Abstract
The aim of the study was to evaluate wheat flour fractions and the relationships between the rheological properties of wheat flour mill streams with arabinoxylans content to predict flour functionality. The tested wheat variety was IS Laudis and an industrial milling station with several roll sections was employed to reach 30 passages of flour streams. Each mill stream fraction was analyzed separately. Several physiochemical (moisture, ash content, falling number, wet gluten content, gluten index, and damaged starch content) and rheological properties were evaluated by means of utilizing various test apparatus and techniques. The total content of non-starch polysaccharides and arabinoxylans, as well as soluble and insoluble fractions were investigated. Results showed significant differences between the mill streams in terms of the content of physicochemical parameters and rheological properties, as well as in soluble and insoluble fractions of non-starch polysaccharides and arabinoxylans. The relationships between the tested parameters and PCA analysis can be useful for millers who can then select and blend several flour streams to obtain a maximum amount of flour with specified characteristics. A better understanding of the origin and function of different fractions and the role of arabinoxylans and their fractions in the milling process will allow the development of wheat flour blends with the desired functionality. Flours from late reduction and sizing passages (C and R) as well as from sorting filter (V) streams showed high ash content as well as T-NSP and T-AX levels, so the final content of NSP in flour blends may be balanced by the application of the proper amount of C6–C7 and R5 stream flours.
1. Introduction
Wheat plays a very important role in human nutrition, as it is the main ingredient of many food products produced around the world. The main benefits of the intake of these products are due to their main components, i.e., starch and protein. The functionality and mutual interactions between starch and protein enable the formation of a specific viscoelastic network of links called ‘gluten’. Gluten makes it possible to produce a wide range of products based on wheat flour [1].
Wheat milling is a mechanical, multi-stage, and complex process of gradual grinding, in which the endosperm is first separated from the bran layers and then, through a series of grinding operations, creates mill streams of wheat flour [2,3,4]. Wheat milling induces the separation of the floury endosperm from the bran and the reduction of the endosperm particles into flours. Roller milling is the principal commercial milling method as it has a very high capacity. Roller milling is conducted on break rolls (which are designed to break and remove the endosperm and germ from the bran coat, and gradually grind endosperm into flour), sizing rolls (so as to generate ‘middling’ with various particle sizes), and reduction rolls (which reduce the middling to flour).
Each mill produces from a few to even dozens of flour streams, with large differences in the physicochemical properties of individual flours. These are mixed at the end of the process to produce so-called ‘composite flours’. These stream mixes are dedicated to restaurants and bakeries for producing breads, cakes, and pizzas, or to various food plants for producing pasta, extruded products, or biscuits [5].
A wide range of wheat flour is produced, as a result of various combinations of flour stream mixing possibilities. Indeed, not every flour stream is equally useful for generating a composition of specialized wheat flour. Significant differences in the parameters of flour streams affect the difficulty in optimizing flour production. Therefore, it becomes very important to precisely test and predict the quality of flour streams, regardless of the ultimate applications of the final composed flour. Determining the distribution of beneficial and harmful components in mill streams is important for aspects such as assessing the quality of wheat milling [6], and the optimal combination of flour streams is of great importance to obtain the best baking quality [4].
Research on the differences between individual flour streams has been continuous. These differences concern such features as rheological properties [3,4,7,8,9,10,11], or physicochemical characteristics such as content and distribution of protein, protein composition or ash content [2,3,8,9,11,12,13,14,15], distribution, amount of enzymes [16,17,18,19], fat content [2,7,20], starch damage degree [3,11,14], pentosans and its fractions [6,21], or antioxidants content [22].
Apart from protein and starch, arabinoxylans (AX) are important components of wheat grain. These significantly affect the properties of flour. Arabinoxylans are non-starch polysaccharides that are present in the endosperm (3–5% of the total endosperm), aleurone, and bran cell walls (about 60–70% of the total cell wall). Arabinoxylans are built of a single main chain consisting of xylose residues linked by a β-1,4 chain, to which single arabinose residues are attached in positions C-3 and simultaneously C-2 and C-3 [23]. In the case of wheat bran, AX constitutes 10.9 to 26% of all bran fractions [24]. As with protein and ash, arabinoxylans are not evenly distributed in the wheat grain. The concentration of AX in the middle endosperm is much lower compared to the outer layers of the wheat grains [6].
Despite the low content of AX in flour, they have an important impact on the quality of gluten and dough, affecting especially bread quality [25,26]. A special feature of AX is the binding of very large amounts of water. Hence, it plays an important role in water management during bread production [27]. The interactions of proteins and AX affect the properties of gluten and dough [28]. WEAX have unique physical properties such as the ability to bind 10 times their own weight of water [29,30], forming highly viscous solutions and gels due to their covalent cross-linking [31,32]. All these properties have a direct functional impact on the formation of gluten and the properties of the dough. In general, it is believed that WEAX have a positive effect on bread quality [33] and WUAX have a negative effect [34,35].
The total arabinoxylan (TAX) content can be empirically divided into water extractable arabinoxylans (WEAX) or water non-water extractable arabinoxylans (WUAX) fractions. WUAX and WEAX have different physicochemical properties [6]. WUAX impinges upon the molecular mobility of water [23] and negatively influences the quality of bread by binding large amounts of water, which prevents proper hydration of starch and gluten. WUEX also affects the proper formation of air bubbles during fermentation in bread dough [18]. In the WEAX fraction, ferulic acid residues are available for oxidative crosslinking induced by free radicals and are partly responsible for changes in dough viscosity [6]. It is therefore of interest to determine the distribution of arabinoxylans in the flour streams in order to compose proper flour stream blends to obtain a functional flour with specific properties without the use of additives or further treatments.
Earlier studies on the distribution of arabinoxylans in mill streams characterized the relationship between the content of AX in individual flour fractions and the technological suitability of specific flour passages to a limited extent [4,11,16,17,21,36]. These studies have often been more focused on the structural characterization of AX from various mill streams and less on their comparison with the most commonly used methods of quality and technological suitability assessment. Understanding the differences between the functional characteristics of different mill streams would improve the efficiency of their composition and the quality of the final blend for various applications [6].
The aim of the study was to evaluate several physiochemical and rheological properties of wheat flour mill streams, including arabinoxylans content and fractions structure, to analyze its distribution so as to optimize flour passage composition.
2. Materials and Methods
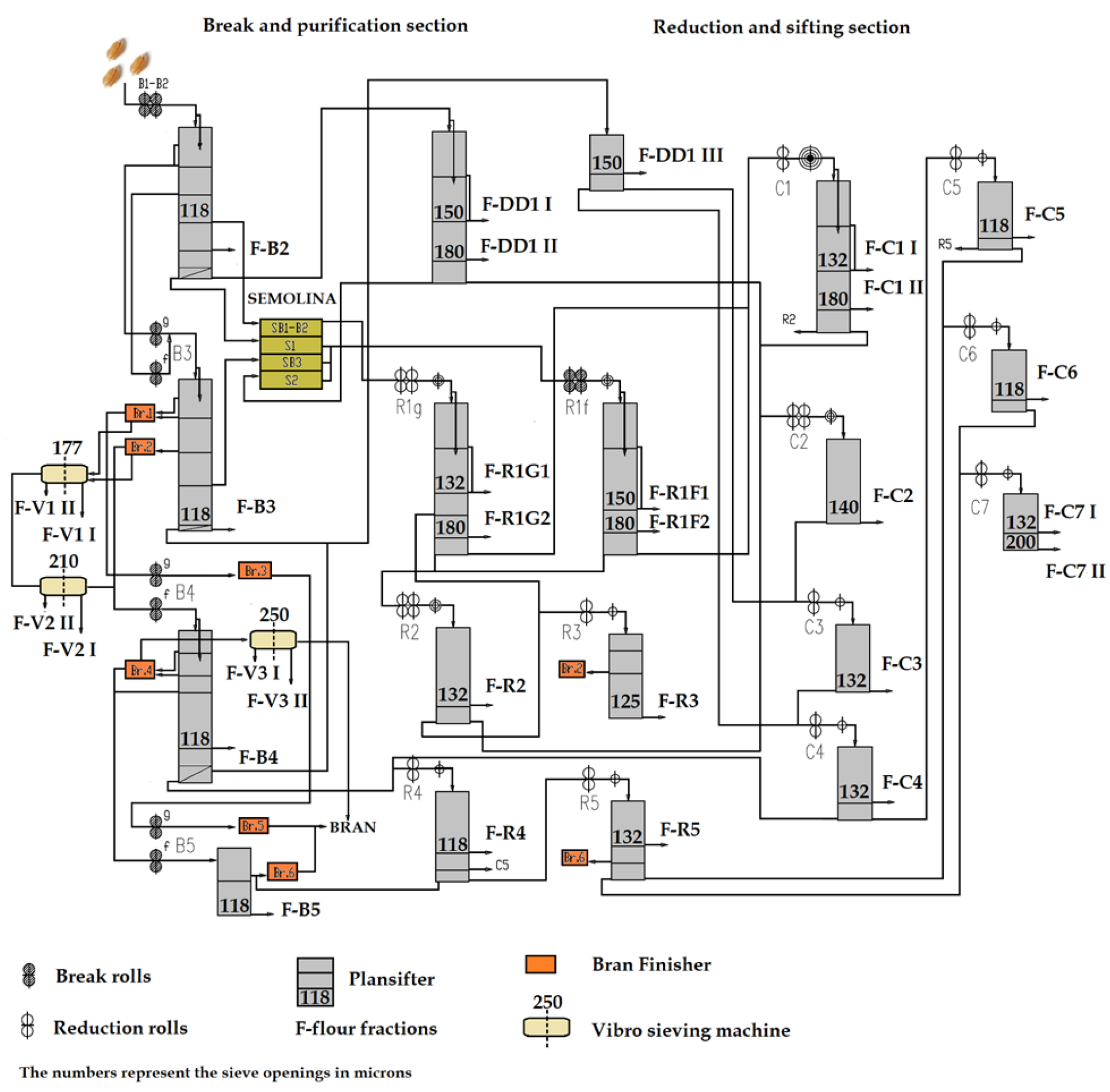
IS Laudis wheat variety, characterized by a high content of non-starch polysaccharides, was used for the milling test. The wheat was cleaned and conditioned to 16% of moisture content and milled in an industrial scale Roller Mill (in PZZ LUBELLA GMW Sp. z o.o., Lublin, Poland) with a throughput of 11,800 kg/h and an extraction rate of 78%. The milling process consisted of breaking, reduction, sizing, sifting, and sorting. A schematic diagram of the industrial milling stages is shown in Figure 1.
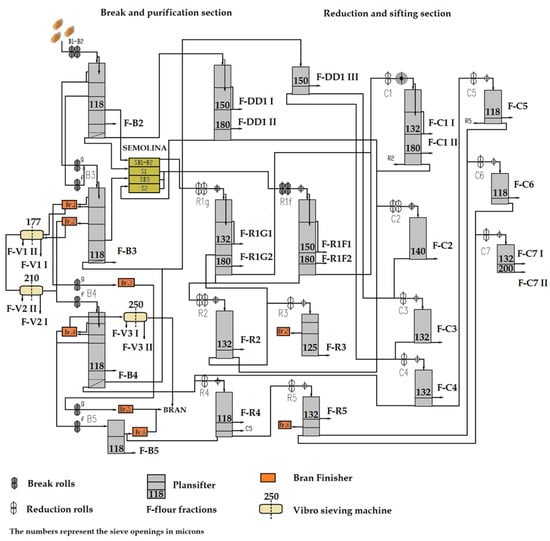
Figure 1.
Schematic diagram of industrial milling station applied.
Indexes I and II refer to passages produced in the same grinding section that differ in granulation, wherein Index I refers to a fine flour and Index II to a coarse one. For V1–V3 streams in each vibro-sifter, two separate fractions (I and II) differing in quality, but with similar particle size, were collected in half of the sifter length. After milling, 30 different flour streams were obtained, marked as “F—flour” on the scheme, consisting of 4 breaking flour streams (B2, B3, B4, B5), 17 reduction and sizing mill flour streams (C1I, C1II, C2, C3, C4, C5, C6, C7I, C7II, R1F1, R1F2, R1G1, R1G2, R2, R3, R4, R5), 3 sifting flour streams (DD1I, DD1II, DD1III) and 6 sorting filter streams flour (V1I, V1II, V2I, V2II, V3I, V3II) as shown in the Scheme (Figure 1) for individual streams. All samples were collected separately and were kept in tight plastic bags prior to further analyzes.
The selected physicochemical properties of the flour mill streams were determined as follows according to ICC Standard Methods [37]: moisture content (MC) according to PN-ISO 712:2002 (ICC 110/1), ash content (A) according to PN-EN ISO 2171:2010 (ICC 104/1), falling number (FN) determined according to PN-EN ISO 3093:2010 (ICC 107/1) standard method, as well as wet gluten content (G) and gluten index (GI) according to PN-EN ISO 21415-2:2008 (ICC 155) by using a Perten Glutomatic 2200 (PerkinElmer Inc., Waltham, MA, USA). Damaged starch (SD) content of the flour mill streams samples was determined by means of an SD-Matic (Chopin Technologies, Villeneuve la Garenne, France) which provides results in AACC units [38] (PN-EN ISO 17715:2015-01).
Rheological tests were performed with the following devices: Alveograph (Chopin Technologies, Villeneuve-la-Garenne, France) according to PN-EN ISO 27971:2015-07 (ICC 121), Brabender Farinograph-E apparatus (Duisburg, Germany) according to PN-EN ISO 5530-1:2015-01 (ICC 115/1), and Mixolab (Chopin Technologies, Villeneuve-la-Garenne, France) according to PN-EN ISO 17718-1:2015-01 (ICC 173) [37].
Standard Alveograph procedure was applied to evaluate the baking strength (W) as the surface area under the curve, dough strength (P) as the maximum pressure needed to blow the dough bubble expressing dough resistance, extensibility (L) as the length of the curve expressing dough extensibility, elasticity index (Ie) [39] and strain hardening index (SH) [40].
The rheological properties of the dough prepared from each mill stream were determined using Farinograph standard procedure. Water absorption (WA) (% of the water needed to obtain a dough consistency of 500 BU), dough development time (DT) (time to reach a consistency of 500 BU), DoS—degree of softening, Farinograph quality number (QN), and dough stability (S) were recorded.
Rheological properties of dough prepared from each mill stream were also studied using the Chopin Mixolab based on the Chopin+ flour protocol with the following settings: mixing speed—80 rpm, total analysis time—45 min, dough weight—75 g, hydration water temperature 30 °C. Flour and water were added accordingly to obtain a dough with a maximum consistency of 1.10 Nm (±0.05) during the first test phase. The Mixolab test was performed using a standard protocol: 8 min at 30 °C, heating for 15 min at a rate of 4 °C/min, holding at 90 °C for 7 min, cooling for 10 min to 50 °C at a rate of 4 °C/min and holding at 50 °C for 5 min [41]. The following rheological features were tested with Mixolab: (i) primary readings: water absorption (M-WA), protein weakening (M-C2), starch gelatinization (M-C3), amylase activity (M-C4), starch retrogradation (M-C5), slope M-α—between the end of 30 °C period and M-C2 as the speed of the protein weakening under heating effect, slope M-β—between M-C2 and as an indicator of pasting (gelatinization) speed, slope M-γ—between M-C3 and M-C4 as enzymatic (α-amylase) degradation speed [42]; (ii) secondary parameters: M-C2–C1—protein weakening range, M-C3–C2—starch gelatinization range (pasting), M-C4–C3—cooking stability range, and M-C5–C4—cooling setback (gelling) [43].
Solvent Retention Capacity (SRC) tests were performed according to an approved AACC 56-11.02 method [38]. SRC is the weight of solvent retained by the swollen flour deposit after centrifugation and is expressed as a percentage of the original flour weight (adjusted to 14% moisture). Solvents were: deionized water, 50 wt% sucrose in water, 5 wt% lactic acids in the water, 5 wt% sodium carbonate in water (WaSRC—water retention capacity; SuSRC—sucrose solvent retention capacity; LaSRC—lactic acid solvent retention capacity; ScSRC—sodium carbonate solvent retention capacity). Herein, a flour sample (5 ± 0.050 g) was transferred to a 50 mL centrifuge tube and mixed with 25 g of solvent [44]. In the next step, the sample was left to solvate for 20 min with shaking every 5 min for 5 s. The tubes were then centrifuged at 2500 rpm for 15 min. The supernatant was poured off and the tubes were allowed to dry for 10 min. The sample was subsequently weighed and the SRC was calculated [45]. Additionally, GPI (gluten performance index) was calculated as described by Vukić et al. [4] based on the ICC method [37] by dividing the LaSRC value by the combined values of SuSRC and ScSRC.
The content of non-starch polysaccharides (NSP) was determined by gas chromatography according to Englyst and Cummings [46] and AOAC 994.13 [47]. The total NSP (T-NSP) content is the amount of sugars: arabinose, xylose, mannose, galactose, and glucose [47]. This analysis allows us to separate the non-starch polysaccharides into two fractions: soluble (S-NSP) and insoluble (I-NSP), and to determine the composition of polysaccharides in both fractions. Total arabinoxylans content (T-AX) and soluble (S-AX) and insoluble (I-AX) fractions were assessed.
All analyzes were performed in triplicate. Data were subjected to one-way analysis of variance (ANOVA) using Statistica 13.3 software (StatSoft, Inc., Tulsa, OK, USA) followed by the Fisher’s least significant difference (LSD) post hoc test to compare means at the 0.05 significance level. Statistica software (version 12.0, StatSoft Inc., Tulsa, OK, USA) was applied for Principle Component Analysis (PCA) and determination of correlation coefficients at the level of significance 0.05. The analysis of the main components was employed to determine the relationship between physiochemical and rheological features and arabinoxylans in the individual flour streams. PCA input data matrix was scaled automatically. The optimal number of principal components obtained in the analysis was determined on the basis of the Cattel criterion.
3. Results
The obtained physiochemical and rheological properties of the individual mill streams are presented in Table 1, Table 2, Table 3 and Table 4. The ash content in the individual mill streams was one of the most differentiating components that demonstrated dependence upon the obtained fraction. Low ash content (around 0.6 or less) of flour fractions indicates the absence of bran in the flour (i.e., more white flour), which is often desired by industry and consumers. In contrast, high ash content (1.6 and above) reveals high content of bran, dietary fiber, antioxidants, and minerals in the flour [22]. Various passages of the tested flour showed significant differences between the obtained mill streams. The lowest content of ash was found in flour streams B2–B3, C1–C5, DD1, and R1–R3 (Table 1) which is evident that from these passages, the flour was white and came from the endosperm. In contrast, the highest ash content was observed in B5, C6–C7, R4–R5, and V1–V3 flour streams. This demonstrates that the feed was rich in the outer layer and bran fractions of wheat grains. Although B2 flour often has the lowest ash content among broken flour, B2 flour was characterized by a higher content of ash compared to the flour stream B3, probably due to the release of accumulated surface dust from the wheat [48] and the presence of some bran fraction in first break streams.

Table 1.
Selected physiochemical properties of IS Laudis wheat flour streams.

Table 2.
Alveograph features of IS Laudis wheat flour streams.

Table 3.
Farinograph features of IS Laudis wheat flour streams.

Table 4.
Mixolab® primary features of IS Laudis wheat flour streams.
In agreement with Pojić et al. [11], we determined that the ash content in the wheat flour streams ranged from 0.440 to 0.755%, with a noticeable increase from B2 to B4 due to the gradual reduction of the gap between the rollers, which resulted in the release of the aleurone layer, fine bran, and germ particles together with the endosperm. In the IS Laudis variety flour, we noted that the ash content tends to increase gradually in successive breaking streams B1–B5 (from 0.671 to 1.282%, respectively), followed by a decrease in fraction streams R1–R3 and early reducing flour streams (C1–C5) with a further increase in later reduction streams C6 (2.352%) and C7 (3.931%), which is consistent with the findings reported by others [2,14]. The increase in the ash content also revealed an increase in contamination with non-endosperm tissue in a later break and reduction streams. Ash content may be related to flour yield, as reported by Banu et al. [3], as we saw around 70% yield, with a mean of 0.44% ash content from the first reduction passages, followed by a final extraction with yield over 76% with 0.51% of ash. As suggested by Every et al. [12,17], flour streams with high ash content may be characterized by a high content of lipoxygenase and dehydroascorbate reductase, and these components may negatively affect the bread quality [9]. When the ash content was higher than 2%, we found it not possible to evaluate the gluten content and gluten index due to the inability of equipment settings.
The IS Laudis wheat variety is a high-gluten wheat, so each of the passages obtained during milling was characterized by a high content of wet gluten in the range of 23.84–44.62%. Wet gluten content is often used as a parameter of protein quality to determine bread dough fermentation tolerance [11]. The high content of this ingredient in the obtained passages is considered an indicator of high quality within the obtained flours.
In the breaking passages B2–B5, which are suited to a gradual grinding of wheat grain and then separation of endosperm from bran, the content of wet gluten increased with progressing milling from 35.99 to 43.56% (Table 1). In the flour stream from the B3 break passage, the gluten content was at a higher level than in the B2 and B4 passages, which was probably due to the fact that the flour from this passage was characterized by a lower content of minerals. A similar dependence was observed in sorting systems DD1I–DD1II and DD1III, these being an extension of wheat meal screening from passages B2 and B3, respectively (Figure 1). Similar observations were made by Banu et al. [3] and Pojić et al. [11].
The increase in the protein content in various flour streams is affected by the increase in the presence of peripheral endosperm and protein-rich bran particles [15]. Flour streams of sorting passages coming from plansifters R1 to R4 contained comparable wet gluten content. Those with an ash content over 1%, such as R5, did not contain enough gluten proteins to perform the test. Streams of reducing passages were characterized by a lower content of wet gluten in comparison to breaking passages, fluctuating in the range of 27.05–30.26%, respectively. Lower values were achieved by flours separated from R1F2 and R1G2, which were characterized by a higher (coarse) particle size. Generally, due to the lower ash content and the lack of peripheral parts of the endosperm, they can be defined as originating from the central parts of the endosperm. The reducing passages C1–C7 are used to break up the endosperm to the largest possible amount of flour. Thus, flour streams from C1–C6 demonstrated wheat gluten that ranged from 23.84–32.10% and, similarly to the sorting passages, at a higher level of ash, they either did not form gluten (passages C7I and C7II), or the amount of wet gluten was very low (passages C5 and C6). The correlation coefficient calculated between A and G was only 0.34 at p < 0.05.
As reported by Pojić et al. [11], the differences in wet gluten content are due to the protein content and the sourcing of the bran and fat. As the grinding progresses, the protein content increases. In the endosperm, the gluten-forming protein predominates over the non-gluten-forming protein. An increase in protein content is therefore correlated with an increase in wet gluten content [49]. The inclusion of bran and aleurone, rich in non-gluten protein, increases the protein content, but not the wet gluten content. Flours with a high proportion of these outer layers tend to have a low wet gluten content. In addition, fat and bran particles can interfere with the gluten network, weakening the network and making precise determination difficult or impossible [11]. Such relationships are shown by the gluten index (GI) determined in this study, which is related to the content of wet gluten and shows the ratio of glutenin and gliadin in wheat flour. If GI shows a higher value, this means that the wheat quality is better.
This outcome is not always correlated with gluten content in several streams. We found the correlation coefficient between A and GI to be −0.61 at p < 0.05. As shown in Table 1, flour streams B5 and V3II were characterized by high gluten content (G), but the gluten index (GI) was low, as the gluten quality was poor. Other relationships were found in V2II, where gluten content, as well as GI, were low (28.64 and 59.0, respectively). This relationship can be explained by the fact that the flour from this passage is another screening of the same filter flour and may be characterized by a reduced quality of gluten proteins. In addition, similar to passages C7I, C7II, and R5, it contains a high content of insoluble arabinoxylans, which may hinder the formation of the gluten network. As observed by Curić et al. [50], flours for baking, in which the GI was above 90%, contained gluten too strong for baking, the resulting bread is characterized by reduced volume, similarly, when the GI was below 75%, the bread is of poor quality. Flours with a GI in the range of 75 to 90% give the best bread with good sensory properties and volumes [50]. Therefore, it seems important to create mixtures of baking flour from passages with different GI in order to obtain flour with the above-mentioned optimal parameters GI.
Differences in protein content and composition have been known for many years, with sub-aleurone cells being richer in protein and having fewer regular starch granules than other starch endosperm cells [49]. The most detailed study to explain protein and gluten relationships in wheat grains was conducted using sequential pearling to remove six outside fractions, each representing on average about 8% of grain weight [51]. A comparison of these fractions and the milled core (corresponding to about 50% of the grain weight) showed that although the total protein content decreased from the outer layers to the center of the grain, the proportion of gluten proteins increased from about 50–55% to about 75% of the total protein in the grain [52].
A characteristic feature of the tested IS Laudis wheat is a high falling number (FN) characterized by a low activity of amylolytic enzymes. In passage flours derived from the central, ventral, and dorsal endosperm, the variability of the falling number was small, 380–460 s, and resulted rather from different content of damaged starch, as explained by Banu et al. [3] and Rani et al. [19]. In our work, only flour streams containing a high content of minerals from the outer layers, such as flour streams R5 and C7II, were distinguished by increased α-amylase activity and the FN results were very low (301 and 74 s, respectively). According to results presented by Rani et al. [19], Every et al. [12], and Dornez et al. [16], amylolytic enzymes are located mainly in the peripheral parts of the grain and the ash content and α-amylase are similarly distributed in the flour passages [3]. In our study, we calculated the correlation coefficient to be −0.75 at p < 0.05 between A and FN.
A measure of the flour’s functionality is the amount of damaged starch (SD) that can be attributed to the mechanical impact acting on grain particles during grinding. Progressive milling causes an increase in the degree of starch damage, which was also confirmed by Banu et al. [53] and Pojić et al. [11]. In the research of Tian et al. [54] on the grinding of wheat endosperm (hard red winter wheat) in a ball mill, an upward trend in the content of damaged starch was shown with the progress of flour fractions extraction, but it slowed down with an increase in the grinding time. In our research, the flour streams coming from breaking and sorting passages showed a lower degree of starch damage than from the reducing passages. According to the results presented in Table 1, SD content increased with the subsequent passage of individual sections. Lower SD values were observed for the initial sorting and reducing passages with lower roller pressure, especially those for which the particle size was coarse (C1II, C2, R1G2, R1F2). The grain is broken down by grooved break rollers which exert less pressure on the endosperm than smooth reduction rollers. In the case of reduction passes, the size of the gap decreases and the pressure of the rolls increases, increasing the starch damage [11].
Table 2 shows the selected properties of wheat flour streams as measured by Alveograph. Several properties were tested in most flour streams: baking strength (W), dough tenacity (P), extensibility (L), elasticity index (Ie), and strain hardening index (SH). High values of dough tenacity (P) (90–151 mm) were noted mainly for the flour streams from the first fractions of the sorting passages (R1–R2), the final sorting and reducing passages (R4–R5, C4–C7I) and the filtration passages from vibro-sifter (V1I–V2I, V3). The lowest p values were obtained by flours of breaking passages and passage-supporting sieving (B2–B5, DD1). These differences result from the origin of the wheat flour stream fraction from the grain zone: the amount of gluten from the middle endosperm is lower than that from the outer aleurone layers [7,9].
The different rheological behavior of the breaking and reducing flour streams can be explained by the higher ratio of polymeric to monomeric proteins in the breaking streams than in the reducing streams [15]. Breaking passages, especially early ones, release relatively clean endosperm particles, while final passages tend to scrape residual endosperm particles from the peripheral endosperm layer, along with fine bran and germ particles [48]. In addition, the final reducing passages (C4–C6) contain more damaged starch and have higher water absorption, which improves the elasticity of the dough. When performing the alveographic test, which is carried out at constant dough moisture, flour containing a higher content of damaged starch would not be fully hydrated, resulting in higher dough tenacity [11]. Conversely, higher extensibility (L) values were obtained for the flour fraction of breaking passages (B2–B5) and intermediate flours of reducing and sorting passages (C1–C3, R3).
Resistance to extension was found to be negatively correlated with damaged starch content [10]. Hence, lower L values were obtained for the flour streams from the final passages. The above characteristic determines the value of the dough configuration index P/L. High P/L indicates a resistant and inextensible dough, while low P/L indicates a weak and extensible dough [40]. The lowest were found for flour streams coming from breaking passages (B2–B5), initial reducing (C1I, C2, C3), and then sorting (DD1, R3) passages. According to the observations of Banu et al. [3], streams fractions derived from dorsal and ventral endosperm were characterized by a higher baking strength (W) and elasticity index (Ie), while lower values were recorded for fractions from the sub-aleurone zone. The higher W will be, the stronger the flour will be during kneading, but the elasticity index has to be considered. The relationships between water absorption and parameters P, W, and P/L can be achieved during measurement, and relationships between P and absorption capacity are very often due to starch damage and lowering of the strength of the flour. The higher W values would suggest more stable flour during kneading [55].
Strain Hardening Index (SH) is related to the properties of the gluten network, especially large glutenin molecules, which are responsible for the branching and entanglement of the gluten polymers and thus the strength and development of the gluten network [40]. Van Vliet [56] found that a high rate of strain hardening is crucial for bread dough development and yield by it facilitating the inflation of dough bubbles into larger volumes and thinner cell walls. Many studies have shown a correlation between bread volume and SH [56,57,58]. The results presented in Table 2 show that the highest SH values and, at the same time, suitability for bread baking, are shown by the first breaking (B2, B3), sorting (R1F1–R1F2), and reducing (C1–C3) passage flour streams. Interestingly, the V1I and V1II filtration passages flour streams obtained from the vibro-sifter are also characterized by high SH values. The R4 and R5 flour streams showed the lowest values of the SH index, so the application of these streams is not recommended for bread flour composition. Samples containing high ash content (C5–C7II) did not show the ability to form gluten networks, so results are missing in these flour streams. These results were not included in PCA analysis due to the absence of alveographic results for some flour streams, i.e., P, L, W, and P/L could not be evaluated in the C7II stream, Ie was not found in C7I and C7II stream flours, and SH index was not available for C6, C7I, and C7II streams flour. These flour streams included very high amounts of ash content (Table 1) because of the presence of external grain layers which excluded gluten from the obtained flour. Thus, it was not possible to test selected properties using the Alveograph procedure.
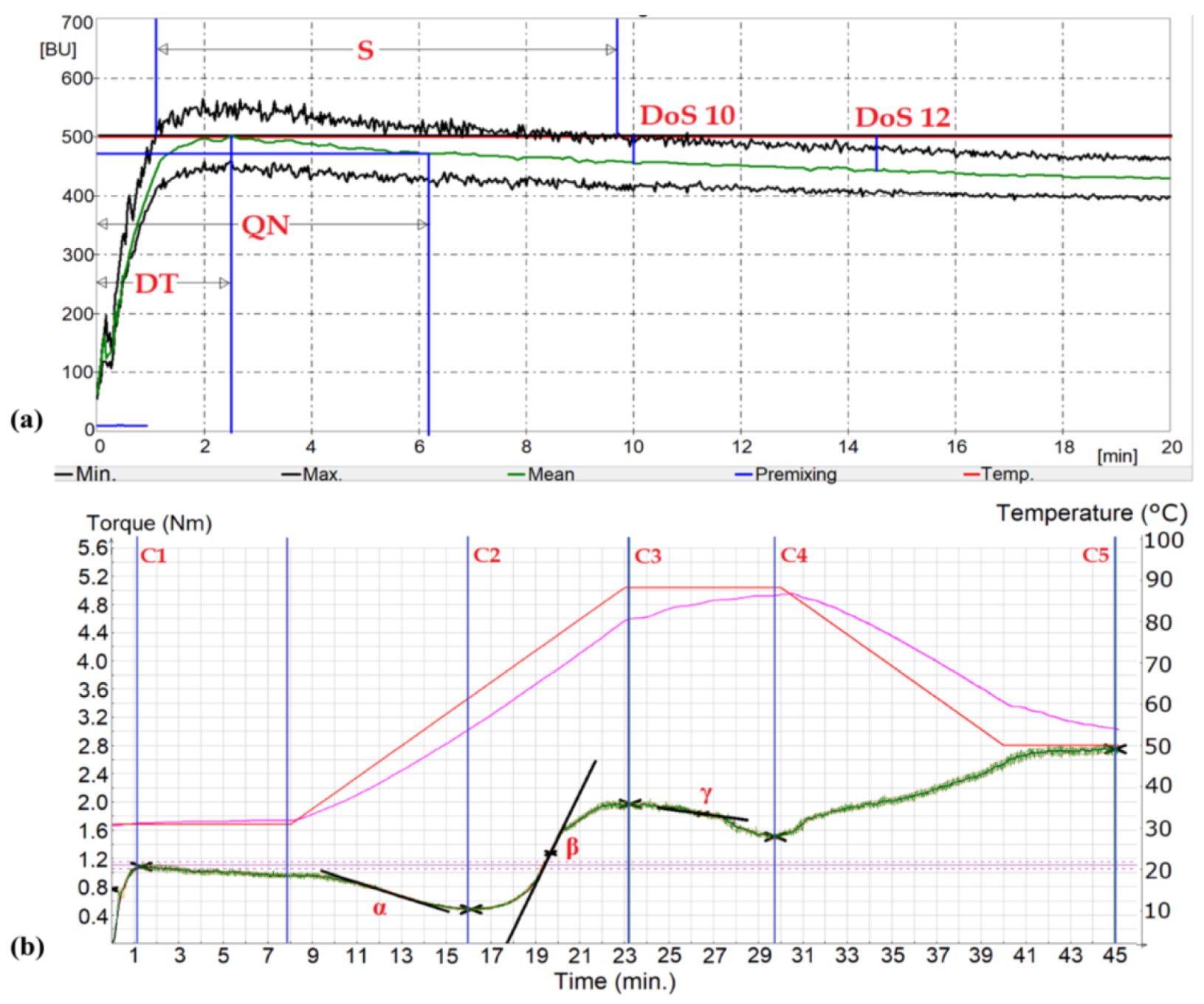
The results of selected features measured with the Farinograph are presented in Table 3. From the individual numerical values of farinographic parameters read from the chart (Figure 2a), conclusions can be drawn about the baking quality of individual flours and the direction of their use. The farinographic assessment of flour allows for testing the dough in conditions similar to the production conditions, thanks to which it enables a more complete determination of flour quality and suitability for mechanical processing than basic methods, such as protein content or gluten amount. The differences in farinographic results indices of passage flours depend on the grain fraction from which it is extracted in the technological scheme of the mill.
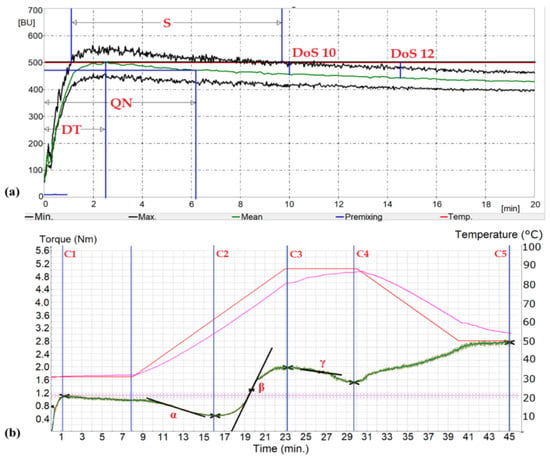
Figure 2.
Example graphs of Farinograph (a) and Mixolab® (b) obtained for R1F1 flour mill stream: DT—development time; QN—quality number; S—stability, DoS10,12—dough softening in time 10 and 12 min; C2—protein weakening; C3—starch gelatinization; C4—amylase activity; C5—starch retrogradation; α slope—speed of the protein weakening under heating effect; β slope—an indicator of pasting (gelatinization) speed; γ slope—enzymatic (α-amylase) degradation speed.
In the tested flour streams from various passages, higher water absorption (WA) was observed in particular in the final breaking (B4, B5), reducing (C4–C7), and sorting (R4, R5) passages, as well as filter flour streams (V1–V3). High WA is most likely due to an increase in the content of ash [3] and damaged starch [59] with high correlation coefficients of 0.86 and 0.72, respectively (p < 0.05). Consecutive passages were characterized by increasing content of non-starch polysaccharides, such as arabinoxylans, which can be found in larger amounts in bran, and the increasing damage in starch granules, which are able to retain larger amounts of water in these flours than in those with low ash. Upon performing tests following the Farinograph procedure, and correcting to a constant moisture content (14%) of flour, we concluded that streams containing higher SD content would not be completely hydrated resulting in higher dough strength. Here, the higher the water absorption (WA), the higher the yield of the prepared dough. In turn, long development time (DT) and low dough softening (DoS) characterize resistance to weakening of the dough with greater tolerance to mechanical processing.
We noted that dough development time (DT) had the highest values for breaking stream flours, but also for filter flours and final sorting and reducing passages with higher ash content. Our work also indicated that the granularity of the fraction also affects the development time of the dough. Therefore, very thick (above 180 µm) fractions (R1F2, R1F2, C1II) had a shortened development time (2.1–2.3 min). In our research, dough stability (S) decreased significantly from 17.9 to 2.7 min with successive flour streams for breaking, sorting, and reducing passages. The highest stability was achieved by the initial breaking flour streams (B2, B3) and the sieving passages (DD1) supporting fraction separation. Thus, the decrease in stability results from low-quality gluten or when other ingredients of the flour start to affect the dough system.
Destabilization occurs when mechanically damaged starch is in the gluten network, breaking the disulfide bond and softening the dough. The lowest values of DoS, both in the 10th and 12th minute of the test, were characterized by breaking passages, especially the initial (B2–B3) and, as in the case of dough stability, additional sorting passages (DD1). Interestingly, low dough softening was also found in flour streams from passages of filter flours (V1–V3). The quality number (QN) was related to the results of other farinographic results as the softening and stability parameters described above [1]. Higher S was positively correlated with higher QN with a correlation coefficient of 0.86 at p < 0.05 but negatively correlated with DoS results obtained both for 10 and 12 min (−0.88 and −0.84 at p < 0.05, respectively). Flour streams B2 and B3 as well as DD1 or V1–V3 flours were characterized by high QN results.
Table 4 and Table 5 show Mixolab® primary and secondary features of IS Laudis wheat flour streams. Several properties can be evaluated and calculated via the Mixolab® procedure. As in the farinographic analysis, fractions from the middle endosperm showed low dough water hydration (M-WA), measured by obtaining a consistency of 1.1 Nm (M-C1), in direct correlation with the water absorption (WA) parameter determined in the farinographic analysis (0.98 at p < 0.05). This proves the possibility of using Mixolab® as an alternative instrument for the analysis of flour water absorption parameters, with less sample use needed and greater possibilities of analysis.

Table 5.
Mixolab® secondary features of IS Laudis wheat flour streams.
Mixolab® allows to evaluate the rheological properties of the dough (testing of the protein-starch complex) at a variable temperature. Using Mixolab®, the flour’s water absorption is determined, and the characteristics characterizing the susceptibility of the dough to proteolytic enzymes (C2), the activity of amylolytic enzymes (C3), and starch retrogradation (C5) are read from the graph. Testing the rheological properties of the dough using the Mixolab® apparatus is carried out in two stages. In the first stage, the water absorption of the flour is determined, corresponding to the dough consistency of 1.1 ± 0.05 Nm. In the second stage, changes in the characteristics of the dough during its formation and further mixing under changing temperature conditions for 45 min are examined [42]. The graph (Figure 2b), which can be divided into five phases, records the changes in the resistance of the dough to the stirrers when mixing the dough. In the first phase, lasting 8 min at a constant dough temperature (30 °C), the properties of the dough during its formation are determined. In the second phase, during further mixing and at the same time increasing the temperature at a rate of 4 °C/min, the consistency of the dough decreases. When the temperature reaches the initial gelatinization temperature (phase 3), gelatinization of the starch begins, which is manifested by an increase in the consistency of the dough. In the fourth phase, a further increase in temperature to 90 °C causes the liquefaction of the starch paste and thus the reduction of the resistance of the dough to the stirrers. Lowering the temperature to 50 °C in phase 5 causes recrystallization of amylose, which in the graph is manifested by an increase in the consistency of the dough, referred to as retrogradation. In the third, fourth, and fifth phases of the graph, the properties of the starch complex are examined [43]. The graph also reads indicators describing the rate of dough consistency reduction during the initial temperature increase in the second phase (α), dough consistency increase due to starch gelatinization (β), and consistency reduction due to enzymatic hydrolysis (γ).
The lowest values of the M-C2 (protein weakening) parameter, excluding passage C7II (with extremely high bran content), were found in flour streams with increasing ash content (−0.86 at p < 0.05). Fractions from the periphery of the endosperm were found to be characterized by proteins from these zones of the grain, which may result in greater proteolytic activity [3]. These M-C2 results corresponded to the M-C2−C1 parameter (protein weakening range) (0.89 at p < 0.05) and were not related to the M-α slope (protein breakdown rate) (Figure 2b).
Flours from the breaking and initial sorting and reduction passages, derived from the central endosperm, were characterized by high values of the M-β slope (Table 4) found on the curve (Figure 2b), which characterize the starch gelatinization rate [3,60,61]. These fractions have reduced enzymatic activity. Passages originating from the outer zone have a lower M-β slope due to the higher content of amylolytic enzymes. We noted that the increased presence of peripheral kernel particles rich in protein, minerals, and amylolytic enzymes affected the decrease in viscosity. The fact that α-amylase is mostly located in the peripheral parts of a wheat kernel indicated the similar distribution of α-amylase and ash content among flour mill streams, where final reduction flours are characterized by higher α-amylase activity [12,16,19].
Accordingly, more intense shear stress of flour streams during reduction passages induced stronger mechanical damage of starch granules than during breaking stages. Since the recorded peak viscosity is an indirect measure of the present α-amylase status along with the starch granule quality, the higher α-amylase activity and higher quantity of mechanically damaged starch in the final reduction passages resulted in lower peak viscosity [62].
High values of the M-C3 point are associated with reduced M-WA (−0.95 at p < 0.05) and less SD (−0.82 at p < 0.05) and are characteristic of the middle endosperm flour fraction. For parameter M-C4 (amylase activity), the final passages from each section showed the lowest parameters (Table 4), while flour streams from the middle endosperm and filter flour (V2) revealed the highest M-C4 values. Low values of M-γ slope, characterized by a small difference for M-C4−C3 (cooking stability range), were found in V1–V3 filter flours, but also in the DD1 sorting flour streams. M-C5 values indicate starch retrogradation [60,61,63] and were lower for breaking passages (B2–B5) and sorting passages flours (R4–R5) and, as in the studies of Banu et al. [3], in the reduction passages (C5–C6) of flour streams from peripheral parts of the kernel. This relationship was associated with the cooling setback M-C5−C4 index (Table 5) at the correlation level of 0.93 (p < 0.05).
There is a strong correlation between the results obtained through the application of the different procedures assessed in this study. In the Mixolab® analysis, a decrease in the M-C4 torque and an increase in the M-C4−C3 differential is generally seen as a decrease in the falling number in the tested flour [55]. Herein, FN and viscosity assessments of M-C4−C3 from Mixolab®, as well as WA and SD allowed analysis of some properties tested by means of the Alveograph procedure. Of note, the alveographic procedure and selected values provide little predictive information about dough behavior, unlike the Mixolab® ‟hot‟ stage (M-C2, M-C3, M-C4, and M-C5). This Mixolab® data is therefore extremely useful. Moreover, Mixolab® data shows good differentiation between the mill streams, allowing inferences on the properties of both protein and starch complex, and the amylolysis/retrogradation phase. A further advantage of the test device is that it gives researchers the ability to analyze flours with a high content of bran fraction and ash, which is difficult or impossible when utilizing other devices evaluating such rheological properties. Hence, using Mixolab® gives the possibility to assess the similarities of various mill streams of flours so as to study the complete milling process and produce reproducible flour blends [55].
Table 6 shows the results of wheat flour stream SRC as determined utilizing a variety of liquids. The Solvent Retention Capacity (SRC) method indicates the hydration capacity of wheat flour, and the flour’s ability to absorb various solutions is measured depending on the chemical composition, i.e., the amount and quality of gluten, starch, and pentosans. This attribute is assessed utilizing distilled water (Wa), 50% sucrose (Su), 5% lactic acid (La), and 5% sodium carbonate (Sc). Water absorption depends on all of the flour components listed above, thus providing an overall picture of the water-holding capacity of the dough system. The absorbed sucrose solution determines the properties of pentoses, which are formed as a result of pentosan hydrolysis. The volume of the absorbed lactic acid solution characterizes the hydration properties of gluten depending on the quality and quantity of gluten proteins, while the absorbed Na2CO3 solution provides information on the degree of starch damage [45]. Large differences in SRC values were found in the tested flour streams.

Table 6.
SRC values of IS Laudis wheat flour streams.
In the data we derived, WaSRC ranged from 61.867 to 159.775%, SuSRC from 92.951 to 178.502%, LaSRC from 96.788 to 180.035%, and ScSRC from 74.394 to 193.406%. According to the results listed in Table 6, the lowest water absorption (WaSRC) was found in the DD1 sorting passages, the breaking passages, and the initial sorting and reduction passages. Comparing the WaSRC results with water absorption measured via the application of farinograph and Mixolab® procedures, the correlation coefficients were 0.93 and 0.92, respectively, at p < 0.05.
The SuSRC values indicate the amount of arabinoxylans in the analyzed flour sample. Unlike in the studies of Vukić et al. [4], we noted higher SuSRC values in breaking stream flours. These were probably brought about by the presence of endosperm adjacent to the aleurone layer. High values of SuSRC were also obtained in the reduction and filter flour streams (V2–V3). Our work indicates that the highest ScSRC values (indicating damaged starch) are in the flour streams from the final reducing passages (C4–C7II) and sorting passages (R3–R5), as well as in the filter and sorting flour streams (V1I, V1II, and R1G1), for which also the starch damage DS measured by the amperometric method was high (Table 1).
LaSRC value is an indicator of gluten quality and functionality and reveals the amount of glutenin protein in passage flours [45]. The high values of this index are related mainly to the content of middle endosperm fractions derived in reducing C1–C5, initial sorting with fine granulation (R1F1 and R1G1), breaking passages (B2, B3) and supporting sorting flour streams (DD1I, DD1III). The C7II flour is also characterized by a high level of LaSRC, but this outcome was probably due to the very high content of the bran hydrophilic fraction, but not the quality and quantity of glutenin. This notion is confirmed by the low value of the GPI parameter for this passage flour stream (Table 6).
Gluten Performance Index (GPI) was considered by Kweon et al. [45] as being a better indicator of the predictability of gluten functionality than LaSRC alone. It describes the general ability of glutenin to function among other modulating networks such as damaged starch or pentosans/arabinoxylans. Lindgren and Simsek [64] confirmed in their research the existence of a positive correlation between GPI and selected passage flour rheological parameters. Similar relationships were noted in this study. GPI was negatively correlated with WA and dough softening at 12 min (DoS12) of the farinograph (−0.77 and −0.62 at p < 0.05, respectively), and strongly positively correlated with L, W, Ie, and alveograph SH (0.73, 0.62, 0.83, 0.83 at p < 0.05, respectively). The GPI in the tested flour streams ranged from 0.42 to 0.91 (average 0.66). As with the LaSRC results, central endosperm flours, also those with increased granulation (R1F2, R1G2) demonstrated the highest values. In line with earlier research by Lindgren and Simsek [64] and Kweon et al. [45], the GPI value can be used to determine the baking quality of flour, in particular, bread.
Polysaccharides content, arabinoxylans content, and fractions in the obtained flour streams are listed in Table 7. The total content of non-starch polysaccharides (T-NSP) was determined by gas chromatography and is the sum of the sugars: arabinose, xylose, mannose, galactose, and glucose. This analysis allows separating non-starch polysaccharides into two fractions: soluble and insoluble, and determining the composition of polysaccharides in both fractions. In the tested flour streams, the total amount of non-starch polysaccharides ranged from 2.70 to 24.70% and was the highest mainly in the final fractions of reduction and sorting passages and in filtration streams flours. We saw that the T-NSP content was strongly related to the ash content (A) in the tested flours, and the calculated correlation coefficient was at the level of 0.85 (at p < 0.05). We also observed a high content of T-NSP for the R1F1 passage (4.39%), the flour of which comes from the middle endosperm and, however, contains a small amount of ash.

Table 7.
Non-starch polysaccharides and arabinoxylans content in IS Laudis wheat flour streams.
Upon analyzing the insoluble (I) and soluble (S) NSP fractions, S-NSP stood at 2.58%. A similarly high content of T-NSP was found in the DD1I and C1II fractions, respectively, 4.66% and 4.11%, in which the amount of both I-NSP and S-NSP fractions was also high. The highest amount of T-NSP was found in flour stream C7II (24.70%), which contains the highest amount of bran fraction, and thus ash, of all the tested fractions. The content of I-NSP in this passage is almost 90%. In the insoluble fraction (I-NSP), the growth gradient is similar to that of T-NSP and generally takes the highest values for flour streams from passages with a higher degree of extraction and thus ash content (0.86 at p < 0.05) from the outer parts of the kernel. The S-NSP fractions were characterized by a more even distribution in all the tested passage flours and were only slightly related to the ash content (0.43 at p < 0.05).
Table 7 also shows the insoluble (I-AX) and soluble (S-AX) fractions of arabinoxylans. These are part of the sum of non-starch polysaccharides that contribute to better bread production [25,26,27]. The values for I-AX were from 0.93% for flour stream B3, to 15.07% for flour stream C7II. Similarly, for S-AX, values ranged from 0.55% in the B3 flour stream, to 1.37% in C7II. Over all, the sum of both fractions ranged from 1.48 to 16.45%. The gradient of the value increase for the individual fractions was generally similar to that of the I-NSP and S-NSP fractions. For I-AX and T-AX, the values increased significantly with the amount of ash (0.85 at p < 0.05), except for flours from the R1F1 and DD1I streams. We also found that the S-AX distribution was more even and less related to ash content (0.45 at p < 0.05) and extraction level. Similar observations were reported by Delcour et al. [36] where the values of the NSP fraction increased with the increase of ash. A clear gradient was observed for T-AX while a fuzzy one was noted for S-AX. Moreover, we discovered that S-AX can increase only when the flour is enriched with a greater amount of very fine bran fraction, which is observed in this study for flour streams C7II, C7I, and R5. As reported by Li et al. [65] intensive grinding of the bran layer leading to obtaining finely divided fragments of these fractions may contribute to increasing the content of soluble fiber, also by breaking glycosidic bonds in cellulose and insoluble hemicellulose, especially in S-AX.
As presented in previous studies, the content of T-AX, I-AX, and I-NSP, as confirmed by the PCA analysis (Figure 3), was strongly positively correlated with the flour stream ash content and water absorption. We observed that the high content of these components effectively prevented the formation of a gluten network and the appropriate consistency of the dough in rheological analyses. As in the studies of Ramseyer et al. [6], we noted an increasing amount of S-AX, I-AX, and T-AX, along with the progressing flour extraction. At the same time, we saw that the increase in the content of S-AX in individual passages was different than the results of ash content, I-AX, and T-AX.
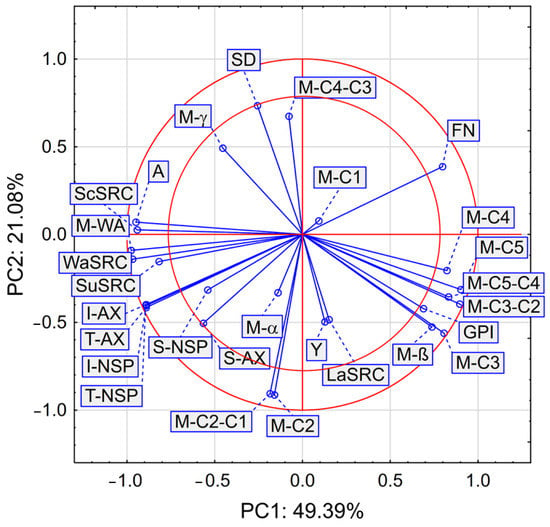
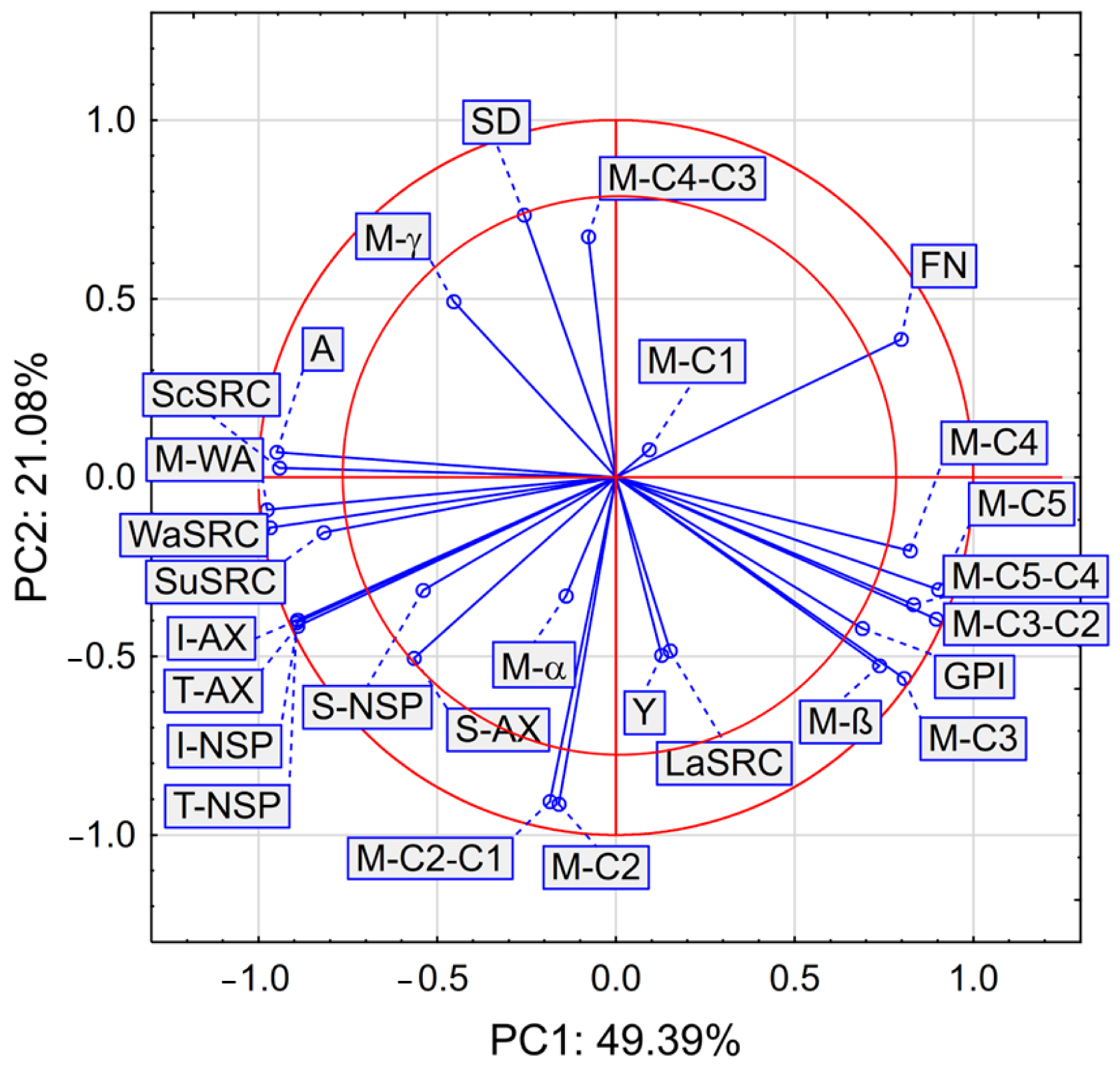
Figure 3.
PCA analysis of selected properties of wheat flour streams.
We therefore conclude that the testing of flour mill streams coming from various passages using rapid rheological analyses, such as the Mixolab® analysis, compared to time-consuming chemical analyzes of the isolation of polysaccharides fractions, allows (to some extent) the possibility of indicating the content of these fractions in flours. Michniewicz et al. [26] demonstrated, for example, the effects of water-soluble and water-insoluble wheat pentosans and water-soluble rye pentosans on certain baking characteristics of wheat flour. In their work, all three pentosan preparations markedly increased the farinograph water absorption, while the addition of water-soluble pentosans (at 2%, w/w) increased the specific loaf volume. However, insoluble fractions did not significantly affect this parameter. According to Michniewicz et al. [26], at a constant dough consistency, pentosan-supplemented breads had higher moisture contents and water activity values. Moreover, in their study, higher retrogradation rates of the amylopectin, as measured by DSC, were shown for breads supplemented with pentosans, presumably due to their higher moisture content. The conclusion was that water-soluble pentosans retarded the aggregation process between amylose molecules, as evidenced by the amount and type of water-extractable carbohydrates from bread crumbs.
Figure 3 presents the results of the PCA analysis of selected flour streams. Here, certain physiochemical and rheological properties, as well as polysaccharides and arabinoxylans were taken into account.
The PCA analysis shows that the first two main components PC1 and PC2 describe the variability of the system to a level of 70.47%, however, the parameters that are contained between the two red circles in Figure 3 have the greatest impact on the variability of the system. In our study, PCA showed that A, M-WA, WaSRC, SuSRC, ScSRC, I-AX, T-AX, I-NSP, and T-NSP are strongly and positively correlated with each other. Hence, the results obtained from instrumental measurements, especially ash content, water absorption measured with the Mixolab® procedure, and solvent retention capacity in water, 50% sucrose, and 5% sodium carbonate solutions may be useful for the prediction of the content of total polysaccharides and arabinoxylans, as well as their insoluble fractions.
We also found a positive and strong correlation between the parameters GPI, the slope of M-β, and certain characteristics measured by means of Mixolab® and labeled as M-C3, M-C4, M-C5, M-C3−C2, M-C5−C4. Figure 3 reveals a strong and positive correlation between the parameters M-C2−C1 and M-C2. Based on the results of PCA analysis we observed a negative and strong correlation between A, M-WA, ScSRC, WaSRC, SuSRC, I-AX, T-AX, I-NSP, T-NSP and FN, GPI, M-β, M-C3, M-C4, M-C5, M-C3−C2, M-C5−C4. We also noted a strong negative correlation between M-C2, M-C2−C1, and SD values. That parameter SD is indicative of the amount of damaged starch best describes the passages B4, B5, C4, C6, C7I, R4, R5, R1G1, V1I, V1II, V3I, V3II which are the final flour fractions from the specific milling scheme used in the experiment, and give flour fractions after intensive treatment by breaking, reducing and sifting passages. The presented comparison of flour mill stream passages, as well as other comparisons [1,2,6,11,21], shows the possibilities of using such data to compose specialized flour mixtures. Hence, flour mixing to achieve a particular flour functionality can be based on instrumental methods instead of long and costly chemical analyzes. As was shown in previous studies, the parameters most differentiating individual flours, such as the content of ash, gluten proteins, amylolytic enzymes, or non-starch polysaccharides and arabinoxylans, had a direct impact on the characterized rheological parameters used to direct the fraction in accordance with the assumed technological usefulness. In our work, we observed that the high content of T-AX, I-AX, and I-NSP effectively prevented the formation of the gluten network and the appropriate consistency of the dough in rheological analyses. Although the results of this study apply only to a specific variety of IS Laudis common wheat and a specific milling scheme, it can be assumed that the results will also be consistent for other varieties. A better understanding of the origin of different fractions and the role of arabinoxylans and their fractions in the milling process will allow the development of wheat flour blends with the desired functionality.
4. Conclusions
In conclusion, there are large differences between the mill streams in terms of the content of physicochemical parameters and rheological properties, as well as soluble and insoluble fractions of non-starch polysaccharides and arabinoxylans. These differences result directly from the origin of specific fractions from the anatomical parts of the kernel and the impact of grinding processes, mechanical damage to starch, and sieving during grain milling. All these operations greatly affect the overall quality of the flours. From the point of view of using the passages, it seems important to know about the subtle differences in the content of these components in the final fractions of the milling scheme. Flour passage tests using rapid rheological analyses, such as the Mixolab® analysis and the combination of principal component analysis with Pearson’s correlation coefficients for the analysis of these relationships, allows for the identification of strict relationships between the tested parameters. Based on this information, millers can select and blend several flour streams for the maximum amount of flour at specified characteristics, especially ash content and non-starch polysaccharides.
Author Contributions
Conceptualization, P.L. and A.W.; methodology, P.L. and A.W.; software, P.L., A.W. and. M.G.; validation, P.L. and A.W.; formal analysis, P.L.; investigation, P.L.; resources, P.L.; data curation, P.L., A.W. and M.G.; writing—original draft preparation, P.L. and A.W.; writing—review and editing, P.L. and A.W.; visualization, P.L. and M.G.; supervision, P.L. and A.W.; project administration, A.W.; funding acquisition, P.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by a grant from the Ministry of Science and Higher Education in Poland (grant number DWD/4/84/2020 “Implementation doctorate”).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data presented in this study are available on request from the corresponding author.
Acknowledgments
Special thanks to Krzysztof Gaczkowski, the manager of wheat mill in PZZ Lubella GMW Sp. z o.o., for his kind support during this research.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Brütsch, L.; Huggler, I.; Kuster, S.; Windhab, E.J. Industrial roller milling process characterisation for targeted bread quality optimization. Food Bioprocess. Technol. 2017, 10, 710–719. [Google Scholar] [CrossRef]
- Prabhasankar, P.; Sudha, M.L.; Rao, P.H. Quality characteristics of wheat flour milled streams. Food Res. Int. 2000, 33, 381–386. [Google Scholar] [CrossRef]
- Banu, I.; Stoenescu, G.; Ionescu, V.; Aprodu, I. Physicochemical and rheological analysis of flour mill streams. Cereal Chem. 2010, 87, 112–117. [Google Scholar] [CrossRef]
- Vukić, M.; Janić Hajnal, E.; Mastilović, J.; Vujadinović, D.; Ivanović, M.; Šoronja-Simović, D. Application of solvent retention capacity tests for prediction of rheological parameters of wheat flour mill streams. Hem. Ind. 2020, 74, 37–49. [Google Scholar] [CrossRef]
- Ahmed, R.; Ali, R.; Saeed, S.A.; Ghufran Saeed, S.M.; Mobin, L. Impact of distinct compositional variations in flours of various milled streams on dough behavior and end quality of baked products. Pak. J. Bot. 2017, 49, 383–387. [Google Scholar]
- Ramseyer, D.; Bettge, A.; Morris, C.F. Distribution of total, water-unextractable, and water-extractable arabinoxylans in wheat flour mill streams. Cereal Chem. 2011, 88, 209–216. [Google Scholar] [CrossRef]
- Indrani, D.; Rajiv, J.; Prabhasankar, P.; Rao, G. Chemical, rheological and parotta making characteristics of flourmill streams. Eur. Food Res. Technol. 2003, 217, 219–223. [Google Scholar] [CrossRef]
- Gómez, M.; Ruiz-París, E.; Oliete, B. Influence of flour mill streams on cake quality. Int. J. Food Sci. Technol. 2010, 45, 1794–1800. [Google Scholar] [CrossRef]
- Liu, Y.; Ohm, J.B.; Hareland, G.; Wiersma, J.; Kaiser, D. Sulfur, protein size distribution, and free amino acids in flour mill streams and their relationship to dough rheology and breadmaking traits. Cereal Chem. 2011, 88, 109–116. [Google Scholar] [CrossRef]
- Pojić, M.; Hadnađev, M.; Dapčević Hadnađev, T. Gelatinization properties of wheat flour as determined by empirical and fundamental rheometric method. Eur. Food Res. Technol. 2013, 237, 299–307. [Google Scholar] [CrossRef]
- Pojić, M.; Spasojević, N.; Atlas, M. Chemometric approach to characterization of flour mill streams: Chemical and rheological properties. Food Bioprocess. Technol. 2014, 7, 1298–1309. [Google Scholar] [CrossRef]
- Every, D.; Simmons, L.; Al-Hakkak, J.; Hawkins, S.; Ross, M. Amylase, falling number, polysaccharide, protein and ash relationships in wheat millstreams. Euphytica 2002, 126, 135–142. [Google Scholar] [CrossRef]
- Iqbal, Z.; Pasha, I.; Abrar, M.; Hanif, M.S.; Arif, A.M.; Masih, S. Protein concentration, composition and distribution in wheat flour mill streams. Annals. Food Sci. Technol. 2015, 16, 104–114. [Google Scholar]
- Sutton, K.H.; Simmons, L.D. Molecular level protein composition of flour mill streams from a pilot-scale flour mill and its relationship to product quality. Cereal Chem. 2006, 83, 52–56. [Google Scholar] [CrossRef]
- Wang, Y.G.; Khan, K.; Hareland, G.; Nygard, G. Distribution of protein composition in bread wheat flour mill streams and relationship to breadmaking quality. Cereal Chem. 2007, 84, 271–275. [Google Scholar] [CrossRef]
- Dornez, E.; Gebruers, K.; Wiame, S.; Delcour, J.; Courtin, C. Insight into the distribution of arabinoxylans, endoxylanases, and endoxylanase inhibitors in industrial wheat roller mill streams. J. Agric. Food Chem. 2006, 54, 8521–8529. [Google Scholar] [CrossRef]
- Every, D.; Simmons, L.; Ross, M. Distribution of redox enzymes in millstreams and relationships to chemical and baking properties of flour. Cereal Chem. 2006, 83, 62–68. [Google Scholar] [CrossRef]
- Gebruers, K.; Courtin, C.; Goesaert, H.; Campenhout, S.; Delcour, J. Endoxylanase inhibition activity in different european wheat cultivars and milling fractions. Cereal Chem. 2002, 79, 613–616. [Google Scholar] [CrossRef]
- Rani, K.; Prasada Rao, U.J.S.; Leelavathi, K.; Haridas Rao, P. Distribution of enzymes in wheat flour mill streams. J. Cereal Sci. 2001, 34, 233–242. [Google Scholar] [CrossRef]
- Abdel-Haleem, A. Influence of heat treatment for some wheat milling fractions on fino bread quality. J. Food Sci. Technol. 2019, 56, 2639–2650. [Google Scholar] [CrossRef]
- Wang, M.; Sapirstein, H.D.; Machet, A.S.; Dexter, J.E. Composition and distribution of pentosans in millstreams of different hard spring wheats. Cereal Chem. 2006, 83, 161–168. [Google Scholar] [CrossRef]
- Engelsen, M.M.; Hansen, Å. Tocopherol and tocotrienol content in commercial wheat mill streams. Cereal Chem. 2009, 86, 499–502. [Google Scholar] [CrossRef]
- Izydorczyk, M.; Biliaderis, C.; Bushuk, W. Physical properties of water-soluble pentosans from different wheat varieties. Cereal Chem. 1991, 68, 145–150. [Google Scholar]
- Zannini, E.; Bravo Núñez, Á.; Sahin, A.W.; Arendt, E.K. Arabinoxylans as functional food ingredients: A Review. Foods 2022, 11, 1026. [Google Scholar] [CrossRef] [PubMed]
- Delcour, J.; Vanhamel, S.; Hoseney, R. Physicochemical and functional properties of rye nonstarch polysaccharides. II. Impact of a fraction containing water-soluble pentosans and proteins on gluten-starch loaf volumes. Cereal Chem. 1991, 68, 72–76. [Google Scholar]
- Michniewicz, J.; Biliaderis, C.; Bushuk, W. Effect of added pentosans on some properties of wheat bread. Food Chem. 1992, 43, 251–257. [Google Scholar] [CrossRef]
- Labat, E.; Morel, M.H.; Rouau, X. Effects of laccase and ferulic acid on wheat flour doughs. Cereal Chem. 2000, 77, 823–828. [Google Scholar] [CrossRef]
- Wang, M.W. Effect of Pentosans on Gluten Formation and Properties. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2003. [Google Scholar]
- Jelaca, S.L.; Hlynca, I. Water binding capacity of wheat flour crude pentosans and their relation to mixing characteristics of dough. Cercal Chem. 1971, 48, 211–222. [Google Scholar]
- Patil, S.K.; Tsen, C.C.; Lineback, D.R. Water soluble pentosans of wheat flour. l. Viscosity properties and molecular weights estimated by gel filtration. Cereal Chem. 1975, 52, 44–56. [Google Scholar]
- Hoseney, R.C.; Faubion, J.M. A mechanism for the oxidative gelation of wheat flour water-soluble pentosans. Cereal Chem. 1981, 58, 421–424. [Google Scholar]
- Izydorczyk, M.S.; Biliaderis, C.G.; Bushuk, W. Physical properties of water-soluble pentosans from wheat. J. Cereal Sci. 1990, 11, 153–169. [Google Scholar] [CrossRef]
- Rouau, X.; EI-Hayek, M.L.; Moreau, D. Effect of an enzyme preparation containing pentosanases on the bread making quality of flours in relation to changes in pentosans properties. J. Cereal Sci. 1994, 19, 259–272. [Google Scholar] [CrossRef]
- Jelaca, S.L.; Hlynka, I. Effect of wheat-flour pentosans in dough, gluten, and bread. Cereal Chem. 1972, 49, 489–495. [Google Scholar]
- Kim, S.K.; D’Appolonia, B.L. Bread staling studies. I. Effect of protein content on staling rate and bread crumb pasting properties. Cereal Chem. 1977, 54, 207–215. [Google Scholar]
- Delcour, J.; Win, H.; Grobet, P. Distribution and structural variation of arabinoxylans in common wheat mill streams. J. Agric. Food Chem. 1999, 47, 271–275. [Google Scholar] [CrossRef] [PubMed]
- ICC. Standard Methods, on-line version; International Association for Cereal Science and Technology: Vienna, Austria, 2018. [Google Scholar]
- AACC. Approved Methods of Analysis; American Association of Cereal Chemists: St. Paul, MN, USA, 2009. [Google Scholar]
- Codină, G.G.; Mironeasa, S.; Bordei, D.; Leahu, A. Mixolab versus Alveograph and Falling Number. Czech J. Food Sci. 2010, 28, 185–191. [Google Scholar] [CrossRef]
- Jødal, A.S.S.; Larsen, K.L. Investigation of the relationships between the alveograph parameters. Sci. Rep. 2021, 11, 5349. [Google Scholar] [CrossRef] [PubMed]
- Szafrańska, A.; Rachoń, L.; Szumiło, G. Estimation of protein-starch complex of selected wheat species depending on production technology intensity. Zesz. Probl. Postępów Nauk Rol. 2015, 582, 81–90. [Google Scholar]
- Dubat, A. A New AACC International Approved Method to measure rheological properties of a dough sample. Cereal Foods World 2010, 55, 150–153. [Google Scholar] [CrossRef]
- Dubat, A.; Rosell, C.M.; Gallagher, E. Mixolab: A New Approach to Rheology, 1st ed.; American Associate of Cereal Chemists International: St. Paul, MN, USA, 2013. [Google Scholar]
- Guzmán, C.; Posadas-Romano, G.; Hernández-Espinosa, N.; Morales-Dorantes, A.; Peña, R.J. A new standard water absorption criteria based on solvent retention capacity (SRC) to determine dough mixing properties, viscoelasticity, and bread-making quality. J. Cereal Sci. 2015, 66, 59–65. [Google Scholar] [CrossRef]
- Kweon, M.; Slade, L.; Levine, H. Solvent Retention Capacity (SRC) testing of wheat flour: Principles and value in predicting flour functionality in different wheat-based food processes and in wheat breeding-A Review. Cereal Chem. 2011, 88, 537–552. [Google Scholar] [CrossRef]
- Englyst, H.; Cummings, J. Simplified method for the measurement of total non-starch polysaccharides by gas-liquid chromatography of constituent sugars as alditol acetates. Analyst 1984, 109, 937–942. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Herlich, K., Ed.; The Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Sakhare, S.D.; Inamdar, A.A. The cumulative ash curve: A best tool to evaluate complete mill performance. J. Food Sci. Technol. 2014, 51, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Hoseney, C. Principles of Cereal Science and Technology, 2nd ed.; American Association of Cereal Chemists: St. Paul, MN, USA, 1994. [Google Scholar]
- Curić, D.; Karlović, D.; Tušak, D.; Petrović, B.; Dugum, J. Gluten as a standard of wheat flour quality. Food Technol. Biotechnol. 2001, 39, 353–361. [Google Scholar]
- He, J.; Penson, S.; Powers, S.; Hawes, C.; Shewry, P.R.; Tosi, P. Spatial patterns of gluten protein and polymer distribution in wheat grain. J. Agric. Food Chem. 2013, 61, 6207–6215. [Google Scholar] [CrossRef]
- Shewry, P.R.; Wan, Y.; Hawkesford, M.J.; Tosi, P. Spatial distribution of functional components in the starchy endosperm of wheat grains. J. Cereal Sci. 2020, 91, 102869. [Google Scholar] [CrossRef]
- Banu, I.; Aprodu, I. Association of physicochemical with technological properties of wheat. Int. J. Food Sci. Technol. 2015, 5, 1644–1650. [Google Scholar] [CrossRef]
- Tian, X.; Wang, Z.; Wang, X.; Ma, S.; Sun, B.; Wang, F. Mechanochemical effects on the structural properties of wheat starch during vibration ball milling of wheat endosperm. Int. J. Biol. Macromol. 2022, 206, 306–312. [Google Scholar] [CrossRef]
- Rheological and enzyme analyses. In Mixolab Applications Handbook; Chopin Applications Laboratory: Villeneuve-la-Garenne, France, 2012.
- van Vliet, T. Strain hardening as an indicator of bread-making performance: A review with discussion. J. Cereal Sci. 2008, 48, 1–9. [Google Scholar] [CrossRef]
- Dobraszczyk, B.J.; Salmanowicz, B.P. Comparison of predictions of baking volume using large deformation rheological properties. J. Cereal Sci. 2008, 47, 292–301. [Google Scholar] [CrossRef]
- Sroan, B.; Bean, S.; Macritchie, F. Mechanism of gas cell stabilization in bread making. I. The primary gluten—starch matrix. J. Cereal Sci. 2009, 49, 32–40. [Google Scholar] [CrossRef]
- Di Stasio, M.; Vacca, P.; Piciocchi, N.; Meccariello, C.; Volpe, M.G. Particle size distribution and starch damage in some soft wheat cultivars. Int. J. Food Sci. Technol. 2007, 42, 246–250. [Google Scholar] [CrossRef]
- Collar, C.; Bollain, C.; Rosell, C.M. Rheological behaviour of formulated bread doughs during mixing and heating. Food Sci. Technol. Int. 2007, 13, 99–107. [Google Scholar] [CrossRef]
- Rosell, C.M.; Collar, C.; Haros, M. Assessment of hydrocolloid effects on the thermo-mechanical properties of wheat using the Mixolab. Food Hydrocoll. 2007, 21, 452–462. [Google Scholar] [CrossRef]
- Fustier, P.; Castaigne, F.; Turgeon, S.L.; Biliaderis, C.G. Impact of commercial soft wheat flour streams on dough rheology and quality attributes of cookies. J. Food Eng. 2009, 90, 228–237. [Google Scholar] [CrossRef]
- Haros, M.; Ferrer, A.; Rosell, M.C. Rheological behaviour of whole wheat flour. In Proceedings of the IUFoST 13th World Congress of Food Sciences Technology, Nantes, France, 17–21 September 2006; pp. 1139–1148. [Google Scholar] [CrossRef]
- Lindgren, A.; Simsek, S. Evaluation of hard red spring wheat mill stream fractions using solvent retention capacity test. J. Food Process Preserv. 2016, 40, 131–139. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Wang, H.; Li, Z.; Qiu, J.; Wang, L. Correlation of microstructure, pore characteristics and hydration properties of wheat bran modified by airflow impact mill. Innov. Food Sci. Emerg. Technol. 2022, 77, 102977. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).