Advances in Chronic Kidney Disease in Africa
Abstract
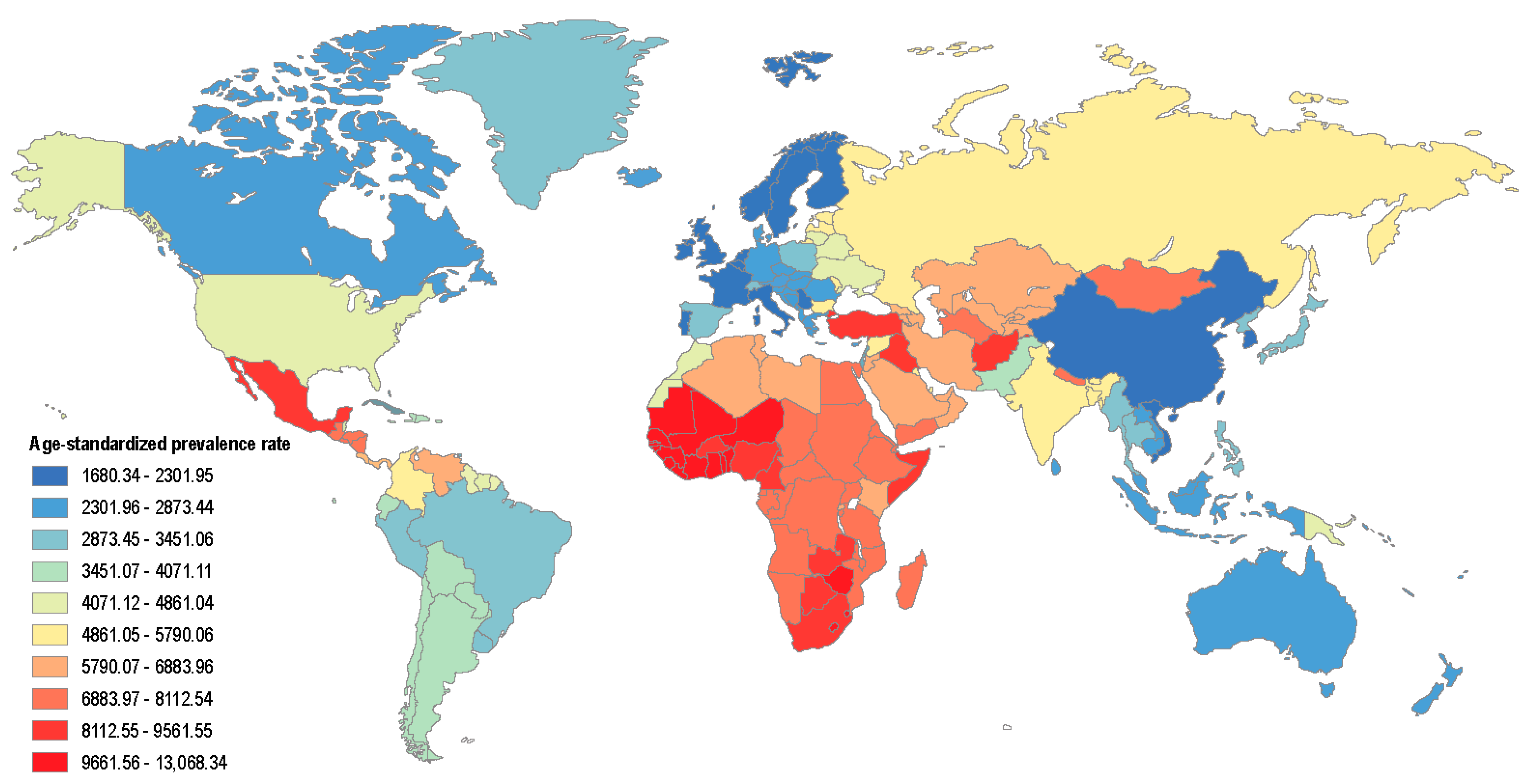
1. Introduction
2. Challenges of Estimating GFR in SSA
3. The Importance of APOL1 and CKD
4. Diabetic Kidney Disease
5. Hypertension and CKD
6. Pregnancy Related AKI and Its Causes—A Growing Concern of CKD in Africa
7. Pre-Eclampsia as a Future Risk for CKD
8. Newer Findings into the Pathogenesis of PET
9. What Is New in HIV and CKD in SSA
10. CKD Unknown (CKDu) in Africa
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Available online: https://ourworldindata.org/causes-of-death (accessed on 15 February 2023).
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; GBD Chronic Kidney Disease Collaboration; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Bowe, B.; Mokdad, A.H.; Xian, H.; Yan, Y.; Li, T.; Maddukuri, G.; Tsai, C.-Y.; Floyd, T.; Al-Aly, Z. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018, 94, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Elhafeez, S.A.; Bolignano, D.; D’Arrigo, G.; Dounousi, E.; Tripepi, G.; Zoccali, C. Prevalence and burden of chronic kidney disease among the general population and high-risk groups in Africa: A systematic review. BMJ Open 2018, 8, e015069. [Google Scholar] [CrossRef] [PubMed]
- Atta, M.G.; Zook, K.; Brown, T.T.; Vaidya, D.; Tao, X.; Maier, P.; Schwartz, G.J.; Lucas, G.M. Racial Adjustment Adversely Affects Glomerular Filtration Estimates in Black Americans Living with HIV. J. Am. Soc. Nephrol. 2021, 32, 2143–2147. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. New Creatinine- and Cystatin C–Based Equations to Estimate GFR without Race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef]
- Fabian, J.; Kalyesubula, R.; Mkandawire, J.; Hansen, C.H.; Nitsch, D.; Musenge, E.; Nakanga, W.P.; E Prynn, J.; Dreyer, G.; Snyman, T.; et al. Measurement of kidney function in Malawi, South Africa, and Uganda: A multicentre cohort study. Lancet Glob. Health 2022, 10, e1159–e1169. [Google Scholar] [CrossRef]
- Genovese, G.; Friedman, D.J.; Ross, M.D.; Lecordier, L.; Uzureau, P.; Freedman, B.I.; Bowden, D.W.; Langefeld, C.D.; Oleksyk, T.K.; Knob, A.L.U.; et al. Association of Trypanolytic ApoL1 Variants with Kidney Disease in African Americans. Science 2010, 329, 841–845. [Google Scholar] [CrossRef]
- Hung, R.K.; Binns-Roemer, E.; Booth, J.W.; Hilton, R.; Harber, M.; Santana-Suarez, B.; Campbell, L.; Fox, J.; Ustianowski, A.; Cosgrove, C.; et al. Genetic Variants of APOL1 Are Major Determinants of Kidney Failure in People of African Ancestry With HIV. Kidney Int. Rep. 2022, 7, 786–796. [Google Scholar] [CrossRef]
- Limou, S.; Nelson, G.W.; Kopp, J.B.; Winkler, C.A. APOL1 Kidney Risk Alleles: Population Genetics and Disease Associations. Adv. Chronic Kidney Dis. 2014, 21, 426–433. [Google Scholar] [CrossRef]
- Genovese, G.; Friedman, D.J.; Pollak, M.R. APOL1 variants and kidney disease in people of recent African ancestry. Nat. Rev. Nephrol. 2013, 9, 240–244. [Google Scholar] [CrossRef]
- Kasembeli, A.N.; Duarte, R.; Ramsay, M.; Mosiane, P.; Dickens, C.; Dix-Peek, T.; Limou, S.; Sezgin, E.; Nelson, G.W.; Fogo, A.B.; et al. APOL1 Risk Variants Are Strongly Associated with HIV-Associated Nephropathy in Black South Africans. J. Am. Soc. Nephrol. 2015, 26, 2882–2890. [Google Scholar] [CrossRef] [PubMed]
- Dummer, P.D.; Limou, S.; Rosenberg, A.Z.; Heymann, J.; Nelson, G.; Winkler, C.A.; Kopp, J.B. APOL1 Kidney Disease Risk Variants: An Evolving Landscape. Semin. Nephrol. 2015, 35, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Atta, M.G.; Estrella, M.M.; Kuperman, M.; Foy, M.C.; Fine, D.M.; Racusen, L.C.; Lucas, G.M.; Nelson, G.W.; Warner, A.C.; Winkler, C.A.; et al. HIV-associated nephropathy patients with and without apolipoprotein L1 gene variants have similar clinical and pathological characteristics. Kidney Int. 2012, 82, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.J.; Ma, L.; Freedman, B.I. Treatment potential in APOL1-associated nephropathy. Curr. Opin. Nephrol. Hypertens. 2022, 31, 442–448. [Google Scholar] [CrossRef]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef]
- Tuttle, K.R.; Bakris, G.L.; Bilous, R.W.; Chiang, J.L.; de Boer, I.H.; Goldstein-Fuchs, J.; Hirsch, I.B.; Kalantar-Zadeh, K.; Narva, A.S.; Navaneethan, S.D.; et al. Diabetic Kidney Disease: A Report From an ADA Consensus Conference. Diabetes Care 2014, 37, 2864–2883. [Google Scholar] [CrossRef]
- Pálsson, R.; Patel, U.D. Cardiovascular Complications of Diabetic Kidney Disease. Adv. Chronic Kidney Dis. 2014, 21, 273–280. [Google Scholar] [CrossRef]
- Noubiap, J.J.N. Diabetic nephropathy in Africa: A systematic review. World J. Diabetes 2015, 6, 759–773. [Google Scholar] [CrossRef]
- Pillay, S.; Aldous, C.; Mahomed, F. A deadly combination—HIV and diabetes mellitus: Where are we now? South Afr. Med. J. 2016, 106, 378. [Google Scholar] [CrossRef]
- Ekrikpo, U.E.; Kengne, A.P.; Bello, A.K.; Effa, E.E.; Noubiap, J.J.; Salako, B.L.; Rayner, B.L.; Remuzzi, G.; Okpechi, I.G. Chronic kidney disease in the global adult HIV-infected population: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0195443. [Google Scholar] [CrossRef]
- Gæde, P.; Lund-Andersen, H.; Parving, H.-H.; Pedersen, O. Effect of a Multifactorial Intervention on Mortality in Type 2 Diabetes. N. Engl. J. Med. 2008, 358, 580–591. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- The EMPA-KIDNEY Collaborative Group; Herrington, W.G.; Staplin, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, J.R.; Preiss, D.; Judge, P.; Mayne, K.J.; et al. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Szarek, M.; Pitt, B.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Inzucchi, S.E.; Kosiborod, M.N.; et al. Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease. N. Engl. J. Med. 2021, 384, 129–139. [Google Scholar] [CrossRef]
- Baigent, C.; Emberson, J.; Haynes, R.; Herrington, W.G.; Judge, P.; Landray, M.J.; Mayne, K.J.; Ng, S.Y.; Preiss, D.; Roddick, A.J.; et al. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: Collaborative meta-analysis of large placebo-controlled trials. Lancet 2022, 400, 1788–1801. [Google Scholar] [CrossRef]
- de Boer, I.H.; Caramori, M.L.; Chan, J.C.; Heerspink, H.J.; Hurst, C.; Khunti, K.; Liew, A.; Michos, E.D.; Navaneethan, S.D.; Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group; et al. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020, 98, S1–S115. [Google Scholar] [CrossRef]
- Vart, P.; Correa-Rotter, R.; Hou, F.F.; Jongs, N.; Chertow, G.M.; Langkilde, A.M.; McMurray, J.J.; Rossing, P.; Sjöström, C.D.; Stefansson, B.V.; et al. Efficacy and Safety of Dapagliflozin in Patients With CKD across Major Geographic Regions. Kidney Int. Rep. 2022, 7, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; Lee, M.M.Y.; Kristensen, S.L.; Branch, K.R.H.; Del Prato, S.; Khurmi, N.S.; Lam, C.S.P.; Lopes, R.D.; McMurray, J.J.V.; Pratley, R.E.; et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021, 9, 653–662. [Google Scholar] [CrossRef]
- de Boer, I.H.; Khunti, K.; Sadusky, T.; Tuttle, K.R.; Neumiller, J.J.; Rhee, C.M.; Rosas, S.E.; Rossing, P.; Bakris, G. Diabetes management in chronic kidney disease: A consensus report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2022, 102, 974–989. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdollahi, M.; Abedi, P.; Abedi, A.; GBD 2019 Viewpoint Collaborators; et al. Five insights from the Global Burden of Disease Study 2019. Lancet 2020, 396, 1135–1159. [Google Scholar] [CrossRef]
- Ataklte, F.; Erqou, S.; Kaptoge, S.; Taye, B.; Echouffo-Tcheugui, J.B.; Kengne, A.P. Burden of Undiagnosed Hypertension in Sub-Saharan Africa: A systematic review and meta-analysis. Hypertension 2015, 65, 291–298. [Google Scholar] [CrossRef]
- Kohli-Lynch, C.N.; Erzse, A.; Rayner, B.; Hofman, K.J. Hypertension in the South African public healthcare system: A cost-of-illness and burden of disease study. BMJ Open 2022, 12, e055621. [Google Scholar] [CrossRef]
- Davari, M.; Sorato, M.M.; Kebriaeezadeh, A.; Sarrafzadegan, N. Cost-effectiveness of hypertension therapy based on 2020 International Society of Hypertension guidelines in Ethiopia from a societal perspective. PLoS ONE 2022, 17, e0273439. [Google Scholar] [CrossRef] [PubMed]
- Comparison of Dual Therapies for Lowering Blood Pressure in Black Africans; Ojji, D.B.; Mayosi, B.; Francis, V.; Badri, M.; Cornelius, V.; Smythe, W.; Kramer, N.; Barasa, F.; Damasceno, A.; et al. Comparison of Dual Therapies for Lowering Blood Pressure in Black Africans. N. Engl. J. Med. 2019, 380, 2429–2439. [Google Scholar] [CrossRef]
- Gaye, B.; Janeczek, A.-L.; Narayanan, K.; N’Guetta, R.; Vignac, M.; Gallardo, V.; Jouven, X.; Luu, D.; Marijon, E. Prevalence of severe hypertension in a Sub-Saharan African community. Int. J. Cardiol. Hypertens. 2019, 2, 100016. [Google Scholar] [CrossRef]
- Chillo, P.; Ismail, A.; Sanyiwa, A.; Ruggajo, P.; Kamuhabwa, A. Hypertensive retinopathy and associated factors among nondiabetic chronic kidney disease patients seen at a tertiary hospital in Tanzania: A cross-sectional study. Int. J. Nephrol. Renov. Dis. 2019, 12, 79–86. [Google Scholar] [CrossRef]
- Akinbodewa, A.A.; Adejumo, A.O.; Koledoye, O.V.; Kolawole, J.O.; Akinfaderin, D.; Lamidi, A.O.; Gbakinro, G.O.; Ogunduyile, C.; Osungbemiro, W.B. Community screening for pre-hypertension, traditional risk factors and markers of chronic kidney disease in Ondo State, South-Western Nigeria. Niger. Postgrad. Med. J. 2017, 24, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Kebede, K.M.; Abateneh, D.D.; Teferi, M.B.; Asres, A. Chronic kidney disease and associated factors among adult population in Southwest Ethiopia. PLoS ONE 2022, 17, e0264611. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Owusu, I.K.; Geng, Q.; Folson, A.A.; Zheng, Z.; Adu-Boakye, Y.; Dong, X.; Wu, W.; Agyekum, F.; Fei, H.; et al. Cardiometabolic Risk Factors and Preclinical Target Organ Damage among Adults in Ghana: Findings from a National Study. J. Am. Heart Assoc. 2020, 9, e017492. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Tiv, S.; Anand, S.; Mohan, D.; Garcia, G.G.; Padilla, J.A.G.; Klarenbach, S.; Blackaller, G.N.; Seck, S.; Wang, J.; et al. Diagnostic Yield of Population-Based Screening for Chronic Kidney Disease in Low-Income, Middle-Income, and High-Income Countries. JAMA Netw. Open 2021, 4, e2127396. [Google Scholar] [CrossRef] [PubMed]
- Ajaegbu, O.C.; Ezeonwu, B.U.; Emeagui, O.D.; Okafor, H.U. Modifiable Risk Factors for Chronic Kidney Disease in Adulthood seen among School Children in Asaba. West Afr. J. Med. 2021, 38, 674–678. [Google Scholar]
- Kruger, R.; Kruger, H.S.; Monyeki, M.A.; Pienaar, A.E.; Roux, S.B.-L.; Gafane-Matemane, L.F.; Smith, W.; Mels, C.M.C.; Lammertyn, L.; Brits, J.S.; et al. A demographic approach to assess elevated blood pressure and obesity in prepubescent children: The ExAMIN Youth South Africa study. J. Hypertens. 2021, 39, 2190–2199. [Google Scholar] [CrossRef]
- Nojilana, B.; Abdelatif, N.; Cois, A.; E Schutte, A.; Wentzel-Viljoen, E.; Turuwa, E.B.; A Roomaney, R.; Awotiwon, O.F.; Neethling, I.; Pacella, R.; et al. Estimating the changing burden of disease attributable to high sodium intake in South Africa for 2000, 2006 and 2012. South Afr. Med. J. 2022, 112, 627–638. [Google Scholar] [CrossRef]
- Strauss-Kruger, M.; Wentzel-Viljoen, E.; Ware, L.J.; Van Zyl, T.; Charlton, K.; Ellis, S.; Schutte, A.E. Early evidence for the effectiveness of South Africa’s legislation on salt restriction in foods: The African-PREDICT study. J. Hum. Hypertens. 2023, 37, 42–49. [Google Scholar] [CrossRef]
- Vinturache, A.; Popoola, J.; Watt-Coote, I. The Changing Landscape of Acute Kidney Injury in Pregnancy from an Obstetrics Perspective. J. Clin. Med. 2019, 8, 1396. [Google Scholar] [CrossRef]
- Bentata, Y.; Housni, B.; Mimouni, A.; Azzouzi, A.; Abouqal, R. Acute kidney injury related to pregnancy in developing countries: Etiology and risk factors in an intensive care unit. J. Nephrol. 2012, 25, 764–775. [Google Scholar] [CrossRef]
- Najar, M.S.; Shah, A.R.; Wani, I.A.; Reshi, A.R.; Banday, K.A.; Bhat, M.A.; Saldanha, C.L. Pregnancy related acute kidney injury: A single center experience from the Kashmir Valley. Indian J. Nephrol. 2008, 18, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, A.S.; Shemies, R.S. Pregnancy-related acute kidney injury in the African continent: Where do we stand? A systematic review. J. Nephrol. 2022, 35, 2175–2189. [Google Scholar] [CrossRef] [PubMed]
- Organization WHO (2015) Trends in Maternal Mortality: 1990–2015: Estimates from WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division: World Health Organization. Available online: https://www.unfpa.org/publications/trends-maternal-mortality-1990-2015 (accessed on 14 February 2023).
- Gaber, T.Z.; Shemies, R.S.; Baiomy, A.A.; Aladle, D.A.; Mosbah, A.; Abdel-Hady, E.S.; Sayed-Ahmed, N.; Sobh, M. Acute kidney injury during pregnancy and puerperium: An Egyptian hospital-based study. J. Nephrol. 2021, 34, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Adejumo, O.; Akinbodewa, A.; Enikuomehin, O.; Lawal, O.; Abolarin, O.; Alli, O. Pregnancy-related acute kidney injury: Etiologies and short-term outcomes in a tertiary hospital in Southwest Nigeria. Saudi J. Kidney Dis. Transplant. 2019, 30, 1423–1430. [Google Scholar] [CrossRef]
- Awowole, I.O.; Omitinde, O.S.; Arogundade, F.A.; Bola-Oyebamiji, S.B.; Adeniyi, O.A. Pregnancy-related acute kidney injury requiring dialysis as an indicator of severe adverse maternal morbidity at a tertiary center in Southwest Nigeria. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 225, 205–209. [Google Scholar] [CrossRef]
- Cooke, W.R.; Hemmilä, U.K.; Craik, A.L.; Mandula, C.J.; Mvula, P.; Msusa, A.; Dreyer, G.; Evans, R. Incidence, aetiology and outcomes of obstetric-related acute kidney injury in Malawi: A prospective observational study. BMC Nephrol. 2018, 19, 25. [Google Scholar] [CrossRef]
- Kabbali, N.; Tachfouti, N.; Arrayhani, M.; Harandou, M.; Tagnaouti, M.; Bentata, Y.; Laouad, I.; Ramdani, B.; Bayahia, R.; Oualim, Z.; et al. Outcome assessment of pregnancy-related acute kidney injury in Morocco: A national prospective study. Saudi J. Kidney Dis. Transplant. 2015, 26, 619–624. [Google Scholar] [CrossRef]
- Arrayhani, M.; El Youbi, R.; Sqalli, T. Pregnancy-Related Acute Kidney Injury: Experience of the Nephrology Unit at the University Hospital of Fez, Morocco. ISRN Nephrol. 2012, 2013, 109034. [Google Scholar] [CrossRef]
- Abdelkader, F.; Conte, A.B.; Saleh, A.M. Insuffisance rénale aigue du post partum: À propos de 102 cas au centre hospitalier National de Nouakchott, Mauritanie. PAMJ Clin. Med. 2020, 4, 48. [Google Scholar] [CrossRef]
- Msehli, M.; Jbali, H.; Ikram, M.; Ben Kaab, B.; Ben Hamida, F.; Rais, L.; BEN Fatma, L.; Karim, Z. Pregnancy-related acute kidney injury in Tunisia: A clinical challenge. Nephrol. Dial. Transplant. 2021, 36, gfab082-0024. [Google Scholar] [CrossRef]
- Elshinnawy, H.A.; Aref, H.M.; Rezk, K.M.; Elkotb, A.M.; Mohamed, A.Y. Study of Pregnancy related AKI in Egyptian patients: Incidence, Risk factors and Outcome. QJM Int. J. Med. 2020, 113, hcaa052-016. [Google Scholar] [CrossRef]
- Muhammad, A.S.; Usman, M.; Garba, B.I.; Abdullahi, U.; Mohammed, B.A.; Garba, S.; Liman, H.M.; Muhammad, A.M.; Bello, K.S. Pregnancy Related Acute Kidney Injury, Clinical profile and Outcome of management: An experience from 3 years retrospective review in a specialist hospital in Gusau, North-Western Nigeria. Trop. J. Nephrol. 2017, 12, 17–21. [Google Scholar]
- Conti-Ramsden, F.I.; Nathan, H.L.; De Greeff, A.; Hall, D.R.; Seed, P.T.; Chappell, L.; Shennan, A.H.; Bramham, K. Pregnancy-Related Acute Kidney Injury in Preeclampsia: Risk Factors and Renal Outcomes. Hypertension 2019, 74, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Akuse, R.M.; Bosan, I.B.; Mohammed, H.H.; Eno, H. Outcome of pregnancy related acute kidney injury requiring haemodialysis in a Nigerian teaching hospital. Ther. RRT 2013, 8, 11. [Google Scholar]
- Bouaziz, M.; Chaari, A.; Turki, O.; Dammak, H.; Chelly, H.; Ammar, R.; Nasri, A.; Ben Algia, N.; Bahloul, M.; Ben Hamida, C. Acute renal failure and pregnancy: A seventeen-year experience of a Tunisian intensive care unit. Ren. Fail. 2013, 35, 1210–1215. [Google Scholar] [CrossRef]
- Oluseyi, A.; Ayodeji, A.; Ayodeji, F. Aetiologies and Short-term Outcomes of Acute Kidney Injury in a Tertiary Centre in Southwest Nigeria. Ethiop. J. Health Sci. 2016, 26, 37–44. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, X.; Zheng, J.; Liu, X.; Yan, T. Pregnancy outcomes in patients with acute kidney injury during pregnancy: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2017, 17, 235. [Google Scholar] [CrossRef]
- Prasad, N.; Gupta, A.; Bhadauria, D.; Kaul, A.; Sharma, R.; Kapoor, D.; Singh, R.; Krishna, A. Maternal, fetal and renal outcomes of pregnancy-associated acute kidney injury requiring dialysis. Indian J. Nephrol. 2015, 25, 77–81. [Google Scholar] [CrossRef]
- Prakash, J.; Pant, P.; Prakash, S.; Sivasankar, M.; Vohra, R.; Doley, P.; Pandey, L.; Singh, U. Changing picture of acute kidney injury in pregnancy: Study of 259 cases over a period of 33 years. Indian J. Nephrol. 2016, 26, 262–267. [Google Scholar] [CrossRef]
- Belayev, L.Y.; Palevsky, P.M. The link between acute kidney injury and chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2014, 23, 149–154. [Google Scholar] [CrossRef]
- Parr, S.K.; Matheny, M.E.; Abdel-Kader, K.; Greevy, R.A.; Bian, A.; Fly, J.; Chen, G.; Speroff, T.; Hung, A.M.; Ikizler, T.A.; et al. Acute kidney injury is a risk factor for subsequent proteinuria. Kidney Int. 2018, 93, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Coca, S.G.; Singanamala, S.; Parikh, C.R. Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int. 2012, 81, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.C.; Best, K.E.; Pearce, M.S.; Waugh, J.; Robson, S.C.; Bell, R. Cardiovascular disease risk in women with pre-eclampsia: Systematic review and meta-analysis. Eur. J. Epidemiol. 2013, 28, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Barrett, P.M.; McCarthy, F.P.; Evans, M.; Kublickas, M.; Perry, I.J.; Stenvinkel, P.; Khashan, A.S.; Kublickiene, K. Hypertensive disorders of pregnancy and the risk of chronic kidney disease: A Swedish registry-based cohort study. PLoS Med. 2020, 17, e1003255. [Google Scholar] [CrossRef]
- Covella, B.; Vinturache, A.E.; Cabiddu, G.; Attini, R.; Gesualdo, L.; Versino, E.; Piccoli, G.B. A systematic review and meta-analysis indicates long-term risk of chronic and end-stage kidney disease after preeclampsia. Kidney Int. 2019, 96, 711–727. [Google Scholar] [CrossRef] [PubMed]
- Khashan, A.S.; Evans, M.; Kublickas, M.; McCarthy, F.P.; Kenny, L.C.; Stenvinkel, P.; Fitzgerald, T.; Kublickiene, K. Preeclampsia and risk of end stage kidney disease: A Swedish nationwide cohort study. PLoS Med. 2019, 16, e1002875. [Google Scholar] [CrossRef] [PubMed]
- Kattah, A. Preeclampsia and Kidney Disease: Deciphering Cause and Effect. Curr. Hypertens. Rep. 2020, 22, 91. [Google Scholar] [CrossRef]
- Say, L.; Chou, D.; Gemmill, A.; Tunçalp, Ö.; Moller, A.-B.; Daniels, J.; Gülmezoglu, A.M.; Temmerman, M.; Alkema, L. Global causes of maternal death: A WHO systematic analysis. Lancet Glob. Health 2014, 2, E323–E333. [Google Scholar] [CrossRef]
- Musarandega, R.; Nyakura, M.; Machekano, R.; Pattinson, R.; Munjanja, S.P. Causes of maternal mortality in Sub-Saharan Africa: A systematic review of studies published from 2015 to 2020. J. Glob. Health 2021, 11, 04048. [Google Scholar] [CrossRef]
- Raguema, N.; Gannoun, M.B.A.; Zitouni, H.; Ben Letaifa, D.; Seda, O.; Mahjoub, T.; Lavoie, J.L. Contribution of -1031T/C and -376G/A tumor necrosis factor alpha polymorphisms and haplotypes to preeclampsia risk in Tunisia (North Africa). J. Reprod. Immunol. 2022, 149, 103461. [Google Scholar] [CrossRef]
- Bombell, S.; McGUIRE, W. Tumour necrosis factor (-308A) polymorphism in pre-eclampsia: Meta-analysis of 16 case-control studies. Aust. N. Z. J. Obstet. Gynaecol. 2008, 48, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Heiskanen, J.; Romppanen, E.-L.; Hiltunen, M.; Iivonen, S.; Mannermaa, A.; Punnonen, K.; Heinonen, S. Polymorphism in the Tumor Necrosis Factor-α Gene in Women with Preeclampsia. J. Assist. Reprod. Genet. 2002, 19, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Thakoordeen-Reddy, S.; Winkler, C.; Moodley, J.; David, V.; Binns-Roemer, E.; Ramsuran, V.; Naicker, T. Maternal variants within the apolipoprotein L1 gene are associated with preeclampsia in a South African cohort of African ancestry. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 246, 129–133. [Google Scholar] [CrossRef]
- Hong, X.; Rosenberg, A.Z.; Zhang, B.; Binns-Roemer, E.; David, V.; Lv, Y.; Hjorten, R.C.; Reidy, K.J.; Chen, T.K.; Wang, G.; et al. Joint Associations of Maternal-Fetal APOL1 Genotypes and Maternal Country of Origin with Preeclampsia Risk. Am. J. Kidney Dis. 2021, 77, 879–888.e1. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.; Azhibekov, T.; O’Toole, J.F.; Sedor, J.R.; Williams, S.M.; Redline, R.W.; Bruggeman, L.A. Association of preeclampsia with infant APOL1 genotype in African Americans. BMC Med. Genet. 2020, 21, 110. [Google Scholar] [CrossRef] [PubMed]
- Dhanjal, M.; Owen, E.; Anthony, J.; Davidson, J.; Rayner, B. Short communication: Association of pre-eclampsia with the R563Q mutation of the β-subunit of the epithelial sodium channel. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Basic Statistics: HIV 2022. Available online: https://www.cdc.gov/hiv/basics/statistics.html (accessed on 21 June 2022).
- The Global HIV/AIDS Epidemic. Available online: https://www.hiv.gov/hiv-%20basics/overview/dataand-trends/global-%20statistics (accessed on 6 August 2022).
- Wong, C.; Gange, S.J.; Buchacz, K.; Moore, R.D.; Justice, A.C.; Horberg, M.A.; Gill, M.J.; Koethe, J.R.; Rebeiro, P.F.; Silverberg, M.J.; et al. First Occurrence of Diabetes, Chronic Kidney Disease, and Hypertension among North American HIV-Infected Adults, 2000–2013. Clin. Infect. Dis. 2016, 64, 459–467. [Google Scholar] [CrossRef]
- Ahuja, T.S.; Grady, J.; Khan, S. Changing Trends in the Survival of Dialysis Patients with Human Immunodeficiency Virus in the United States. J. Am. Soc. Nephrol. 2002, 13, 1889–1893. [Google Scholar] [CrossRef]
- Fabian, J.; Maher, H.A.; Clark, C.; Naicker, S.; Becker, P.; Venter, W.D.F. Morbidity and mortality of black HIV-positive patients with end-stage kidney disease receiving chronic haemodialysis in South Africa. South Afr. Med. J. 2015, 105, 110–114. [Google Scholar] [CrossRef]
- Halle, M.P.; Edjomo, A.M.; Fouda, H.; Djantio, H.; Essomba, N.; Ashuntantang, G.E. Survival of HIV infected patients on maintenance hemodialysis in Cameroon: A comparative study. BMC Nephrol. 2018, 19, 166. [Google Scholar] [CrossRef]
- Davids, M.R.; Jardine, R.; Marais, N.; Sebastian, S.; Davids, T.; Jacobs, J.C. South African Renal Registry Annual Report 2019. Afr. J. Nephrol. 2021, 24, 95–106. [Google Scholar] [CrossRef]
- Bookholane, H.; Wearne, N.; Surapaneni, A.; Ash, S.; Berghammer-Böhmer, R.; Omar, A.; Spies, R.; Grams, M.E. Predictors and Prognosis of HIV-Associated Nephropathy on Kidney Biopsy in South Africa. Kidney Int. Rep. 2020, 5, 1799–1804. [Google Scholar] [CrossRef] [PubMed]
- Diana, N.E.; Davies, M.; Mosiane, P.; Vermeulen, A.; Naicker, S. Clinicopathological correlation of kidney disease in HIV infection pre- and post- ART rollout. PLoS ONE 2022, 17, e0269260. [Google Scholar] [CrossRef] [PubMed]
- Kudose, S.; Santoriello, D.; Bomback, A.S.; Stokes, M.B.; Batal, I.; Markowitz, G.S.; Wyatt, C.M.; D’Agati, V.D. The spectrum of kidney biopsy findings in HIV-infected patients in the modern era. Kidney Int. 2020, 97, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Berliner, A.R.; Fine, D.M.; Lucas, G.M.; Rahman, M.H.; Racusen, L.C.; Scheel, P.J.; Atta, M.G. Observations on a Cohort of HIV-Infected Patients Undergoing Native Renal Biopsy. Am. J. Nephrol. 2008, 28, 478–486. [Google Scholar] [CrossRef]
- Wearne, N.; Swanepoel, C.R.; Boulle, A.; Duffield, M.S.; Rayner, B.L. The spectrum of renal histologies seen in HIV with outcomes, prognostic indicators and clinical correlations. Nephrol. Dial. Transplant. 2012, 27, 4109–4118. [Google Scholar] [CrossRef]
- Wearne, N.; Manning, K.; Price, B.; Rayner, B.L.; Davidson, B.; Jones, E.S.; Spies, R.; Cunningham, C.; Omar, A.; Ash, S.; et al. The Evolving Spectrum of Kidney Histology in HIV-Positive Patients in South Africa. Kidney Int. Rep. 2023, in press. [Google Scholar] [CrossRef]
- Available online: https://www.who.int/news/item/27-10-2022-tuberculosis-deaths-and-disease-increase-during-the-covid-19-pandemic (accessed on 14 February 2023).
- Gelaw, Y.A.; Williams, G.; Magalhães, R.J.S.; Gilks, C.F.; Assefa, Y. HIV Prevalence Among Tuberculosis Patients in Sub-Saharan Africa: A Systematic Review and Meta-analysis. AIDS Behav. 2019, 23, 1561–1575. [Google Scholar] [CrossRef]
- Lanjewar, D.N.; Duggal, R. Pulmonary pathology in patients with AIDS: An autopsy study from Mumbai. HIV Med. 2001, 2, 266–271. [Google Scholar] [CrossRef]
- Soriano-Rosas, J.; Avila-Casado, M.; Carrera-Gonzalez, E.; Chavez-Mercado, L.; Cruz-Ortiz, H.; Rojo, J. AIDS-associated Nephropathy: 5-year Retrospective Morphologic Analysis of 87 Cases. Pathol. Res. Pract. 1998, 194, 567–570. [Google Scholar] [CrossRef]
- Wearne, N.; Hung, R.; Bohmer, R.; Spies, R.; Omar, A.; Ash, S.; Ibrahim, F.; Miller, R.F.; Booth, J.W.; Lucas, S.B.; et al. Kidney disease in Africans with HIV and tuberculosis. Aids 2019, 33, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B.; Nel, D.; Jones, E.S.W.; Manning, K.; Spies, R.; Bohmer, R.; Omar, A.; Ash, S.; Wearne, N. Granulomatous interstitial nephritis on renal biopsy in human immunodeficiency virus positive patients: Prevalence and causes in Cape Town, South Africa. Nephrology 2019, 24, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Lawn, S.D.; Bekker, L.-G.; Miller, R.F. Immune reconstitution disease associated with mycobacterial infections in HIV-infected individuals receiving antiretrovirals. Lancet Infect. Dis. 2005, 5, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.A.; He, G.-X.; Eisenberg, E.; Cihlar, T.; Swaminathan, S.; Mulato, A.; Cundy, K.C. Selective Intracellular Activation of a Novel Prodrug of the Human Immunodeficiency Virus Reverse Transcriptase Inhibitor Tenofovir Leads to Preferential Distribution and Accumulation in Lymphatic Tissue. Antimicrob. Agents Chemother. 2005, 49, 1898–1906. [Google Scholar] [CrossRef]
- E Sax, P.; Wohl, D.; Yin, M.T.; Post, F.; DeJesus, E.; Saag, M.; Pozniak, A.; Thompson, M.; Podzamczer, D.; Molina, J.M.; et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: Two randomised, double-blind, phase 3, non-inferiority trials. Lancet 2015, 385, 2606–2615. [Google Scholar] [CrossRef]
- Atta, M.G.; De Seigneux, S.; Lucas, G.M. Clinical Pharmacology in HIV Therapy. Clin. J. Am. Soc. Nephrol. 2019, 14, 435–444. [Google Scholar] [CrossRef]
- Walmsley, S.L.; Antela, A.A.; Clumeck, N.; Duiculescu, D.; Eberhard, A.A.; Gutiérrez, F.; Hocqueloux, L.L.; Maggiolo, F.F.; Sandkovsky, U.U.; Granier, C.C.; et al. Dolutegravir plus Abacavir–Lamivudine for the Treatment of HIV-1 Infection. N. Engl. J. Med. 2013, 369, 1807–1818. [Google Scholar] [CrossRef]
- Muller, E.; Barday, Z. HIV-Positive Kidney Donor Selection for HIV-Positive Transplant Recipients. J. Am. Soc. Nephrol. 2018, 29, 1090–1095. [Google Scholar] [CrossRef]
- Muller, E.; Barday, Z.; Mendelson, M.; Kahn, D. HIV-Positive–to–HIV-Positive Kidney Transplantation—Results at 3 to 5 Years. N. Engl. J. Med. 2015, 372, 613–620. [Google Scholar] [CrossRef]
- Selhorst, P.; Combrinck, C.E.; Manning, K.; Botha, F.C.; Labuschagne, J.P.; Anthony, C.; Matten, D.L.; Breaud, A.; Clarke, W.; Quinn, T.C.; et al. Longer-Term Outcomes of HIV-Positive–to–HIV-Positive Renal Transplantation. N. Engl. J. Med. 2019, 381, 1387–1389. [Google Scholar] [CrossRef]
- Barday, Z.; Manning, K.; Freercks, R.; Bertels, L.; Wearne, N.; Muller, E. Retrospective Review of ART Regimens in HIV-Positive to HIV-Positive Kidney Transplant Recipients. Kidney Int. Rep. 2022, 7, 2039–2046. [Google Scholar] [CrossRef] [PubMed]
- Nobakht, E.; Cohen, S.D.; Rosenberg, A.Z.; Kimmel, P.L. HIV-associated immune complex kidney disease. Nat. Rev. Nephrol. 2016, 12, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Swanepoel, C.R.; Atta, M.G.; D’Agati, V.D.; Estrella, M.M.; Fogo, A.B.; Naicker, S.; Post, F.A.; Wearne, N.; Winkler, C.A.; Cheung, M.; et al. Kidney disease in the setting of HIV infection: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2018, 93, 545–559. [Google Scholar] [CrossRef]
- Booth, J.W.; Hamzah, L.; Jose, S.; Horsfield, C.; O’Donnell, P.; McAdoo, S.; Kumar, E.A.; Turner-Stokes, T.; Khatib, N.; Das, P.; et al. Clinical characteristics and outcomes of HIV-associated immune complex kidney disease. Nephrol. Dial. Transplant. 2016, 31, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Inusah, A.-J.; Coetzee, L.; Bates, W.; Chothia, M.-Y. Kidney outcomes of immune-complex associated mesangiocapillary glomerulonephritis in patients with and without HIV. J. Nephropathol. 2022, 11, e17269. [Google Scholar] [CrossRef]
- Fabian, J.; Gondwe, M.; Mayindi, N.; Chipungu, S.; Khoza, B.; Gaylard, P.; Wade, A.N.; Gómez-Olivé, F.X.; A Tomlinson, L.; Ramsay, M.; et al. Chronic kidney disease (CKD) and associated risk in rural South Africa: A population-based cohort study. Wellcome Open Res. 2022, 7, 236. [Google Scholar] [CrossRef]
- Aseneh, J.B.; Kemah, B.-L.A.; Mabouna, S.; Njang, M.E.; Ekane, D.S.M.; Agbor, V.N. Chronic kidney disease in Cameroon: A scoping review. BMC Nephrol. 2020, 21, 409. [Google Scholar] [CrossRef] [PubMed]
- Abdissa, D. Purposeful Review to Identify Risk Factors, Epidemiology, Clinical Features, Treatment and Prevention of Chronic Kidney Disease of Unknown Etiology. Int. J. Nephrol. Renov. Dis. 2020, 13, 367–377. [Google Scholar] [CrossRef]
- Magombo, M.; Barregard, L.; Kgalamono, S.; George, J.; Naicker, S.; Dorkin, E.; Made, F.; Rees, D. O4D.4 Changes in kidney function among sugarcane cutters on a moderately hot sugar plantation in South Africa. Occup. Environ. Med. 2019, 76, A38. [Google Scholar] [CrossRef]
- Ajayi, S.; Raji, Y.R.; Michael, O.S.; Adewole, D.; Akande, T.; Abiola, B.; Aminu, S.; Olugbenga-Bello, A.; Arije, A. Exposure to Agrochemicals and Markers of Kidney Damage among Farmers in Rural Communities in Southwestern Nigeria. West Afr. J. Med. 2021, 38, 48–53. [Google Scholar]
- Olowogbon, T.S.; O Babatunde, R.; Asiedu, E.; Yoder, A.M. Agrochemical Health Risks Exposure and Its Determinants: Empirical Evidence among Cassava Farmers in Nigeria. J. Agromed. 2020, 26, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.A.; Nakanga, W.P.; Prynn, J.E.; Crampin, A.C.; Fecht, D.; Vineis, P.; Caplin, B.; Pearce, N.; Nyirenda, M.J. Prevalence and risk factors for chronic kidney disease of unknown cause in Malawi: A cross-sectional analysis in a rural and urban population. BMC Nephrol. 2020, 21, 387. [Google Scholar] [CrossRef] [PubMed]
- Hodel, N.C.; Hamad, A.; Praehauser, C.; Mwangoka, G.; Kasella, I.M.; Reither, K.; Abdulla, S.; Hatz, C.F.R.; Mayr, M. The epidemiology of chronic kidney disease and the association with non-communicable and communicable disorders in a population of sub-Saharan Africa. PLoS ONE 2018, 13, e0205326. [Google Scholar] [CrossRef] [PubMed]
- Conroy, A.L.; Opoka, R.O.; Bangirana, P.; Idro, R.; Ssenkusu, J.M.; Datta, D.; Hodges, J.S.; Morgan, C.; John, C.C. Acute kidney injury is associated with impaired cognition and chronic kidney disease in a prospective cohort of children with severe malaria. BMC Med. 2019, 17, 98. [Google Scholar] [CrossRef] [PubMed]
- George, C.; Yako, Y.Y.; Okpechi, I.G.; E Matsha, T.; Folefack, F.J.K.; Kengne, A.P. An African perspective on the genetic risk of chronic kidney disease: A systematic review. BMC Med. Genet. 2018, 19, 187. [Google Scholar] [CrossRef] [PubMed]
- Cave, E.M.; Prigge, K.L.; Crowther, N.J.; George, J.A.; Padoa, C.J. A Polymorphism in the Gene Encoding the Insulin Receptor Binding Protein ENPP-1 Is Associated with Decreased Glomerular Filtration Rate in an Under-Investigated Indigenous African Population. Kidney Blood Press. Res. 2020, 45, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Ekrikpo, U.E.; Mnika, K.; Effa, E.E.; Ajayi, S.O.; Okwuonu, C.; Waziri, B.; Bello, A.; Dandara, C.; Kengne, A.P.; Wonkam, A.; et al. Association of Genetic Polymorphisms of TGF-β1, HMOX1, and APOL1 With CKD in Nigerian Patients With and Without HIV. Am. J. Kidney Dis. 2020, 76, 100–108. [Google Scholar] [CrossRef]
- Fatumo, S.; Chikowore, T.; Kalyesubula, R.; Nsubuga, R.N.; Asiki, G.; Nashiru, O.; Seeley, J.; Crampin, A.C.; Nitsch, D.; Smeeth, L.; et al. Discovery and fine-mapping of kidney function loci in first genome-wide association study in Africans. Hum. Mol. Genet. 2021, 30, 1559–1568. [Google Scholar] [CrossRef]
| Analysis | PRAKI n (%) | No of Deliveries/Total Cohort | Percentage PRAKI Caused by PET | Time of PRAKI | AKI Stage 3 | Dialysis | Mortality in PRAKI | Full Recovery | |
|---|---|---|---|---|---|---|---|---|---|
| Gaber Egypt (2021) [55] | Prospective referral nephrology | 40 (1%) | 4500 | 35% | 45% 3 32% PP | - | - | 22.5% | 62.5% 35% D |
| Adejumo Nigeria (2019) [56] | Retrospective review of PRAKI | 32 | - | 18.8% | 34.4% 3 56.3% PP | 71.9% R | 5.4% (25% ND) | 34.4% | 53.1% 3.1% D |
| Awolwale Nigeria (2018) [57] | Retrospective PRAKI requiring HD | 43 (3.8%) D | 11,242 | 40% | 22.5% 3 50% PP | all | 100% (9.3% WD) | 17.5% | 72.5% |
| Cooke Malawi (2018) [58] | Prospective | 26 (1.1%) | 2300 | 73.1% | - | 23% K | 0 | 0 | 84.6% |
| Kabbali Morocco (2015) [59] | Prospective based on referrals | 44 | - | 63.6% | 40% 3 52.3% PP | 61% R | 38.6% | 11.4% | 66% |
| Arrayhani Morocco (2013) [60] | Prospective | 37 (0.66%) | 5600 | 66.6% | 61.1% 3 22% PP | 33% R | 16.2% | 1 | 76% 5.4% D |
| Abdelkader Mauritius (2020) [61] | Retrospective referral nephrology | 102 (6.9%) | 1675 | 61.2% | - | - | 38.2% | 9.8% | 39.2% |
| Msheli, M Tunisia 2021 [62] | Retrospective | 96 (100%) | - | 40% | - | - | 23.9% | 13% | 75% |
| Elshinnawy Egypt 2020 [63] | Prospective observational | 78 (0.5%) | 13,050 | - | - | - | 15.3% | 14% | 60% 7.6% D |
| Aminu, MS. Nigeria 2017 [64] | Retrospective review | 26 | - | 23% | 77% 3 | 26.9% | 30.7% | 65.3% 3.8% D | |
| Studies related to PRAKI secondary to HDP cohort | |||||||||
| Conti-Ramsden South Africa (2019) [65] | Retrospective analysis of cohort with PET | 237 (15.3%) | Total in cohort 1547 | 100% | - | 4.1% K | 12.5% | 3% in total cohort | 80.4% |
| Studies related to PRAKI in defined cohorts ICU/AKI or dialysis cohorts | |||||||||
| Bentata Morocco 2012 [51] | Retrospective analysis of PRAKI in Obstetric ICU | 43 | Total PRAKI cohort in ICU 43 | 60% | - | 27.9% R | 11.6% | 25.6% in total cohort | 84.4% |
| Akuse Nigeria [66] | Retrospective audit on acute dialysis obstetric cohort | 8 (5.3%) D | 483 | 50% | - | 100% | 7 (87.2%) 1(12.5% ND) | 25% | 12.5% |
| Bouaziz Tunisia [67] | Retrospective review of ICU admissions | 313 (56.9%) | 550 ICU cohort | 66.5% | - | 6% R | 1.7% | 9.3% | 0.4% D |
| Oluyesi Nigeria [68] | Retrospective review of AKI cohort | 18 (19.8%) | 91 AKI patients | 33% | - | * 62.2% K | 38.5% | 38.5% ** | 52.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rayner, B.L.; Jones, E.S.W.; Davidson, B.; Wearne, N. Advances in Chronic Kidney Disease in Africa. Appl. Sci. 2023, 13, 4924. https://doi.org/10.3390/app13084924
Rayner BL, Jones ESW, Davidson B, Wearne N. Advances in Chronic Kidney Disease in Africa. Applied Sciences. 2023; 13(8):4924. https://doi.org/10.3390/app13084924
Chicago/Turabian StyleRayner, Brian L., Erika S. W. Jones, Bianca Davidson, and Nicola Wearne. 2023. "Advances in Chronic Kidney Disease in Africa" Applied Sciences 13, no. 8: 4924. https://doi.org/10.3390/app13084924
APA StyleRayner, B. L., Jones, E. S. W., Davidson, B., & Wearne, N. (2023). Advances in Chronic Kidney Disease in Africa. Applied Sciences, 13(8), 4924. https://doi.org/10.3390/app13084924







