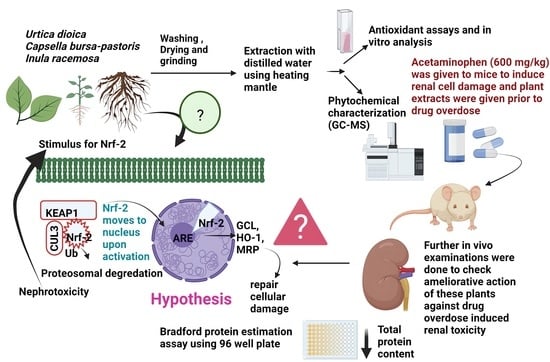
Investigation of the Protective Effects of Urtica dioica, Capsella bursa-pastoris and Inula racemosa on Acetaminophen-Induced Nephrotoxicity in Swiss Albino Male Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Equipment
2.2. Sample Collection and Identification of Plant Materials
2.3. Extraction of Plant Material
2.4. Analysis of Antioxidant Activity and Quantification of Phytochemicals in Selected Plants
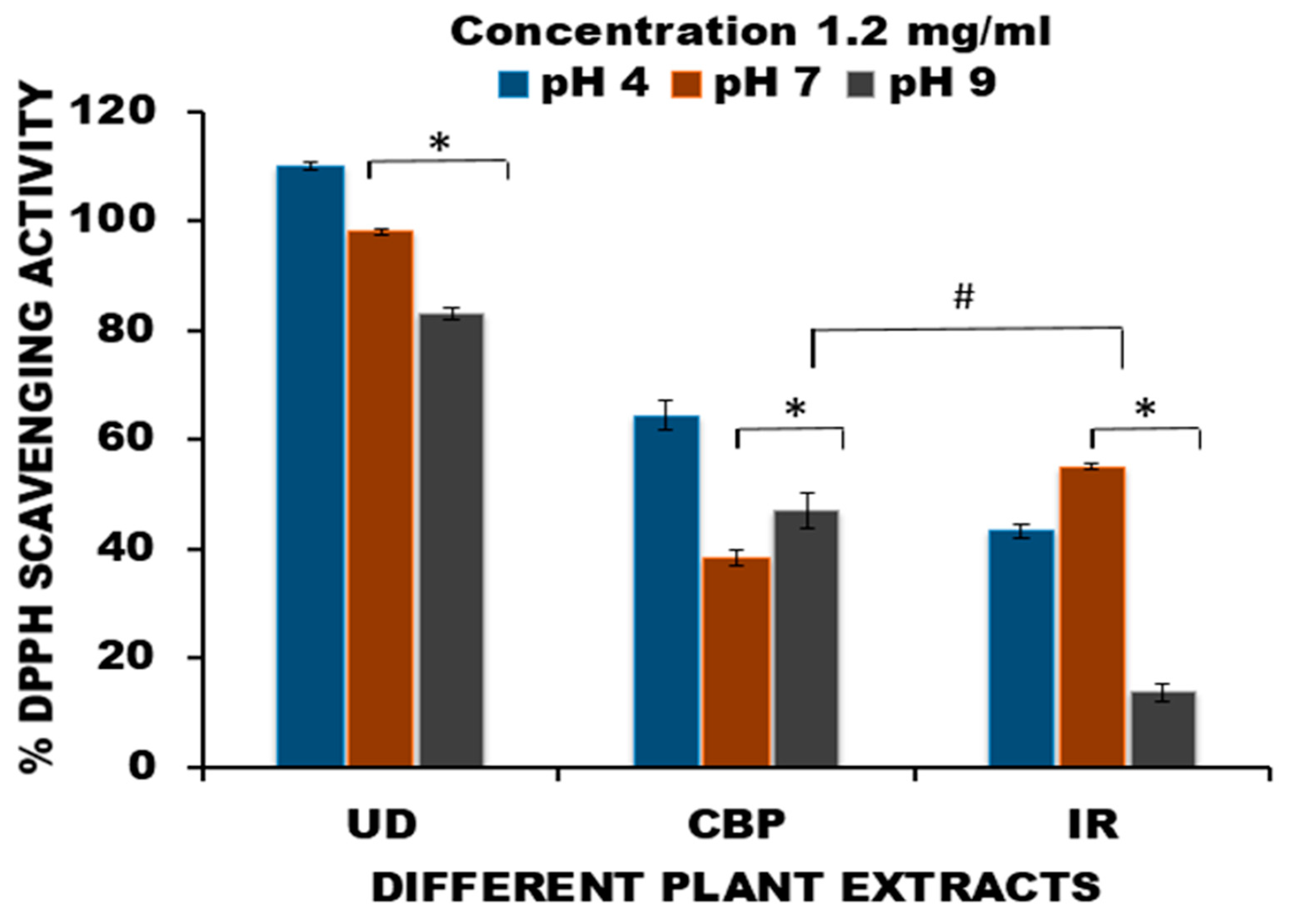
2.5. pH Stability Test of Herbs
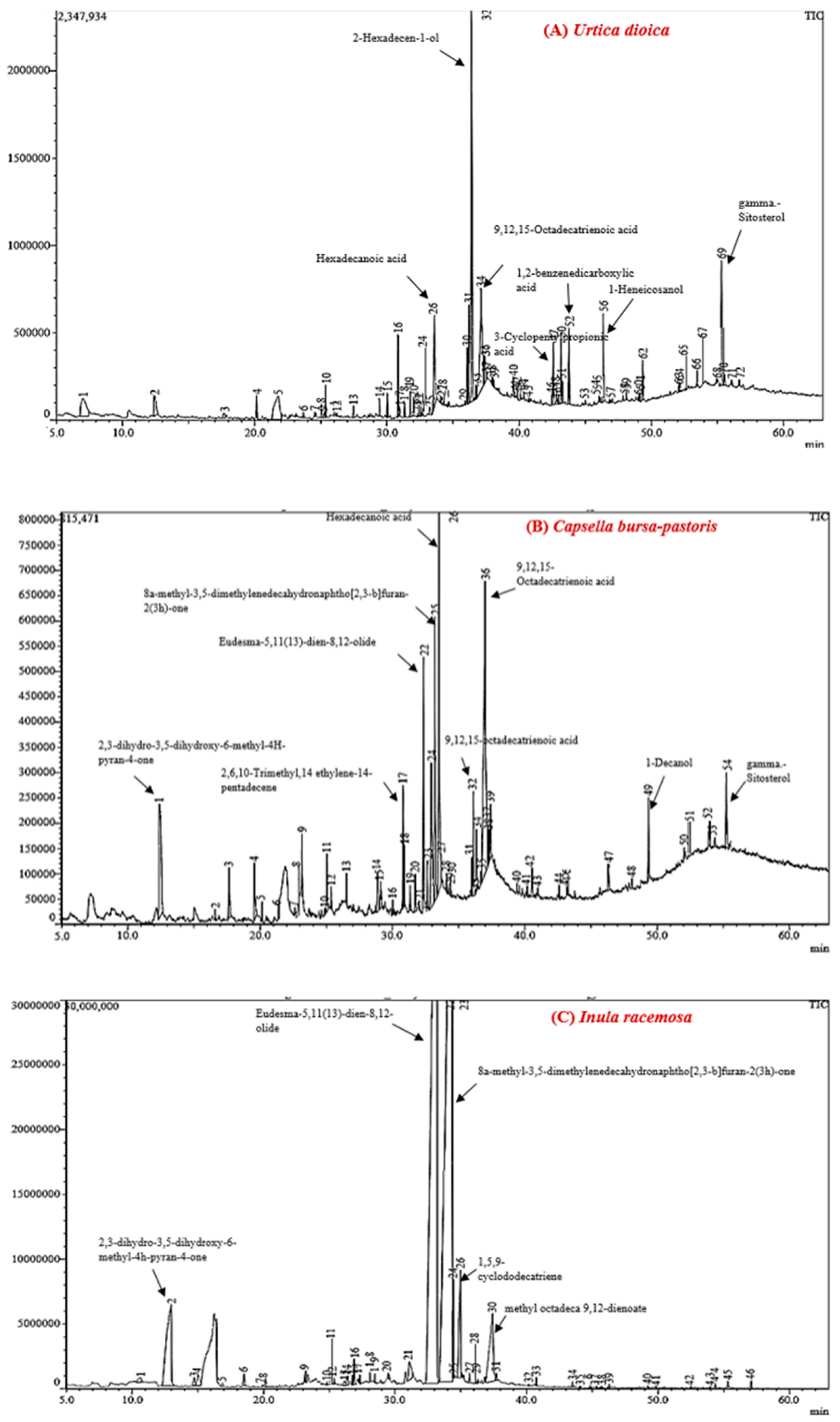
2.6. Gas Chromatography-Mass Spectrometry (GC–MS) Analysis
2.7. Animal Experimentation Procedure
2.8. Experimental Design of Acetaminophen-Induced Nephrotoxicity In Vivo
2.9. Histopathological Analysis
2.10. Total Protein Estimation
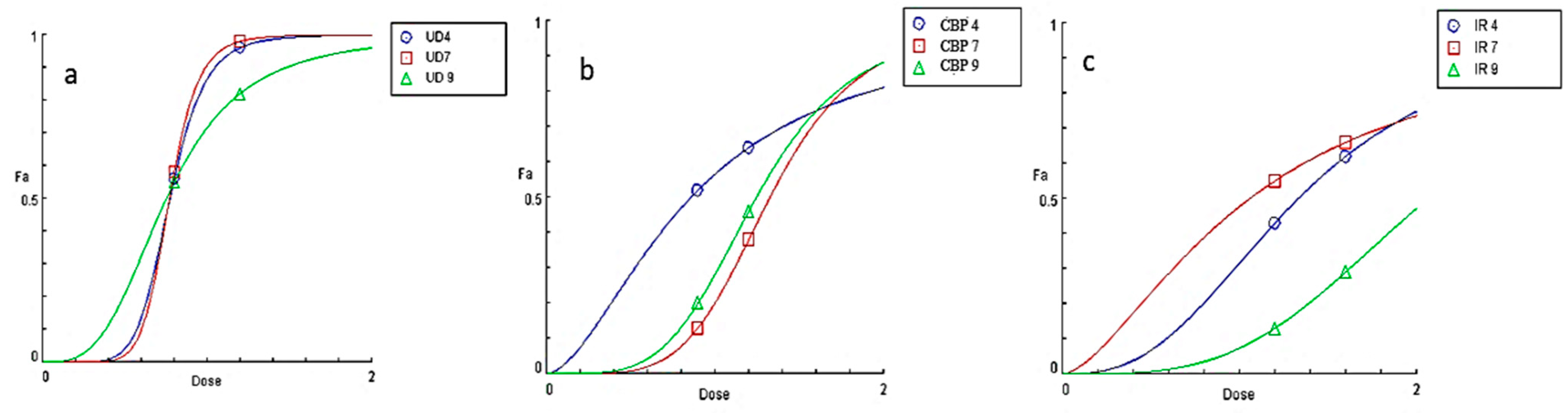
2.11. EC50 (Effective Concentration) Calculation of Aqueous Extracts of Plant Materials Used
2.12. Statistical Analysis
3. Results
3.1. Determination of pH Stability of Extracts
3.2. Phytochemical Fingerprinting via GC–MS
3.2.1. Compound Profiling of Urtica dioica
3.2.2. Compound Profiling of Capsella bursa-pastoris
3.2.3. Compound Profiling of Inula racemosa
3.3. In Vivo Analysis of Acetaminophen-Induced Renal Toxicity
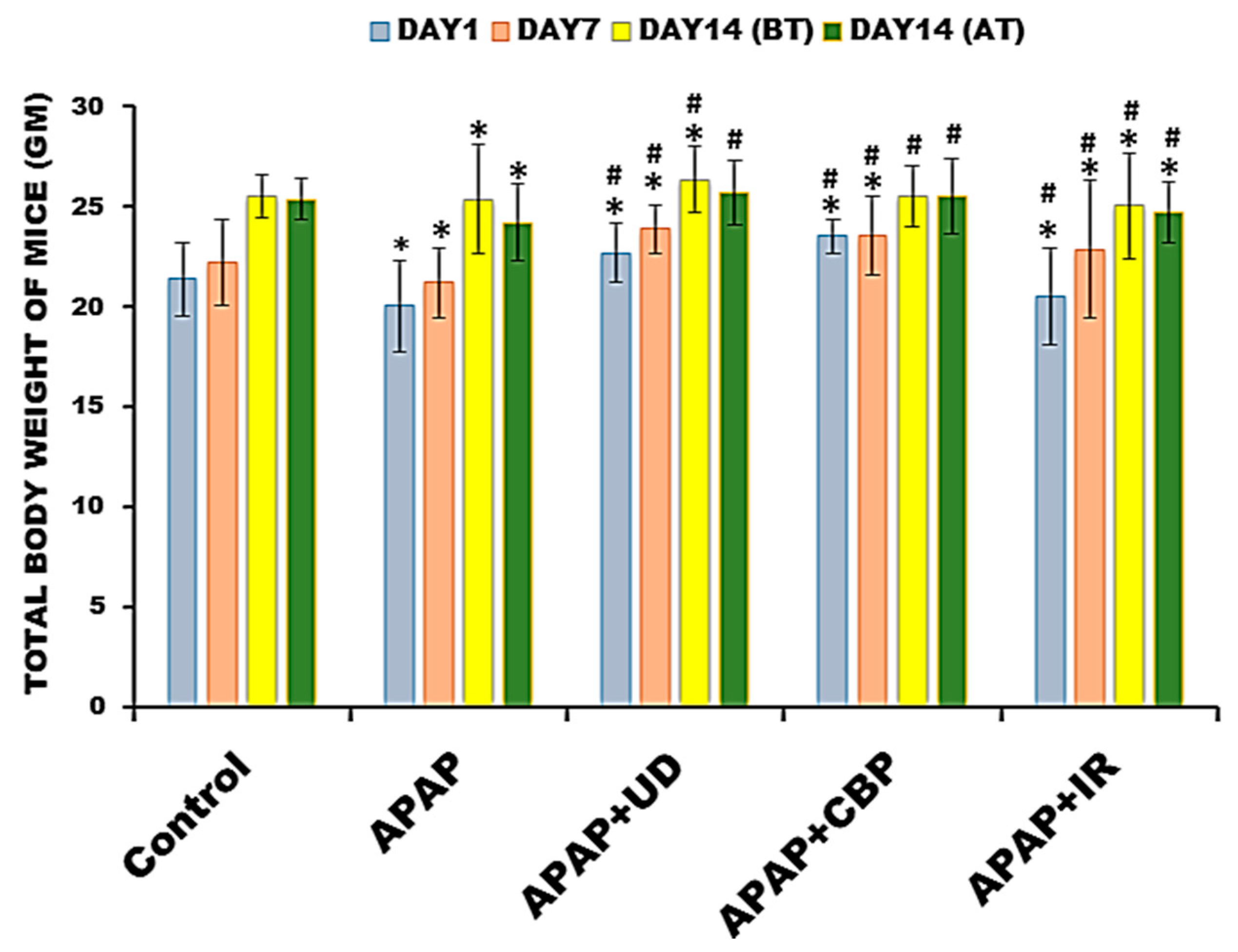
3.3.1. Assessment of Change in the Body Weight of the Experimental Animals
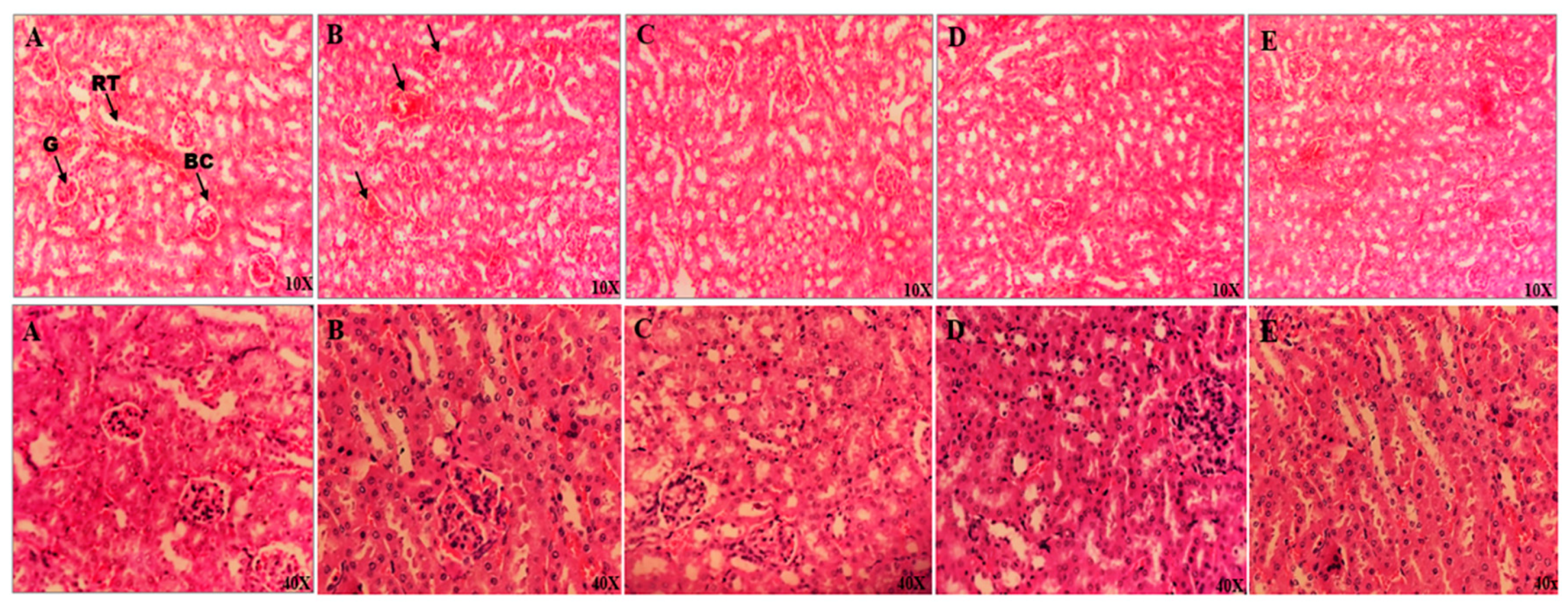
3.3.2. Histopathological Observations of Mouse Kidneys
3.4. Degenerated Protein Concentrations in the Mice Exposed to Acetaminophen Overdose
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahi-Birjand, M.; Yaghoubi, S.; Abdollahpour-Alitappeh, M.; Keshtkaran, Z.; Bagheri, N.; Pirouzi, A.; Karimzadeh, I. Protective effects of pharmacological agents against aminoglycoside-induced nephrotoxicity: A systematic review. Expert Opin. Drug Saf. 2020, 19, 167–186. [Google Scholar] [CrossRef] [PubMed]
- Rabani, M.R.; Azarmehr, N.; Moslemi, Z.; Sadeghi, H.; Amini-Khoei, H.; Doustimotlagh, A.H. Protective effects of hydroalcoholic extract of Stachys pilifera on paracetamol-induced nephrotoxicity in female rats. Res. Pharm. Sci. 2021, 16, 643. [Google Scholar]
- Ansari, S.; Azarmehr, N.; Barmoudeh, Z.; Moslemi, Z.; Ghahremani, H.; Mirzaei, A.; Doustimotlagh, A.H. Evaluation of the protective potential of hydroalcoholic extract of Thymus daenensis on acetaminophen-induced nephrotoxicity in rats. Heliyon 2020, 6, e03898. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, J.; Das, J.; Manna, P.; Sil, P.C. Acetaminophen induced renal injury via oxidative stress and TNF-α production: Therapeutic potential of arjunolic acid. Toxicology 2010, 268, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Dua, T.K.; Palai, S.; Roy, A.; Paul, P. Protective effect of probiotics against acetaminophen induced nephrotoxicity. Res. Pharm. Sci. 2022, 49, 8139–8143. [Google Scholar] [CrossRef]
- Hussain, Z.; Khan, J.A.; Arshad, A.; Asif, P.; Rashid, H.; Arshad, M.I. Protective effects of Cinnamomum zeylanicum L. (Darchini) in acetaminophen-induced oxidative stress, hepatotoxicity and nephrotoxicity in mouse model. Biomed. Pharm. 2019, 109, 2285–2292. [Google Scholar] [CrossRef]
- Dallak, M.; Dawood, A.F.; Haidara, M.A.; Abdel Kader, D.H.; Eid, R.A.; Kamar, S.S.; Eldeen, A.M.S.; Al-Ani, B. Suppression of glomerular damage and apoptosis and biomarkers of acute kidney injury induced by acetaminophen toxicity using a combination of resveratrol and quercetin. Drug Chem. Toxicol. 2022, 45, 1–7. [Google Scholar] [CrossRef]
- Akakpo, J.Y.; Ramachandran, A.; Curry, S.C.; Rumack, B.H.; Jaeschke, H. Comparing N-acetylcysteine and 4-methylpyrazole as antidotes for acetaminophen overdose. Arch. Toxicol. 2022, 96, 453–465. [Google Scholar] [CrossRef]
- Najafizadeh, A.; Kaeidi, A.; Rahmani, M.; Hakimizadeh, E.; Hassanshahi, J. The protective effect of carvacrol on acetaminophen-induced renal damage in male rats. Mol. Biol. Rep. 2022, 49, 1763–1771. [Google Scholar] [CrossRef]
- Krishnan, V.; Loganathan, C.; Thayumanavan, P. Green synthesized selenium nanoparticles using Spermacoce hispida as carrier of s-allyl glutathione: To accomplish hepatoprotective and nephroprotective activity against acetaminophen toxicity. Artif. Cells Nanomed. Biotechnol. 2019, 47, 56–63. [Google Scholar] [CrossRef]
- Dhouibi, R.; Affes, H.; Salem, M.B.; Charfi, S.; Marekchi, R.; Hammami, S.; Ksouda, K. Protective effect of Urtica dioica in induced neurobehavioral changes, nephrotoxicity and hepatotoxicity after chronic exposure to potassium bromate in rats. Environ. Pollut. 2021, 287, 117657. [Google Scholar] [CrossRef] [PubMed]
- Guil-Guerrero, J.L.; Rebolloso-Fuentes, M.M.; Isasa, M.T. Fatty acids and carotenoids from Stinging Nettle (Urtica dioica L.). J. Food Compos. Anal. 2003, 16, 111–119. [Google Scholar] [CrossRef]
- Zeipiņa, S.; Alsiņa, I.; Lepse, L.; Dūma, M. Antioxidant activity in nettle (Urtica dioica L.) and garden orache (Atriplex hortensis L.) leaves during vegetation period. Chem. Technol. 2015, 66, 29–33. [Google Scholar] [CrossRef]
- Vajić, U.J.; Grujić-Milanović, J.; Živković, J.; Šavikin, K.; Gođevac, D.; Miloradović, Z.; Mihailović-Stanojević, N. Optimization of extraction of stinging nettle leaf phenolic compounds using response surface methodology. Ind. Crops Prod. 2015, 74, 912–917. [Google Scholar] [CrossRef]
- Kregiel, D.; Pawlikowska, E.; Antolak, H. Urtica spp.: Ordinary plants with extraordinary properties. Molecules 2018, 23, 1664. [Google Scholar] [CrossRef]
- Di Sotto, A.; Mazzanti, G.; Savickiene, N.; Staršelskytė, R.; Baksenskaite, V.; Di Giacomo, S.; Vitalone, A. Antimutagenic and antioxidant activity of a protein fraction from aerial parts of Urtica dioica. Pharm. Biol. 2015, 53, 935–938. [Google Scholar] [CrossRef]
- Gutowska, I.; Jakubczyk, K.; Dec, K.; Baranowska-Bosiacka, I.; Drozd, A.; Janda, K.; Chlubek, D. Effect of the extract from nettle (Urtica dioica L.) fruit cluster on the synthesis of pro-inflammatory agents in hepatocytes treated with fluoride. Fluoride 2014, 47, 109–118. [Google Scholar]
- Das, R.; Mitra, S.; Tareq, A.M.; Emran, T.B.; Hossain, M.J.; Alqahtani, A.M.; Simal-Gandara, J. Medicinal plants used against hepatic disorders in Bangladesh: A comprehensive review. J. Ethnopharmacol. 2022, 282, 114588. [Google Scholar] [CrossRef]
- Razak, S.; Afsar, T.; Al-Disi, D.; Almajwal, A.; Arshad, M.; Alyousef, A.A.; Chowdary, R.A. GCMS fingerprinting, in vitro pharmacological activities, and in vivo anti-inflammatory and hepatoprotective effect of selected edible herbs from Kashmir valley. J. King Saud. Univ. Sci. 2020, 32, 2868–2879. [Google Scholar] [CrossRef]
- Namazi, F.; Shomali, T.; Taghikhani, P.; Nazifi, S. Protective effect of Urtica dioica leaf hydro alcoholic extract against experimentally induced atherosclerosis in rats. Avicenna J. Phytomed. 2018, 8, 254. [Google Scholar]
- Al-Snafi, A.E. The chemical constituents and pharmacological effects of Capsella bursa-pastoris—A review. Int. J. Pharmacol. Toxicol. 2015, 5, 76–81. [Google Scholar]
- Ma, Q.; Guo, Y.; Wei, R.; Sang, Z.; Liu, W.; Gao, L.; Liu, T. Flavonoids from Capsella bursa-pastoris and their hepatoprotective activities in vitro. Rev. Bras. Farmacogn. 2016, 26, 710–713. [Google Scholar] [CrossRef]
- Mohan, S.; Gupta, D. Phytochemical analysis and differential in vitro cytotoxicity assessment of root extracts of Inula racemosa. Biomed. Pharm. 2017, 89, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Lateef, R.; Bhat, K.A.; Chandra, S.; Banday, J.A. Antioxidant Activity of Chloroform Extract of Inula racemosa from Kashmir Himalayas. Chem. J. 2018, 1, 179–185. [Google Scholar]
- Rao, K.S.; Mishra, S.H. Hepatoprotective activity of Inuta racemosa root. Fitoterapia 1997, 68, 510–514. [Google Scholar]
- Basak, P.; Sadhukhan, P.; Sarkar, P.; Sil, P.C. Perspectives of the Nrf-2 signaling pathway in cancer progression and therapy. Toxicol. Rep. 2017, 4, 306–318. [Google Scholar] [CrossRef]
- Bai, Z.; Wang, Z. Genistein protects against doxorubicin-induced cardiotoxicity through Nrf-2/HO-1 signaling in mice model. Environ. Toxicol. 2019, 34, 645–651. [Google Scholar] [CrossRef]
- James, S.A.; Soltis, P.S.; Belbin, L.; Chapman, A.D.; Nelson, G.; Paul, D.L.; Collins, M. Herbarium data: Global biodiversity and societal botanical needs for novel research. Appl. Plant Sci. 2018, 6, e1024. [Google Scholar] [CrossRef]
- Shabir, S.; Yousuf, S.; Singh, S.K.; Vamanu, E.; Singh, M.P. Ethnopharmacological Effects of Urtica dioica, Matricaria chamomilla, and Murraya koenigii on Rotenone-Exposed D. melanogaster: An Attenuation of Cellular, Biochemical, and Organismal Markers. Antioxidants 2022, 11, 1623. [Google Scholar] [CrossRef]
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reis, A.S.; Santos TC, D.; Coube, C.S.; Leitão, S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, S.; Shabir, S.; Kauts, S.; Minocha, T.; Obaid, A.A.; Khan, A.A.; Singh, M.P. Appraisal of the Antioxidant Activity, Polyphenolic Content, and Characterization of Selected Himalayan Herbs: Anti-Proliferative Potential in HepG2 Cells. Molecules 2022, 27, 8629. [Google Scholar] [CrossRef] [PubMed]
- Roy, L.G.; Urooj, A. Antioxidant potency, pH and heat stability of selected plant extracts. J. Food Biochem. 2013, 37, 336–342. [Google Scholar] [CrossRef]
- Yusoff NA, H.; Rukayadi, Y.; Abas, F.; Khatib, A.; Hassan, M. Antimicrobial stability of Cosmos caudatus extract at varies pH and temperature, and compounds identification for application as food sanitizer. Food Res. 2021, 5, 83–91. [Google Scholar] [CrossRef]
- Chakraborty, P.; Roy, S.S.; Sk, U.H.; Bhattacharya, S. Amelioration of cisplatin-induced nephrotoxicity in mice by oral administration of diphenylmethyl selenocyanate. Free Radic. Res. 2011, 45, 177–187. [Google Scholar] [CrossRef]
- Shertzer, H.G.; Schneider, S.N.; Kendig, E.L.; Clegg, D.J.; D’Alessio, D.A.; Genter, M.B. Acetaminophen normalizes glucose homeostasis in mouse models for diabetes. Biochem. Pharmacol. 2008, 75, 1402–1410. [Google Scholar] [CrossRef]
- Singh, M.P.; Kim, K.Y.; Kim, H.Y. Methionine sulfoxide reductase A deficiency exacerbates acute liver injury induced by acetaminophen. Biochem. Biophys. Res. Commun. 2017, 484, 189–194. [Google Scholar] [CrossRef]
- Johnson, S.J.; Hick, P.M.; Robinson, A.P.; Rimmer, A.E.; Tweedie, A.; Becker, J.A. The impact of pooling samples on surveillance sensitivity for the megalocytivirus Infectious spleen and kidney necrosis virus. Transbound. Emerg. Dis. 2019, 66, 2318–2328. [Google Scholar] [CrossRef]
- Chowdhury, S.; Ghosh, S.; Das, A.K.; Sil, P.C. Ferulic acid protects hyperglycemia-induced kidney damage by regulating oxidative insult, inflammation and autophagy. Front. Pharmacol. 2019, 10, 27. [Google Scholar] [CrossRef]
- Subedi, P.; Schneider, M.; Philipp, J.; Azimzadeh, O.; Metzger, F.; Moertl, S.; Tapio, S. Comparison of methods to isolate proteins from extracellular vesicles for mass spectrometry-based proteomic analyses. Analyt. Biochem. 2019, 584, 113390. [Google Scholar] [CrossRef]
- Sherovski, P.; Stojković, G.; Ristovska, N. Development, validation and application of first derivative spectroscopy ratio method for estimation of Bradford assay. Analyt. Biochem. 2018, 558, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.P.; Shabir, S.; Deopa, A.S.; Raina, S.R.; Bantun, F.; Jalal, N.A.; Abdel-Razik, N.E.; Jamous, Y.F.; Alhumaidi, M.S.; Altammar, K.A.; et al. Synthesis of Green Engineered Silver Nanoparticles through Urtica dioica: An Inhibition of Microbes and Alleviation of Cellular and Organismal Toxicity in Drosophila melanogaster. Antibiotics 2022, 11, 1690. [Google Scholar] [CrossRef] [PubMed]
- Abdulhafiz, F.; Mohammed, A.; Kayat, F.; Bhaskar, M.; Hamzah, Z.; Podapati, S.K.; Reddy, L.V. Xanthine oxidase inhibitory activity, chemical composition, antioxidant properties and GC–MS Analysis of Keladi Candik (Alocasia longiloba Miq). Molecules 2020, 25, 2658. [Google Scholar] [CrossRef] [PubMed]
- Nagao, K.; Yanagita, T. Conjugated fatty acids in food and their health benefits. J. Biosci. Bioeng. 2005, 100, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Vijayalakshmi, M.; Umamaheswari, A.; Agalya, B.; Jegasubramaniam, S.N.; Prabu, S.L. Foresight on Phytoconstituents and Associated Pharmacological Activities of Traditional Medicinal Plant: Saussurea costus (Falc.) Lipschitz. Curr. Pharmacol. Rep. 2022, 8, 281–289. [Google Scholar] [CrossRef]
- Singh, M.P.; Reddy, M.K.; Mathur, N.; Saxena, D.K.; Chowdhuri, D.K. Induction of hsp70, hsp60, hsp83 and hsp26 and oxidative stress markers in benzene, toluene and xylene exposed Drosophila melanogaster: Role of ROS generation. Toxicol. App. Pharmacol. 2009, 235, 226–243. [Google Scholar] [CrossRef]
- Paul, I.M.; Walson, P.D. Acetaminophen and ibuprofen in the treatment of pediatric fever: A narrative review. Curr. Med. Res. Opin. 2021, 37, 1363–1375. [Google Scholar] [CrossRef]
- Akakpo, J.Y.; Ramachandran, A.; Orhan, H.; Curry, S.C.; Rumack, B.H.; Jaeschke, H. 4-methylpyrazole protects against acetaminophen-induced acute kidney injury. Toxicol. Appl. Pharmacol. 2020, 409, 115317. [Google Scholar] [CrossRef]
- Mazer, M.; Perrone, J. Acetaminophen-induced nephrotoxicity: Pathophysiology, clinical manifestations, and management. J. Med. Toxicol. 2008, 4, 2–6. [Google Scholar] [CrossRef]
- Eraky, S.M.; El-Magd NF, A. Omega-3 fatty acids protect against acetaminophen-induced hepatic and renal toxicity in rats through HO-1-Nrf2-BACH1 pathway. Arch. Biochem. 2020, 687, 108387. [Google Scholar] [CrossRef]
- Seok, P.R.; Kim, J.H.; Kwon, H.R.; Heo, J.S.; Choi, J.R.; Shin, J.H. Protective effects of Gastrodia elata Blume on acetaminophen-induced liver and kidney toxicity in rats. Food Sci. Biotech. 2018, 27, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.M.; Lee, S.G.; Oh, C.S.; Kim, S.G. Hypergravity Load Modulates Acetaminophen Nephrotoxicity via Endoplasmic Reticulum Stress in Association with Hepatic microRNA-122 Expression. Int. J. Mol. Sci. 2021, 22, 4901. [Google Scholar] [CrossRef] [PubMed]
- Çoban, F.K.; İnce, S.; Demirel, H.H.; İslam, İ.; Aytuğ, H. Acetaminophen-Induced Nephrotoxicity: Suppression of Apoptosis and Endoplasmic Reticulum Stress Using Boric Acid. Biol. Trace Elem. Res. 2022, 201, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.T.; Lin, W.C.; Yu, B.; Lee, T.T. Antioxidant capacity of phytochemicals and their potential effects on oxidative status in animals—A review. Asian-Australas J. Anim. Sci. 2017, 30, 299. [Google Scholar] [CrossRef]
- Cabrera, C.; Artacho, R.; Giménez, R. Beneficial effects of green tea—A review. J. Am. Coll. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef]
- Yousuf, S.; Shabir, S.; Singh, M.P. Protection against drug-induced liver injuries through nutraceuticals via amelioration of Nrf-2 signaling. J. Am. Nutr. Assoc. 2022, 1–21. [Google Scholar] [CrossRef]
- Caglar, H.G.; Selek Şahabettin Koktasoglu, F.; Koyuncu, I.; Demirel, M.; Sarikaya, A.; Meydan, S. Effect of Camellia sinensis, Hypericum perforatum and Urtica dioica on kidney and liver injury induced by carbon tetrachloride in rats. Cell Mol. Biol. 2019, 65, 79–86. [Google Scholar] [CrossRef]
- Dar, M.A.; Mir, R.H.; Mohi-ud-din, R.; Mir, P.A.; Masoodi, M.H.; Akbar, S.; Sawhney, G. Capsella bursa-pastoris (L.) Medic: An Insight into its Pharmacology, Expository Traditional Uses and Extensive Phytochemistry. Curr. Tradit. Med. 2021, 7, 168–179. [Google Scholar] [CrossRef]
- Seth, R.; Devi, A.; Sharma, B.; Masand, M.; Singh, G.; Pal, P.; Sharma, R.K. An Integrative Transcriptional Network Revealed Spatial Molecular Interplay Underlying Alantolactone and Inulin Biosynthesis in Inula racemosa Hook f. Int. J. Mol. Sci. 2022, 23, 11213. [Google Scholar] [CrossRef]
- Olivia, N.U.; Goodness, U.C.; Obinna, O.M. Phytochemical profiling and GC-MS analysis of aqueous methanol fraction of Hibiscus asper leaves. Future J. Pharm. Sci. 2021, 7, 59. [Google Scholar] [CrossRef]
- Gu, I.; Howard, L.; Lee, S.O. Volatiles in Berries: Biosynthesis, Composition, Bioavailability, and Health Benefits. Appl. Sci. 2022, 12, 10238. [Google Scholar] [CrossRef]
- Grauso, L.; Emrick, S.; Bonanomi, G.; Lanzotti, V. Metabolomics of the alimurgic plants Taraxacum officinale, Papaver rhoeas and Urtica dioica by combined NMR and GC–MS analysis. Phytochem. Anal. 2019, 30, 535–546. [Google Scholar] [CrossRef]
- Farag, M.A.; Weigend, M.; Luebert, F.; Brokamp, G.; Wessjohann, L.A. Phytochemical, phylogenetic, and anti-inflammatory evaluation of 43 Urtica accessions (stinging nettle) based on UPLC–Q-TOF-MS metabolomic profiles. Phytochemistry 2013, 96, 170–183. [Google Scholar] [CrossRef]
- Rathore, S.; Raj, Y.; Debnath, P.; Kumar, M.; Kumar, R. Ethnopharmacology, phytochemistry, agrotechnology, and conservation of Inula racemosa Hook f.–A critically endangered medicinal plant of the western Himalaya. J. Ethnopharmacol. 2022, 283, 114613. [Google Scholar] [CrossRef]
- Garcìa, L.M.; Ceccanti, C.; Negro, C.; De Bellis, L.; Incrocci, L.; Pardossi, A.; Guidi, L. Effect of drying methods on phenolic compounds and antioxidant activity of Urtica dioica L. Leaves. Horticulturae 2021, 7, 10. [Google Scholar] [CrossRef]
- Apaydin Yildirim, B.; Aydin, T.; Kordali, S.; Yildirim, S.; Cakir, A.; Yildirim, F. Antihemorrhoidal activity of organic acids of Capsella bursa-pastoris on croton oil-induced hemorrhoid in rats. J. Food Biochem. 2020, 44, e13343. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Pangloli, P.; Perumal, R.; Cox, S.; Noronha, L.E.; Dia, V.P.; Smolensky, D. A comparative study on phenolic content, antioxidant activity and anti-inflammatory capacity of aqueous and ethanolic extracts of sorghum in lipopolysaccharide-induced RAW 264.7 macrophages. Antioxidants 2020, 9, 1297. [Google Scholar] [CrossRef]
- Adeneye, A.A.; Benebo, A.S. Protective effect of the aqueous leaf and seed extract of Phyllanthus amarus on gentamicin and acetaminophen-induced nephrotoxic rats. J. Ethnopharmacol. 2008, 118, 318–323. [Google Scholar] [CrossRef]
- Grauso, L.; de Falco, B.; Lanzotti, V.; Motti, R. Stinging nettle, Urtica dioica L.: Botanical, phytochemical and pharmacological overview. Phytochem. Rev. 2020, 19, 1341–1377. [Google Scholar] [CrossRef]
- Cha, J.M.; Suh, W.S.; Lee, T.H.; Subedi, L.; Kim, S.Y.; Lee, K.R. Phenolic glycosides from Capsella bursa-pastoris (L.) Medik and their anti-inflammatory activity. Molecules 2019, 22, 1023. [Google Scholar] [CrossRef]
- Kalachaveedu, M.; Raghavan, D.; Telapolu, S.; Kuruvilla, S.; Kedike, B. Phytoestrogenic effect of Inula racemosa Hook f–A cardioprotective root drug in traditional medicine. J. Ethnopharmacol. 2018, 210, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Rjeibi, I.; Feriani, A.; Saad, A.B.; Ncib, S.; Sdayria, J.; Hfaiedh, N.; Allagui, M.S. Lycium europaeum Linn as a source of polysaccharide with in vitro antioxidant activities and in vivo anti-inflammatory and hepato-nephroprotective potentials. J. Ethnopharmacol. 2018, 225, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, A.; Abdelkader, A.; Abdo, M.; Wareth, G.; Aboubakr, M.; Aleya, L.; Abdel-Daim, M. Protective effect of cinnamon against acetaminophen-mediated cellular damage and apoptosis in renal tissue. Environ. Sci. Pollut. Res. 2019, 26, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Parameshappa, B.; Ali Basha, M.S.; Sen, S.; Chakraborty, R.; Kumar, G.V.; Sagar, G.V.; Lakshmi AV, S.M. Acetaminophen-induced nephrotoxicity in rats: Protective role of Cardiospermum halicacabum. Pharm. Biol. 2012, 50, 247–253. [Google Scholar] [CrossRef]
- Epure, A.; Pârvu, A.E.; Vlase, L.; Benedec, D.; Hanganu, D.; Gheldiu, A.M.; Oniga, I. Phytochemical profile, antioxidant, cardioprotective and nephroprotective activity of Romanian chicory extract. Plants 2020, 10, 64. [Google Scholar] [CrossRef]
- Marin, M.; Drăgotoiu, D.; Nicolae, C.G.; Diniţă, G. Research on the influence of the oregano oil use over the productive performances and quality of duck meat. AgroLife Sci. J. 2015, 4, 48–51. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousuf, S.; Shabir, S.; Mehdi, M.M.; Srivastav, S.; Mohammedsaleh, Z.M.; Bassfar, Z.; Jalal, M.M.; Moawadh, M.S.; Jamous, Y.F.; Singh, S.K.; et al. Investigation of the Protective Effects of Urtica dioica, Capsella bursa-pastoris and Inula racemosa on Acetaminophen-Induced Nephrotoxicity in Swiss Albino Male Mice. Appl. Sci. 2023, 13, 3925. https://doi.org/10.3390/app13063925
Yousuf S, Shabir S, Mehdi MM, Srivastav S, Mohammedsaleh ZM, Bassfar Z, Jalal MM, Moawadh MS, Jamous YF, Singh SK, et al. Investigation of the Protective Effects of Urtica dioica, Capsella bursa-pastoris and Inula racemosa on Acetaminophen-Induced Nephrotoxicity in Swiss Albino Male Mice. Applied Sciences. 2023; 13(6):3925. https://doi.org/10.3390/app13063925
Chicago/Turabian StyleYousuf, Sumaira, Shabnam Shabir, Mohammad Murtaza Mehdi, Shailesh Srivastav, Zuhair M. Mohammedsaleh, Zaid Bassfar, Mohammed M. Jalal, Mamdoh S. Moawadh, Yahya F. Jamous, Sandeep Kumar Singh, and et al. 2023. "Investigation of the Protective Effects of Urtica dioica, Capsella bursa-pastoris and Inula racemosa on Acetaminophen-Induced Nephrotoxicity in Swiss Albino Male Mice" Applied Sciences 13, no. 6: 3925. https://doi.org/10.3390/app13063925
APA StyleYousuf, S., Shabir, S., Mehdi, M. M., Srivastav, S., Mohammedsaleh, Z. M., Bassfar, Z., Jalal, M. M., Moawadh, M. S., Jamous, Y. F., Singh, S. K., Vamanu, E., & Singh, M. P. (2023). Investigation of the Protective Effects of Urtica dioica, Capsella bursa-pastoris and Inula racemosa on Acetaminophen-Induced Nephrotoxicity in Swiss Albino Male Mice. Applied Sciences, 13(6), 3925. https://doi.org/10.3390/app13063925