolism Differences of the Pacific Abalone Haliotis discus Hannai. J. Photochem. Photobiol. B Biol. 2020, 211, 12. [Google Scholar] [CrossRef]
- Gao, X.L.; Pang, G.W.; Luo, X.; You, W.W.; Ke, C.H. Effects of Light Cycle on Circadian Feeding Activity and Digestive Physiology in Haliotis discus Hannai. Aquaculture 2021, 539, 10. [Google Scholar] [CrossRef]



| Equipment | Parameter Name | Parameter Information |
|---|---|---|
| Song Meter SM4BAT FS Ultrasonic Recorder | Channels | 1 |
| Recording Format | 16-bit PCM WAV | |
| Supported Sample Rates (kHz) | 192, 256, 384, and 500 | |
| Amplifier Gain | 0 or 12 dB | |
| High Pass Filter | Selectable 2-pole at 16 kHz | |
| Anti-alias Filter | 2-pole at 156 kHz | |
| Equivalent Input Noise | in dBVrms (>10 kHz, 0 dB gain) | |
| Shenyou 5 megapixel high-definition infrared camera | lens size | 3.6 mm |
| video format | H.265 | |
| SNR | ≥48 dB | |
| pixel | 5 million |
| Hardware | Version |
| CPU | AMD Ryzen 5 3500U |
| GPU | Radeon Vega 8 Graphics |
| RAM | 8G |
| Software | Version |
| Matlab | R2021a |
| OriginPro | 2022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.; Qian, Y.; Chen, J.; Gao, X.; Zhang, M.; You, W.; Zhang, R. Acoustic Characterization for The Feeding Activities of Haliotis discus Hannai. Appl. Sci. 2023, 13, 5559. https://doi.org/10.3390/app13095559
Lin H, Qian Y, Chen J, Gao X, Zhang M, You W, Zhang R. Acoustic Characterization for The Feeding Activities of Haliotis discus Hannai. Applied Sciences. 2023; 13(9):5559. https://doi.org/10.3390/app13095559
Chicago/Turabian StyleLin, Hongyue, Yiyang Qian, Jia Chen, Xiaolong Gao, Mo Zhang, Weiwei You, and Rongxin Zhang. 2023. "Acoustic Characterization for The Feeding Activities of Haliotis discus Hannai" Applied Sciences 13, no. 9: 5559. https://doi.org/10.3390/app13095559
APA StyleLin, H., Qian, Y., Chen, J., Gao, X., Zhang, M., You, W., & Zhang, R. (2023). Acoustic Characterization for The Feeding Activities of Haliotis discus Hannai. Applied Sciences, 13(9), 5559. https://doi.org/10.3390/app13095559







