Forest Fragmentation and Landscape Connectivity Changes in Ecuadorian Mangroves: Some Hope for the Future?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Compilation and Acquisition of Cartographic Information
2.3. Calculation of Deforestation and Fragmentation
2.4. Fragmentation Patterns
2.5. Connectivity Analysis
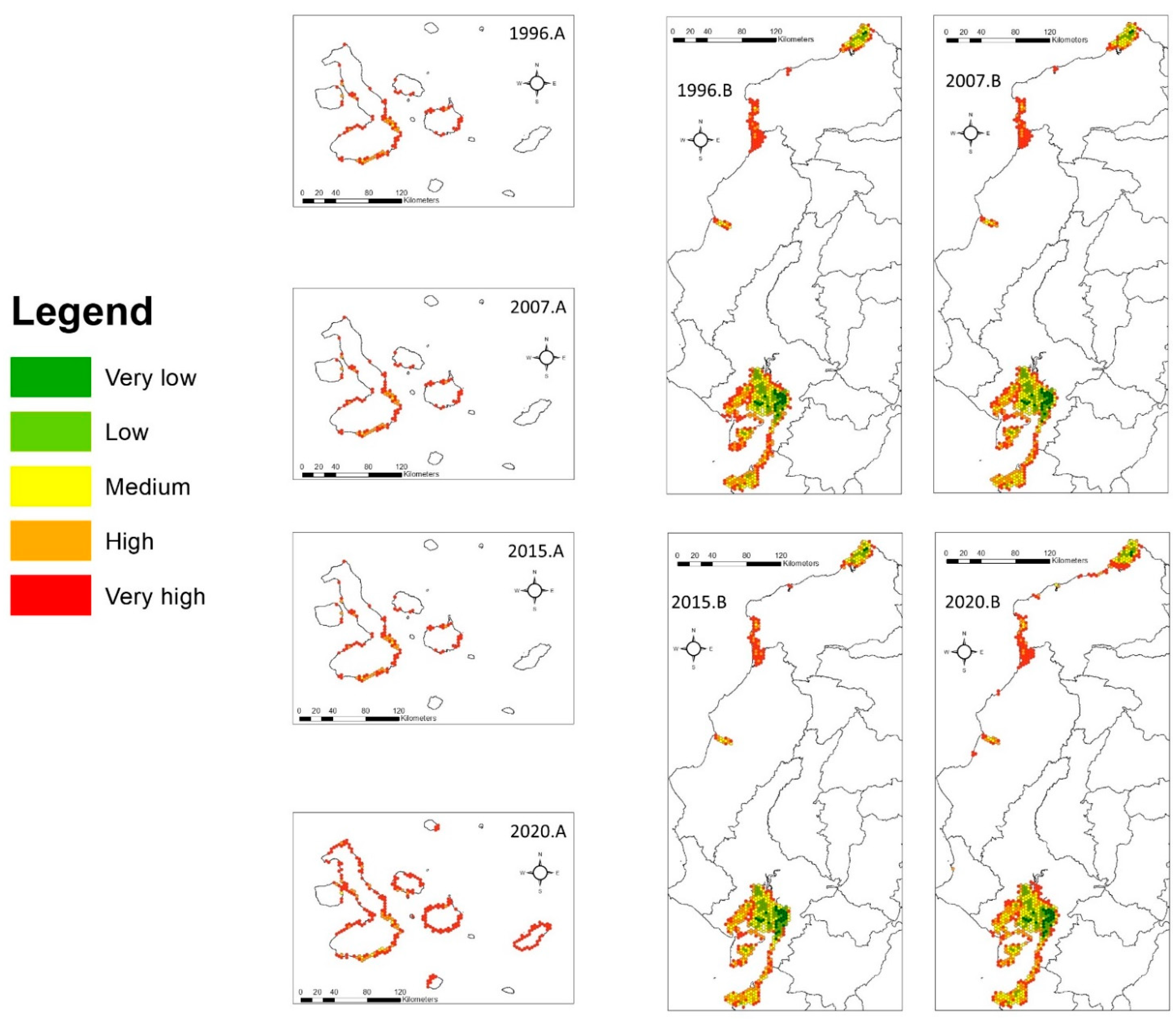
3. Results
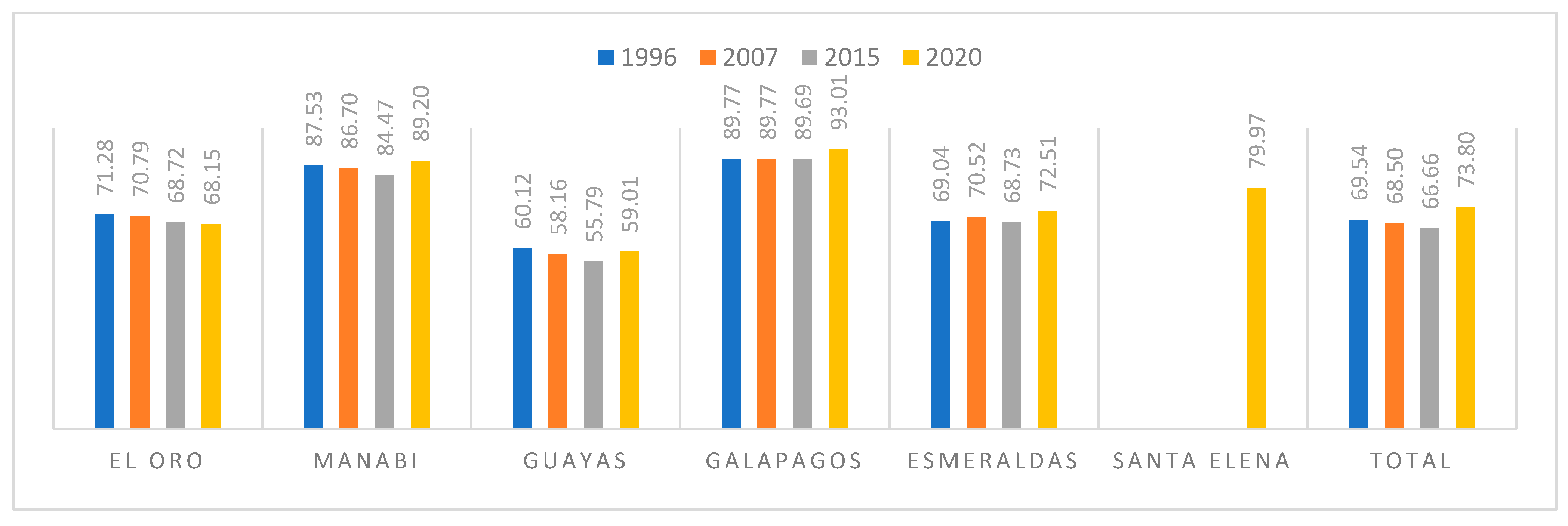
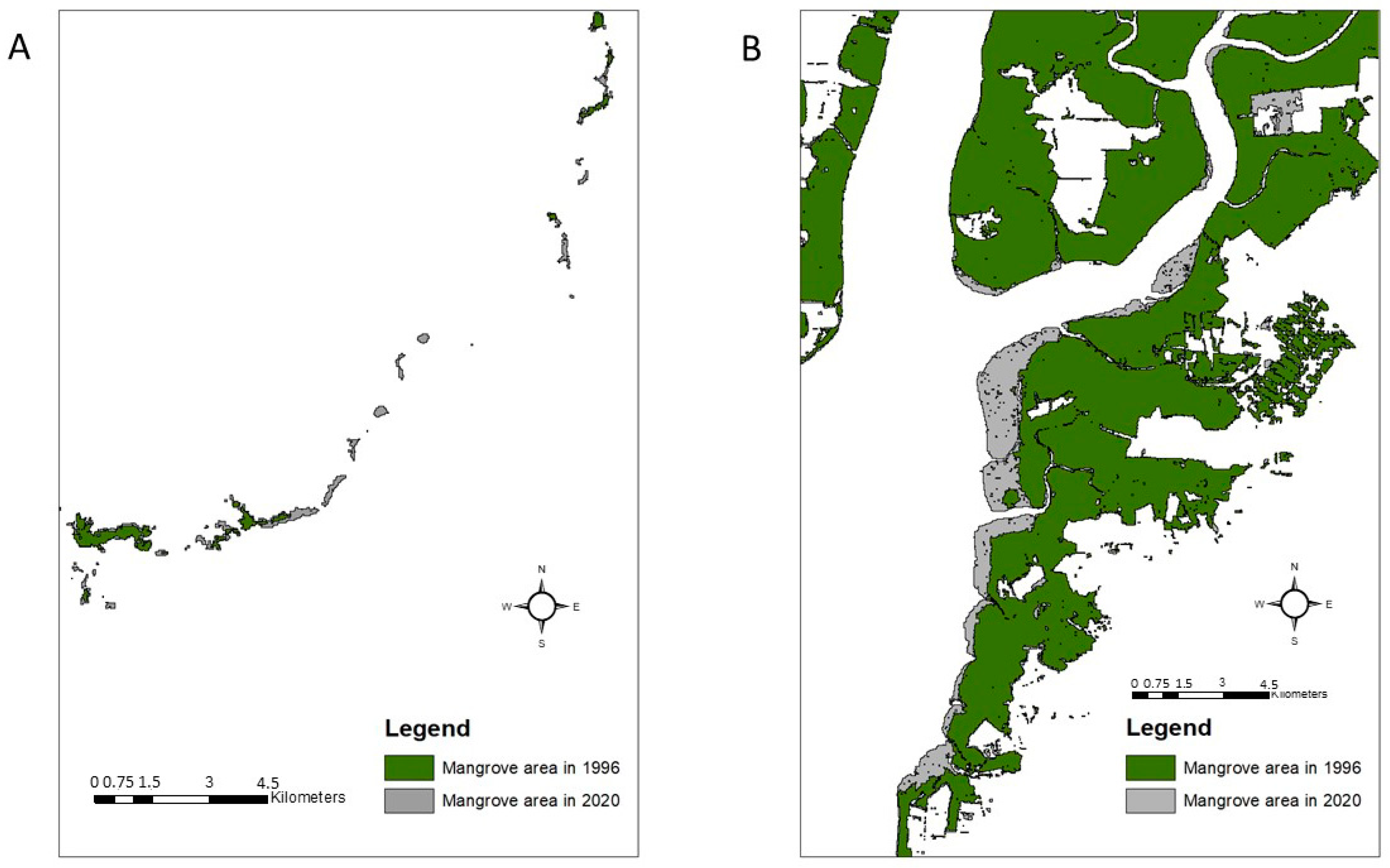
3.1. Deforestation and Fragmentation
3.2. Fragmentation Patterns
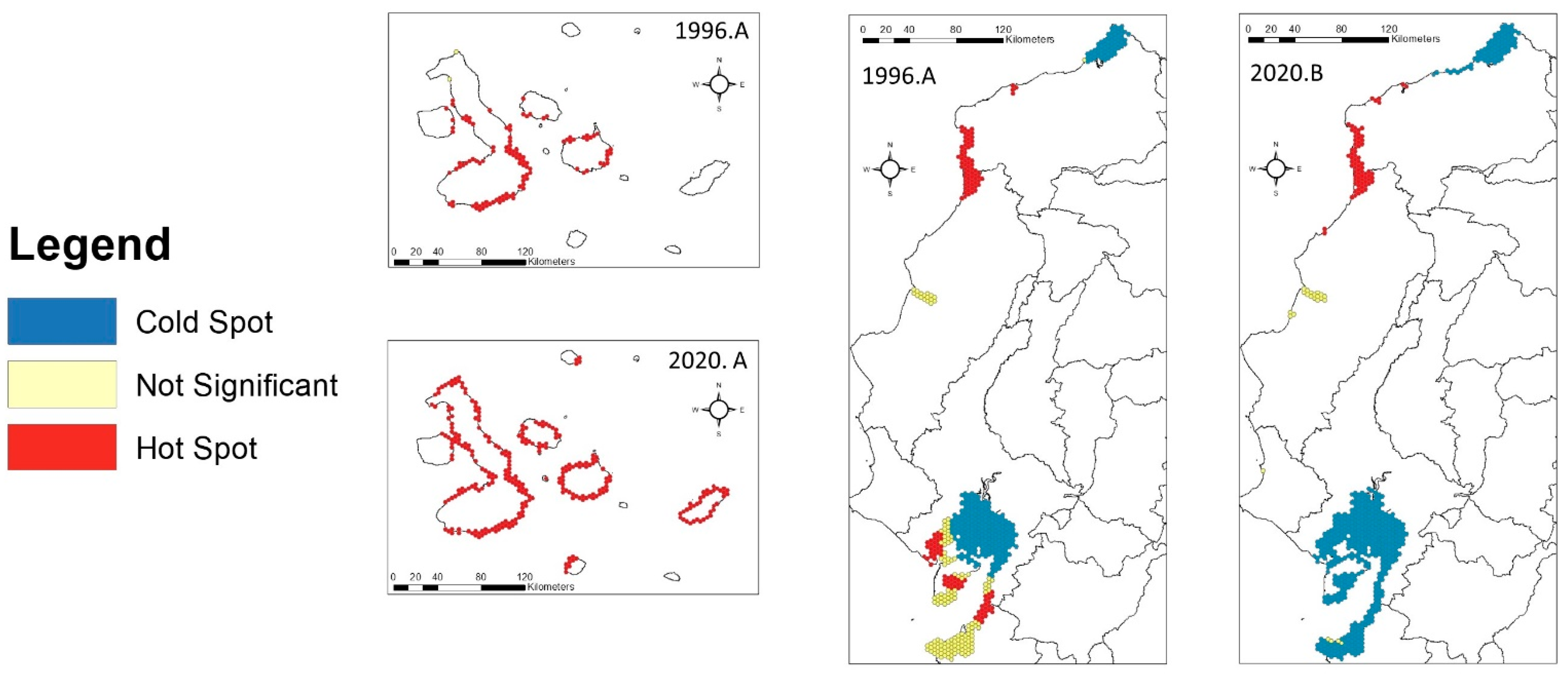
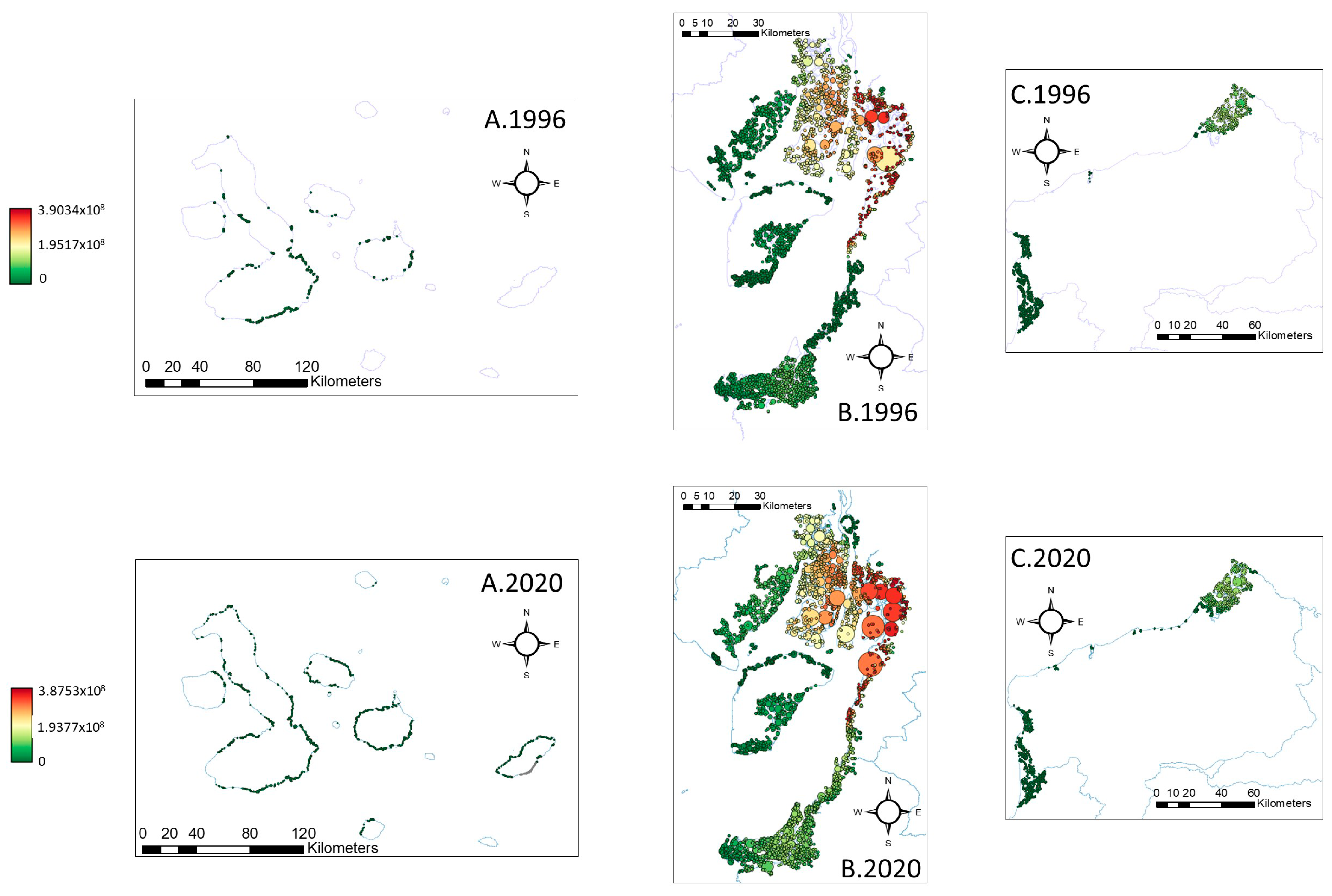
3.3. Connectivity Analysis
4. Discussion
4.1. Deforestation and Fragmentation
4.2. Fragmentation Metrics
4.3. Connectivity of Mangrove Forests in Ecuador
4.4. Implications for Mangrove Conservation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taubert, F.; Fischer, R.; Groeneveld, J.; Lehmann, S.; Müller, M.S.; Rödig, E.; Wiegand, T.; Huth, A. Global patterns of tropical forest fragmentation. Nature 2018, 554, 519–522. [Google Scholar] [CrossRef]
- Grantham, H.S.; Duncan, A.; Evans, T.D.; Jones, K.R.; Beyer, H.L.; Schuster, R.; Walston, J.; Ray, J.C.; Robinson, J.G.; Callow, M.; et al. Anthropogenic modification of forests means only 40% of remaining forests have high ecosystem integrity. Nat. Commun. 2020, 11, 5978. [Google Scholar] [CrossRef]
- Ramírez-Delgado, J.P.; Di Marco, M.; Watson, J.E.; Johnson, C.J.; Rondinini, C.; Corredor Llano, X.; Arias, M.; Venter, O. Matrix condition mediates the effects of habitat fragmentation on species extinction risk. Nat. Commun. 2022, 13, 595. [Google Scholar] [CrossRef]
- Anjos, L.J.; de Souza, E.B.; Amaral, C.T.; Igawa, T.K.; de Toledo, P.M. Future projections for terrestrial biomes indicate widespread warming and moisture reduction in forests up to 2100 in South America. Glob. Ecol. Conserv. 2021, 25, e01441. [Google Scholar] [CrossRef]
- Duke, N.C.; Meynecke, J.O.; Dittmann, S.; Ellison, A.M.; Anger, K.; Berger, U.; Cannicci, S.; Diele, K.; Ewel, K.C.; Field, C.D.; et al. A world without mangroves? Science 2007, 317, 41–42. [Google Scholar] [CrossRef] [PubMed]
- Giri, C.; Ochieng, E.; Tieszen, L.L.; Zhu, Z.; Singh, A.; Loveland, T.; Masek, J.; Duke, N. Status and distribution of mangrove forests of the world using earth observation satellite data. Glob. Ecol. Biogeogr. 2011, 20, 154–159. [Google Scholar] [CrossRef]
- Leal, M.; Spalding, M.D. The State of the World’s Mangroves. 2022. Available online: https://www.mangrovealliance.org/wp-content/uploads/2022/09/The-State-of-the-Worlds-Mangroves-Report_2022.pdf (accessed on 13 November 2022).
- Tomlinson, P.B. The Botany of Mangroves; Cambridge University Press: Cambridge, UK, 2016. [Google Scholar]
- Hilty, J.; Worboys, G.L.; Keeley, A.; Woodley, S.; Lausche, B.J.; Locke, H. Guidelines for Conserving Connectivity through Ecological Networks and Corridors; IUCN: Gland, Switzerland, 2020. [Google Scholar]
- Hogarth, P.J. The Biology of Mangroves; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Hamilton, S.E.; Friess, D.A. Global carbon stocks and potential emissions due to mangrove deforestation from 2000 to 2012. Nat. Clim. Chang. 2018, 8, 240–244. [Google Scholar] [CrossRef]
- Gorman, D. Historical losses of mangrove systems in South America from human-induced and natural impacts. In Threats to Mangrove Forests: Hazards, Vulnerability, and Management; Springer: Berlin/Heidelberg, Germany, 2018; pp. 155–171. [Google Scholar]
- Blanco-Libreros, J.F.; Estrada-Urrea, E.A. Mangroves on the edge: Anthrome-dependent fragmentation influences ecological condition (Turbo, Colombia, Southern Caribbean). Diversity 2015, 7, 206–228. [Google Scholar] [CrossRef]
- Polidoro, B.A.; Carpenter, K.E.; Collins, L.; Duke, N.C.; Ellison, A.M.; Ellison, J.C.; Farnsworth, E.J.; Fernando, E.S.; Kathiresan, K.; Koedam, N.E.; et al. The loss of species: Mangrove extinction risk and geographic areas of global concern. PLoS ONE 2010, 5, e10095. [Google Scholar] [CrossRef]
- Bunting, P.; Rosenqvist, A.; Lucas, R.M.; Rebelo, L.M.; Hilarides, L.; Thomas, N.; Hardy, A.; Itoh, T.; Shimada, M.; Finlayson, C.M. The global mangrove watch—A new 2010 global baseline of mangrove extent. Remote Sens. 2018, 10, 1669. [Google Scholar] [CrossRef]
- Murray, N.J.; Worthington, T.A.; Bunting, P.; Duce, S.; Hagger, V.; Lovelock, C.E.; Lucas, R.; Saunders, M.I.; Sheaves, M.; Spalding, M.; et al. High-resolution mapping of losses and gains of Earth’s tidal wetlands. Science 2022, 376, 744–749. [Google Scholar] [CrossRef]
- Blanco-Libreros, J.F.; Ramírez-Ruiz, K. Threatened mangroves in the Anthropocene: Habitat fragmentation in urban coastalscapes of Pelliciera spp.(Tetrameristaceae) in northern South America. Front. Mar. Sci. 2021, 8, 670354. [Google Scholar] [CrossRef]
- Bryan-Brown, D.N.; Connolly, R.M.; Richards, D.R.; Adame, F.; Friess, D.A.; Brown, C.J. Global trends in mangrove forest fragmentation. Sci. Rep. 2020, 10, 7177. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.A.; DeFries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global consequences of land use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Morocho, R.; González, I.; Ferreira, T.O.; Otero, X.L. Mangrove forests in ecuador: A two-decade analysis. Forests 2022, 13, 656. [Google Scholar] [CrossRef]
- Ward, R.D.; Friess, D.A.; Day, R.H.; Mackenzie, R.A. Impacts of climate change on mangrove ecosystems: A region by region overview. Ecosyst. Health Sustain. 2016, 2, e01211. [Google Scholar] [CrossRef]
- Sócola Sánchez, J.; Corgne, S.; González Bonilla, M. Mangroves of Latin America. In Manglares de América; Moreira, N., Galvis, F., Eds.; Universidad Espíritu Santo: Guayaquil. Ecuador, 2020. [Google Scholar]
- López-Rodríguez, F. Mangrove in Ecuador: Conservation and management strategies. In Coastal Environments; IntechOpen: London, UK, 2021. [Google Scholar]
- Dahdouh-Guebas, F.; Friess, D.A.; Lovelock, C.E.; Connolly, R.M.; Feller, I.C.; Rogers, K.; Cannicci, S. Cross-cutting research themes for future mangrove forest research. Nat. Plants 2022, 8, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, L.D.; Conde, J.E.; Kjerfve, B.; Alvarez-León, R.; Alarcón, C.; Polanía, J. American mangroves. In Mangrove Ecosystems: Function and Management; Springer: Berlin/Heidelberg, Germany, 2002; pp. 1–62. [Google Scholar]
- Puyravaud, J.P. Standardizing the calculation of the annual rate of deforestation. For. Ecol. Manag. 2003, 177, 593–596. [Google Scholar] [CrossRef]
- Birch, C.P.; Oom, S.P.; Beecham, J.A. Rectangular and Hexagonal Grids Used for Observation, Experiment and Simulation in Ecology. Ecol. Model. 2007, 206, 347–359. [Google Scholar]
- Rempel, R.S.; Kaukinen, D.; Carr, A.P. Patch Analyst and Patch Grid Ontario; Ontario Ministry of Natural Resources, Centre for Northern Forest Ecosystem Research: Thunder Bay, ON, Canada, 2012. [Google Scholar]
- Leautaud Valenzuela, P. Fragmentación Forestal de la Reserva Monarca: Cuantificación, Caracterización, y Correlaciones (1990–2010); Universidad Nacional Autónoma de México: Ciudad de México. México, 2014. [Google Scholar]
- Rivas, C.A.; Guerrero-Casado, J.; Navarro-Cerillo, R.M. Deforestation and fragmentation trends of seasonal dry tropical forest in Ecuador: Impact on conservation. For. Ecosyst. 2021, 8, 46. [Google Scholar] [CrossRef]
- McGarigal, K. FRAGSTATS: Spatial Pattern Analysis Program for Quantifying Landscape Structure; US Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 1995; Volume 351.
- Ord, J.K.; Getis, A. Local Spatial Autocorrelation Statistics: Distributional Issues and an Application. Geogr. Anal. 1995, 27, 286–306. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, X.; Gao, F.; Liu, Y. Impacts of Changing Scale on Getis-Ord Gi* Hotspots of CPUE: A Case Study of the Neon Flying Squid (Ommastrephes bartramii) in the Northwest Pacific Ocean. Acta Oceanol. Sin. 2018, 37, 67–76. [Google Scholar] [CrossRef]
- Cetin, M. Sustainability of urban coastal area management: A case study on Cide. J. Sustain. For. 2016, 35, 527–541. [Google Scholar] [CrossRef]
- Foltête, J.C.; Clauzel, C.; Vuidel, A. software tool dedicated to the modelling of landscape networks. Environ. Model. Softw. 2012, 38, 316–327. [Google Scholar] [CrossRef]
- Urban, D.; Keitt, T. Landscape connectivity: A graph-theoretic perspective. Ecology 2001, 82, 1205–1218. [Google Scholar] [CrossRef]
- Saura, S.; Torné, J. Conefor Sensinode 2.2: A software package for quantifying the importance of habitat patches for landscape connectivity. Environ. Model. Softw. 2009, 24, 135–139. [Google Scholar] [CrossRef]
- CLIRSEN. Update of the Multitemporal Study of Mangroves, Shrimp Farms and Saline Areas on the Ecuadorian Continental Coast; Coastal Resource Management Programme (PMRC): Quito, Ecuador, 2006. [Google Scholar]
- Bravo, M. Alianza público privada para la gestión de los manglares del Ecuador; USAID Sustainable Coasts and Forests: Quito, Ecuador, 2013.
- Ferrer-Paris, J.R.; Zager, I.; Keith, D.A.; Oliveira-Miranda, M.A.; Rodríguez, J.P.; Josse, C.; González-Gil, M.; Miller, R.M.; Zambrana-Torrelio, C.; Barrow, E. An ecosystem risk assessment of temperate and tropical forests of the Americas with an outlook on future conservation strategies. Conserv. Lett. 2019, 12, e12623. [Google Scholar] [CrossRef]
- Santillán, X.; Rosero, P. Proceso histórico de creación del Plan de Acción Nacional para la conservación de los manglares del Ecuador continental como herramienta de gestión. In Primer Congreso Manglares de América; Universidad Espíritu Santo: Guayaquil, Ecuador, 2019. [Google Scholar]
- Rodríguez, F.V. Mangrove concessions: An innovative strategy for community mangrove conservation in Ecuador. In Threats to Mangrove Forests: Hazards, Vulnerability, and Management; Springer: Berlin/Heidelberg, Germany, 2018; pp. 557–578. [Google Scholar]
- Thornton, H.; Branch, L.C.; Sunquist, E. The influence of landscape, patch, and within-patch factors on species presence and abundance: A review of focal patch studies. Landsc. Ecol. 2011, 26, 7–18. [Google Scholar] [CrossRef]
- Turschwell, M.P.; Tulloch, V.J.; Sievers, M.; Pearson, R.M.; Andradi-Brown, D.A.; Ahmadia, G.N.; Connolly, R.M.; Bryan-Brown, D.; Lopez-Marcano, S.; Adame, M.F.; et al. Multi-scale estimation of the effects of pressures and drivers on mangrove forest loss globally. Biol. Conserv. 2020, 247, 108637. [Google Scholar] [CrossRef]
- Kanniah, K.D.; Kang, C.S.; Sharma, S.; Amir, A.A. Remote sensing to study mangrove fragmentation and its impacts on leaf area index and gross primary productivity in the South of Peninsular Malaysia. Remote Sens. 2021, 13, 1427. [Google Scholar] [CrossRef]
- Rivas, C.A.; Guerrero-Casado, J.; Navarro-Cerrillo, R.M. A new combined index to assess the fragmentation status of a forest patch based on its size, shape complexity, and isolation. Diversity 2022, 14, 896. [Google Scholar] [CrossRef]
- Zulfiqar, A.; Ghaffar, M.M.; Shahzad, M.; Weis, C.; Malik, M.I.; Shafait, F.; Wehn, N. AI-ForestWatch: Semantic segmentation based end-to-end framework for forest estimation and change detection using multi-spectral remote sensing imagery. J. Appl. Remote Sens. 2021, 15, 024518. [Google Scholar] [CrossRef]
- Fahrig, L. Effects of Habitat Fragmentation on Biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef]
- Collins, S.; Hamilton, E. Livelihood responses to mangrove deforestation in the northern provinces of Ecuador. Bosque 2013, 34, 143–153. [Google Scholar]
- Cohen, M.C.; Rodrigues, E.; Rocha, D.O.; Freitas, J.; Fontes, N.A.; Pessenda, L.C.; de Souza, A.V.; Gomes, V.L.; França, M.C.; Bonotto, D.M.; et al. Southward migration of the austral limit of mangroves in South America. Catena 2020, 195, 104775. [Google Scholar] [CrossRef]
- Ortega-Pacheco, D.; Mendoza-Jimenez, M.J.; Herrera, P. Mangrove Conservation Policies in the Gulf of Guayaquil. In Handbook of Climate Change and Biodiversity; Springer: Berlin/Heidelberg, Germany, 2019; pp. 25–43. [Google Scholar]
- Kool, J.T.; Moilanen, A.; Treml, E.A. Population connectivity: Recent advances and new perspectives. Landsc. Ecol. 2013, 28, 165–185. [Google Scholar] [CrossRef]
- Tabor, G.; Bankova-Todorova, M.; Correa Ayram, C.A.; Garcia, L.C.; Kapos, V.; Olds, A.; Stupariu, I. Ecological connectivity: A bridge to preserving biodiversity. In Frontiers 2018/19 Emerging Issues of Environmental Concern; Emerging, I., Ed.; United Nations Environment Programme: Nairobi, Kenya, 2019. [Google Scholar]
- Bailey, S. Increasing connectivity in fragmented landscapes: An investigation of evidence for biodiversity gain in woodlands. For. Ecol. Manag. 2007, 238, 7–23. [Google Scholar] [CrossRef]
- Navarro-Cerrillo, R.M.; Rivas, C.A.; Quinto, L.; Navarro, S.H.; Varo-Martínez, M.Á.; Palacios-Rodríguez, P. Afforestation on agricultural land in southern Spain: An important driver to improve forest landscape connectivity. New For. 2022, 1–24. [Google Scholar] [CrossRef]
- Tulloch, A.I.; Barnes, M.D.; Ringma, J.; Fuller, R.A.; Watson, J.E. Understanding the importance of small patches of habitat for conservation. J. Appl. Ecol. 2016, 53, 418–429. [Google Scholar] [CrossRef]
- Field, R.D.; Parrott, L. Multi-ecosystem services networks: A new perspective for assessing landscape connectivity and resilience. Ecol. Complex. 2017, 32, 31–41. [Google Scholar] [CrossRef]
- Li, F.; Liu, X.; Zhang, X.; Zhao, D.; Liu, H.; Zhou, C.; Wang, R. Urban ecological infrastructure: An integrated network for ecosystem services and sustainable urban systems. J. Clean. Prod. 2017, 163, S12–S18. [Google Scholar] [CrossRef]
- Primavera, H. Mangroves, Fishponds, and the Quest for Sustainability. Science 2000, 310, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Ellison, A.M.; Farnsworth, E.J. Anthropogenic Disturbance of Caribbean Mangrove Ecosystems: Past Impacts, Present Trends, and Future Predictions. Biotropica 1996, 28, 549–565. [Google Scholar] [CrossRef]
- Hamilton, S.E.; Lovette, J. Ecuador’s mangrove forest carbon stocks: A spatiotemporal analysis of living carbon holdings and their depletion since the advent of commercial aquaculture. PLoS ONE 2015, 10, e0118880. [Google Scholar] [CrossRef] [PubMed]
- Magris, R.A.; Barreto, R. Mapping and assessment of protection of mangrove habitats in Brazil. Pan Am. J. Aquat. Sci. 2010, 5, 546–556. [Google Scholar]
- López-Portillo, J.; Lewis, R.R., III; Saenger, P.; Rovai, A.; Koedam, N.; Dahdouh-Guebas, F. Mangrove Ecosystems: A Global Biogeographic Perspective; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Brander, M.; Wagtendonk, J.; Hussain, S.; McVittie, H.; Verburg, H.; De Groot, S. Ecosystem service values for mangroves in Southeast Asia: A meta-analysis and value transfer application. Ecosyst. Serv. 2012, 12, 62–69. [Google Scholar] [CrossRef]
- Wang, L.; Jia, M.; Yin, D.; Tian, J. A review of remote sensing for mangrove forests: 1956–2018. Remote Sens. Environ. 2019, 231, 111223. [Google Scholar] [CrossRef]


| Parameter | Name | Definition | Unit |
|---|---|---|---|
| Number of Patches | NumP | Total number of patches inside the tiles. The more patches there are, the more fragmented the forest is considered to be. | Number |
| Class area | CA | Sum of areas of all patches belonging to a hexagon. | m2 |
| Median patch size | MPS | The average patch size of the forest within the tile. A smaller average forest patch size is considered indicative of a more fragmented forest. | m2 |
| Total edge | TE | Perimeter of patches within each tile. The greater the perimeter, the more exposed to disturbances. Greater TE patches may be associated with more fragmented forests (if the fragmentation is related to an anthropogenic disturbance). | m |
| Edge density | ED | Amount of edge (km) relative to the forest area (km2) within the tile. ED = TE/CA A high ratio of perimeter to forest patch area may be associated with more fragmented forests (if fragmentation is related to anthropogenic disturbance). | m/m2 |
| Edge density percentage | ED | Edge percentage relative to landscape area. A high ratio of perimeter to forest patch area may be associated with more fragmented forests (if fragmentation is related to anthropogenic disturbance). | % |
| Percentage without forest | PSB | Non-mangrove area (%) without forest within the tile. Higher percentage of area without forest within the tile would indicate greater fragmentation. | % |
| Reticular fragmentation index | RFI | Reticular fragmentation index of each tile. RFI = A higher RFI means a greater percentage of fragmentation within the tile. | % |
| Metric | Level | Formula | Meaning | References |
|---|---|---|---|---|
| Flux (F) | Global level and component level | Sum of potential dispersion from all patches. | [32,33,34] | |
| Equivalent probability (EC) | Global level | Square root of the sum of the products of the capacity of all pairs of patches weighted by their interaction probability. | [35] | |
| Probability of connectivity (PC) | Global level | Sum of the products of the capacity of all pairs of patches weighted by their interaction probability, divided by the square of the area of the study zone. This ratio is equivalent to the probability that two points randomly placed in the study area are connected. | [36] | |
| Number of components (NC) | Global level | NC = nc | Number of components of the graph. | [37] |
| Year | |||||
|---|---|---|---|---|---|
| Provinces | 1996 | 2007 | 2015 | 2020 | |
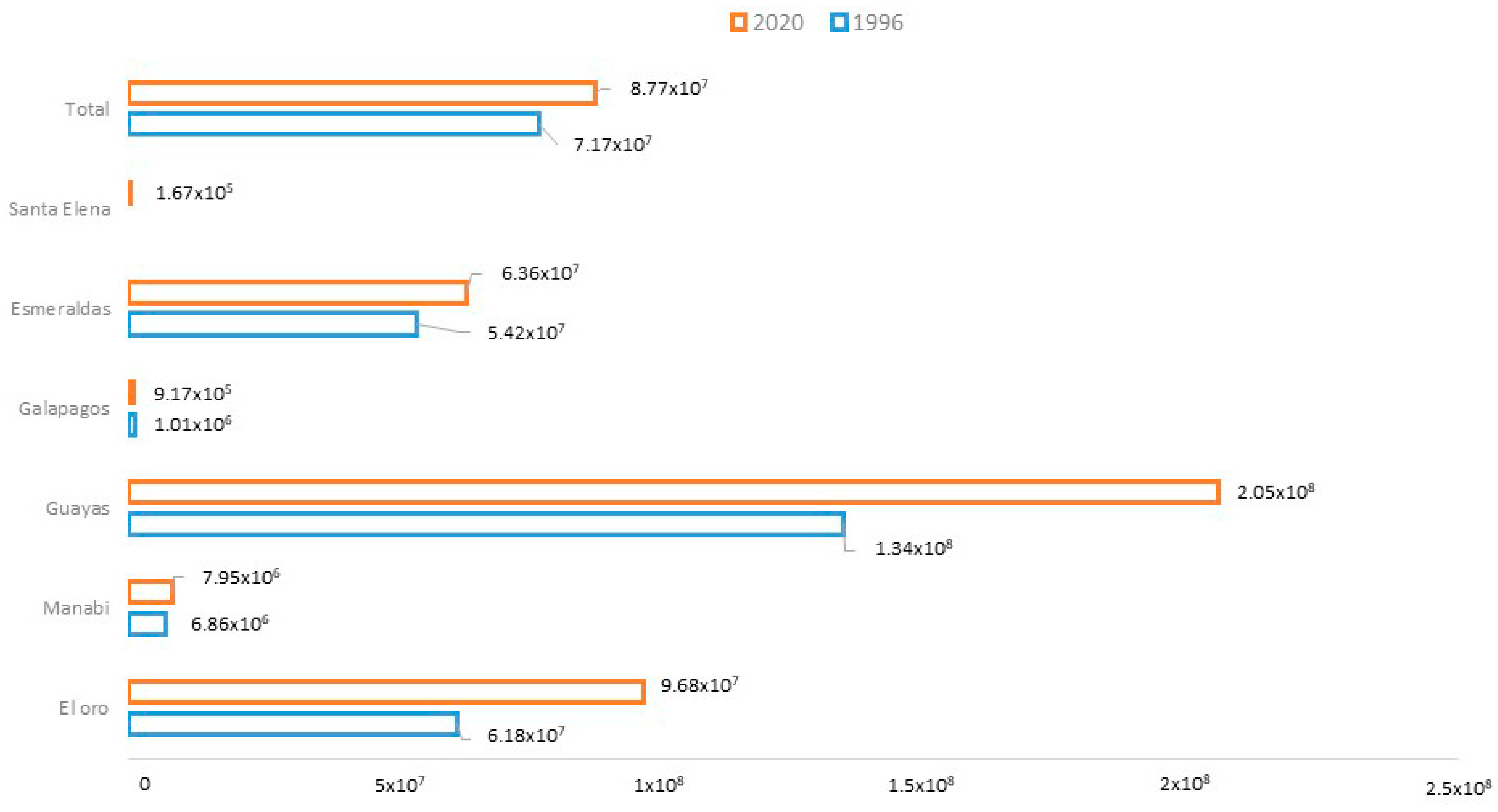
| El Oro | Area (km2) | 177.30 | 153.81 | 152.09 | 189.18 |
| Deforestation rate | −1.29 | −0.14 | 4.36 | ||
| Manabí | Area (km2) | 35.83 | 28.08 | 24.94 | 31.70 |
| Deforestation rate | −2.22 | −1.48 | 4.80 | ||
| Guayas | Area (km2) | 973.24 | 958.63 | 954.77 | 1008.16 |
| Deforestation rate | −0.14 | −0.05 | 1.09 | ||
| Galapagos | Area (km2) | 25.17 | 25.17 | 25.10 | 51.72 |
| Deforestation rate | 0.00 | −0.03 | 14.46 | ||
| Esmeraldas | Area (km2) | 235.13 | 223.51 | 222.32 | 240.31 |
| Deforestation rate | −0.46 | −0.07 | 1.56 | ||
| Santa Elena | Area (km2) | 0.00 | 0.00 | 0.00 | 0.23 |
| Deforestation rate | |||||
| Total | Area (km2) | 1446.68 | 1389.20 | 1379.22 | 1521.30 |
| Deforestation rate | −0.37 | −0.09 | 1.96 | ||
| El Oro | Esmeraldas | Manabí | Guayas | Galapagos | Santa Elena | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1996 | 2020 | 1996 | 2020 | 1996 | 2020 | 1996 | 2020 | 1996 | 2020 | 1996 | 2020 | ||
| No. of tesserae | 92 | 94 | 104 | 124 | 49 | 53 | 363 | 379 | 112 | 285 | 1 | ||
| No. of patches | Media | 25.21 | 21.43 | 19.11 | 17.39 | 35.79 | 22.34 | 11.86 | 12.56 | 3.73 | 8.21 | 4.00 | |
| Median | 23.00 | 18.00 | 11.00 | 13.00 | 26.00 | 18.00 | 8.00 | 10.00 | 3.00 | 6.00 | |||
| D.E | 17.45 | 15.02 | 24.38 | 17.10 | 34.09 | 18.56 | 12.70 | 10.62 | 2.69 | 7.52 | |||
| CA | Media | 1,946,635.91 | 2,010,785.00 | 2,227,188.18 | 1,903,931.99 | 802,363.64 | 652,096.31 | 2,681,125.61 | 2,660,761.05 | 224,724.32 | 181,408.21 | 230,058.70 | |
| Median | 1,696,904.73 | 1,913,977.32 | 1,132,075.13 | 781,943.33 | 635,734.56 | 414,483.58 | 1,970,581.36 | 1,932,550.15 | 106,937.56 | 65,810.02 | |||
| D.E | 1,593,176.85 | 1,506,126.98 | 2,297,435.83 | 2,238,564.03 | 819,943.25 | 736,706.88 | 2,573,196.57 | 2,529,074.78 | 316,142.77 | 299,890.39 | |||
| MPS | Media | 120,265.85 | 131,660.95 | 217,648.19 | 150,965.32 | 43,065.63 | 31,498.59 | 505,207.61 | 397,775.08 | 58,117.42 | 33,335.31 | 57,514.67 | |
| Median | 64,866.77 | 78,361.81 | 78,157.16 | 42,940.87 | 19,371.30 | 18,717.56 | 150,966.99 | 150,113.02 | 34,562.55 | 11,424.89 | |||
| D.E | 238,725.94 | 167,385.28 | 325,148.44 | 224,003.10 | 70,260.64 | 35,848.34 | 1,018,677.41 | 682,903.92 | 86,536.65 | 95,691.39 | |||
| Total edge | Media | 37,849.21 | 33,280.29 | 35,647.83 | 27,103.36 | 24,768.89 | 31,498.59 | 25,837.57 | 24,884.24 | 5351.22 | 5178.30 | 5718.37 | |
| Median | 34,134.56 | 35,437.50 | 29,639.05 | 20,507.57 | 22,274.17 | 18,717.56 | 25,575.47 | 23,836.67 | 3929.99 | 2986.24 | |||
| D.E | 27,322.80 | 20,924.47 | 28,386.32 | 23,556.53 | 20,868.24 | 35,848.34 | 18,518.45 | 17,091.42 | 5252.64 | 5483.67 | |||
| Edge density | Media | 0.03 | 0.03 | 0.03 | 0.03 | 0.05 | 0.05 | 0.02 | 0.03 | 0.17 | 0.06 | 0.02 | |
| Median | 0.02 | 0.02 | 0.03 | 0.03 | 0.04 | 0.04 | 0.02 | 0.01 | 0.04 | 0.05 | |||
| D.E | 0.02 | 0.02 | 0.02 | 0.03 | 0.04 | 0.06 | 0.04 | 0.04 | 1.29 | 0.05 | |||
| 1996 | 2020 | Δ 2020−1996 | |
|---|---|---|---|
| Flux | 7.06 × 1011 | 9.37 × 1011 | 32.62 |
| EC | 5.38 × 108 | 5.52 × 108 | 2.51 |
| PC | 4.86 × 10−7 | 5.01 × 10−7 | 3.15 |
| NC | 30 | 35 | 16.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaramillo, J.J.; Rivas, C.A.; Oteros, J.; Navarro-Cerrillo, R.M. Forest Fragmentation and Landscape Connectivity Changes in Ecuadorian Mangroves: Some Hope for the Future? Appl. Sci. 2023, 13, 5001. https://doi.org/10.3390/app13085001
Jaramillo JJ, Rivas CA, Oteros J, Navarro-Cerrillo RM. Forest Fragmentation and Landscape Connectivity Changes in Ecuadorian Mangroves: Some Hope for the Future? Applied Sciences. 2023; 13(8):5001. https://doi.org/10.3390/app13085001
Chicago/Turabian StyleJaramillo, Julio J., Carlos A. Rivas, José Oteros, and Rafael M. Navarro-Cerrillo. 2023. "Forest Fragmentation and Landscape Connectivity Changes in Ecuadorian Mangroves: Some Hope for the Future?" Applied Sciences 13, no. 8: 5001. https://doi.org/10.3390/app13085001
APA StyleJaramillo, J. J., Rivas, C. A., Oteros, J., & Navarro-Cerrillo, R. M. (2023). Forest Fragmentation and Landscape Connectivity Changes in Ecuadorian Mangroves: Some Hope for the Future? Applied Sciences, 13(8), 5001. https://doi.org/10.3390/app13085001








