Abstract
The aim of the present study was to investigate the photoinduced properties of nitrogen-doped titanium dioxide (N-TiO2) against the Salmonella ser. Typhimurium bacterial biofilm, under visible-light irradiation. The capability of N-TiO2 nanoparticles working as multipurpose materials with antimicrobial applications, as well as environmental ones, was therefore investigated. The sol–gel method was used to synthesize N-TiO2 particles, which were then characterized by Fourier-transform infrared (FTIR) spectroscopy, dynamic light scattering (DLS), Brunauer–Emmett–Teller (BET) analysis of surface area, scanning electron microscopy with energy-dispersive X-ray spectroscopy (SEM/EDS), and transmission electron microscopy (TEM). The results showed that the particles formed were nano-sized and had the expected Ti-O bonds and the presence of elemental N. The as-produced N-TiO2 nanoparticles (NPs) were tested for their antimicrobial activity. The antibacterial photocatalytic testing was performed under visible-light irradiation, on Salmonella Typhimurium biofilm. To form the biofilm, stainless steel (ss) coupons were incubated with three different strains of Salmonella Typhimurium bacteria for 48 h at 15 °C in tryptone soy broth (TSB). After the biofilm’s formation, the coupons were placed on a horizontal, rectangular, batch, equipped with a vis-LED irradiation source reactor in the presence of N-TiO2 NPs. After 1, 2, and 3 h of irradiation, sampling of the bacterial population was assessed. The results showed an evident inhibition of proliferation under light irradiation when the N-TiO2 was present, compared to the non-irradiated NPs. It is noteworthy that, during the first 2 h, the TiO2 NPs specimens tended to attract more bacteria on their surface then the control specimens, due to their higher available surface area, which worked as a shelter. There were ~6% viable (remaining) Salmonella cells after the first hour of visible-light irradiation with N-TiO2 NPs.
1. Introduction
In recent years, there has been increased scientific interest in how to control bacterial infections in health as well as in the food industry, due to the outbreak of the recent COVID-19 pandemic, along with the longstanding issue of antibiotic resistance in pathogens [1,2]. To achieve this, various organic and inorganic compounds have been evaluated in terms of their antimicrobial properties—especially nanoparticles, as they are a low-cost and effective solution to the problem of bacterial contamination [3]. These nanoparticles can be carbon-based [4] or metal- and metal-oxide-based [5]. Among the latter, titanium dioxide (TiO2) is a widely studied semiconductor with various antimicrobial and self-cleaning properties [6,7]. It is found in three crystalline phases that have differences in stability and catalytic performance. Brookite is orthorhombic and more unstable, whereas rutile and anatase are tetragonal and are more effective catalytically [8]. A TiO2 catalyst can exhibit different physicochemical properties, depending on the shape or size of the NPs and the dominant crystalline phase present [9]. The energy gap between the valence and conduction bands (Eg) is ~3.2 eV for anatase, which makes it easy to sensitize via ultraviolet (UV) irradiation and, consequently, imparts it with self-cleaning and antibacterial behavior [10]. In order to extend these features under visible-light irradiation, TiO2 can be chemically modified or doped with non-metal or metal elements [11,12].
Regarding the antimicrobial properties of doped TiO2, there has been extensive scientific research testing TiO2 doped with metals such as Cu [13], Zn, Ag [14], Fe [15], and Ni [16]. However, it has been observed that metal doping leads to biological and environmental issues due to their toxicity and low biocompatibility [17]. Consequently, a more eco-friendly choice needs to be found [18]. A low-cytotoxicity solution is the syncretism of TiO2 with carbon (quantum dots) [19] or other non-metal elements. The non-metal doping approach, especially with elemental N, is an effective, low-cost, and environmentally safe way to deal with bacterial spreading. Another biocompatible approach is the combination of TiO2 with organic polymers such as chitosan [20]. The antimicrobial properties of N-TiO2 have always attracted the interest of the scientific community due to its easy use, testing, and low cost [21]. A recent study focused on the investigation of nitrogen and bismuth co-doping for implant-biofilm-related diseases [22]. The form of N-TiO2 tested is either a film [23] or a powder [24]. However, most of these studies tested the antimicrobial properties of TiO2 on single-standing bacteria and not on biofilms, which are the natural form of surface arrangement for microbes in nature [25,26].
Salmonella is recognized as one of the most significant enteric foodborne bacterial pathogens. Salmonella is a Gram-negative bacterium that can form biofilms on many biotic (e.g., human or animal tissues) and abiotic (e.g., glass or stainless steel) surfaces [27,28]. In recent years, the resistance of pathogens to biocides and other environmental stresses—especially when they are embedded in biofilm structures—has led to the search for and development of novel antimicrobial strategies capable of displaying both high efficiency and high safety [29]. Different parameters affecting the biofilm formation—such as temperature, pH, and quorum-sensing compounds—have been widely explored in previous studies; thus, the optimal conditions for Salmonella biofilm formation are well known and can be selected for testing [30,31]. In this direction, the aims of the present work were to evaluate the antimicrobial activity of N-TiO2 NPs under visible-light irradiation. It should be noted that this research actually extends the findings of a recent publication form our research group [32], in order to explore the capability of such particles to have end-use biomedical applications, along with environmental ones, illustrating that they can work as multifunctional materials.
To achieve this, N-TiO2 nanoparticles were synthesized by the sol–gel method. Full characterization of the particles followed, utilizing instrumental analysis techniques such as Fourier-transform infrared (FTIR) spectroscopy, dynamic light scattering (DLS), Brunauer–Emmett–Teller (BET) analysis of surface area, scanning electron microscopy with energy-dispersive X-ray spectroscopy (SEM/EDS), and transmission electron microscopy (TEM). All of the abovementioned techniques were applied to examine the physicochemical properties and morphology of the synthesized NPs, as well as to ensure that the particles were nano-sized. Salmonella biofilms were then conveyed and developed, so that the antimicrobial activity of the NPs could be evaluated under visible-light irradiation. This is a strong advantage in comparison with the established antimicrobial activity of TiO2, which is evident only under UV irradiation. Consequently, the novelty of this research is that this is the first study to investigate the antimicrobial properties of N-TiO2 NPs on biofilms—which are the natural state of the microbes found in nature—under visible-light irradiation (i.e., close to real-life conditions). These results can contribute to the problem of bacterial infection in the food industry or in hospitals and other public places.
2. Materials and Methods
2.1. Synthesis of N-Doped TiO2 NPs
The preparation method followed for the N-TiO2 nanoparticles has been previously published by our research group [32,33], where the sol–gel preparation method was utilized. Firstly, nitric acid (HNO3 65%, Penta, Prague, Czech Republic) was added to 100 × 10−6 m3 of deionized water so that the pH became acidic and hydrolysis phenomena could evolve. After that, 15 × 10−6 m3 of the titanium butoxide alkoxide precursor (titanium(IV) butoxide, C16H36O4Ti, 97%, Sigma-Aldrich, Darmstadt, Germany) was added. The solution was sub-white and was stirred vigorously for 5 h. Then, 30 × 10−6 m3 of 2-propanol (C3H8O 99.8%, Sigma-Aldrich, Darmstadt, Germany) was added, and the solution became transparent. This was the final titania sol–gel. For the doping, we added 30 × 10−3 kg of urea (CH4N2O 99%, Sigma-Aldrich, Darmstadt, Germany), under vigorous stirring. The solution was then heated so that all of the solvents were volatilized, and the gel produced was calcined for 4 h at 450 °C. The produced powder was triturated and centrifuged so that the impurities were removed, resulting in a yellow powder.
2.2. Characterization
The produced N-TiO2 powder was physicochemically and structurally analyzed by various techniques, including FTIR, DLS, BET, SEM/EDS, and TEM analysis. Each FTIR (model Spectrum 100, U-ATR, ZnSe Ri 2.4 crystal, PerkinElmer, Waltham, MA, USA) spectrum was obtained by performing 16 scans, with a 4 cm−1 resolution. The size distribution and zeta potential of the NPs were estimated with a dynamic light scattering apparatus (Zeta Sizer nano S, Malvern Inst., Malvern, UK). All of the measurements were performed at 25 °C and pH 6, on aqueous suspensions of the powder. The Brunauer–Emmett–Teller (BET) method (ChemBET 3000 Instrument, Yumpu, Diepoldsau, Switzerland) was applied to analyze the surface area, the average pore diameter, and the pore volume of the Ν-TiO2 NPs. The micropore surface area was calculated via t-plot analysis, according to the Harkins–Jura model; the cumulative volume of pores between 1.7 and 300 nm was calculated from N2-sorption data and the BJH desorption method, and the average pore diameter was calculated by the 4 V/σ method, where V was set to be equal to the maximum volume of N2 adsorbed along the isotherm as P/Po→1.0. The morphological characteristics and surface of the N-TiO2 powder were examined using a scanning electron microscope with energy-dispersive X-ray spectroscopy (SEM/EDS, JSM-6390, JEOL, Tokyo, Japan), along with a transmission electron microscope (TEM, CM20, Philips, Amsterdam, The Netherlands).
2.3. Antimicrobial Testing
In order to test the antibacterial properties of N-TiO2, Salmonella enterica subsp. enterica serovar Typhimurium strains from three different origins (FMCC B-137, human isolate epidemic; FMCC B-193, isolated from calf bowel—kindly provided by Prof. P. Skandamis; FMCC B-194, mutant derived from B-193—kindly provided by Prof. P. Skandamis) were used. The microorganisms were previously stored at −80 °C and were then resuscitated by adding brain heart infusion (BHI) broth (BH broth; Merck, Darmstadt, Germany). These pre-cultures were incubated for 24 h at 37 °C, and then an amount of them was added to BHI broth and incubated for another 18 h. These final cultures were centrifuged (5000 rpm, 10 min, 4 °C), washed and, finally, all of the strains of different origins were suspended in Ringer’s solution (Ringer’s tablets; Merck, Darmstadt, Germany). Stainless steel coupons (3 × 1 × 0.1 cm) were soaked in acetone and autoclaved at 121 °C for 15 min so that they were cleaned and sterilized. To achieve the formation of the biofilm on these coupons, Ringer’s solution containing the inocula of previously prepared strains was incubated with the ss coupons for 3 h at 15 °C, in order for the bacteria to attach to the ss surface [26]. The coupons were removed from the bacterial suspension with sterile forceps and were transferred to a half-tube containing Ringer’s solution [26]. The test tube rack was shaken, so that the loosely attached bacteria were removed, and the coupons were transferred into a half-tube with tryptone soy broth (TSB; LABM; International Diagnostics Group Plc, Bury, Lancashire, UK). Then, they were incubated for 48 h at 15 °C [26].
After the biofilm formation, the coupons were placed into a batch, rectangular, and horizontal photocatalytic reactor (dimensions 0.5 × 0.4 × 0.3 m), equipped on top with a white LED light tape of 6 W/m, 870 lm/m, and 12 V, with and without N-TiO2 NPs. The photoreactor was built with reflective walls (lCutoff = 320 nm), and all of the experiments were conducted under visible-light irradiation. After 1, 2, and 3 h of irradiation, the coupons were removed from the reactor with sterile forceps, so that they were removed aseptically, and then they were rinsed by pipetting twice with an 10 mL of quarter-strength Ringer’s solution. Afterwards, the coupons were immersed for 5 min in a sterile Falcon tube containing 5 mL of Ringer’s solution and 10 sterile glass beads, and they were shaken so that the attached cells were removed via bead-vortexing for 2 min. These detached cells were then enumerated by the agar plating method, after which they were serially diluted. An appropriate amount of the bacterial Ringer’s solution was distributed on tryptone soy agar (TSA, LAB M, Bury, Lancashire, UK) plates and then incubated at 37 °C for 24 h to assess the remaining biofilm cells, with the help of a cell counting device [26].
Coupons with Salmonella biofilms without the presence of N-TiO2, under visible-light irradiation and in the dark, as well as coupons with N-TiO2 NPs in the dark for 0, 1, 2, and 3 h of irradiation, were used as controls, for a more holistic approach to the study.
Every set of experiments was repeated twice (two independent experiments) and included three replicate samples (coupons) to ensure repeatability (a total of six different replicate samples).
3. Results and Discussion
3.1. FTIR Analysis
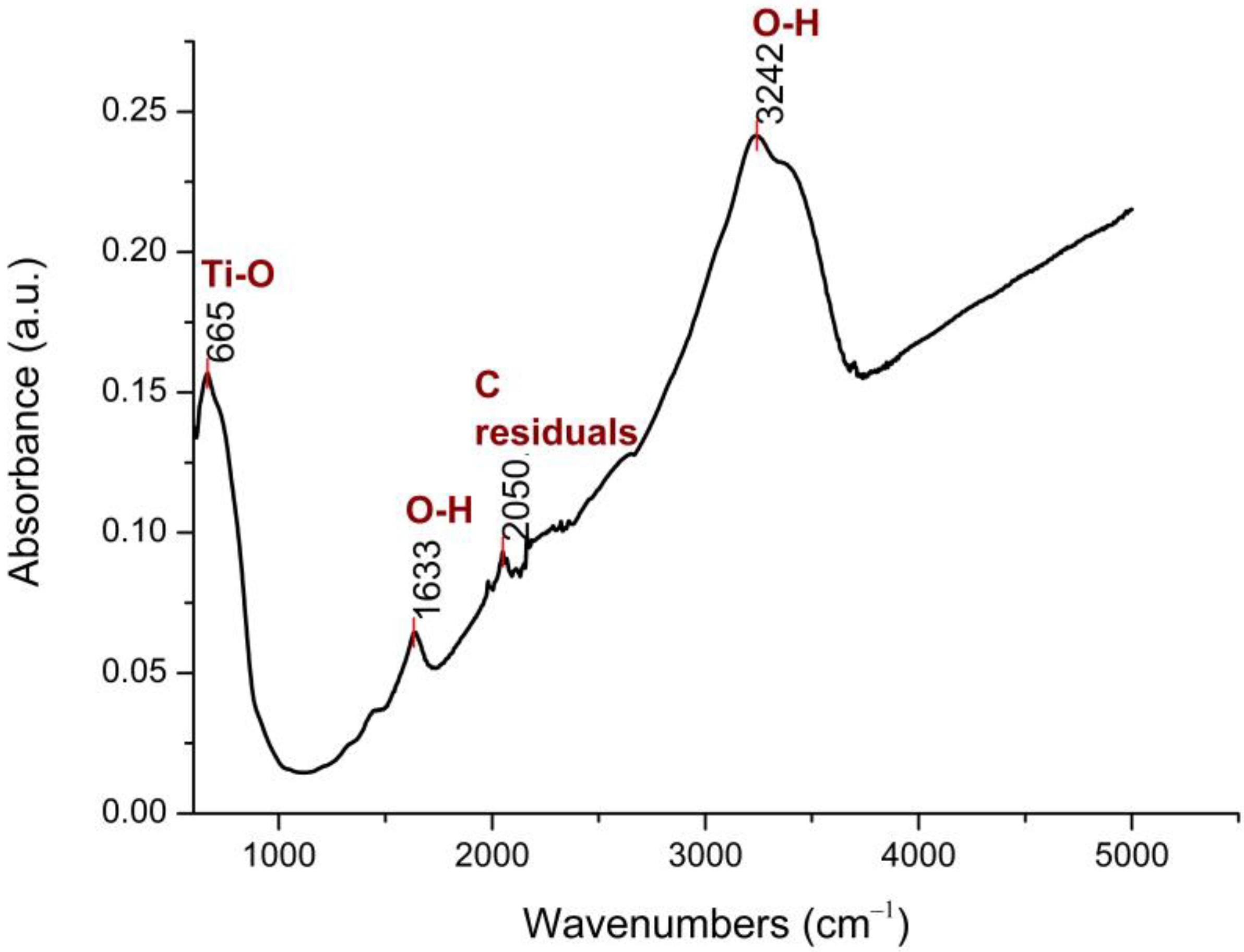
The FTIR spectrum of the N-TiO2 NPs is presented in Figure 1.
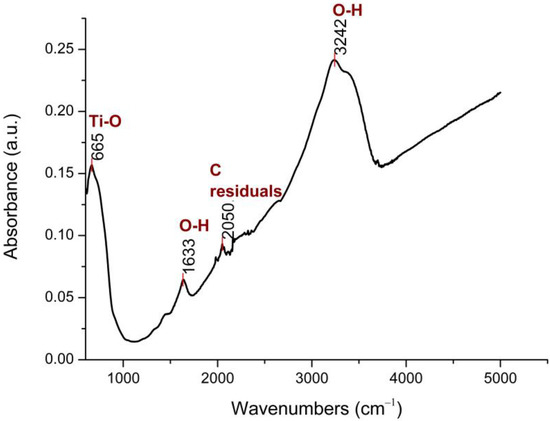
Figure 1.
FTIR spectrum of N-TiO2 powder, at 25 °C.
As shown in Figure 1, four major peaks were found, with the two broad bands at 3242 cm−1 and 1633 cm−1 being assigned to the O–H stretching and bending vibrations, respectively. The presence of these hydroxyl groups was due to water that was absorbed on the N-TiO2 NPs’ surface. The vibrational peak at 2050 cm−1 was a result of using organic solvents during the synthesis process and was attributed to C-residuals. The peak at 665 cm−1 was related to the Ti–O stretching mode of Ti–O–Ti bonds [34].
3.2. DLS Analysis
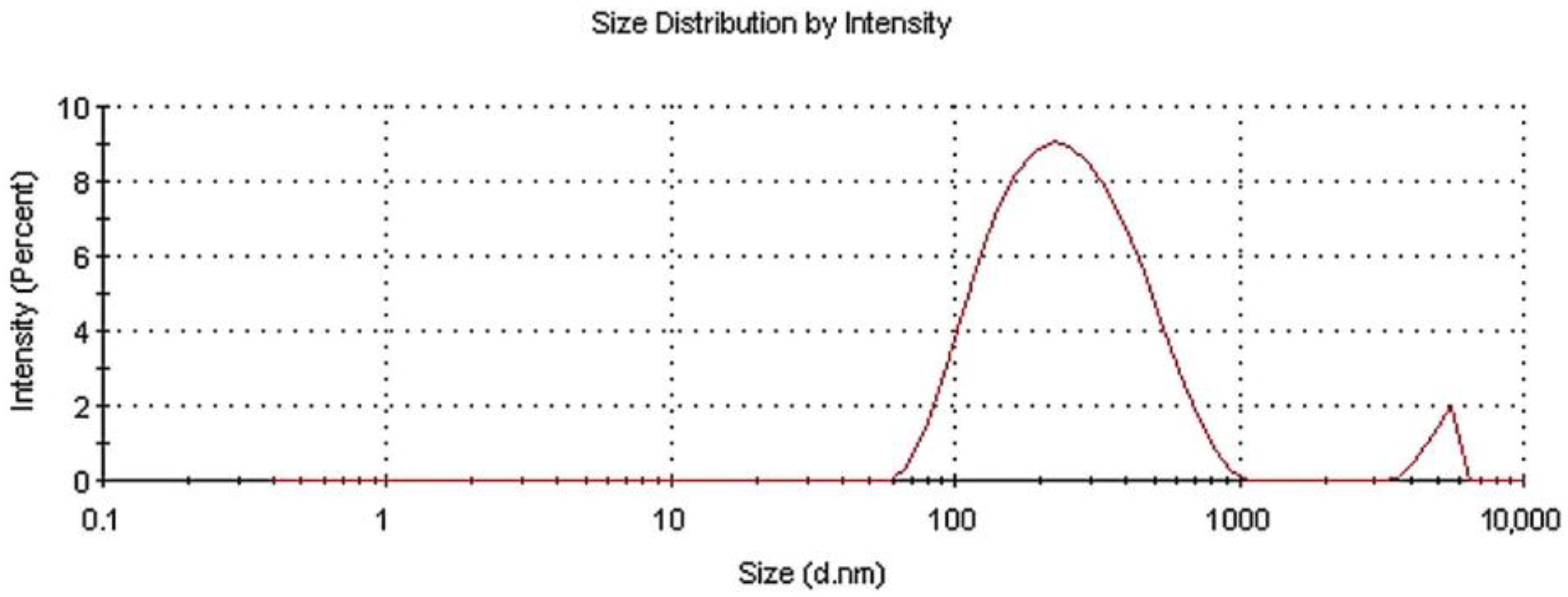
The size distribution of the as-prepared powder in aqueous colloidal dispersion was measured via DLS. Figure 2 presents the experimental size results.
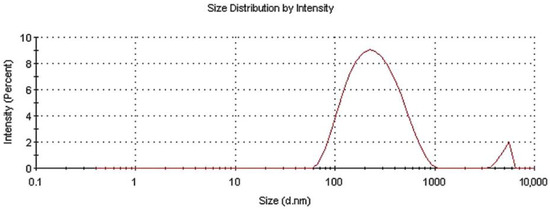
Figure 2.
Size distribution for N-TiO2 powder dispersed in water.
After the analysis, two different diffusing species with a distinct hydrodynamic radius distribution were revealed. The average hydrodynamic radius was estimated at ~237 nm, with two main peaks at 275.7 nm and 5076 nm being present. The first peak was attributed to nanoscale N-TiO2 particles, whereas the second was attributed to nanoparticles tending to agglomerate. The intensities of these peaks were 96.1% and 3.9%, respectively, while the polydispersity index (PDI) was 0.327. It is noteworthy that the size measured via this technique was that of the hydrodynamic radius in an aquatic environment; therefore, the particles tended to form colloids, aggregate into larger particles, and exhibit a larger average size than when in dry powder form [35].
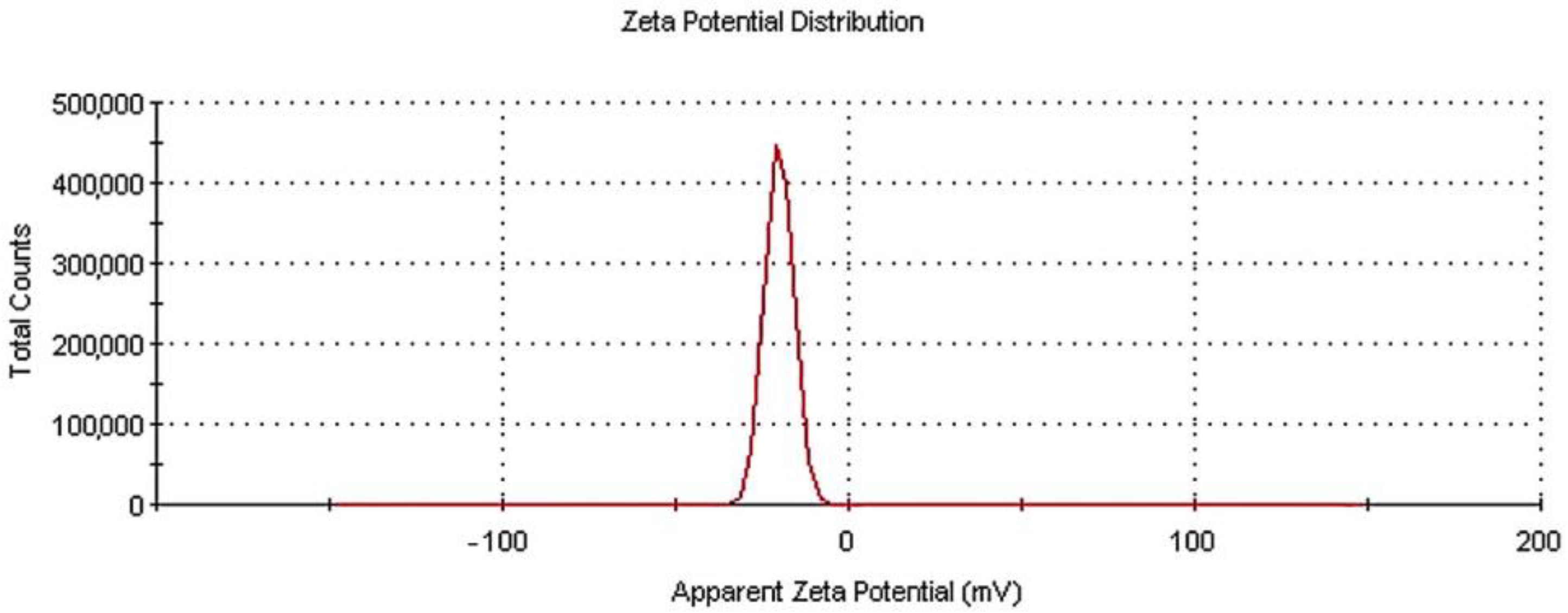
Figure 3 depicts the zeta potential distribution measured for the N-TiO2 particles dispersed in an aquatic environment.
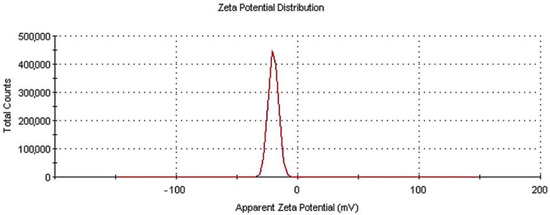
Figure 3.
Zeta potential distribution for N-TiO2 particles in water dispersion.
Through the zeta potential measurement, the electronic charge of the particles was estimated. When TiO2 or metal oxides in general are dispersed in water, there is a negative surface charge. The higher the zeta potential value, the grater the stability of the dispersion will be, so these measurements are of critical importance in the field of toxicology and related applications. The exact charge on the particle is dependent on the isoelectric point (IEP) in the aqueous suspension [36,37]. Here, the zeta potential result for the N-TiO2 powder was −20 mV, with a zeta deviation of 4.07 mV—a result considered to be moderately stable in literature [38]. All of the DLS experiments were repeated three times to ensure the reproducibility of the results.
3.3. BET Analysis
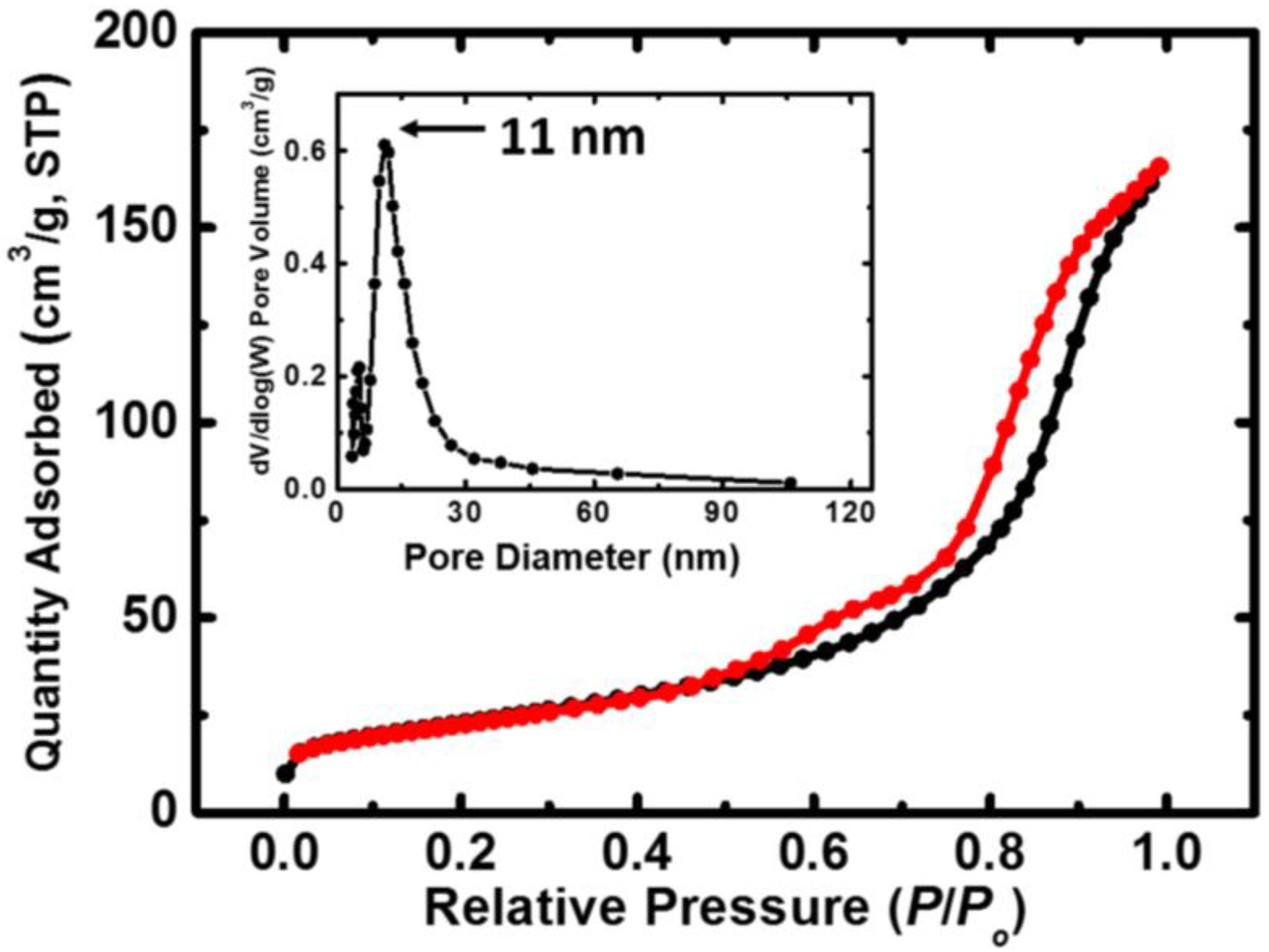
The isotherms obtained via the BET method on N-TiO2 powder, together with the pore size distribution, are given in Figure 4. The properties of the N-TiO2 powder as extracted from the BET analysis are presented in Table 1.
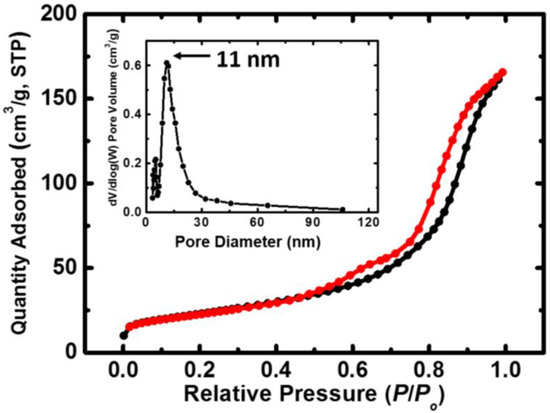
Figure 4.
N2-sorption diagram for the N-TiO2 nanoparticles. The black line represents adsorption and the red line represents desorption. The inset shows the pore size distribution as determined by the BJH method.

Table 1.
BET analysis and properties of N-TiO2 powder.
As we are investigating a catalyst (TiO2), surface area and adsorption–desorption phenomena are of great importance. In more detail, the smaller the particles, the more the surface area increases, leading to more active sites being available, as well as to higher photoactivity [39]. The surface area of the as-prepared N-TiO2 powder was 82 m2 g−1, which is higher than that of the Degussa P25 commercial powder (plain TiO2, 53 m2 g−1 surface area) [40]. The space between the adsorption and desorption isotherms in Figure 4 is linked to the porosity of the specimen and is expected for materials with pores ranging from 1.5 to 100 nm in size [41]. The estimated average pore diameter was 13 nm, and the powder was porous, with a 0.3 cm3 g−1 pore volume. All of the abovementioned results are in accordance with the relevant literature [40].
3.4. SEM/EDS and TEM Analysis
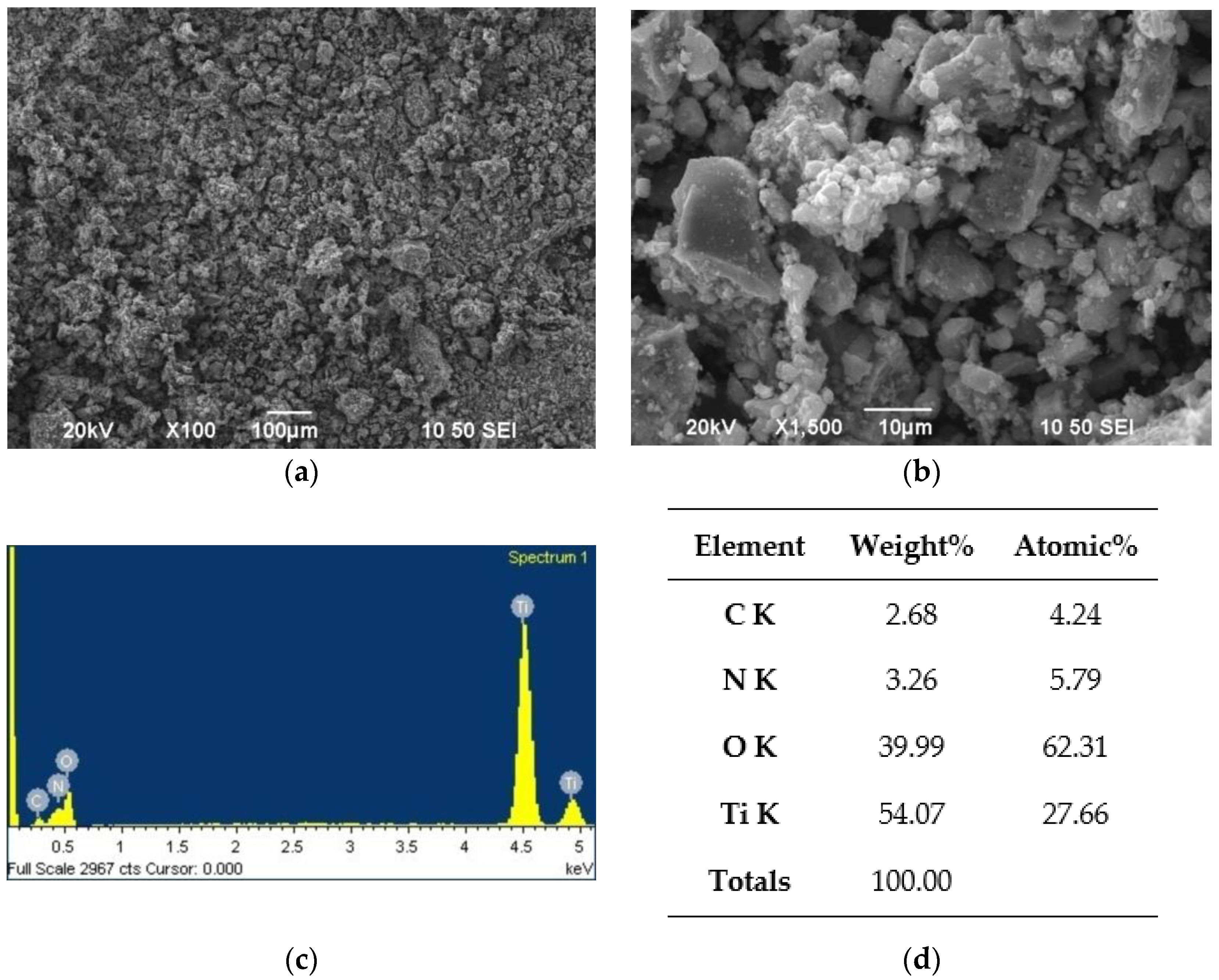
The N-TiO2 nanoparticles were morphologically tested by SEM/EDS. The images and elementary analysis are presented in Figure 5.
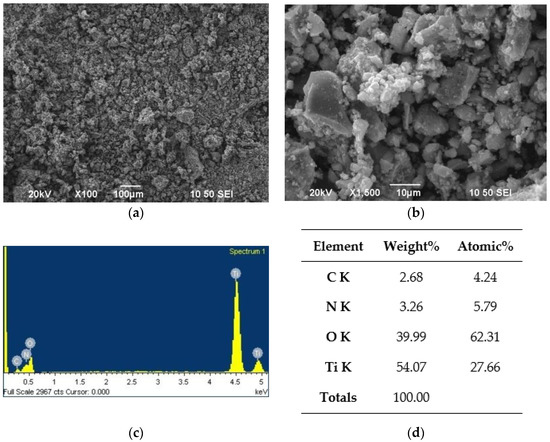
Figure 5.
SEM/EDS analysis for the N-TiO2 NPs: (a) ×100 magnification image of the powder, (b) ×1500 magnification image of the powder, (c) EDS spectrum of the powder, and (d) EDS analysis results of the powder.
The SEM images reveal a microstructural morphology, with particles of spherical shape. The EDS analysis (Figure 5d) revealed that the N dopant was present at 3.26 wt.%, with the expected Ti percentage being 54.07 wt.%. Oxygen and carbon were also found at 39.99 and 2.68 wt.%, respectively. This was due to the oxygen present in TiO2, and to carbon residuals from the final annealing step of the powder.
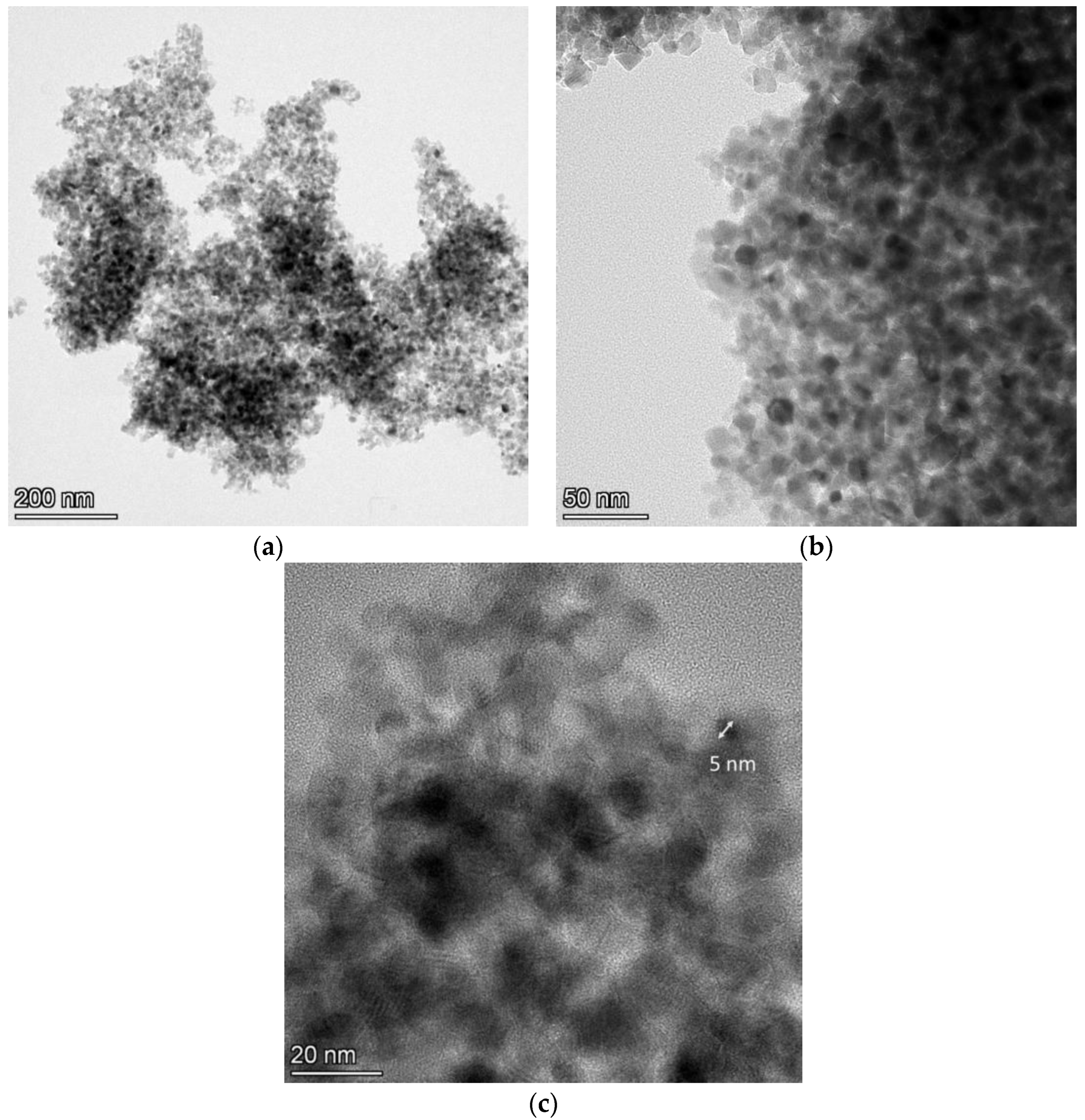
As the distinctive ability of the SEM apparatus is of a certain level, further examination of the powder via TEM analysis followed. Figure 6 shows the TEM images for the N-TiO2 NPs.
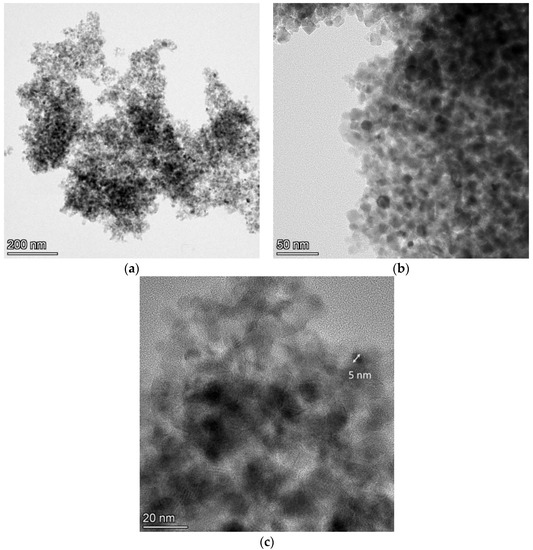
Figure 6.
TEM images at (a) ×58,000, (b) ×190,000, and (c) ×500,000 magnification for the N-TiO2 NPs.
The TiO2-based particles exhibited a size ranging between 5 and 15 nm, and they had a spherical shape with different crystalline orientations. The color difference in these particles was due to the different shade effects created by the electrons when hitting the surface of the variably oriented particles. These size results differed from those obtained by the DLS technique, as nanoparticles in solution tend to aggregate into larger particles and, therefore, their size in solution is larger than that in the dry powder form [35]. This overestimation of the DLS size compared to the TEM size is commonly found in the literature [42]. Light-scattering techniques provide information regarding the hydrodynamic diameter, whereas TEM measures the particles’ dense core [43].
All of the abovementioned results are in accordance with a recent similar study conducted by our research group, where the N-TiO2 particles were fully characterized by applying various techniques, including X-ray powder diffraction (XRD) (see Figure 1 in [32]), micro-Raman analysis (see Figure 2 in [32]), X-ray photoelectron spectroscopy (XPS) (see Figure 3 in [32]), ultraviolet–visible (UV-Vis) spectroscopy (see Figure 4 and Figure 5 in [32]), and transmission electron microscopy (TEM) (see Figure 7 in [32]). This research is an extension of the findings of our recent publication, aimed at exploring the capability of such particles to have end-use biomedical applications, along with environmental ones.
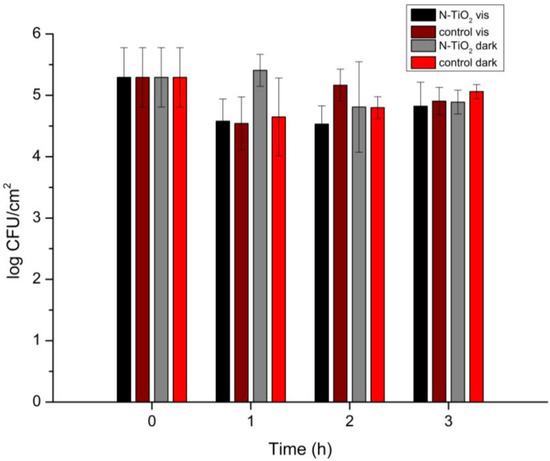
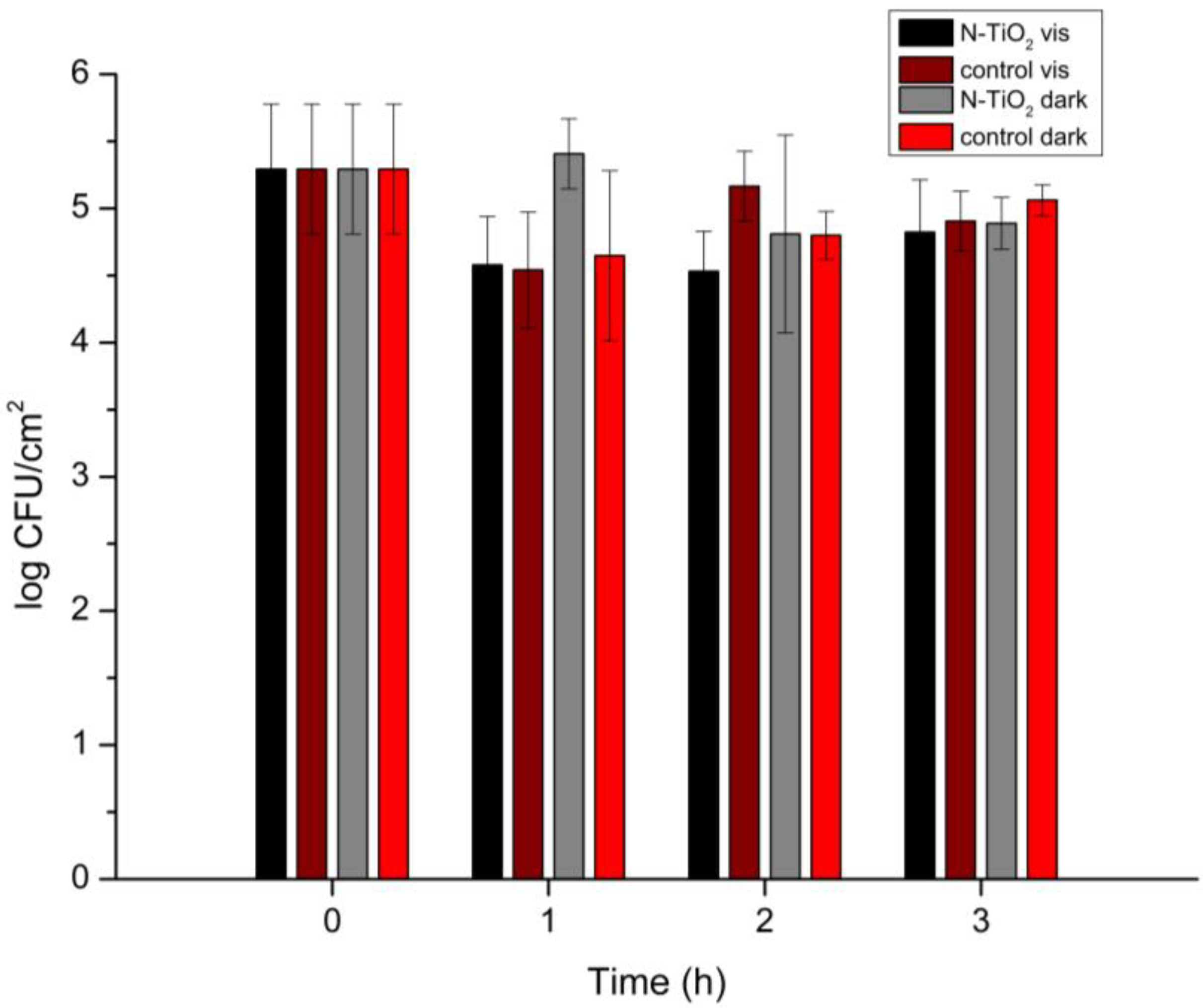
Figure 7.
Salmonella Typhimurium biofilm population (log CFU/cm2) with the presence of N-TiO2 NPs under visible-light irradiation and in the dark for 0, 1, 2, and 3 h of irradiation. Control specimens are included (no N-TiO2 presence), and the reported data are the mean values of two replicates with three samples each, ±standard deviation.
3.5. Antimicrobial Assessment
The results of the antimicrobial testing for the N-TiO2 NPs under visible-light irradiation are presented in Figure 7.
An effect of the presence of N-TiO2 on the Salmonella Typhimurium strains was observed. In detail, under visible-light irradiation, the N-TiO2 NPs led to a one-logarithm decrease in the biofilm population. The inhibition of proliferation was more obvious after 1 and 2 h of irradiation, whereas after 3 h the populations gradually started to increase again. This was the case for all of the biofilm specimens, as Salmonella Typhimurium shows great resistance as a bacterium. When compared to the biofilm in a dispersion of N-TiO2 under no irradiation, the NPs’ photoactivity was always evident, for all three different times of irradiation.
It is noteworthy that, in some cases, there was a tendency for the N-TiO2 NPs to attract greater biofilm populations. This occurred both under visible-light radiation and under no irradiation. For example, in the first hour of irradiation, the biofilm population was slightly higher when N-TiO2 was present, compared with the control specimen in the photoreactor. Similar results were obtained for the biofilms left in the dark for 1 h. The reason for this was that the NPs, due to their morphology and small size (i.e., higher surface area), offered a wider space for the biofilms to grow on. Moreover, they created “trapping” sites that worked as shelters for the cells.
The abovementioned results contrast somewhat with the recently published literature [17,24,44], since all of the published studies included testing in free-standing bacterial strains. This is a preliminary attempt to test the antimicrobial properties of N-TiO2 NPs under visible-light irradiation in biofilms. The need to further investigate N-TiO2 NPs is of high importance in the biomedical and dental fields [45,46], and previous research from our study group has already investigated the effects of nanoparticles on the formation and surface modification of Salmonella biofilms [47].
Consequently, the morphology of the N-TiO2 photocatalyst needed to be further investigated so that the biofilm testing protocol could be optimized. This was due to the existence of antimicrobial properties of N-TiO2—even in that form—when the visible light source was present, compared to when it was missing. The percentage of remaining Salmonella Typhimurium biofilm cells for every irradiation time is shown in Figure 8.
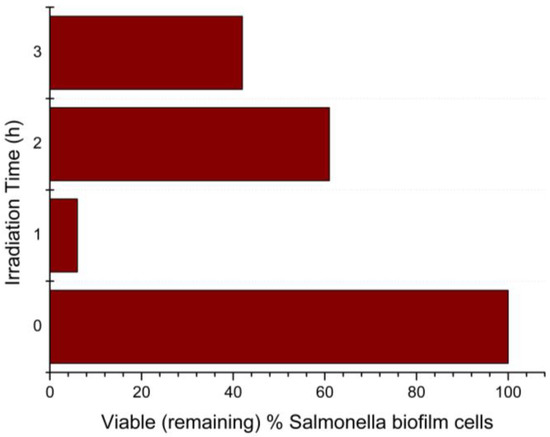
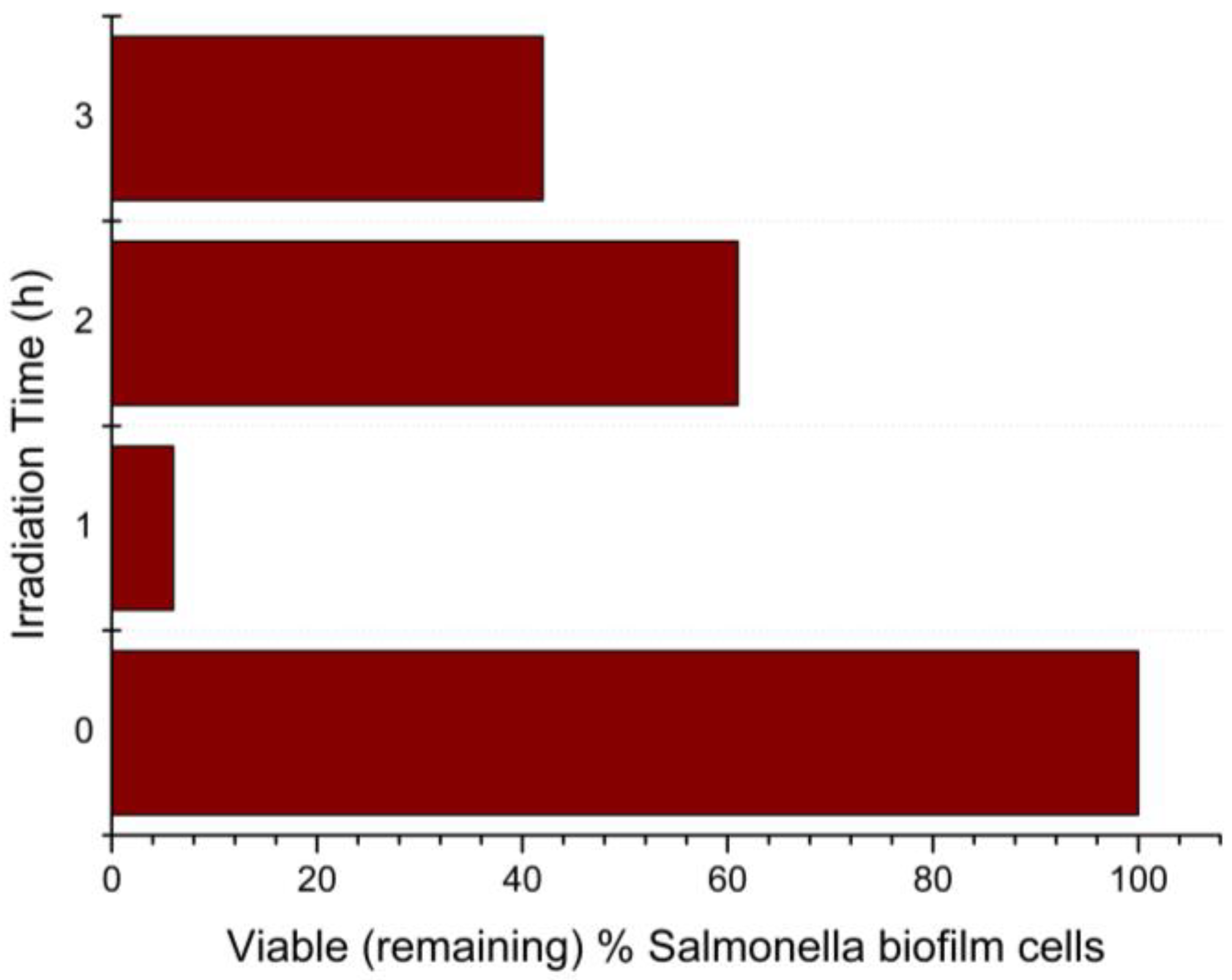
Figure 8.
Percentage of remaining viable Salmonella Typhimurium biofilm cells after 1, 2, and 3 h of visible-light irradiation, with the presence of N-TiO2 NPs.
The results presented in Figure 8 were derived from comparing the viable cells on the biofilms with N-TiO2 NPs irradiated with visible light to the biofilms with N-TiO2 NPs in dark. After 1 h of visible-light irradiation, the percentage of viable (remaining) biofilm cells was 6%, which means that there was a 94% killing of Salmonella Typhimurium biofilm cells by the N-TiO2 NPs. As the Salmonella Typhimurium biofilm manifested its resistance to cell death, the percentage of remaining cells after two hours of irradiation decreased to 61%, or 39% killing. After three hours of irradiation, 42% of cells remained, i.e., 58% killing of biofilm cells.
4. Conclusions
In the present study, the antimicrobial properties of N-TiO2 nanoparticles under visible-light irradiation on Salmonella Typhimurium biofilms were investigated. To achieve that, N-TiO2 particles were synthesized via the sol–gel method. The particles were fully characterized by FTIR, DLS, BET, SEM/EDS, and TEM, before assessing their antibacterial behavior. It was revealed that the particles formed were nanoscale (5–15 nm), and the expected Ti-O bonds and elemental N were present. When in water dispersion, the particles tended to form agglomerates and had a zeta potential of −20 mV, making the suspensions moderately stable. They had a high surface area, making them more photoactive, and they were porous, with a 0.3 cm3 g−1 pore volume and 13 nm average pore diameter.
The antimicrobial photocatalytic testing was conducted under visible-light irradiation, on Salmonella Typhimurium biofilms that were formed on stainless steel coupons. After the biofilm development, the coupons were placed on a horizontal, batch, rectangular reactor equipped with a vis-LED irradiation source, in the presence of N-TiO2 NPs. After 1, 2, and 3 h of irradiation, the biofilm population was assessed. An inhibition of proliferation under visible-light irradiation was noticed when the N-TiO2 was present, compared to when the light source was missing. It should be noted that the TiO2 NPs tend to attract bacteria on their surface, so in some cases the biofilm population was increased compared to the control coupons. This was due to the morphology of the NPs, which created “trapping” sites working as shelters, along with their higher surface area available for the bacteria. However, the antibacterial activity of the N-TiO2 NPs was noticeable, as only 6% of Salmonella biofilm cells remained viable after the first hour of visible-light irradiation. Therefore, N-TiO2 can be considered to be a promising multipurpose material with biomedical and environmental photoinduced properties, able to reduce biofilm formation under visible-light irradiation.
Author Contributions
Conceptualization, M.-E.K., N.C. and E.A.P.; methodology, M.-E.K., N.C. and E.A.P.; software, M.-E.K.; validation, N.C., E.A.P. and G.-J.N.; formal analysis, M.-E.K. and N.C.; investigation, M.-E.K., N.C. and E.A.P.; resources, E.A.P. and G.-J.N.; data curation, M.-E.K.; writing—original draft preparation, M.-E.K.; writing—review and editing, N.C. and E.A.P.; visualization, E.A.P. and G.-J.N.; supervision, E.A.P. and G.-J.N.; project administration, E.A.P. and G.-J.N.; funding acquisition, E.A.P. and G.-J.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to acknowledge Nikolaos Boukos and Elias Sakellis (Institute of Nanoscience and Nanotechnology, National Center for Scientific Research “Demokritos”, Agia Paraskevi, Greece) for the TEM images and analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the Natural environment to infectious diseases. Nat. Rev. Genet. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Ceramella, J.; Iacopetta, D.; Catalano, A.; Cirillo, F.; Lappano, R.; Sinicropi, M.S. A Review on the Antimicrobial Activity of Schiff Bases: Data Collection and Recent Studies. Antibiotics 2022, 11, 191. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Mennati, A.; Jafari, S.; Khezri, K.; Adibkia, K. Review. Antimicrobial activity of carbon-based nanoparticles. Adv. Pharm. Bull. 2015, 1, 19–23. [Google Scholar]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Review. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.A.; Khatun, F.; Monir, M.U.; Ching, S.L.; Hon, L.K. TiO2: A Semiconductor Photocatalyst. In Titanium Dioxide—Advances and Applications; IntechOpen: London, UK, 2021; pp. 1–16. [Google Scholar]
- Yadav, S.; Jaiswar, G. Review on Undoped/Doped TiO2 Nanomaterial; Synthesis and Photocatalytic and Antimicrobial Activity. J. Chin. Chem. Soc. 2017, 64, 103–116. [Google Scholar] [CrossRef]
- Wold, A. Photocatalytic Properties of TiO2. Chem. Mater. 1993, 5, 280–283. [Google Scholar] [CrossRef]
- Bavykin, D.; Friedrich, J.; Walsh, F. Protonated Titanates and TiO2 Nanostructured Materials: Synthesis, Properties and Applications. Adv. Mater. 2006, 18, 2807–2824. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Al-Mamun, M.; Kader, S.; Islam, M.; Khan, M. Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 103248. [Google Scholar] [CrossRef]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Cho, M.H. Nitrogen-doped titanium dioxide (N-doped TiO2) for visible light photocatalysis. New J. Chem. 2016, 40, 3000–3009. [Google Scholar] [CrossRef]
- Mathew, S.; Ganguly, P.; Rhatigan, S.; Kumaravel, V.; Byrne, C.; Hinder, S.J.; Bartlett, J.; Nolan, M.; Pillai, S.C. Cu-Doped TiO2: Visible Light Assisted Photocatalytic Antimicrobial Activity. Appl. Sci. 2018, 8, 2067. [Google Scholar] [CrossRef]
- Kubacka, A.; Muñoz-Batista, M.J.; Ferrer, M.; Fernández-García, M. UV and visible light optimization of anatase TiO2 antimicrobial properties: Surface deposition of metal and oxide (Cu, Zn, Ag) species. Appl. Catal. B Environ. 2013, 140–141, 680–690. [Google Scholar] [CrossRef]
- He, R.-L.; Wei, Y.; Cao, W.-B. Preparation of (Fe, N)-Doped TiO2 Powders and Their Antibacterial Activities Under Visible Light Irradiation. J. Nanosci. Nanotechnol. 2009, 9, 1094–1097. [Google Scholar] [CrossRef]
- Ananpattarachai, J.; Boonto, Y.; Kajitvichyanukul, P. Visible light photocatalytic antibacterial activity of Ni-doped and N-doped TiO2 on Staphylococcus aureus and Escherichia coli bacteria. Environ. Sci. Pollut. Res. 2016, 23, 4111–4119. [Google Scholar] [CrossRef]
- Yu, B.; Lau, W.M.; Yang, J. Preparation and characterization of N–TiO2 photocatalyst with high crystallinity and enhanced photocatalytic inactivation of bacteria. Nanotechnology 2013, 24, 335705. [Google Scholar] [CrossRef]
- Seneviratne, K.L.; Munaweera, I.; Peiris, S.E.; Peiris, C.N.; Kottegoda, N. Recent Progress in Visible-Light Active (VLA) TiO2 Nano-Structures for Enhanced Photocatalytic Activity (PCA) and Antibacterial Properties: A Review. Iran. J. Catal. 2021, 11, 217–245. [Google Scholar]
- Yan, Y.; Kuang, W.; Shi, L.; Ye, X.; Yang, Y.; Xie, X.; Shi, Q.; Tan, S. Carbon quantum dot-decorated TiO2 for fast and sustainable antibacterial properties under visible-light. J. Alloys Compd. 2019, 777, 234–243. [Google Scholar] [CrossRef]
- Raut, A.; Yadav, H.; Gnanamani, A.; Pushpavanam, S.; Pawar, S. Synthesis and characterization of chitosan-TiO2: Cu nanocomposite and their enhanced antimicrobial activity with visible light. Colloids Surf. B Biointerfaces 2016, 148, 566–575. [Google Scholar] [CrossRef]
- Wong, M.-S.; Chu, W.-C.; Sun, D.-S.; Huang, H.-S.; Chen, J.-H.; Tsai, P.-J.; Lin, N.-T.; Yu, M.-S.; Hsu, S.-F.; Wang, S.-L.; et al. Visible-Light-Induced Bactericidal Activity of a Nitrogen-Doped Titanium Photocatalyst against Human Pathogens. Appl. Environ. Microbiol. 2006, 72, 6111–6116. [Google Scholar] [CrossRef]
- Nagay, B.E.; Dini, C.; Cordeiro, J.M.; Ricomini-Filho, A.P.; de Avila, E.D.; Rangel, E.C.; da Cruz, N.C.; Barao, V.A.R. Visible-Light-Induced Photocatalytic and Antibacterial Activity of TiO2 Codoped with Nitrogen and Bismuth: New Perspectives to Control Implant-Biofilm-Related Diseases. ACS Appl. Mater. Interfaces 2019, 11, 18186–18202. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Liu, B.; Fan, L.; Yue, Z.; Liu, B.; Cao, B. Highly antibacterial activity of N-doped TiO2 thin films coated onstainless steel brackets under visible light irradiation. Appl. Surf. Sci. 2014, 309, 119–127. [Google Scholar] [CrossRef]
- Lee, H.U.; Lee, S.C.; Choi, S.; Son, B.; Lee, S.M.; Kim, H.J.; Lee, J. Efficient visible-light induced photocatalysis on nanoporous nitrogen-doped titanium dioxide catalysts. Chem. Eng. J. 2013, 228, 756–764. [Google Scholar] [CrossRef]
- Prakash, B.; Veeregowda, B.M.; Krishnappa, G. Biofilms: A Survival Strategy of Bacteria. Curr. Sci. 2003, 85, 1299–1307. [Google Scholar]
- Kostaki, M.; Chorianopoulos, N.; Braxou, E.; Nychas, G.-J.; Giaouris, E. Differential Biofilm Formation and Chemical Disinfection Resistance of Sessile Cells of Listeria monocytogenes Strains under Monospecies and Dual-Species (with Salmonella enterica) Conditions. Appl. Environ. Microbiol. 2012, 78, 2586–2595. [Google Scholar] [CrossRef]
- Steenackers, H.; Hermans, K.; Vanderleyden, J.; De Keersmaecker, S.C. Salmonella biofilms: An overview on occurrence, structure, regulation and eradication. Food Res. Int. 2012, 45, 502–531. [Google Scholar] [CrossRef]
- Gkana, E.N.; Giaouris, E.D.; Doulgeraki, A.I.; Kathariou, S.; Nychas, G.-J.E. Biofilm formation by Salmonella Typhimurium and Staphylococcus aureus on stainless steel under either mono- or dual-species multi-strain conditions and resistance of sessile communities to sub-lethal chemical disinfection. Food Control. 2017, 73, 838–846. [Google Scholar] [CrossRef]
- Joseph, B.; Otta, S.; Karunasagar, I. Biofilm formation by Salmonella spp. on food contact surfaces and their sensitivity to sanitizers. Int. J. Food Microbiol. 2001, 64, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Giaouris, E.; Chorianopoulos, N.; Nychas, G.-J. Effect of Temperature, pH, and Water Activity on Biofilm Formation by Salmonella enterica Enteritidis PT4 on Stainless Steel Surfaces as Indicated by the Bead Vortexing Method and Conductance Measurements. J. Food Prot. 2005, 68, 2149–2154. [Google Scholar] [CrossRef]
- Chorianopoulos, N.G.; Giaouris, E.D.; Kourkoutas, Y.; Nychas, G.-J.E. Inhibition of the Early Stage of Salmonella enterica Serovar Enteritidis Biofilm Development on Stainless Steel by Cell-Free Supernatant of a Hafnia alvei Culture. Appl. Environ. Microbiol. 2010, 76, 2018–2022. [Google Scholar] [CrossRef] [PubMed]
- Kassalia, M.-E.; Nikolaou, Z.; Pavlatou, E.A. Photocatalytic Testing Protocol for N-Doped TiO2 Nanostructured Particles under Visible Light Irradiation Using the Statistical Taguchi Experimental Design. Appl. Sci. 2023, 13, 774. [Google Scholar] [CrossRef]
- Galata, E.; Georgakopoulou, E.A.; Kassalia, M.-E.; Papadopoulou-Fermeli, N.; Pavlatou, E.A. Development of Smart Composites Based on Doped-TiO2 Nanoparticles with Visible Light Anticancer Properties. Materials 2019, 12, 2589. [Google Scholar] [CrossRef]
- Tan, L.; Ong, W.; Chai, S.; Mohamed, A. Reduced graphene oxide-TiO2 nanocomposite as a promising visible-light-active photocatalyst for the conversion of carbon dioxide. Nanoscale Res. Lett. 2013, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qiu, X.; Burda, C. The Effects of Sintering on the Photocatalytic Activity of N-Doped TiO2 Nanoparticles. Chem. Mater. 2008, 20, 2629–2636. [Google Scholar] [CrossRef]
- Sahu, M.; Biswas, P. Single-step processing of copper-doped titania nanomaterials in a flame aerosol reactor. Nanoscale Res. Lett. 2011, 6, 441. [Google Scholar] [CrossRef]
- Moulahoum, H.; Ghorbanizamani, F.; Sakarya, S.; Timur, S. Lightless catalytic layered chitosan coating film using doped TiO2@metal ions nanoparticles for highly efficient dye degradation in aqueous media and disinfection applications. Prog. Org. Coat. 2022, 169, 106923. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Bhatkhande, D.S.; Pangarkar, V.G.; Beenackers, A.A. Photocatalytic degradation for environmental applications—A review. J. Chem. Technol. Biotechnol. 2002, 77, 102–116. [Google Scholar] [CrossRef]
- Hussain, M.; Ceccarelli, R.; Marchisio, D.; Fino, D.; Russo, N.; Geobaldo, F. Synthesis, characterization, and photocatalytic application of novel TiO2 nanoparticles. Chem. Eng. J. 2010, 157, 45–51. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, With Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051. [Google Scholar] [CrossRef]
- de la Calle, I.; Menta, M.; Klein, M.; Maxit, B.; Séby, F. Towards routine analysis of TiO2 (nano-)particle size in consumer products: Evaluation of potential techniques. Spectrochim. Acta Part B At. Spectrosc. 2018, 147, 28–42. [Google Scholar] [CrossRef]
- Souza, T.G.F.; Ciminelli, V.S.T.; Mohallem, N.D.S. A comparison of TEM and DLS methods to characterize size distribution of ceramic nanoparticles. J. Phys. Conf. Ser. 2016, 733, 012039. [Google Scholar] [CrossRef]
- Ma, W.; Li, J.; Liu, Y.; Ren, X.; Gu, Z.-G.; Xie, Z.; Liang, J. Preparation and characterization of excellent antibacterial TiO2/N-halamines nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 284–290. [Google Scholar] [CrossRef]
- Iwatsu, M.; Kanetaka, H.; Mokudai, T.; Ogawa, T.; Kawashita, M.; Sasaki, K. Visible light-induced photocatalytic and antibacterial activity of N-doped TiO2. J. Biomed. Mater. Res. 2020, 108B, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Kumaravel, V.; Nair, K.M.; Mathew, S.; Bartlett, J.; Kennedy, J.E.; Manning, H.G.; Whelan, B.J.; Leyland, N.S.; Pillai, S.C. Antimicrobial TiO2 nanocomposite coatings for surfaces, dental and orthopaedic implants. Chem. Eng. J. 2021, 416, 129071. [Google Scholar] [CrossRef] [PubMed]
- Gkana, E.N.; Doulgeraki, A.I.; Chorianopoulos, N.G.; Nychas, G.-J.E. Anti-adhesion and Anti-biofilm Potential of Organosilane Nanoparticles against Foodborne Pathogens. Front. Microbiol. 2017, 8, 1295. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).