Abstract
Fish gelatin has been increasingly used as a safe alternative to cattle and pig gelatin due to its similar structure, avoiding the health and socio-cultural issues associated with the use of materials of mammalian origin. Fish gelatin can be produced from processed fish products to achieve a high yield at a low cost. Recent studies show that although fish gelatin comes from a wide range of sources, the protein content and amino acid composition of fish gelatin from different sources are different, and some fish gelatin is soft and unstable transglutaminase (TGase) can catalyze the γ-amide group of glutamine residues and the ε-amino group of lysine residues in proteins to form covalent bonds to form a stable protein network structure, improve the strength of the gel so that it can be applied in a more special environment. In this experiment, after screening the raw materials of cold-water fish gelatin M06 and M08, warm-water fish gelatin M03 and M04, a strong fish gelatin was successfully prepared by catalytic modification of cold-water fish gelatin by transglutaminase (TGase), and the excellent performance of TG enzyme-catalyzed modified gelatin was proved through the application effect of chicken salt soluble protein. In this experiment, the protein content of cold-water fish M08 was the highest, which was up to 99.9%, 1.09 times that of warm-water fish. The gelatin content of cold-water fish M08 was the highest of the four kinds of fish gelatin, with a wide proportion of components and rich amino acid composition. Cold-water fish M08 gelatin-derived gel had the highest strength of 253 ± 1 g/cm at 4 °C. It was found that fish gelatin with protein molecular weight distribution and rich amino acid composition had higher gel strength. M08 gelatin is cross-linked by transglutaminase (TGase), which increases the strength of enzyme gels by approximately 200% compared to self-assembled gels. Fish gelatin catalyzed by the TG enzyme improves the gel strength of raw material and makes it more applicable. M08 gelatin also showed good application performance at low temperatures in compound chicken salt-soluble protein gel, with a water retention rate of 95.84% and gel strength of 198.5 g/cm. This study expanded the application range of fish gelatin by TG enzyme and improved the application potential of fish gelatin.
1. Introduction
Fish gelatin is an important source of gelatin in addition to mammalian gelatin [1]. Fish gelatin is a polypeptide polymer, extracted from fish scales, skin and bones. Due to its unique functional properties, good biocompatibility, good biodegradability and rich extraction sources, gelatin has been widely explored and applied in the fields of food, cosmetics, drug delivery and tissue engineering [1,2,3,4]. Gelatin has a variety of properties [5,6] and is widely used in the food industry. Gelatin forms thermoreversible gels with water and can be used as a gelling agent, stabilizer, emulsifier, crystallization modifier, thickener, foaming agent, glue, dispersant and food clarifying agent. Gelatin can be added to meat products as a gelling agent and used in the production of pork, aspic, canned ham, veal and other meat products to improve the yield and quality of products [7,8]. For example, gelatin with an optimal gel strength of 150–250 (g/cm) is primarily used for preservation of cold (4 °C) meat products and frozen foods (−20 °C) because of its gelatinous, adhesive, stable and protective gel properties. Its film forming, adhesion and protective colloid properties are used for the preservation of frozen foods, where the ideal gel strength is 200–250 (g/cm). Similarly, gelatin is mainly used for gelation, adhesion, thickening, emulsification, stability and protection of colloidal properties in food preservation at 10–20 °C, mainly including candy and dairy products, where the optimal gel strength is 100–250 (g/cm).
Transglutaminase (TGase) is a naturally occurring enzyme that exists in almost all organisms. It is usually used to catalyze the formation of intermolecular and intramolecular covalent cross-linking of proteins, modify proteins and change their functional properties [9,10]. The catalytic mechanism of TGase is to catalyze the formation of ε-gamma (-glutamyl) lysine isopeptide bonds within and between protein molecules by using the gamma-carboxamide group of glutamine residues in the polypeptide chain as the acyl group donor, and the amino group of lysine residues in the ε-polypeptide chain as the acyl group receptor. It catalyzes the formation of stable protein network structures by crosslinking the gamma-amido group of glutamine residues and ε-amino group of lysine residues in proteins to form covalent bonds, which in turn affect their functional properties [11]. The application of fish gelatin in the food sector has been greatly limited by its poor gelation and rheological properties. The gelation properties of fish gelatin, which is a macromolecular hydrocolloid produced by the partial hydrolysis of collagen, can be enhanced through crosslinking and generation of covalent bonds by transglutaminase (TG) enzymes, resulting in the formation of enzymatic gels.
Salt-soluble proteins are those mainly affecting the gelation properties of meat proteins, directly affecting the tissue properties, adhesion and yield of meat products. Gelatin is a common meat product additive that can significantly improve the hardness, elasticity and water holding capacity of salt-soluble proteins. Among the three most common types of meat products, that is, chicken, beef and pork, chicken salt-soluble proteins are known to have better water-holding capacity leading to improved meat texture [12]. However, although China is a major chicken breeding country, the percentage of chicken meat converted into meat products is low. Feng et al. selected chicken salt-soluble proteins as the experimental object and prepared composite salt-soluble proteins by only adding fish gelatin raw material to enhance the application characteristics of the obtained enzymatic gelatin salt-soluble protein composite [13].
In this study, compared with (Fernandez-Diaz et al.) [14], the gel strength of the M08 crosslinked TG enzyme of cold-water cod was increased by 480–500 g/cm and 2.3–2.6 times. Cold water cod gelatin M08 is 480–500 g/cm higher and 2.3–2.6 times higher than the current study Fernandez-Diaz et al. (2001). Through the research of raw materials and new processes, it is compared with (Aidat, Omaima, et al.) [15]. The rate of increase is 150 g/cm (reference). In this experiment, the cold-water fish gum M08 containing more amino acids and different molecular weight proteins was screened from four kinds of gelatin raw materials by measuring protein molecular weight distribution and amino acid analysis. As the raw material of this study, cold-water fish glue is catalyzed by TG enzyme, which improves the gel strength of raw material and makes it more widely applied. TG enzyme can catalyze the cross-linking of γ-amide and glutamine lysine residues in proteins to form covalent bonds, forming a stable protein network structure and improving the gel strength. In addition, fish gelatin was combined with chicken salt-soluble proteins to further investigate its self-assembly and enzymatic gel properties, such as water-holding capacity and texture, and improve the performance of self-assembled and enzymatic gels and their practical application in composite salt-soluble protein gels. Our results provide novel insights for improving the self-assembly and enzymatic gelation performance and practical application of salt-soluble protein gels.
2. Materials and Methods
2.1. Materials and Equipment
2.1.1. Material
Balsa gelatin M03, tilapia gelatin M04, and cod gelatin M06 and M08 were purchased from Shandong, Longkot, China. TG enzyme (6400 U/g), food-grade enzyme preparation, was provided by Shandong Longkot Enzyme Preparation Co. (Linyi, China) Glutaraldehyde, hydrochloric acid, sodium hydroxide, ethanol and other reagents were analytically pure and purchased from Sinopharm Chemical Reagent Co. (Shanghai, China).
2.1.2. Equipment
The BHS-4 digital thermostat water bath was obtained from Fuzhou Taimei Experimental Instrument Co., Ltd., (Fuzhou, China); DV-C digital viscometer from COMPANY (Brookfield, WI, USA); mini-PIII vertical electrophoresis tank from Bio-Rad (Hercules, CA, USA); G:Box gel imager from Syngene (Cambridge, UK); TA-XT Plus mass spectrometer from TA Instruments Ltd. (Eden Prairie, MN, USA); Stable Micro System from UK Stable Micro System (Godalming, UK); and S-4800 electron scanning microscope from Hitachi Manufacturing (Tokyo, Japan); CT 15RE centrifuge from him (Tokyo, Japan); L-8900 amino acid analyzer from Hitachi (Tokyo, Japan); ARES/G2 rotary evaporator from TA Instruments Ltd. (Eden Prairie, MN, USA); from Lichen Technology Corporation FJ200-S homogenizer, FTIR from Nicolet10 Fisher from Thermo (Waltham, MA, USA), ESCALABXi+ X-ray diffractometer from Thermo Fisher (Thermo Fisher, UK), XR11 from Zhongxi Yuanda Technology Co., Ltd. (Beijing, China)-KDN-08C Kjeldahl nitrogen analyzer.
2.2. Gelatin Protein Content and Molecular Weight Determination
2.2.1. Determination of Protein Content
SDS-PAGE analysis was performed according to our previous work (Zhang, Sun, Ding, Tao, et al., 2020) [16]. Protein content was determined using the Kjeldahl method. The gelatin conversion coefficient was 5.55.
2.2.2. Gel Electrophoresis for Protein Molecular Weight Detection
Gel electrophoresis was performed using the Meilun Protein Gel Kit for protein molecular weight detection. The concentration of each gelatin sample was 1 mg/mL, while the loading volume of each sample and protein marker was 15 μL. The protein-loaded gel was electrophoresed at a constant voltage of 100 V for 3 h. The gel was stained with Coomassie blue for 2 h and then rinsed and decolored, and photographed and scanned using the Burroughs gel imager. The Image Lab software (Image Lab 6.1 mac win)was used for protein quantitative analysis.
2.3. Analysis of Amino Acid Composition of Gelatin
2.3.1. Sample Preparation
Briefly, 50 mg of each fish gelatin was dissolved in 10 mL of 6 mol/L hydrochloric acid, ablated at 105 °C for 24 h and concentrated by rotary evaporation. The pH was adjusted to 2.0 and each sample volume was fixed to 100 mL. Then, 40 μL of sample solution was added to 960 μL of 0.02 mol/L hydrochloric acid and filtered.
2.3.2. Amino Acid Analysis
Amino acid analysis was conducted using the Pico. Tag method [17]. The amino acid composition of gelatin was determined using an amino acid analyzer.
2.4. Preparation of Gel
2.4.1. Gelatin Solution Preparation
A 6.67% gelatin aqueous solution was prepared by dissolving 4 g of gelatin in 60 mL of deionized water at 4 °C followed by mixing and beating with a high-speed homogenizer according to the testing standard GB 6783-2013 [18] of the National Standard of the People’s Republic of China—Edible Gelatin.
2.4.2. Self-Assembly and Enzymatic Gelation
Each of the 4 generated 6.67% aqueous gelatin solutions were placed in an incubator at 4 °C to observe their ability for gel self-assembly. Consecutively, 100 U/mL of TG enzyme solution was added to each of the 4 °C 6.67% gelatin aqueous solutions and their enzymatic gelation was detected at 4 °C.
2.5. Determination of Gel Temperature and Gel Melting Point
Briefly, 5 mL of prepared gelatin solution was added in a test tube placed in a constant temperature bath, and warmed up from 4 °C, with a temperature increase range of 0.1 °C/min; after 10 min at each temperature, the test tube was inverted, the flow of gelatin was observed and its ability for gel self-assembly was determined. Following its self-assembly, the gel was returned to 4 °C to be restored to gelatin because gelatin is thermally unstable and then placed back in the water bath to determine the approximate gel temperature; the temperature increase interval was decreased to accurately determine the gel temperature.
The gel-melting temperature of fish gelatin was determined using the method described by (Chen et al.) [19]. The self-assembled and enzymatic gels were poured into a 10 × 100 mm (diameter × length) test tube with a screw cap, leaving some space in the upper part of the test tube, and incubated in a refrigerator at 4 °C for 16–17 h. The test tube was then placed in a constant temperature water bath at 4 °C for 10 min, and the temperature at which the bubbles at the bottom of the test tube started to move upward was recorded as the gel melting temperature.
2.6. Scanning Electron Microscopy Detection of Fish Gelatin
Scanning electron microscopy (SEM) was used to observe the microstructures of self-assembled gels and enzymatic gel-lyophilized samples. Samples were gold-sprayed and magnified 10.00 KX at an accelerating voltage of 10 kV for SEM observation.
2.7. Gel Strength Assay
The gel strength of self-assembled gels at 4 °C versus enzymatic gel samples prepared at 4 °C was determined using a mass spectrometer (XT). The probe diameter was 0.5 in, the trigger force was 5 g, the pretest speed was 1 mm/s, the posttest speed was 1 mm/s and the penetration speed was 1 mm/s. The gel strength was expressed as the maximum force (g), with the test compression being 70%. Each sample was tested thrice, and the average value was calculated.
2.8. Water-Holding and Oil-Holding Tests of Gelatin
WHC and FBC were measured according to a previous work [20]. For this assay, 1 g of gelatin was added to 30 mL of deionized water at 25 °C and stirred well, followed by 2 rounds of standing for 15 min and then stirring for 5 s. After repeating this process 4 times, the sample was centrifuged for 20 min, the upper aqueous phase was poured out, the residual water on the surface was absorbed with filter paper, and the mass of the gel sample (g) was recorded. The water-holding capacity (WHC) of gelatin was calculated using the following formula:
Note: m is the initial gelatin powder mass, g.
Likewise, 1 g gelatin was added to a 50 mL centrifuge tube at 25 °C and 10 mL of soybean oil was added and stirred well, followed by 2 rounds of standing for 15 min and stirring for 5 s. After repeating this process 4 times, the sample was centrifuged for 20 min, the upper oil phase was removed, the residual oil on the surface was absorbed with filter paper, and the mass of the gel sample (g) was recorded. The oil-holding capacity (FBC) of gelatin was calculated using the following formula:
Note: m is the initial gelatin powder mass, g.
2.9. Detection of the Secondary Structure of Self-Assembled and Enzymatic Gels Using Fourier Infrared Spectroscopy (ATR-FTIR)
According to the improved method [21], 2 mg of self-assembled and enzymatic gel samples prepared at 4 °C were weighed, co-milled with 200 mg of potassium bromide, pressed and scanned using Fourier transform infrared spectroscopy at the interval of 400 cm−1~4000 cm−1 at a scanning frequency of 16 times.
2.10. Detection of Tertiary Structure of Self-Assembled and Enzymatic Gels by X-ray Diffraction (XRD)
A small amount of gelatin powder and lyophilized samples of enzymatic gels prepared at 4 °C were placed flat in the groove of the sample table, flattened with slides and put on the X-ray diffractometer carrier table. Parameter settings were as follows: CuKα (1.542 Å) for copper target, voltage 30 kV, current 10 mA, scanning range (2θ) 5~50°, step size 0.02° and scanning speed 2 °min−1.
2.11. Preparation of Chicken Salt-Soluble Proteins and Gels
For the extraction of salt-soluble proteins, chicken breast was cut into pieces, grounded using a meat grinder, followed by the addition of 0.6 mol/L NaCl extraction solution at a volume of 4 times the mass, as described by Chen et al., with slight modifications.
Briefly, 160 mL of 0.6 mol/L NaCl was added to 60 g sample and homogenized, followed by adjusting the pH to 7.0 with 0.2 mol/L NaOH. The homogenate was left for 24 h at 4 °C, filtered using a double layer gauze and centrifuged at 4000 r/min and 4 °C for 10 min. The obtained supernatant contained the salt-soluble proteins.
In addition, 4.5 g of each of the 4 types of fish gelatin was weighed into a beaker and TG enzyme was added at a ratio of 100 U/g. Then, 50 mL of chicken salt-soluble proteins was evenly mixed for 30 s using a high-speed homogenizer at 10,000 r/min, added to gelatin at 25 °C and then stored in the refrigerator at 4 °C. For the control group, 50 mL of ice water was used.
2.12. Statistical Analysis
Each experiment was repeated three times. The results were expressed as mean ± standard deviation. Data analysis and graphical design were performed using SPSS 26 and Origin 2020 software, respectively.
3. Results and Discussion
3.1. Analysis of Protein Content and Composition of Different Fish Gelatins
According to the national food safety standard (GB6783-2013), the protein content index of food additive gelatin is ≥82.0%. We found that the total protein content of the M03, M04, M06 and M08 fish gelatins was 90.9 ± 0.3c%, 95.4 ± 0.5b%, 94.7 ± 0.3b% and 99.9 ± 0.1a% (Table 1), respectively, as measured using the Kjeldahl nitrogen determination method. These results are consistent with the previous literature that compares the protein content composition of cold-water fish and mammalian gelatin (Karim et al.) [22] reported that the collagen contents in the fish skin waste of Japanese sea bass, chub mackerel and bullhead shark were 51.4%, 49.8% and 50.1% (dry basis), respectively. The protein content of the raw material in this experiment is excellent. Although the protein content of all four gelatins met the national standard, we observed large differences in the protein content between different gelatins, which was attributed to the source of gelatins and processing technology.

Table 1.
Gelatin protein content.
Gelatin is generally composed of α chain, β chain and γ chain three kinds of polypeptide chains and between the components of the molecular chain fragments, the β chain is composed of two α chains connected, γ chain is composed of three α chains and the distribution of these chains will affect the reliability of gelatin. According to the study of (SHA X M et al.) [23], it is believed that the gel strength of fish gelatin is related to the distribution of molecular mass, namely the number of α-chains and β-chains. The more α-chains, the more hydrogen bonds of components and the more triple helix structure, resulting in a denser microstructure and greater gel strength. We observed some differences in the molecular weight between the four fish gelatin samples (Figure 1). Fish gelatins with high protein content have a wider distribution of proteins with different molecular weights [24]. In addition to the protein content, the physicochemical properties of gelatin are also influenced by protein composition. These results are consistent with the previous literature that compares the protein content composition of cold-water fish and mammalian gelatin. Fish gelatin mainly contains α1 and α2 peptide chains with molecular weights of approximately 130 and 110 kDa, β peptide chains with molecular weights higher than 200 kDa and a small number of high and low molecular weight components below 110 kDa. Of note, as β chains promote gel formation, the gel strength of fish gelatin has been correlated with the molecular weight distribution and presence of α and β chains, with higher α chain content leading to greater gel strength. We determined that the four fish gelatins had different distributions of α1 and α2 peptide chains of 130 and 110 kDa. In particular, we found that the β-chain content of the two gelatins from cold-water fish, M06 and M08, was higher than that of warm-water fish gelatins, M03 and M04. The high total protein content of gelatin and its wide molecular weight distribution (M08) indicated a higher gel strength.
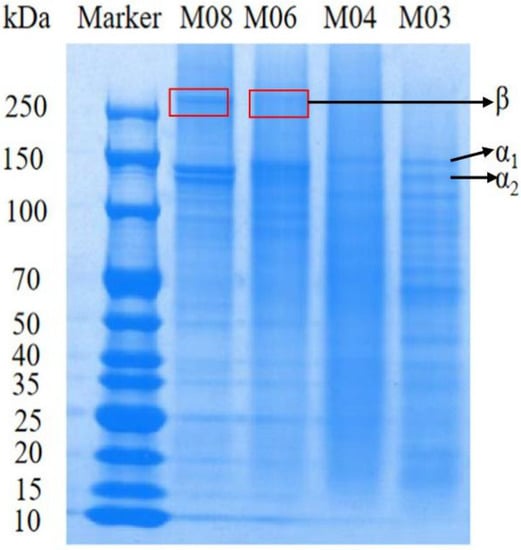
Figure 1.
Molecular weights of four types of fish gelatin.
The physicochemical properties of gelatin have also been closely associated with its amino acid composition. Interestingly, the proline and hydroxyproline subamino acids have been closely correlated to fish gelatin gel strength. We noticed that the content of subamino acids (proline and hydroxyproline) in warm-water fish gelatin was significantly higher than that in cold-water fish gelatin; more specifically, the subamino acid content of warm-water fish gelatin M04 was 4.32% higher than that of cold-water fish gelatin M08. Meanwhile, the content of hydrophobic amino acids (alanine, valine, leucine, isoleucine, proline, hydroxyproline and methionine) in warm-water fish gelatin M03 was 4.4% higher than that in cold-water fish gelatin M06 (Table 2). This might be due to the influence of factors such as the source of fish gelatin. The total amino acid content of cod skin, Alaskan cod skin and fish gum is about 620–630.

Table 2.
Amino acid content of four types of fish gelatin.
According to the gelatinizing study of fish gelatin by (HAUG I J et al.) [25]. The content of proline and hydroxyproline in fish gelatin is low, and fewer hydroxyl groups form hydrogen bonds with water when the gel is formed, thus affecting the gel structure. The result is that fish gelatin has a lower gel temperature and melting temperature than mammalian gelatin.
3.2. Physical and Chemical Properties of Fish Gelatin
The gelation time, temperature and melting point of gelatin are shown in Figure 2 and Table 3. We observed that the gelation time and melting temperature of cold-water fish gelatin were lower than those of warm-water fish gelatin. Among them, M08 was able to gelate at 4–12 °C, whereas warm water fish gelatin M04 gelated at 4–23 °C. Although warm-water fish gelatin exhibited a broader range of gelation temperature than cold-water fish gelatin, M06 and M08 were able to gelate at 4 °C in 10 min, whereas M03 and M04 required 30 min to gelate at 4 °C (Figure 2). We specifically found that at the same temperature and time, the gelation of M08 was better than that of M06; therefore, we selected M08 as the optimal gelatin for low-temperature applications. It has been shown that there is a correlation between the content of proline and hydroxyproline in gelatin and the gel melting temperature (Table 3), and this is also pointed out by Lin, L and Soua, L ’s study [26,27].
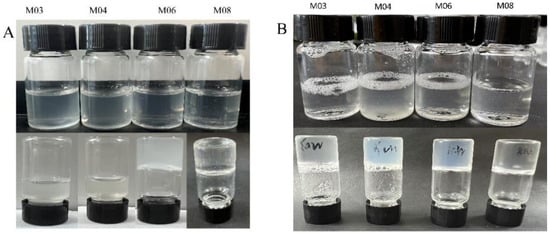
Figure 2.
(A) 4 °C 10 min self-gel (B) 4 °C 30 min self-gel. (Note: (A) is 10 min self-gel at 4 °C, in which M03 and M04 failed to gel when inverted for 10 min, M06 and M08 could gel, (B) is 30 min self-gel at 4 °C.M03, M04, M06 and M08 all became gel).

Table 3.
Gelation temperature and gel melting point of gelatin.
We then characterized the structure of self-assembled and enzymatic gels by scanning electron microscopy (Figure 3). We observed that the surface particles of M03 and M04 gelatins were more tightly arranged in the formed gels, whereas the particles of M06 gelatin were aggregated with fewer adherent particles on the surface of gels. We further detected that among the four fish gelatin sources, the microstructure of M08-derived gels was characterized by a higher number of aggregated particles with nearly non-adherent small particles on the surface of gels compared with those formed by the other three gelatins. This was positively correlated with gel strength. In addition, the enzymatic gel was crosslinked using the TG enzyme, which generated covalent bonds and enhanced the network structure. We observed that the surface of the M08 enzymatic gel was non-porous and flat, and exhibiting the highest gel strength, indicating that the microstructure determined the gel strength. These results are consistent with the previous literature comparing the microstructure of gelatin in cold water fish and mammals.

Figure 3.
Scanning electron microscopy of enzymatic gels and autogels (all magnifications are 10.00 KX) (A) M03; (B) M04; (C) M06; (D) M08; (E) M03+TG; (F) M04+TG; (G) M06+TG; (H) M08+TG.
3.3. Characteristics of Gels Derived from the 4 Fish Gelatins
Gel strength is one of the three most important properties for characterizing the gelatin source and determining its applications. Previous studies have reported gel strengths of 70–270 g/cm and 100–300 g/cm for fish and mammalian gelatins, respectively. Importantly, low gel strength affects the application of gels [28]. We found that the gel strength of M08 was the highest among self-assembled gels (253 ± 1 g/cm), whereas that of M04 was the lowest (35 ± 1 g/cm) (Table 4). Moreover, we noticed that the covalent bonds generated by crosslinking with the TG enzyme enhanced the gel strength of fish gelatin, resulting in the enzymatic M08 gel exhibiting the highest gel strength of 800 ± 2 g/cm, whereas, even the lowest gel strength of the enzymatic M04 gel was increased to 204 ± 6 g/cm (Table 5). Compared with the study of Fernandez-Diaz et al. (2001), the gel strength of M08 crosslinked TG enzyme of cold-water cod was increased by 480–500 g/cm, 2.3–2.6 times This indicated that the gel strength of self-assembled and enzymatic gels was determined by physical and chemical factors, such as the molecular weight of fish gelatin itself. Our results show that the gel strength of fish gelatin mainly depends on the physicochemical properties between the source of the fish and the fish itself: Gelatin itself has self-relatability, but because of the different sources of gelatin, the performance of the gel is not the same, also determines the gelatinization temperature and melting temperature of gelatin, limiting the application of gelatin at different temperatures. Therefore, we used the TG enzyme to catalyze cross-linking to improve the properties of fish gelatin, improve the gel strength and improve the properties of fish gelatin.

Table 4.
Crosslinked gel strength.

Table 5.
Water-holding properties of gelatin and its oil-holding properties.
Water- and oil-holding are important functional properties of gelatin and are closely related to its structure through the interaction of water and oil components [29]. We observed that M04 gelatin had the highest water holding capacity among the four gelatins (245 ± 15%), whereas M06 exhibited the lowest capacity (6 ± 11%), which was proportional to the subamino acid content of gelatin. We found that the oil holding capacity was highest in M08, reaching a maximum of 253 ± 3%, whereas M04 exhibited the lowest capacity at 122 ± 4%, which was proportional to the molecular weight content of gelatin. We determined that crosslinking by the TG enzyme improved the water and oil holding properties of gelatin, resulting in the enzymatic M08 gel exhibiting the lowest water holding capacity (327 ± 12%). TG enzymes can form cross-linking bonds between protein molecules, which can improve the water and oil retention of gelatin. It can be seen from the table that the water and oil retention of gelatin is both improved after the cross-linking of fish gelatin by TG enzymes. Studies have shown that the water holding capacity of commercial fish gelatin on the market is 5.1 ± 1 and the oil holding capacity is 3.45 ± 0.06. By comparison, we concluded that the water- and oil-holding properties of both self-assembled and enzymatic gels were better than those of commercially available fish gelatin [30].
3.4. Structural Detection of Self-Assembled and Enzymatic Gels
Fourier transform infrared spectroscopy (FTIR) is a common technique for determining the secondary structure of proteins. FTIR spectra correlate with the vibrational state of chemical bonds in proteins [31]. As shown in Figure 4, the peaks and bands of amides A, I, II and III were similar between the FTIR spectra of the four gelatin samples, indicating that the four fish gelatin samples had similar secondary structures of proteins.
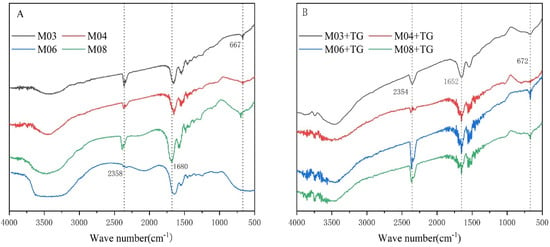
Figure 4.
Infrared map of gelatin raw material and enzymatic gel (A) gelatin raw material self-gel; (B) TG enzyme cross-linked.
We observed that the absorption peak of amide A that is attributed to the N-H stretching vibration was 3400~3440 cm−1. When the N-H group was involved in hydrogen bonding, the absorption peak of amide A shifted to a lower position, with stronger hydrogen bonding causing a greater shift. We found that the position of the amide A absorption peak was essentially the same for all samples. However, as crosslinking by TG enzyme generated more amide bonds and increased covalent bonding energy in gelatin [32], we detected that the amide A absorption peak in enzymatic gels was mainly shifted to a lower position. We specifically observed a greater shift in the distance of the amide A peak between the self-assembled and enzymatic gels, suggesting changes in the physical and chemical properties of gelatin and enhancement of gel strength.
We tested the diffraction effect of gelatin by X-ray diffraction (XRD), as shown in Figure 5. We found that self-assembled gels exhibited two diffraction peaks, a small peak at 7° (2θ), the intensity of which reflected the triple helix content in gelatin, and a stronger broad peak at 20° (2θ) attributed to the large number of polar groups in the gelatin molecular chain, which will form intra- or intermolecular hydrogen bonds. The highly ordered arrangement of these hydrogen bonding networks can transform gelatin into an amorphous structure (non-crystalline structure) with a uniform polymeric network structure. We detected that the position of the diffraction peak at 20° did not change between gels, with the intensity of the amorphous characteristic peaks of these four gelatins being continuously weakened from high to low, thereby preventing the formation of an ordered microcrystalline structure. We further observed that after crosslinking by the TG enzyme, the position and intensity of the small peak at 7° (2θ) of the enzymatic gel did not differ from that of the self-assembled gel, indicating that the triple helix content remained unaltered. However, as crosslinking by the TG enzyme increased the hydrogen bonding between gelatin molecules, we noticed that the peak intensity at 20° in the enzymatic gel was decreased compared with that in the self-assembled gel, suggesting an increased structured order in the enzymatic gel.
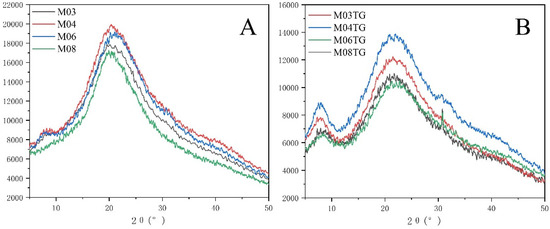
Figure 5.
X-ray diffraction (XRD) plots of self-gel and enzymatic gel (A) self-gel; (B) enzymatic gel.
3.5. Gelatin for Low-Temperature Enzymatic Crosslinking of Chicken Salt-Soluble Proteins
We observed that among the composite salt-soluble protein gels generated from the addition of fish gelatin raw materials, the one derived from M08 had a smooth surface and no flocculent presentation at low temperature. Conversely, we detected that the surfaces of composite gels derived from M03 and M04 gelatins were not smooth but flocculent (Figure 6).
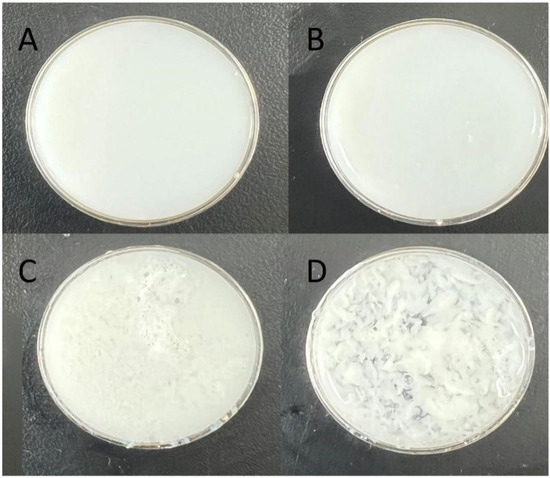
Figure 6.
Characterization of chicken salt-soluble protein ((A): chicken salt-soluble protein+M08 (B): chicken salt-soluble protein+M06 (C): chicken salt-soluble protein+M04 (D): chicken salt-soluble protein+M03).
In addition, we determined that among all composite salt-soluble protein gels, the one derived from M08 exhibited the highest gel strength with 198.5 ± 18 g/cm. Likewise, among the enzymatic composite salt-soluble protein gels, the M08-derived gel had the highest gel strength with 416.7 ± 10 g/cm (Table 6). These findings indicated that the physical and chemical properties of fish gelatin determine the gel strength. Studies have shown that water retention is positively correlated with gel strength. The water retention of hydrogel is significantly improved, and the service life of the gel can be effectively extended.

Table 6.
Gel strength and water holding capacity of enzymatic and self-gelatinized salt-soluble protein gels.
The quality of salt-soluble proteins has been shown to directly affect the gel characteristics of meat products and plays a key role in producing the desired texture and water holding capacity of crushed and reconstituted meat products. The gel water holding capacity of chicken breast was shown to be 90% [33]. In our study, addition of different types of gelatin to salt-soluble proteins improved the water holding capacity of the gel by approximately 5% (Table 6). In particular, we observed that among the composite salt-soluble protein gels, the water holding capacity of M08 was the highest at 95.84%, while among the enzymatic composite salt-soluble protein gels, the water holding capacity of M08 was the highest at 91.53%. Some studies have shown that crosslinking is excessively catalyzed by TG enzymes, leading to the decrease in its water holding performance, which is consistent with the results in this experiment.
4. Conclusions
The source of fish gelatin determines its basic physicochemical properties, such as protein content, molecular weight distribution, amino acid composition and consequently gel strength. The gel strength of warm-water fish gelatin M04 and M03 was significantly lower than that of cold-water fish gelatin M06 and M08 at low temperatures under the same gelation conditions (temperature and time). Because M08 is derived from cold-water fish, it has the characteristics of faster gelation, higher protein content and wider protein molecular weight distribution at low temperatures. Accordingly, because of the good performance of M08 at low temperatures, such as higher gel strength and better stability, the M08 gelatin was selected for the generation of low-temperature composite salt-soluble protein gels. Following TG enzyme-induced modifications, the strength of M08-derived enzymatic gels was enhanced by a minimum of 200% at low temperatures.
At present, to keep the cold-water fish gelatin in a good state, the reaction of TG enzyme crosslinking fish gelatin solution is carried out at 4 °C, but not at the optimum reaction temperature of TG enzyme, which will affect the activity and crosslinking time of TG enzyme to a certain extent.
We improved the gel strength of fish gelatin through TG enzyme reaction so that it can be used in a more special environment. Therefore, this study broadened the application range of fish gelatin and improved its application potential of fish gelatin.
Author Contributions
Conceptualization and methodology: J.W. and J.X.; validation: J.W. and. M.Z.; data analysis: Y.L.; investigation: H.Y.; writing—original draft preparation: J.W. and J.L. (Jingjing Liu); writing—review and editing: J.W. and J.X.; funding acquisition: J.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Major Science and Technology Innovation Project in Shandong Province (2019JZZY011001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All generated and analyzed data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, J.; Euring, M.; Ostendorf, K.; Zhang, K. Biobased materials for food packaging. J. Bioresour. Bioprod. 2022, 7, 1–13. [Google Scholar] [CrossRef]
- Oyeoka, H.C.; Ewulonu, C.M.; Nwuzor, I.C.; Obele, C.M.; Nwabanne, J.T. Packaging and degradability properties of polyvinyl alcohol/gelatin nanocomposite films filled water hyacinth cellulose nanocrystals. J. Bioresour. Bioprod. 2021, 6, 168–185. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Wang, J. Photo-crosslinkable hydrogel and its biological applications. Chin. Chem. Lett. 2021, 32, 1603–1614. [Google Scholar] [CrossRef]
- Wu, T.; Ding, M.; Shi, C.; Qiao, Y.; Wang, P.; Qiao, R.; Wang, X.; Zhong, J. Resorbable polymer electrospun nanofibers: History, shapes and application for tissue engineering. Chin. Chem. Lett. 2020, 31, 617–625. [Google Scholar] [CrossRef]
- Zhou, P.; Mulvaney, S.J.; Regenstein, J.M. Properties of Alaska pollock skin gelatin: A comparison with tilapia and pork skin gelatins. J. Food Sci. 2006, 71, C313–C321. [Google Scholar] [CrossRef]
- Callow, M.E.; Callow, J.A.; Ista, L.K.; Coleman, S.E.; Nolasco, A.C.; López, G.P. Use of self-assembled monolayers of different wettabilities to study surface selection and primary adhesion processes of green algal (Enteromorpha) zoospores. Appl. Environ. Microbiol. 2000, 66, 3249–3254. [Google Scholar] [CrossRef]
- Hidaka, S.; Liu, S.Y. Effects of gelatins on calcium phosphate precipitation: A possible application for distinguishing bovine bone gelatin from porcine skin gelatin. J. Food Compos. Anal. 2003, 16, 477–483. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, J.; Zhang, Y.; Wang, X.; Lorenzo, J.M.; Zhong, J. Gelatins as emulsifiers for oil-in-water emulsions: Extraction, chemical composition, molecular structure, and molecular modification. Trends Food Sci. Technol. 2020, 106, 113–131. [Google Scholar] [CrossRef]
- Gutierrez, E.; Sung, L.A. Interactions of recombinant mouse erythrocyte transglutaminase with membrane skeletal proteins. J. Membr. Biol. 2007, 219, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Ricotta, M.; Iannuzzi, M.; De Vivo, G.; Gentile, V. Physio-pathological roles of transglutaminase-catalyzed reactions. World J. Biol. Chem. 2010, 1, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, A.L.C.; de Góes-Favoni, S.P. Action of microbial transglutaminase (MTGase) in the modification of food proteins: A review. Food Chem. 2015, 171, 315–322. [Google Scholar] [CrossRef]
- Lesiów, T.; Xiong, Y.L. Gelation properties of poultry myofibrillar proteins and comminuted poultry meat—Effect of protein concentration, pH and muscle type—A review. Fleischwirtsch. Int. 2001, 4, 39–44. [Google Scholar]
- Feng, J.; Xiong, Y.L. Interaction of myofibrillar and preheated soy proteins. J. Food Sci. 2010, 67, 2851–2856. [Google Scholar] [CrossRef]
- Fernandez-Dıaz, M.D.; Montero, P.; Gomez-Guillen, M.C. Gel properties of collagens from skins of cod (Gadus morhua) and hake (Merluccius merluccius) and their modification by the coenhancers magnesium sulphate, glycerol and transglutaminase. Food Chem. 2001, 74, 161–167. [Google Scholar] [CrossRef]
- Aidat, O.; Belkacemi, L.; Belalia, M.; Zainol, M.K.; Barhoum, H.S. Physicochemical, rheological, and textural properties of gelatin extracted from chicken by-products (feet-heads) blend and application. Int. J. Gastron. Food Sci. 2023, 32, 100708. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, R.; Ding, M.; Tao, L.; Liu, L.; Tao, N.; Wang, X.; Zhong, J. Effect of extraction methods on the structural characteristics, functional properties, and emulsion stabilization ability of Tilapia skin gelatins. Food Chem. 2020, 328, 127114. [Google Scholar] [CrossRef]
- Zhang, T.; Ding, M.; Tao, L.; Liu, L.; Tao, N.; Wang, X.; Zhong, J. Octenyl succinic anhydride modification of bovine bone and fish skin gelatins and their application for fish oil-loaded emulsions. Food Hydrocoll. 2020, 108, 106041. [Google Scholar] [CrossRef]
- <GB6783-2013>. Available online: http://down.foodmate.net/standard/sort/3/38978.html (accessed on 20 April 2023).
- Chen, T.; Embree, H.D.; Brown, E.M.; Taylor, M.M.; Payne, G.F. Enzyme-catalyzed gel formation of gelatin and chitosan: Potential for in situ applications. Biomaterials 2003, 24, 2831–2841. [Google Scholar] [CrossRef]
- Yang, L.; Yang, M.; Xu, J.; Nie, Y.; Wu, W.; Zhang, T.; Wang, X.; Zhong, J. Structural and emulsion stabilization comparison of four gelatins from two freshwater and two marine fish skins. Food Chem. 2022, 371, 131129. [Google Scholar] [CrossRef]
- Shi, C.; Bi, C.; Ding, M.; Xie, J.; Xu, C.; Qiao, R.; Wang, X.; Zhong, J. Polymorphism and stability of nanostructures of three types of collagens from bovine flexor tendon, rat tail, and tilapia skin. Food Hydrocoll. 2019, 93, 253–260. [Google Scholar] [CrossRef]
- Karim, A.A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Sha, X.M.; Tu, Z.C.; Liu, W.; Wang, H.; Shi, Y.; Huang, T.; Man, Z.Z. Effect of ammonium sulfate fractional precipitation on gel strength and characteristics of gelatin from bighead carp (Hypophthalmichthys nobilis) scale. Food Hydrocoll. 2014, 36, 173–180. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, H.; Chen, Y.; Jiang, J.; Zhang, W. Testing and modeling of the state-dependent behaviors of rockfill material. Comput. Geotech. 2014, 61, 153–165. [Google Scholar] [CrossRef]
- Haug, I.J.; Draget, K.I.; Smidsrød, O. Physical and rheological properties of fish gelatin compared to mammalian gelatin. Food Hydrocoll. 2004, 18, 203–213. [Google Scholar] [CrossRef]
- Lin, L.; Regenstein, J.M.; Lv, S.; Lu, J.; Jiang, S. An overview of gelatin derived from aquatic animals: Properties and modification. Trends Food Sci. Technol. 2017, 68, 102–112. [Google Scholar] [CrossRef]
- Soua, L.; Koubaa, M.; Barba, F.J.; Fakhfakh, J.; Ghamgui, H.K.; Chaabouni, S.E. Water-soluble Polysaccharides from Ephedra alata stems: Structural characterization, functional properties, and antioxidant activity. Molecules 2020, 25, 2210. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, R.; Ding, M.; Li, L.; Tao, N.; Wang, X.; Zhong, J. Commercial cold-water fish skin gelatin and bovine bone gelatin: Structural, functional, and emulsion stability differences. LWT 2020, 125, 109207. [Google Scholar] [CrossRef]
- Pilipenko, N.; Goncalves, O.H.; Bona, E.; Fernandes, I.P.; Pinto, J.A.; Sorita, G.D.; Leimann, F.V.; Barreiro, M.F. Tailoring swelling of alginate-gelatin hydrogel microspheres by crosslinking with calcium chloride combined with transglutaminase. Carbohydr. Polym. 2019, 223, 115035. [Google Scholar] [CrossRef]
- Liu, F.; Chiou, B.S.; Avena-Bustillos, R.J.; Zhang, Y.; Li, Y.; McHugh, T.H.; Zhong, F. Study of combined effects of glycerol and transglutaminase on properties of gelatin films. Food Hydrocoll. 2017, 65, 1–9. [Google Scholar] [CrossRef]
- Wang, H.Y.; Tian, Q.; Ma, Y.Q.; Wu, Y.; Miao, G.J.; Ma, Y.; Cao, D.-H.; Wang, X.-L.; Lin, C.; Pang, J.; et al. Transpositional reactivation of two LTR retrotransposons in rice-Zizania recombinant inbred lines (RILs). Hereditas 2010, 147, 264–277. [Google Scholar] [CrossRef]
- Xiong, Y.L. Structure-function relationships of muscle proteins. In Food Proteins and Their Applications; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Wang, M.; Chen, C.; Sun, G.; Wang, W.; Fang, H. Effects of curdlan on the color, syneresis, cooking qualities, and textural properties of potato starch noodles. Starch-Stärke 2010, 62, 429–434. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).