1. Introduction
Soil is considered an essential natural resource, the equivalent of air and water, that supports plant growth and provides habitats for microorganisms. Changes in soil properties, productivity and inevitably pollution/stress are the result of industrialization and the long-term use of synthetic fertilizers [
1].
The intensification of agricultural production, based mainly on tillage, a lack of proper crop rotation and the long-term use of fertilizers and plant protection products, has significantly reduced the biodiversity of soils, and thus their productivity. For many years, high productivity was maintained mainly due to intensive mineral fertilization.
Therefore, in the last scenario, the reclamation of contaminated/stressed soils has become a potential challenge. To date, several non-standard technologies based on physical, chemical, and biological approaches have been used to rehabilitate contaminated land.
Among them, phytoremediation additionally supported by microbes is considered to be an economical and more ecological alternative [
2,
3].
In addition, due to changing social awareness and the growing expectations of consumers with respect to the quality of food, it is likely that agriculture will evolve towards sustainable and environmentally friendly technologies, i.e., sustainable, ecological agriculture.
Currently, one of the basic premises for the implementation of sustainable agriculture is ensuring high yields and maintaining high food production without increasing the consumption of fertilizers per hectare.
In recent decades, soil microorganisms have been successfully used to improve the ability of plants to tolerate biotic and abiotic stresses. Microorganisms belonging to the PGPR (Plant Growth-Promoting Rhizobacteria) group in agriculture can provide excellent support in counteracting the destructive effects of abiotic stress, such as excessive salinity and drought, replacing expensive, environmentally harmful inorganic fertilizers [
4,
5,
6]. They influence the proper course of vegetation by direct and indirect stimulation of plant growth. Direct stimulation consists of providing plants with minerals, the synthesis of phytohormones affecting the development of plants (auxins, gibberellins, cytokinins), and reducing the level of ethylene, which adversely affects the roots [
7]. Indirect stimulation consists of the biological control of phytopathogens by producing siderophores and the mitigation of other abiotic stress factors. The use of these bacteria as an inoculant increases the level of proline and sugars, which support plants exposed to drought stress by reducing the loss of water through the leaves.
Scientific studies have documented the abovementioned properties of PGPR, which are particularly strongly manifested by
Bacillus and
Pseudomonas bacteria [
8,
9,
10]. These bacteria used as inoculants not only stimulate the development of plants but also interact with soil bacteria, which usually belong to a different taxonomic group. Thanks to these relations, the size and biodiversity of the bacterial population may increase, which will improve the biochemical properties of soil.
Apart from beneficial organisms, catch crops are also an important element of the environment-friendly agricultural policy of the European Union and an important agrotechnical treatment. When they are applied in the right amount, composition, and at the right time, they not only provide nutrients to plants but also catalyze biochemical changes in soil and thus improve its fertility.
Catch crops are perceived as elements that protect the soil from erosion and regenerate the site, especially when there is a large share of cereals in the crop rotation system [
11]. They mobilize soil nutrients other than nitrogen. They reduce greenhouse gas emissions, and thus reduce the causes of global warming. Legume catch crops additionally ensure biological nitrogen fixation, which significantly affects the nitrogen balance in the entire crop rotation. Among catch crops, undersown cover crops are the most important in the organic farming system because they provide a wide range of benefits (ecosystem services) and act as a living protection of the soil surface. As the initial growth of these plants is slow, they can be sown in plantations where cereals are grown as the main crops. The best conditions for the growth and development of undersown crops are in spring barley plantations as the vegetation period of this cereal is short and the plant has short culms that do not overshadow undersown crops [
12].
As results from scientific reports show, both PGPR applied individually and with catch crops influence the biological, chemical, and physical condition of the soil as well as the yield of crops. The use of biofertilization with PGPR and accompanying plants (undersown cover crops) is an innovative approach offering an alternative to sustainable and organic farming in order to maintain soil fertility, protect the climate and ensure global food security.
Soil microorganisms and their biochemical activity are indicators of soil quality and all processes of organic matter degradation and transformation [
13]. Among various soil enzymes, dehydrogenases and catalase, which are classified as oxidoreductases, play an important role in the oxidation of organic matter in soil. The activity of these enzymes is influenced not only by the content of organic matter in soil, but also by its physicochemical properties, such as moisture, temperature, and pH. They are the best indicators of the activity and population size of soil microorganisms. The indicated enzymes are active only in living cells of microorganisms and clearly react to emerging stress factors and their intensity; hence, they are important indicators of soil quality.
The aim of the study was to assess the influence of the constructed consortium of PGPR (Bacillus subtilis, Bacillus amyloliquefaciens, Pseudomonas fluorescens) and catch crops (undersown red clover alone, undersown Italian ryegrass and a mixture of clover and ryegrass) as well as the influence of the interaction of these factors on the count of selected groups of microorganisms and the biochemical activity of the soil under spring barley cultivated in the organic farming system.
2. Materials and Methods
2.1. The Field Experiment
The field experiment was conducted on an organic farm located in the village of Włazy, near the town of Siedlce, Poland (52.1379376 N, 16.0029782 E). The experiment was conducted on Stagnic Luvisol (according to the WRB). The soil pH was neutral (pH in KCl 6.1). The 0–20 cm soil layer contained 1.05% of organic matter; pH 6.2 (measured in 1 M KCl). At the beginning of the experiment, the content of available P, K, and Mg was as follows: P—8.3 mg·100 g−1 soil, K—12.1 mg·100 g−1 soil, and Mg—4.2 mg·100 g−1 soil.
The experiment was conducted in the years 2019–2021. Eight variants were randomly used each year, each in three repetitions:
1—control variant (without undersown crops, without PGPR);
2—red clover catch crop;
3—red clover and Italian ryegrass catch crop;
4—Italian ryegrass catch crop;
5—control variant (without undersown catch crops) + PGPR;
6—red clover catch crop + PGPR;
7—red clover catch crop + Italian ryegrass + PGPR;
8—Italian ryegrass catch crop + PGPR.
The area of the plot was 20 m2 (4 × 5 m).
Spring barley with undersown cover crops was grown at the site where winter rye had been cultivated. After harvesting the forecrop, post-harvest tillage was applied. In late October a field for the experiment was prepared and goat manure was applied with a spreader over the entire area at a dose of 15 t ha−1. The plot was then ploughed before winter and the field was left until spring. Over the three years of the experiment, the average content of nitrogen, phosphorus, potassium, and magnesium in the goat manure was as follows: 0.54%, 0.28%, 0.87%, and 0.15%.
In spring, in early April, a pre-sowing cultivator was used. Afterwards, the plots were delimited, and spring barley seeds were sown in all of them with a plot seeder to the amount of 160 kg ha−1. On the same day, undersown cover crops were sown in the plots: red clover—18 kg ha−1, a mixture of red clover and Italian ryegrass 9 + 15 kg ha−1, and Italian ryegrass 30 kg ha−1.
Spring barley was sown with a grain drill in rows spaced at 12.5 cm and at a depth of 5–6 cm. The companion crops were then sown in the barley rows, at a depth of 1–2 cm, spaced at 12.5 cm. Spring barley seeds were purchased from the ‘STARY FOLWARK’ organic farm. The red clover and Italian ryegrass seeds also came from organic cultivation and were purchased from the seed company DSV Polska Sp. z o.o. (DSV Poland Ltd., Wągrowiec, Poland). The seeds were certified by AGROBIOTEST Sp. z o.o (AG-ROBIOTEST Ltd., Warsaw, Poland). The PGPR consortium (Bacillus subtilis, Bacillus amyloliquefaciens, Pseudomonas fluorescens) protecting crops from molds was applied twice during the growing season (first term ‘0′ on the day of sowing, second term—BBCH 29–30). The inoculant was applied at a dose of 1 L 200 L−1 water ha−1. The bacteria in the consortium were concentrated at 1012 CFU (colony form units) mL−1.
In the control plots, where only spring barley was sown without undersown cover crops, mechanical care (a weeder harrow after the emergence of plants and a medium harrow at the development of 5–6 leaves) was applied for weed control. Mechanical weed control was not applied in the plots with spring barley and undersown cover crops.
Undersown cover crops initially grow slowly and tolerate shading well. Therefore, they can be sown in plantations where cereals are grown as the main crops. After the application of PGPR the increase in the biomass of undersown cover crops can be expected to be greater than in the plots where the bacteria have not been applied because PGPR act as biostimulants. Undersown cover crops are harvested in August and September, after the harvest of the main crop. Therefore, in October, their biomass can be used as green manure.
2.2. Trait and Ability of PGPR
The PGPR consortium used in the field experiment consisted of the following strains of endophytic bacteria: Bacillus subtilis, Bacillus amyloliquefaciens, and Pseudomonas fluorescens.
The bacterial species included in the consortium came from the collection of the Department of Soil Science and Microbiology, Poznań University of Life Sciences, Poland. They were isolated from substrates under crops, on selective media:
Bacillus subtilis—on
Bacillus Chromo Select agar,
Bacillus amyloliquefaciens—on starch agar [
14], and
Pseudomonas fluorescens—on King B agar [
15]. The species were genetically identified on the basis of a fragment of the 16S rRNA gene sequence.
The research enabled a determination of the ability of the endophytic isolates to (i) produce indole-3-acetic acid (IAA) (by spectrophotometry), (ii) produce antifungal metabolites on PDA (Potato Dextrose Agar) in interaction with the
Fusarium,
Alternaria, and
Botrytis genera, (iii) activate phosphorus on a substrate dissolving calcium phosphate Ca
3 (PO4)
2 [
14], and (iv) fix molecular nitrogen by PCR, where the polF/polR primers were used to amplify a specific fragment of the
nifH gene.
Bacillus subtilis bacteria exhibited all of the aforementioned metabolic properties. Bacillus amyloliquefaciens bacteria exhibited the first (i) and second (ii) properties, whereas Pseudomonas fluorescens exhibited the first (i), third (iii) and fourth (iv) properties.
2.2.1. Interactions between Bacteria Used in Consortia
In order to select bacterial strains for the consortium and to test their compatibility, the ring method was used to determine synergism between selected bacterial strains [
16]. The experiment was based on six variants (five replicates in each):
Bacillus subtilis on Bacillus amyloliquefaciens;
Bacillus amyloliquefaciens on Bacillus subtilis;
Bacillus subtilis on Pseudomonas fluorescens;
Pseudomonas fluorescens on Bacillus subtilis;
Bacillus amyloliquefaciens on Pseudomonas fluorescens;
Pseudomonas fluorescens on Bacillus amyloliquefaciens.
The analysis of interactions between the bacterial strains did not reveal any antagonism, as evidenced by the absence of a halo around the wells for all the bacteria. Therefore, these bacteria were used to create a consortium for the phytostimulation of spring barley.
2.2.2. Preparation of Liquid Modifier and Its Application in the Field
Endophytic bacterial isolates were stored in test tubes on agar slants, in a refrigerator, at 8 °C. Before the field experiment, the isolates had been passaged several times onto prepared agar slants with an appropriate medium for a specific bacterial species so that the strains selected for the study could regain their vitality and metabolic activity. Then, for each date of barley inoculation, liquid cultures of selected inoculates were prepared in 100 mL flasks (five replicates). Three-day-old starter cultures of bacteria were suspended by adding 5 mL of saline to each tube diagonally. Next, the microbial cultures were scraped with a loop. The resulting 0.5 mL of bacterial suspension was inoculated with 100 mL of liquid NB medium (Nutrient Broth). The obtained liquid cultures were incubated at 28 °C on a shaker at 70 rpm, for 48 h. The count of microorganisms in 1 mL of the obtained liquid culture was 1012 cells. After the incubation, the cultures of each bacterial species were pooled and concentrated by centrifugation at 4000 rpm.
The prepared bacterial consortium was applied with a sprayer twice throughout the growing season of spring barley:
Date 1—on the day of sowing, applied into the soil;
Date 2—the beginning of the stem formation phase (BBCH 29–30).
The concentrated consortium of 300 mL of the obtained inoculum was dissolved in 60 L of water (according to the applied commercial consortia of 1 L of the preparation to 200 L of water−1 ha−1). The density of bacterial cells in the resulting suspension was assessed with the direct method under a microscope in a Thoma cell counting chamber—108 cells in 1 mL. The inoculates were applied on a warm but cloudy day (18–25 °C).
2.3. Weather Conditions
The weather conditions varied during the experiment (
Table 1). The highest average air temperature occurred in 2021; the total rainfall was 155.3 mm–48.9 mm lower than the long-term average. The lowest rainfall was in 2019. The average monthly temperature was 1.1 °C higher than the long-term average. The highest rainfall was recorded in 2020 and the average temperature in the growing season was 0.9 °C higher than the long-term average.
2.4. Sampling and Measurements
One representative soil sample was collected before the experiment for chemical soil analyses, in order to determine soil pH and the content of basic macro- and micronutrients in the soil. Soil samples for microbial and biochemical analyses were collected from each plot at three dates corresponding to the successive stages of spring barley development: 1—at the spring barley tillering stage (BBCH 18–21), 2—at the grain filling stage (BBCH 74–75), and 3—after the harvesting. The samples were collected with an Egner’s stick. In autumn, after the entire experiment had been set up, 15 soil samples were collected diagonally from a depth of 0–20 cm and thoroughly mixed. Next, one composite sample of about 0.5 kg was collected and placed in a marked plastic bag. The same method was applied to collect soil samples during the growing season of spring barley, although they were taken from the middle of each plot. On collection, the soil samples for microbial analyses were immediately placed in a refrigerator and stored at 2–5 °C. They were then placed in a portable cool box and transported to a laboratory at the Poznań University of Life Sciences.
2.5. Microbiological Analyses
2.5.1. Count of Microorganisms
Soil samples were collected from under the plants, at a depth of 0–20 cm. The count of microorganisms was measured by serial dilutions, on appropriate agars (in five replicates). The average count of colonies was converted to the dry weight of soil. The following values were measured:
- -
The total bacterial count on the ready Merck standard count agar after 5 days of incubation, at 25 °C;
- -
Molds—on Martin’s medium [
17] after 5 days of incubation at 24 °C;
- -
Actinobacteria—on Pochon agar after five days of culturing at 25 °C [
18].
2.5.2. Enzyme Assay
The following methods were applied to assay the enzyme activity in various cultivation variants:
- -
Dehydrogenases (EC 1.1.1.)—the colorimetric method [
19], with 1% TTC (triphenyl tetrazolium chloride) as a substrate, after a 24-h incubation at 30 °C, at a wavelength of 485 nm, expressed as μmol TPF∙g
−1 dm soil·24 h
−1;
- -
Acid phosphatase (EC 3.1.3.2)—the spectrophotometry method [
20], with sodium p-nitrophenylphosphate as a substrate, after 1 hour incubation at 37 °C, at a wavelength of 400 nm, with a Novospac spectrophotometer, expressed as μmol PNP·g
−1 dm soli·h
−1;
- -
Catalase (EC 1.11.1.6)—permanganometry according to Johnson and Temple [
21], with 0.3% H
2O
2 as a substrate, after 20 min incubation at room temperature (approx. 20 °C), titration with 0.02 M KMnO
4 to a light-pink color, expressed as μmol H
2O
2 g
−1 dm soil·min
−1.
2.5.3. BIF Measurement
Various synthetic indicators expressing the relationship between microbial activity and soil fertility may be useful to compare the impact of biological and chemical factors on soil biological activity. One such indicator is BIF (Biological Index Fertility). The value of this indicator, depending on the achieved fertility, ranges from 1 to 40. The higher the value, the better the quality of the soil environment. DHA (dehydrogenase activity) and CAT (catalase activity) were used to calculate the biological index of fertility (BIF) according to the formula: (DHA + kCAT)/2, where k is the coefficient of proportionality and equals 0.01 [
22].
2.6. Statistical Analyses
The R and Statistica 12.0 software package (StatSoft Inc., Poland, Kraków) was used for all statistical analyses. The effect of the experimental factor, i.e., catch crops/inoculation and the time (development phase of barley—BBCH scale) of individual analyses on the soil microbial activity (microbial count and enzyme activity) was investigated with a three-way ANOVA (Equation (1)). Owing to the large variation of weather conditions in individual years of the experiment and due to the fact that the year was a variable describing data samples in years selected from a longer period, a two-way ANOVA was applied for the microbiological parameters analyzed in a given year (Equation (2)).
where:
µ—overall average,
αi—the effect of random factor
year at level
i (
i = 1, 2, 3),
βj—the effect of constant factor
Term at level
j (=1, 2, 3),
γk—the effect of constant factor
inoculation at level
j (=1, 2, …, 8), with the relevant interactions of these factors and errors
eijkl or
eikl.
A heat map with the
heatmaply function on the R computing platform was proposed for the graphic presentation of transformed data. In order to compare and group data of different orders, the data were transformed with the normalization transformation (0–1) in the
heatmaply package on the R platform [
23].
The catch crop and/or inoculation data were represented by colors. Thanks to cluster analysis, it was possible to group the parameters referring to the counts of physiological groups of microorganisms, the enzyme activity with various catch crops, as well as the effect of the inoculation with PGPR in individual years of the experiment (2019–2020) in terms of all parameters, so as to achieve the greatest association between the variants of the experiment within one group and the smallest association between the groups. Ward’s agglomerative hierarchical clustering and the Euclidean distance were used to obtain grouping tree diagrams.
3. Results
The year was a random factor in our study (Equation (1)). Due to the variability of weather conditions in particular years of field experiments, which affected the operation of the tested factors, e.g., used catch crops and inoculations with bacteria belonging to PGPR, the result of soil microbiological activity for each year is presented separately.
In order to investigate the influence of the catch crops alone as well as the catch crops together with the PGPR consortium on the microbiological condition of the soil, the total count of heterotrophic bacteria, actinobacteria and mold; the level of activity of dehydrogenases, acid phosphatase, and catalase; and the biological index of fertility were measured.
The two-way analysis of variance (Equation (2)) revealed that the applied catch crops alone and the catch crops applied together with the PGPR as well as the date of the study (BBCH phase of barley growth) had a highly significant effect (α = 0.001) on the counts of selected groups of soil microorganisms and the level of biochemical activity of soil under organically grown spring barley (
Table 2). There were also statistically significant interactions between the applied catch crop and/or the catch crop with the applied bacterial consortium and the count of the groups of soil microorganisms at individual dates of analyses.
Although there were statistically significant differences, the values of the soil microbial activity parameters were averaged for individual dates of the analyses. As a result, it was possible to observe trends in the influence of the applied catch crops or catch crops with PGPR inoculants on the microbiological parameters in the organic cultivation of spring barley.
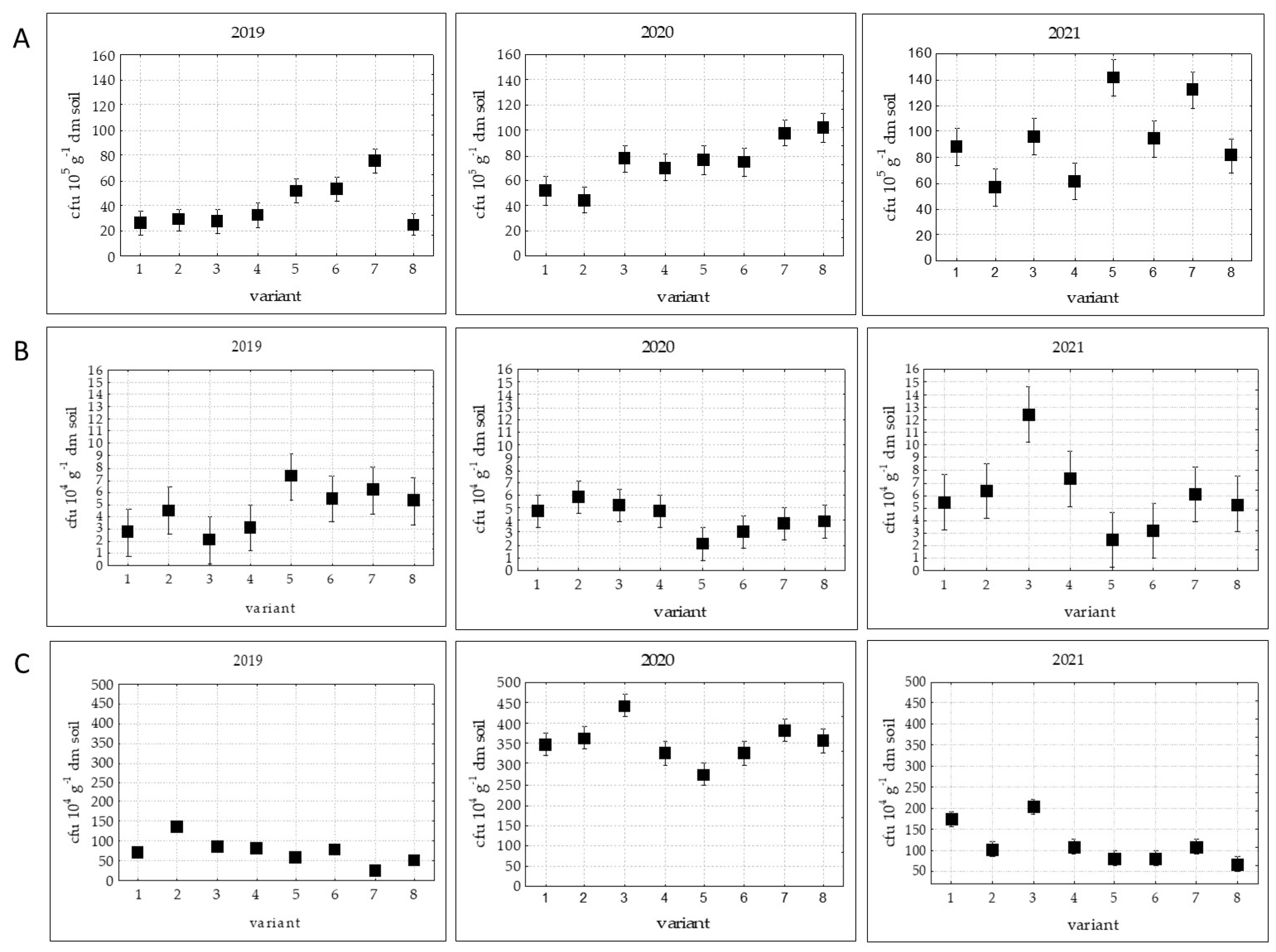
The dynamics of variation in the total count of heterotrophic bacteria in the soil under spring barley cultivated between 2019 and 2021 are shown in
Figure 1A.
Although the count of this group of microorganisms varied considerably in individual years of the experiment, it always increased significantly after the application of PGPR (Bacillus subtilis, Bacillus amyloliquefaciens, Pseudomonas fluorescens), i.e., in variants 5–8. In 2019, the greatest count of these microorganisms was observed after the application of the PGPR together with the catch crops (red clover and Italian ryegrass—variant 7). In 2021, the greatest count was noted after the application of the PGPR only (variant 5), whereas in 2020—after the application of the PGPR together with the Italian ryegrass catch crop (variant 8)—the counts of these microorganisms in the aforementioned variants were, respectively, 197%, 67%, and 97% greater than the count in the control variant.
Like the total bacterial count, the count of molds in the soil under the spring barley also varied and depended on the applied treatments. In the first year of the experiment their count was greater in the variants in which both the catch crops and PGPR had been applied (
Figure 1B). In 2020 and 2021, there were significantly greater counts of molds than in the control variant when only the catch crops had been applied. On the other hand, when the PGPR inoculants were applied alone (variant 5), they reduced the count of molds significantly (
Figure 1B).
The chart shows the counts of actinobacteria in individual years of the experiment. In 2020, their count was high—it ranged from 350 to 450 cfu 10
4 g
−1 dm soil (
Figure 1C). In 2019, their count was lower—it ranged from 50 to 137 cfu 10
4 g
−1 dm soil. During the three years of the experiment the count of actinobacteria in variants 5–8 was significantly lower than in the control variant as well as in the variants where the PGPR consortium had been applied alone or in combination with the catch crops. In 2019, the lowest count of actinobacteria was observed in variant 7, where the catch crops of red clover and Italian ryegrass had been combined with the bacterial consortium. In 2020, the lowest count of actinobacteria was found in variant 5, where the bacterial inoculant had been applied alone. In 2021, the lowest count of actinobacteria was noted in variant 8, where the catch crop of ryegrass had been combined with the PGPR (
Figure 1C).
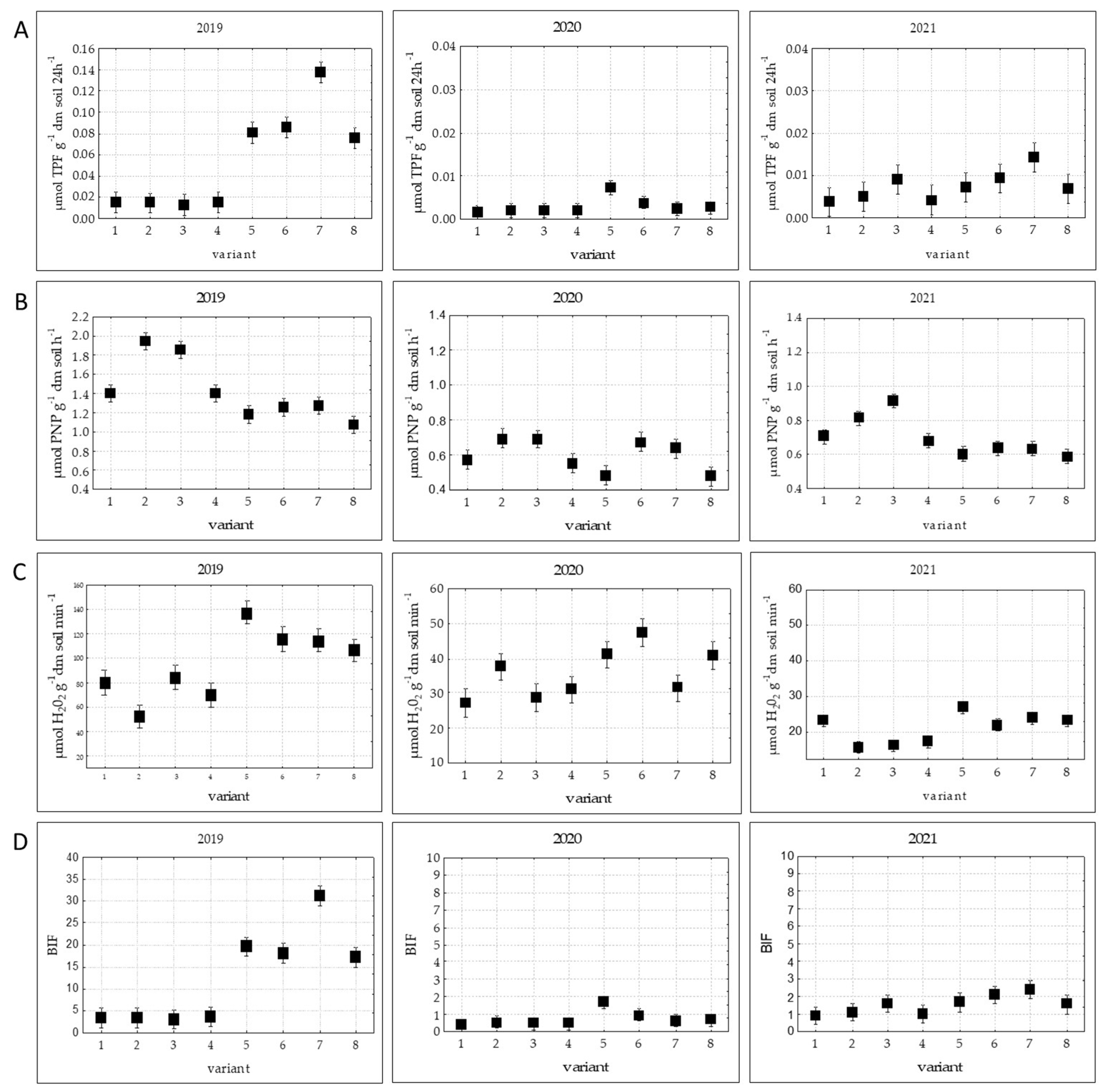
The analysis of the biochemical activity of the soil (the activity of dehydrogenases, acid phosphatase, and catalase) and the biological index of fertility (BIF) showed that these biological parameters were significantly higher in 2019 than in 2020 and 2021 (
Figure 2).
In 2019, the activity of dehydrogenases in the soil under barley ranged from 0.0143 µmol TPF g
−1 dm soil∙24 h
−1 to 0.1378 µmol TPF g
−1 dm soil∙24 h
−1. In 2020, the activity of this enzyme was much lower—it ranged from 0.0014 to 0.0072 µmol TPF g
−1 dm soil∙24 h
−1 (
Figure 2A). The analysis of the dehydrogenase activity showed that in 2019 and 2021 it tended to increase in the variants where the catch crops had been applied simultaneously with the PGPR. In those years the highest metabolic activity of the enzyme was observed in variant 7, where red clover and Italian ryegrass had been applied in combination with the PGPR.
The acid phosphatase activity in the soil under spring barley was similar to the dehydrogenase activity. The activity of this enzyme dropped significantly after the application of the bacterial consortium alone, which consisted of the PGPR only (variant 5), and after the application of these bacteria together with the catch crop of Italian ryegrass (
Figure 2B).
Like the dehydrogenases, catalase exhibited significantly higher activity in the variants in which the barley had been inoculated with the PGPR consortium and in which the undersown crops had been applied simultaneously with the PGPR consortium (variants 5–8). The highest catalase activity was observed in 2019 and 2021 in the variant in which only the PGPR consortium had been applied (
Figure 2C).
The results of the biological index fertility (BIF) were compatible with the activity of the oxidoreductases, i.e., dehydrogenase and catalase. In 2019 and 2021, the highest value of the index was observed in variant 7, where undersown red clover and Italian ryegrass had been applied together with the PGPR. In 2020, the highest value of the index was noted in variant 5, where only the bacterial consortium had been applied (
Figure 2D). In comparison with the control variant, the value of the index in these variants was ten times greater in 2019 and three times greater in 2021.
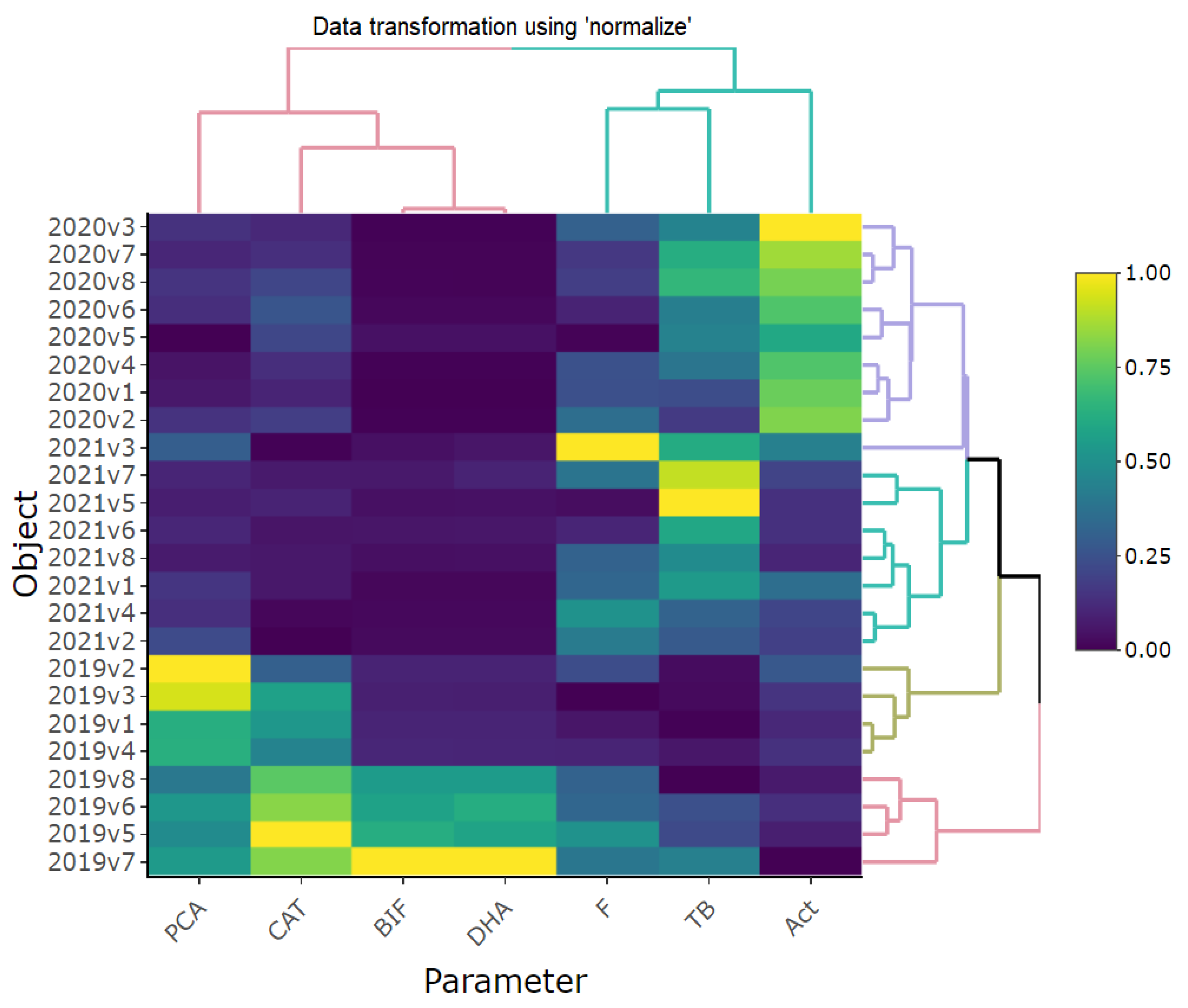
The three-year research on the influence of various catch crops and PGPR, as well as the simultaneous application of PGPR and catch crops, was illustrated with heat maps and dendrograms (
Figure 3). These illustrations enabled the comparison of all soil biological parameters with each other in all variants of the experiment conducted within three years (2019–2021). The analysis showed that the values of these parameters in 2019 differed independently from the values in 2020 and 2021. In 2020 and 2021, the values of such parameters as F, TB, and Act were greater than in 2019. On the other hand, the values of PCA, CAT, BIF, and DHA in 2021 and 2020 were much lower than in 2019. Moreover, the analysis of the heat map revealed a clear division of the variants into two groups in 2019. The first group included the variants in which only the catch crops had been applied, whereas the other group included the variants in which the catch crops had been used together with the PGPR consortium.
It is worth noting that despite the differences between the parameters tested in the experiment, the BIF and the DHA were the traits characterized by the most similar trends regardless of the year and variant.
4. Discussion
Variation in the count of microbial groups is a major indicator of microbial activity in the pedosphere. Qualitative and quantitative changes in microorganisms may result from the method of cultivation, fertilization, chemical crop protection, and sometimes from certain crop inoculation treatments [
24].
The catch crops of undersown red clover and red clover with Italian ryegrass as well as the catch crops in interaction with the prepared PGPR consortium significantly increased or decreased the counts of the groups of microorganisms in the soil under spring barley. The catch crops affected the total count of heterotrophic bacteria, molds, and actinobacteria. The effect depended on the date of the analyses and the year of the experiment.
The analysis of the results of the experiment in individual years (2019–2021) revealed a certain trend. When the bacterial consortium was applied alone (variant 5), the count of actinobacteria (Act) decreased to a lower level than in the control variant, where it was significantly higher. According to the reference publications, the dominance of actinobacteria over bacteria indicates deteriorating soil moisture. The authors of publications describing the influence of various soil cultivation methods on the counts of selected physiological groups of bacteria indicated that the count of actinobacteria increased, e.g., in plough cultivation, where the moisture conditions were worse than in simplified cultivation [
11,
25]. As results from the observations made during the research on the effect of PGPR suggest, these microorganisms might mitigate the water shortage stress in the soil. It is likely that the inoculation of spring barley with the prepared PGPR consortium in variants 5–8 of our experiment reduced the effects of water shortage in 2019. The consortium used in the experiment may have increased the surface area, and thus the root weight of the crops and the amount of root exudates, due to the metabolic properties of the microorganisms contained in it (production of auxins, cytokinins, gibberellins, siderophores, etc.). The metabolic activity of the applied bacteria, especially endophytes, improved the buffer properties of the soil because it stimulated the growth of the other physiological groups of microorganisms under analysis, such as the total count of heterotrophic bacteria. Vardharajul et al. [
26] observed that the inoculation of plants with bacteria of the
Bacillus genus could compensate for the effects of drought. These bacteria improve plant development because they stimulate the production of proline, amino acids, and soluble sugars. In consequence, plants can better absorb water and nutrients from the soil, which mitigates the level of soil salinity caused by water shortage. The accumulation of proline, which acts as an intercellular substance for osmotic regulation during drought stress, has been extensively documented in the scientific literature [
27]. Garcia et al. [
28] found that microorganisms perceive changes in the salt concentration in the soil caused by water deficiency as osmotic changes, which significantly affect changes in the total count of heterotrophic bacteria. It is worth noting that in our study, in 2019, when the rainfall was low, there were high counts of heterotrophic bacteria in the variants where the microbial consortium had been applied alone or in combination with the undersown crops.
In our experiment, the count of actinobacteria also dropped in the variants with undersown catch crops and PGPR in 2019, as well as, in some cases, after the application of catch crops only in 2020. However, researchers are divided in their opinions on the effect of catch crops on the soil moisture level. They found that catch crops had different effects on water relations in the soil. The effects depended on abiotic environmental factors (mainly soil and rainfall), cereal species, the type of catch crop, and the plants sown in it [
29]. Many authors say that catch crops generally cause the soil to dry out when the rainfall is low, whereas in wet seasons they have no effect, or only slightly improve the soil moisture [
30]. This situation was also observed in our study.
Papp et al. [
31] showed that the cultivation of cover crops affects soil microorganism environments that are critically important for maintaining soil functions and ecosystem sustainability as they are involved in the cycling of nutrients and the turn-over of organic matter. It has been reported that cover crops can alter the dynamics of soil bacterial and fungal communities [
32], stimulate beneficial microorganisms [
33], and suppress soil borne pathogens [
34]. The cover-plants produce phytoncides that affect soil microorganisms and limit the occurrence of the pathogen. The literature data indicate [
32,
33,
34] that the catch crops used in the cultivation stimulate the development of antagonistic, autochthonic bacteria
Pseudomonas spp. and
Bacillus spp. as well as saprotrophic fungi, which have an antagonistic effect on, for example, Trichoderma spp. They have a positive effect on the healthiness of root plants by considerably decreasing the infection of roots of the seedlings and later older plants by
Alternaria alternate,
Fusarium oxysporum,
F. culmorum,
Thanatephorus cucumeris, and
Sclerotinia sclerotiorum. PGPR play a similar role.
The stimulation of the development of microorganisms is accompanied by the stimulation of the biochemical activity of the soil. The diversified methods of cover crop cultivation and PGPR use caused noticeable changes in the activity of soil enzymes, which reflected environmental disturbances affecting both the soil and plants [
35]. Dehydrogenase (DHA), catalase (CAT), and acid phosphatase (PAC) are the soil enzymes whose activities are most often studied.
Each habitat is characterized by a specific system of microbiological transformation. Soil bioactivity, which is an inseparable element of this structure, is determined by the transformations of compounds and energy occurring in this habitat. It is most visibly manifested by enzyme activity, which is determined by various factors, such as: soil type, vegetation cover, depth of soil profile, soil pH and temperature, weather conditions, organic matter content and agricultural technology [
36]. Soil enzymes are the first to respond to changes occurring in the soil environment. The level of their expression is closely related to the content of organic matter in the soil, its physical properties, and the activity of microorganisms [
37].
Among various soil enzymes, dehydrogenases, which are classified as oxidoreductases, play an important role in the oxidation of organic matter [
38]. The activity of these enzymes is influenced not only by the content of organic matter in soil but also by its physicochemical properties, such as moisture, temperature, and pH. Research has shown that a change in soil oxygenation significantly modifies the activity of dehydrogenases. In our experiment, in 2019 and 2021, when the rainfall was lower than the long-term average, the highest dehydrogenase activity was noted after the application of PGPR and the use of catch crops together with the prepared consortium.
Singh et al. [
39] also reported that a change in the soil microclimate caused by different residues of the leguminous cover crops influenced the metabolism of microorganisms, thus significantly contributing to the catabolic activity of soil dehydrogenase. According to Morris et al. [
40], the mulch of stubble catch crops retains significant amounts of rainwater, and the soil moisture level affects the activity of this enzyme.
The activity of catalase, which is also an oxidoreductase, was similar to the dehydrogenase activity. The activity of this enzyme can also be used for the monitoring of soil quality. Catalase can be found in the cells of all soil microorganisms that use oxygen for respiration (aerobes, facultative anaerobes). Xu et al. [
41] found a significant relationship between catalase activity and the oxygenation of soil modified by compaction and moisture. In our experiment, the activity of this enzyme increased after the application of the undersown cover crop of red clover, and, above all, after the application of the bacterial consortium. During the growing season of spring barley, the stressful abiotic factors (low rainfall) were mitigated by biofertilization with PGPR and by the application of the bacteria in combination with the undersown cover crops.
Acid phosphatase is another important enzyme indicating soil quality and the availability of phosphorus. When the availability of soil phosphorus is low, the exudation of acid phosphatase (PAC) increases. However, this enzyme is exuded not only by communities of soil microorganisms but also by the plant itself. In consequence, the amount of acid phosphatase in the pedon increases significantly, which is often negatively correlated with the count of microorganisms. In our study, the soil samples collected from the organic spring barley plantation were characterized by lower acid phosphatase activity in the variants where only PGPR as well as PGPR together with undersown ryegrass as a catch crop had been applied. This means that the active biological factor increased the content of assimilable phosphorus in the soil. Such dependencies were presented in the studies conducted by Niewiadomska [
11,
42] and Majchrzak [
43]. In another study, Lemanowicz and Koper [
44] also observed the higher catabolic activity of this enzyme in an experimental treatment without phosphorus fertilization. As results from research on some PGPR of the
Bacillus,
Enterobacter, and
Pseudomonas genera show, these microorganisms stimulate plant growth by dissolving soil phosphates. This effect is regulated by two main mechanisms: the lowering of soil pH through the production of organic acids and the mineralization of organic phosphate by acid phosphatases and phytases [
45].
The results from the observations made in our study show that the biological parameters of the soil under the organic spring barley plantation were stimulated by the PGPR consortium. These bacteria can affect plants both directly and indirectly. Their direct effect consists in making phosphorus and potassium available to plants and in the production of phytohormones. The indirect effect of the PGPR consists in the production of siderophores and hydrolytic enzymes and the protection and promotion of the growth of crops [
46,
47,
48]. The PGPR also stimulate the indigenous soil microbiome.
5. Conclusions
Modern agriculture faces new challenges. Molecular and ecological actions are combined to increase yields and reduce environmental impact. Soil quality and plant growth and resistance can be improved by intercropping and the use of PGPR bacteria. Biological solutions are key strategies to improve crop yields and adapt to environmental changes as well as carbon and energy inputs.
The management of crop residues can offer a way to make use of all the services they provide, such as the effect of green manure or improving the physical properties of soils in agroecological systems, while reducing their potential negative impact on the water balance. Our study highlighted the effect of different cover crop management practices and the use of PGPR microorganisms on microbial activity compared to bare soil. The applied catch crops themselves increased the biochemical activity of the soil (activity of dehydrogenases, catalase and soil fertility index) compared to the bare soil (control). However, higher values of the tested soil microbiological parameters (DHA, CAT, BIF, total number of heterotrophic bacteria) were only obtained after using the group of PGPR bacteria and/or the PGPR consortium with a catch crop, compared to the use of a catch crop alone.
To evaluate this management practice as good, studies in different soil and climate conditions, combining field experiments and simulation modeling, are required, as the problem is also site-specific due to interactions between soil type and local climatic conditions.