Study of a Novel High-Frequency Ultrasound-Guided Integrated System for Varicose Veins Ultrasound Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Design of the Integrated Probe
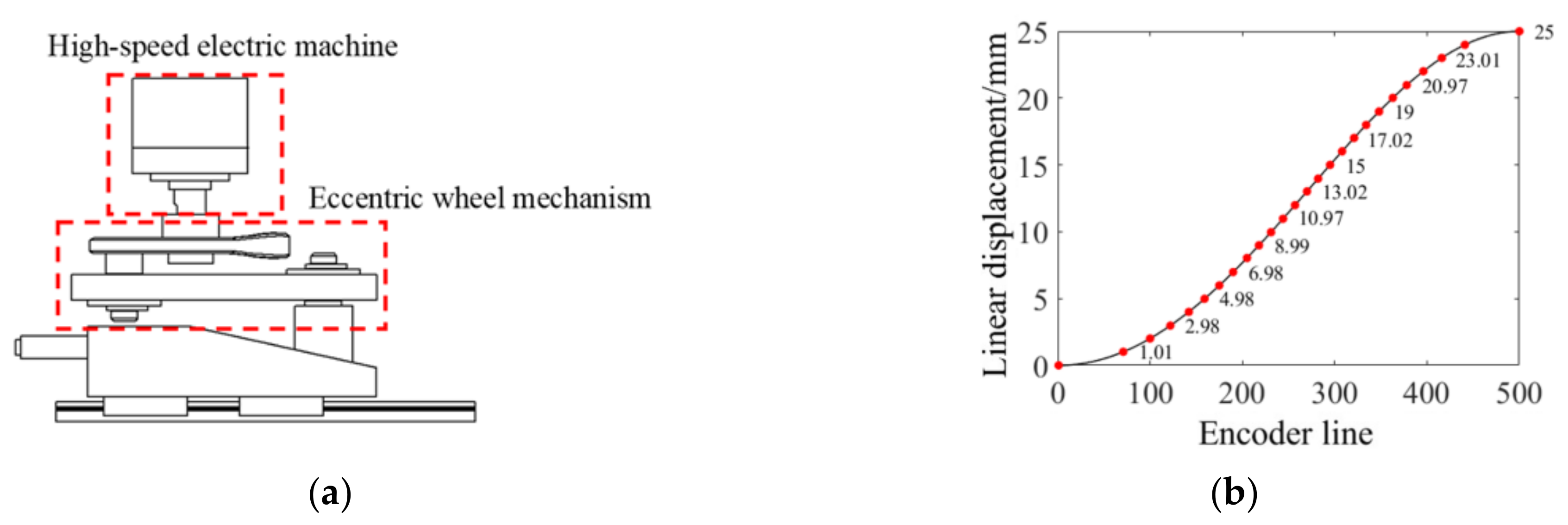
2.2. High-Speed Scanning Positioning Structure
2.3. Verification Experiment of the Treatment System
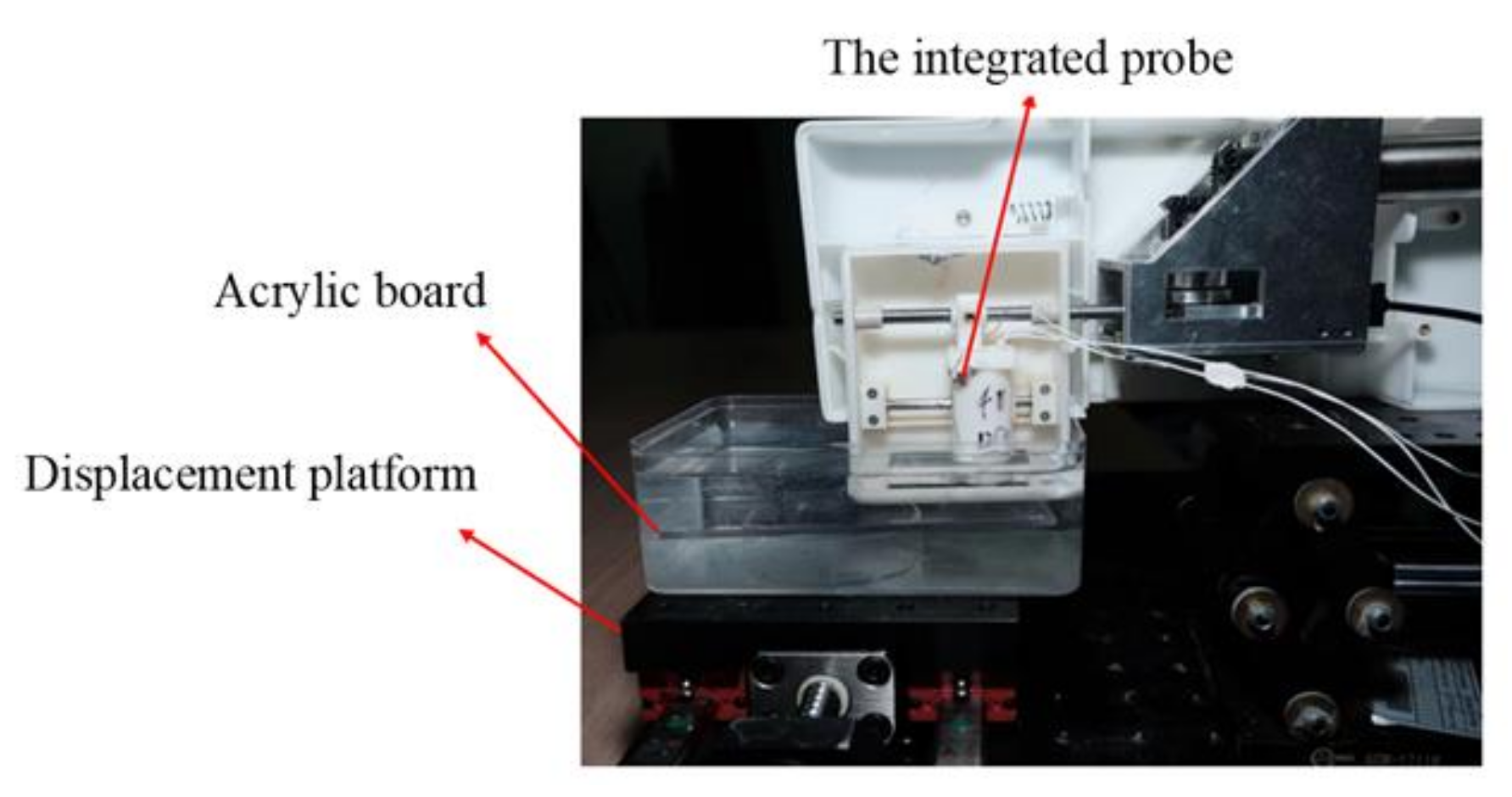
2.3.1. In Vitro Experiment
2.3.2. In Vivo Experiment
2.4. Ex Vivo Tissue Experiment
3. Results and Discussion
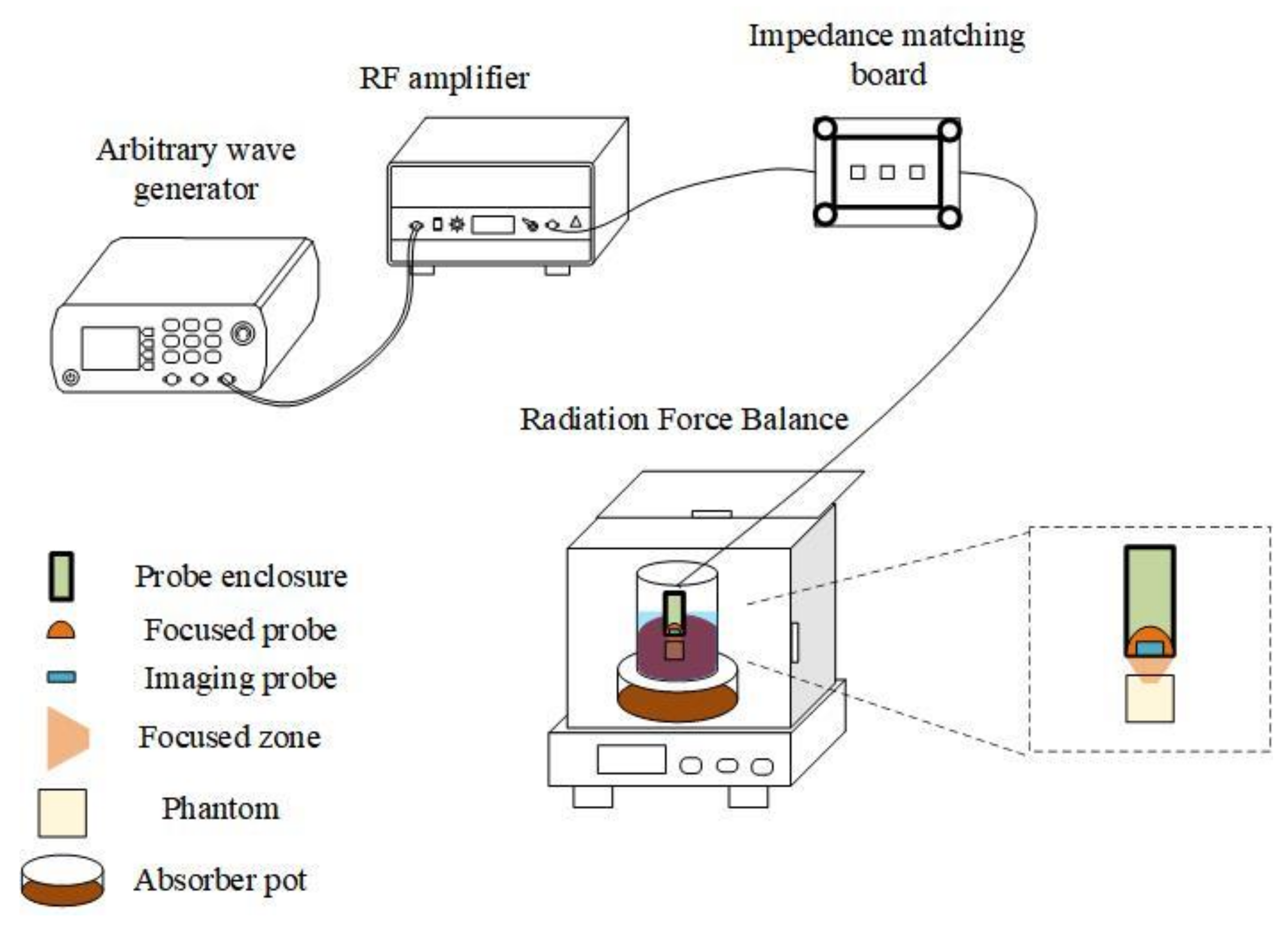
3.1. Results of the Treatment System Validation Experiments
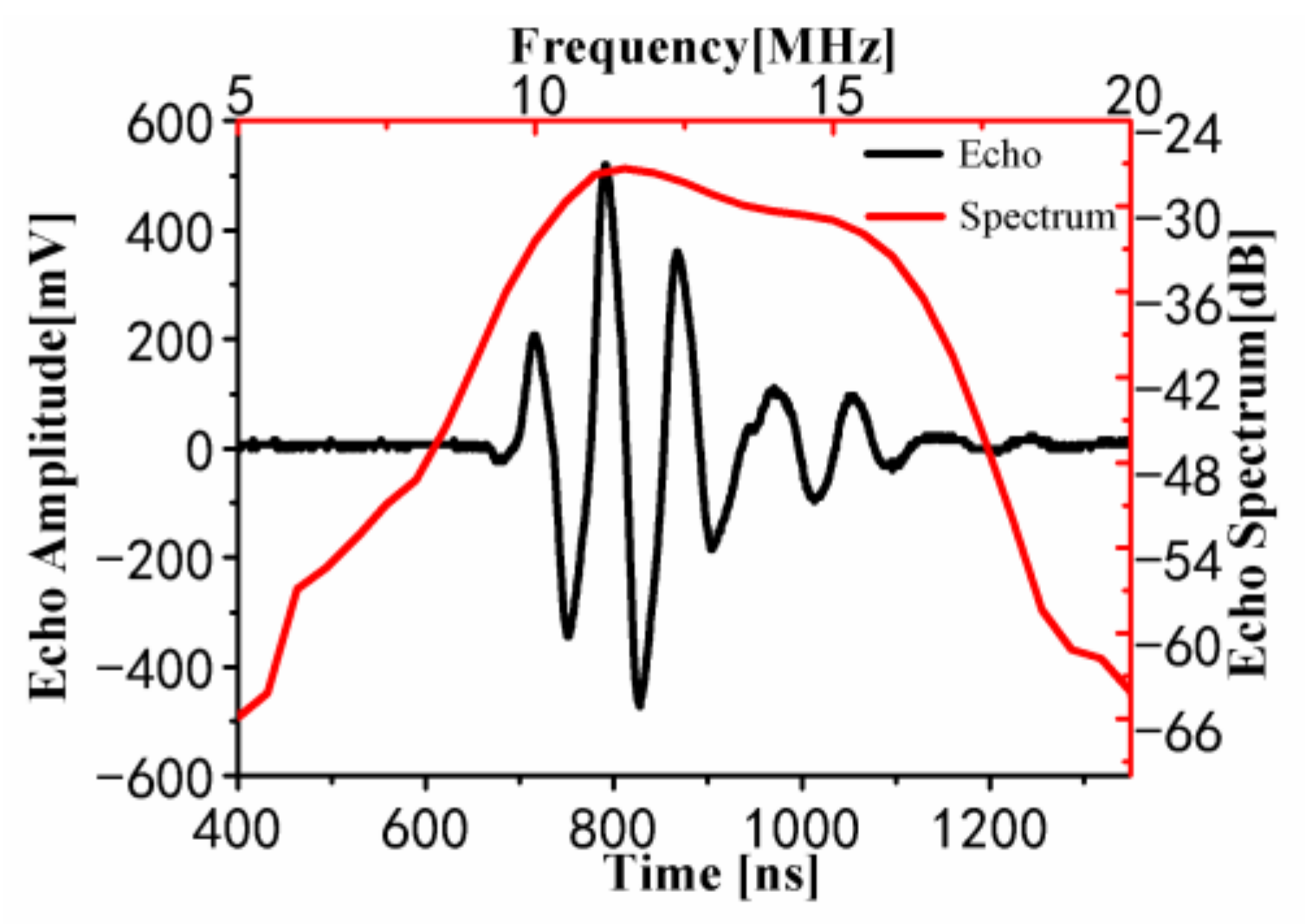
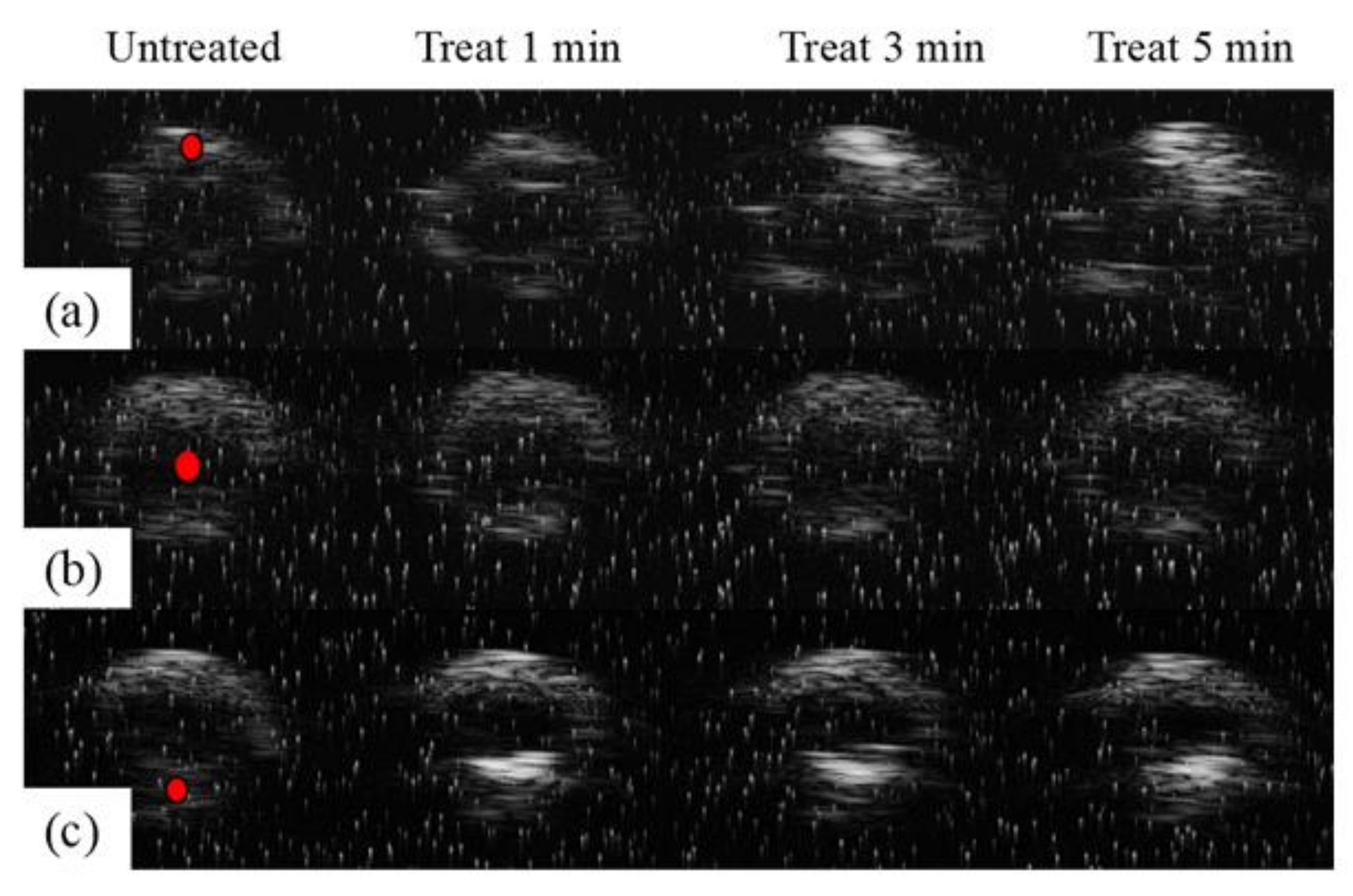
3.1.1. Results of In Vitro Experiment
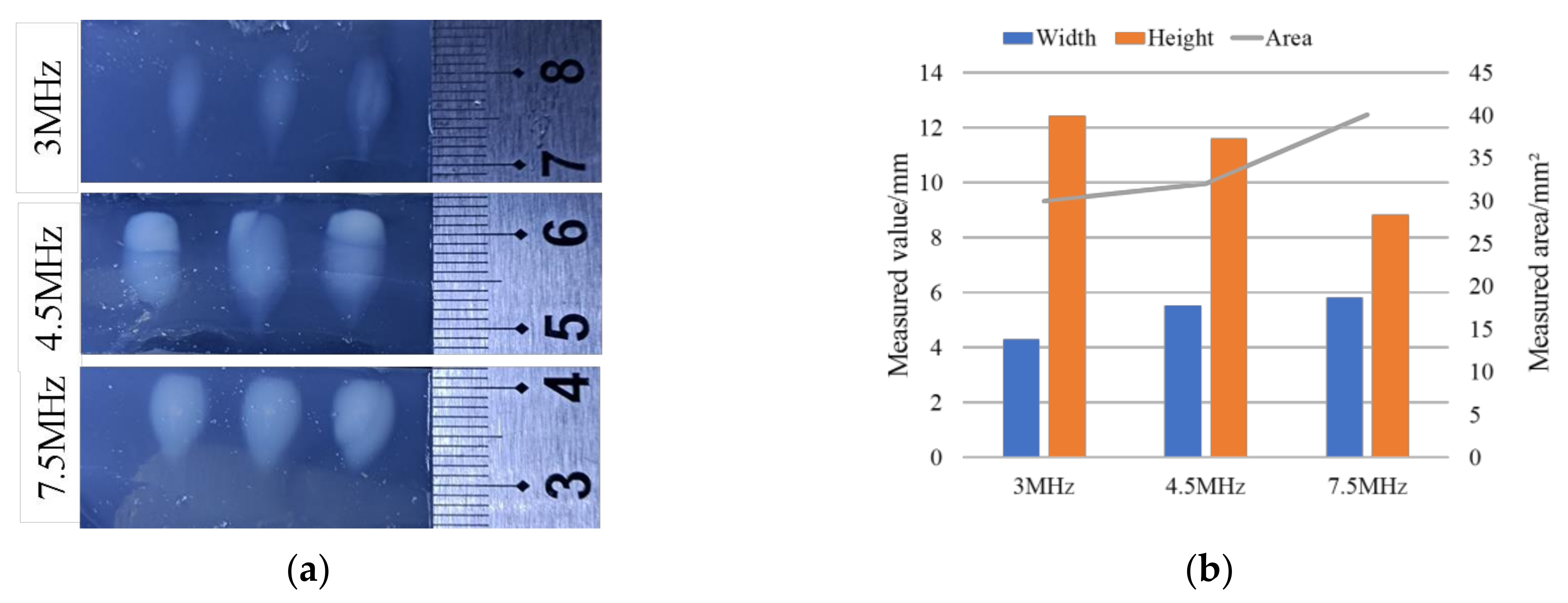
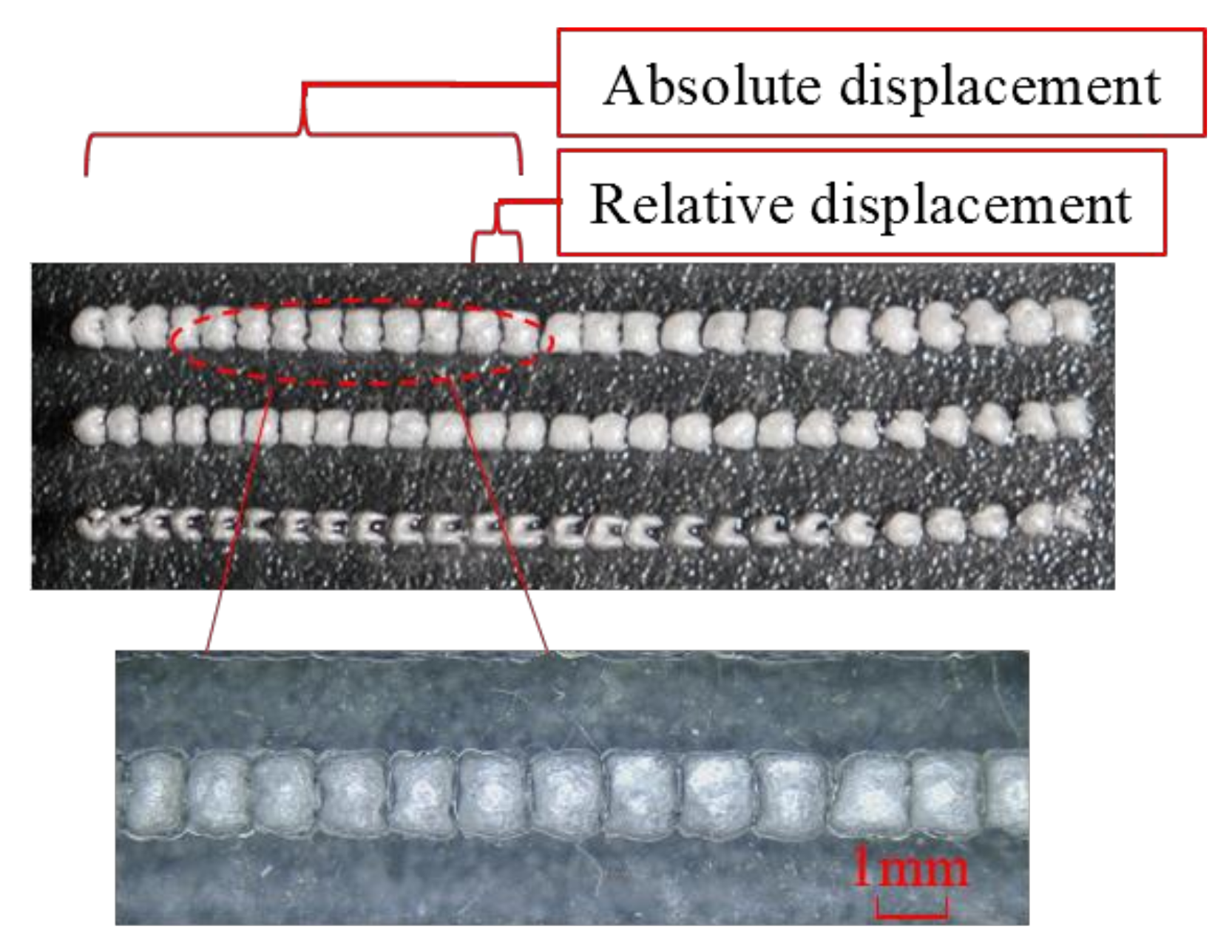
3.1.2. In Vitro Validation Experiments for Treatment Accuracy
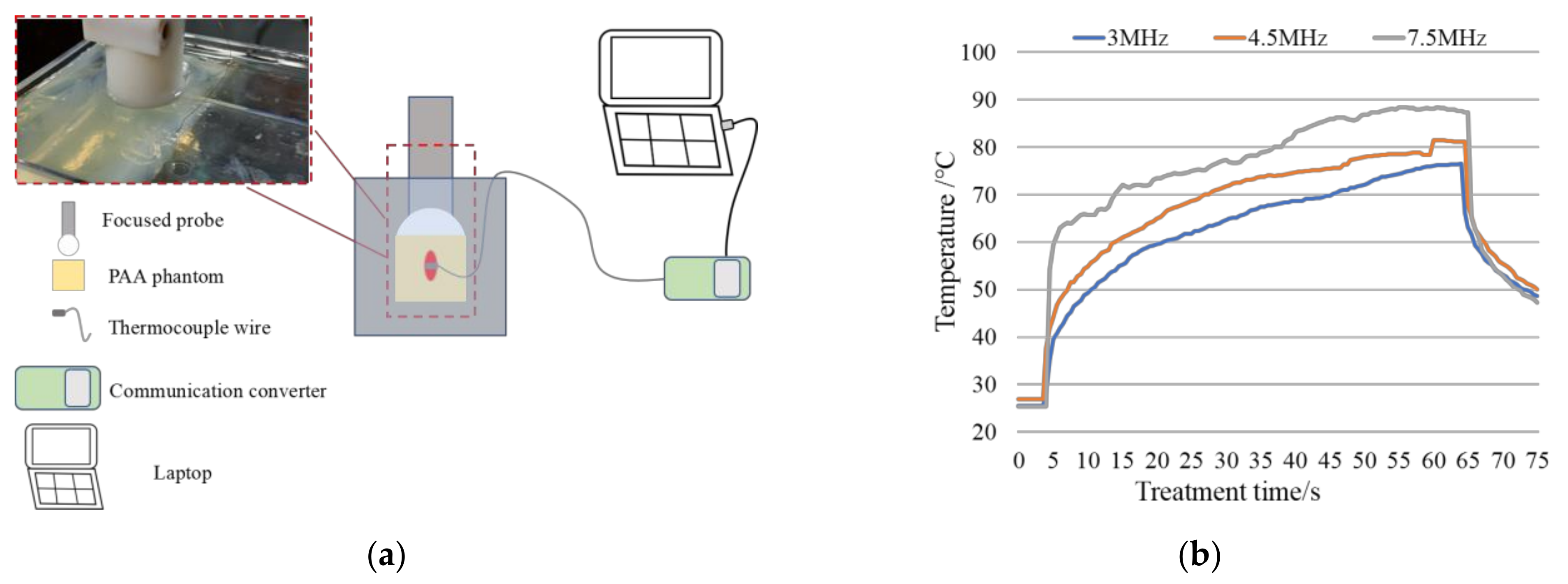
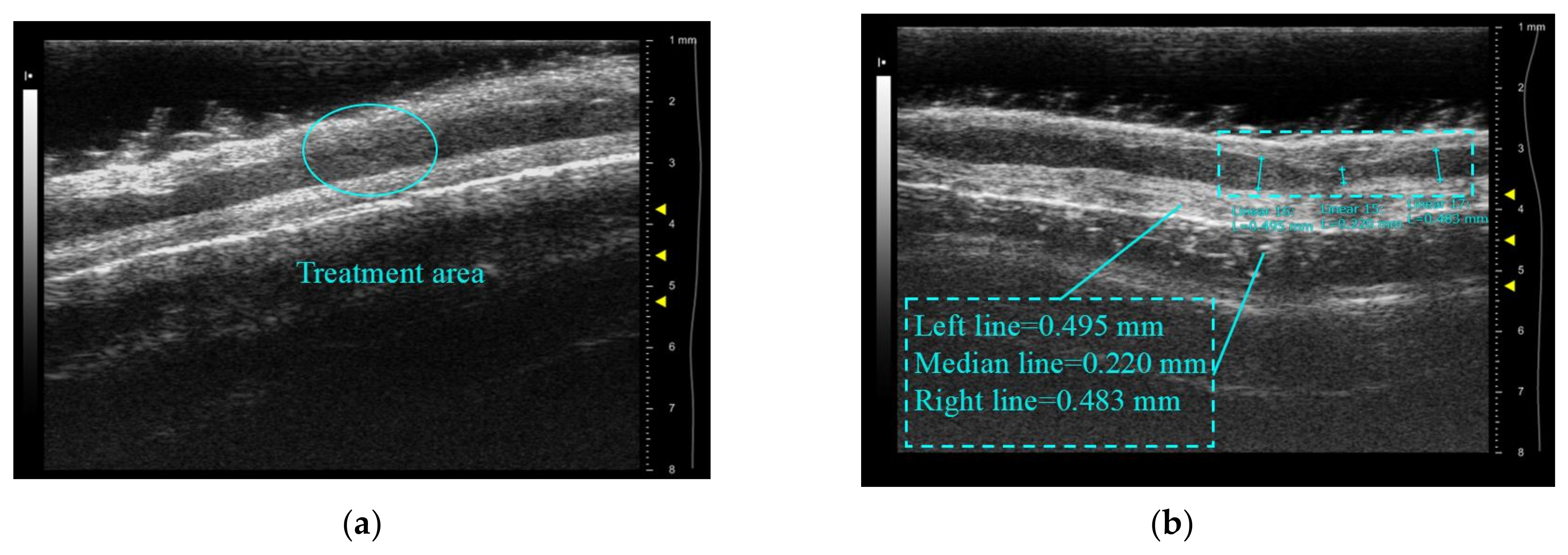
3.2. Results of Porcine Arteries Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leopardi, D.; Hoggan, B.L.; Fitridge, R.A.; Woodruff, P.W.H.; Maddern, G.J. Systematic Review of Treatments for Varicose Veins. Ann. Vasc. Surg. 2009, 23, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Heit, J.A.; Silverstein, M.D.; Mohr, D.N.; Petterson, T.M.; O’Fallon, W.M.; Melton, L.J. Risk factors for deep vein thrombosis and pulmonary embolism—A population-based case-control study. Arch. Intern. Med. 2000, 160, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Y.; Qiu, T.; Bu, X.Q.; Li, X.T.; Liang, G.Z.; Zhang, H.; Niu, L.Y.; Zhao, H.; Zhang, F.X. A national survey on management of varicose veins in China. J. Vasc. Surg.-Venous Lymphat. Disord. 2018, 6, 338–346.e1. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Y.; Zhang, X.J.; Li, J.H.; Huang, M. Comparison of high ligation and stripping of the great saphenous vein combined with foam sclerotherapy versus conventional surgery for the treatment of superficial venous varicosities of the lower extremity. Int. J. Clin. Exp. Med. 2015, 8, 7843–7848. [Google Scholar]
- Wittens, C.; Davies, A.H.; Bwkgaard, N.; Broholm, R.; Cavezzi, A.; Chastanet, S.; De Wolf, M.; Eggen, C.; Giannoukas, A.; Gohel, M.; et al. Editor’s Choice—Management of Chronic Venous Disease Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2015, 49, 678–737. [Google Scholar] [CrossRef]
- Bozkurt, A.K.; Lawaetz, M.; Danielsson, G.; Lazaris, A.M.; Pavlovic, M.; Olariu, S.; Rasmussen, L. European College of Phlebology guideline for truncal ablation. Phlebology 2020, 35, 73–83. [Google Scholar] [CrossRef]
- Dillavou, E.D.; Harlander-Locke, M.; Labropoulos, N.; Elias, S.; Ozsvath, K.J. Current state of the treatment of perforating veins. J. Vasc. Surg.-Venous Lymphat. Disord. 2016, 4, 131–135. [Google Scholar] [CrossRef]
- Levy, J.; Khakoo, S.; Barton, R.; Vicary, R. Fatal injection sclerotherapy of a bleeding peptic-ulcer. Lancet 1991, 337, 504. [Google Scholar] [CrossRef]
- Schuman, B.M. The systemic complications of sclerotherapy of esophageal-varices. Gastrointest. Endosc. 1985, 31, 348–349. [Google Scholar] [CrossRef]
- Strussky, L.P.; Asabaev, A.S.; Vakhidov, A.V. Endoscopic sclerotherapy in treatment of portal-hypertension. Klin. Meditsina 1991, 69, 87–89. [Google Scholar]
- Siribumrungwong, B.; Noorit, P.; Wilasrusmee, C.; Leelahavarong, P.; Thakkinstian, A.; Teerawattananon, Y. Cost-utility analysis of great saphenous vein ablation with radiofrequency, foam and surgery in the emerging health-care setting of Thailand. Phlebology 2016, 31, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Barnat, N.; Grisey, A.; Gerold, B.; Yon, S.; Anquez, J.; Aubry, J.F. Efficacy and safety assessment of an ultrasound-based thermal treatment of varicose veins in a sheep model. Int. J. Hyperth. 2020, 37, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Barnat, N.; Grisey, A.; Gerold, B.; Yon, S.; Anquez, J.; Aubry, J.F. Vein wall shrinkage induced by thermal coagulation with high-intensity-focused ultrasound: Numerical modeling and in vivo experiments in sheep. Int. J. Hyperth. 2020, 37, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
- Obermayer, A.; Aubry, J.F.; Barnat, N. Extracorporeal Treatment with High Intensity Focused Ultrasound of an Incompetent Perforating Vein in a Patient with Active Venous Ulcers. Ejves Vasc. Forum 2021, 50, 1–5. [Google Scholar] [CrossRef]
- Barnat, N.; Grisey, A.; Lecuelle, B.; Anquez, J.; Gerold, B.; Yon, S.; Aubry, J.F. Noninvasive vascular occlusion with HIFU for venous insufficiency treatment: Preclinical feasibility experience in rabbits. Phys. Med. Biol. 2019, 64, 025003. [Google Scholar] [CrossRef]
- Strunk, H.M.; Lutzow, C.; Henseler, J.; Mucke, M.; Rauch, M.; Marx, C.; Schild, H.H.; Marinova, M. Mesenteric Vessel Patency Following HIFU Therapy in Patients with Locally Invasive Pancreatic Cancer. Ultraschall Med. 2018, 39, 650–658. [Google Scholar] [CrossRef]
- Whiteley, M.S. High intensity focused ultrasound (HIFU) for the treatment of varicose veins and venous leg ulcers—A new non-invasive procedure and a potentially disruptive technology. Curr. Med. Res. Opin. 2020, 36, 509–512. [Google Scholar] [CrossRef]
- Charrel, T.; Greillier, B. HIFU Varicose Vein Treatment Using Veinsound Device Feasibility Study on Sheep. FocUS Arch. 2021. [Google Scholar] [CrossRef]
- Henderson, P.W.; Lewis, G.K.; Shaikh, N.; Sohn, A.; Weinstein, A.L.; Olbricht, W.L.; Spector, J.A. A portable high-intensity focused ultrasound device for noninvasive venous ablation. J. Vasc. Surg. 2010, 51, 707–711. [Google Scholar] [CrossRef]
- Hwang, J.H.; Zhou, Y.F.; Warren, C.; Brayman, A.A.; Crum, L.A. Targeted Venous Occlusion Using Pulsed High-Intensity Focused Ultrasound. IEEE Trans. Biomed. Eng. 2010, 57, 37–40. [Google Scholar] [CrossRef]
- Senoo, N.; Suzuki, J.; Yoshinaka, K.; Deguchi, J.; Takagi, S.; Miyata, T.; Matsumoto, Y. Development of Noninvasive Vascular Occlusion Method with HIFU. In Proceedings of the 9th International Symposium on Therapeutic Ultrasound, Aix en Provence, France, 24–26 September 2009; p. 153. [Google Scholar]
- Mitragotri, S. Innovation—Healing sound: The use of ultrasound in drug delivery and other therapeutic applications. Nat. Rev. Drug Discov. 2005, 4, 255–260. [Google Scholar] [CrossRef]
- Hynynen, K.; Jolesz, F.A. Demonstration of potential noninvasive ultrasound brain therapy through an intact skull. Ultrasound Med. Biol. 1998, 24, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Agah, R.; Pearce, J.A.; Welch, A.J.; Motamedi, M. Rate process model for arterial tissue thermal damage: Implications on vessel photocoagulation. Lasers Surg. Med. 1994, 15, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, R.; Sasaki, K.; Ishikawa, T.; Suzuki, M.; Umemura, S.-I.; Kushima, M.; Okai, T. Arterial blood flow occlusion by high intensity focused ultrasound and histologic evaluation of its effect on arteries and surrounding tissues. J. Med. Ultrason. 2002, 29, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Takegami, K.; Kaneko, Y.; Watanabe, T.; Maruyama, T.; Matsumoto, Y.; Nagawa, H. Polyacrylamide gel containing egg white as new model for irradiation experiments using focused ultrasound. Ultrasound Med. Biol. 2004, 30, 1419–1422. [Google Scholar] [CrossRef]
- Hoerig, C.L.; Serrone, J.C.; Burgess, M.T.; Zuccarello, M.; Mast, T.D. Prediction and suppression of HIFU-induced vessel rupture using passive cavitation detection in an ex vivo model. J. Ther. Ultrasound 2014, 2, 14. [Google Scholar] [CrossRef]
- Vaezy, S.; Zderic, V. Hemorrhage control using high intensity focused ultrasound. Int. J. Hyperth. 2007, 23, 203–211. [Google Scholar] [CrossRef]
- Yue, Y.; Chen, W.; Wang, Z. The impact of microbubbles-mediated intermitten HIFU on bloodflow in femoral artery of rabbit. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi = J. Biomed. Eng. 2010, 27, 58–61. [Google Scholar]
- Jiang, C.P. Vessel phantom fabrication using rapid prototyping technique for investigating thermal dosage profile in HIFU surgery. Rapid Prototyp. J. 2010, 16, 417–423. [Google Scholar] [CrossRef]
- Pichardo, S.; Milleret, R.; Curiel, L.; Pichot, O.; Chapelon, J.Y. In vitro experimental study on the treatment of superficial venous insufficiency with high-intensity focused ultrasound. Ultrasound Med. Biol. 2006, 32, 883–891. [Google Scholar] [CrossRef]
- Yang, W.J.; Zhou, Y.F. Effect of pulse repetition frequency of high-intensity focused ultrasound on in vitro thrombolysis. Ultrason. Sonochem. 2017, 35, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Croucher, A.; Whiteley, M.; Abu-Bakr, O. One-year results of treatment of incompetent truncal veins and incompetent perforators using high intensity focused ultrasound (HIFU). Phlebology 2022, 37, 23–24. [Google Scholar]
- Ahmed, T.; Portnoy, R.; Chachati, G.; Chait, J.; Alsheekh, A.; Kibrik, P.; Marks, N.; Hingorani, A.; Ascher, E. Correlation of body mass index with recanalization risk after endovenous thermal ablation. J. Vasc. Surg. Venous Lymphat. Disord. 2022, 10, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.R.; Loson, V.; Coria, A.; Fosser, C.M.; Dotta, M.; Katsini, R.; Pared, C.; Moreno, H.B.; Martínez, H. Secondary Ablation of Recanalized Saphenous Vein after Endovenous Thermal Ablation. Ann. Vasc. Surg. 2020, 68, 172–178. [Google Scholar] [CrossRef]
- Mitton, D.; Thornton, M.; Beard, J. Retrograde stripping of recurrent varicose veins. Eur. J. Vasc. Endovasc. Surg. 2001, 22, 90–91. [Google Scholar] [CrossRef]
- Gao, R.D.; Qian, S.Y.; Wang, H.H.; Liu, Y.S.; Ren, S.Y. Strategies and challenges in treatment of varicose veins and venous insufficiency. World J. Clin. Cases 2022, 10, 5946–5956. [Google Scholar] [CrossRef]






| Probe Configurations | 30 Vpp 1 Excitation Voltage | 40 Vpp Excitation Voltage | 55 Vpp Excitation Voltage | 70 Vpp Excitation Voltage | 80 Vpp Excitation Voltage |
|---|---|---|---|---|---|
| Complete probe with air backing/W | 2.19 2 | 4.88 | 10.12 | 15.15 | 24.07 |
| Hole digged with water backing/W | 0.38 | 0.55 | 1.1 | 1.92 | 2.99 |
| Hole digged with air backing/W | 0.86 | 1.81 | 3.28 | 5.33 | 8.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, J.; Huang, W.; Li, X.; Li, J.; Xu, Y.; Jiao, Y.; Cui, Y. Study of a Novel High-Frequency Ultrasound-Guided Integrated System for Varicose Veins Ultrasound Therapy. Appl. Sci. 2023, 13, 5069. https://doi.org/10.3390/app13085069
Xie J, Huang W, Li X, Li J, Xu Y, Jiao Y, Cui Y. Study of a Novel High-Frequency Ultrasound-Guided Integrated System for Varicose Veins Ultrasound Therapy. Applied Sciences. 2023; 13(8):5069. https://doi.org/10.3390/app13085069
Chicago/Turabian StyleXie, Jing, Wenchang Huang, Xinze Li, Jiaqi Li, Yiwen Xu, Yang Jiao, and Yaoyao Cui. 2023. "Study of a Novel High-Frequency Ultrasound-Guided Integrated System for Varicose Veins Ultrasound Therapy" Applied Sciences 13, no. 8: 5069. https://doi.org/10.3390/app13085069
APA StyleXie, J., Huang, W., Li, X., Li, J., Xu, Y., Jiao, Y., & Cui, Y. (2023). Study of a Novel High-Frequency Ultrasound-Guided Integrated System for Varicose Veins Ultrasound Therapy. Applied Sciences, 13(8), 5069. https://doi.org/10.3390/app13085069







