Prevalence and Antibiotic Resistance of Enterococcus spp.: A Retrospective Study in Hospitals of Southeast Romania
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Collection
2.2. Identification of Bacteria
2.3. Antimicrobial Susceptibility Testing
2.4. Exclusion Criteria of Bacterial Isolates
2.5. Statistical Analysis
3. Results
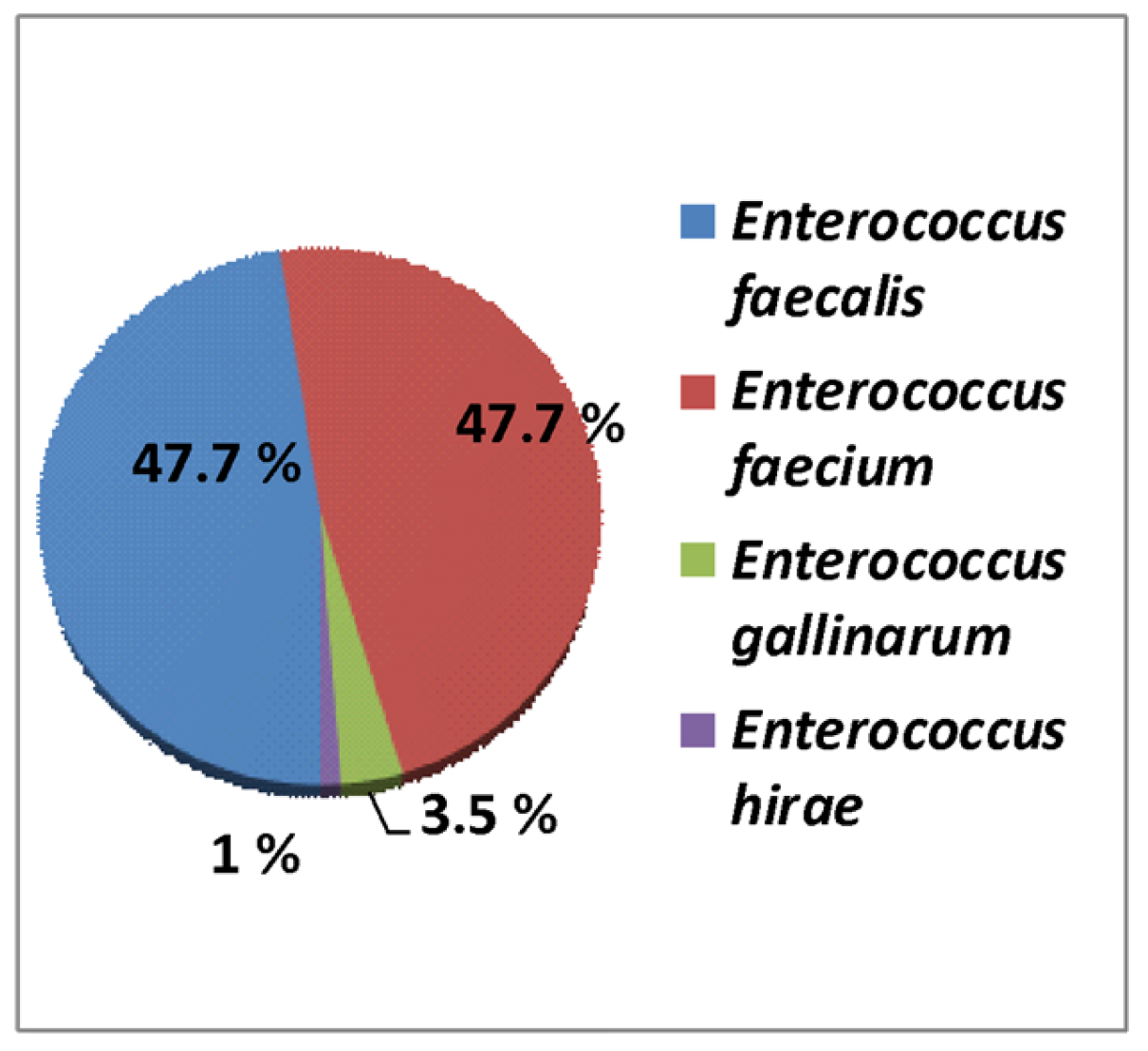
3.1. Prevalence of Enterococcus spp. from Hospital Bacterial Isolates
3.2. Antibiotic Resistance of Enterococcus spp.
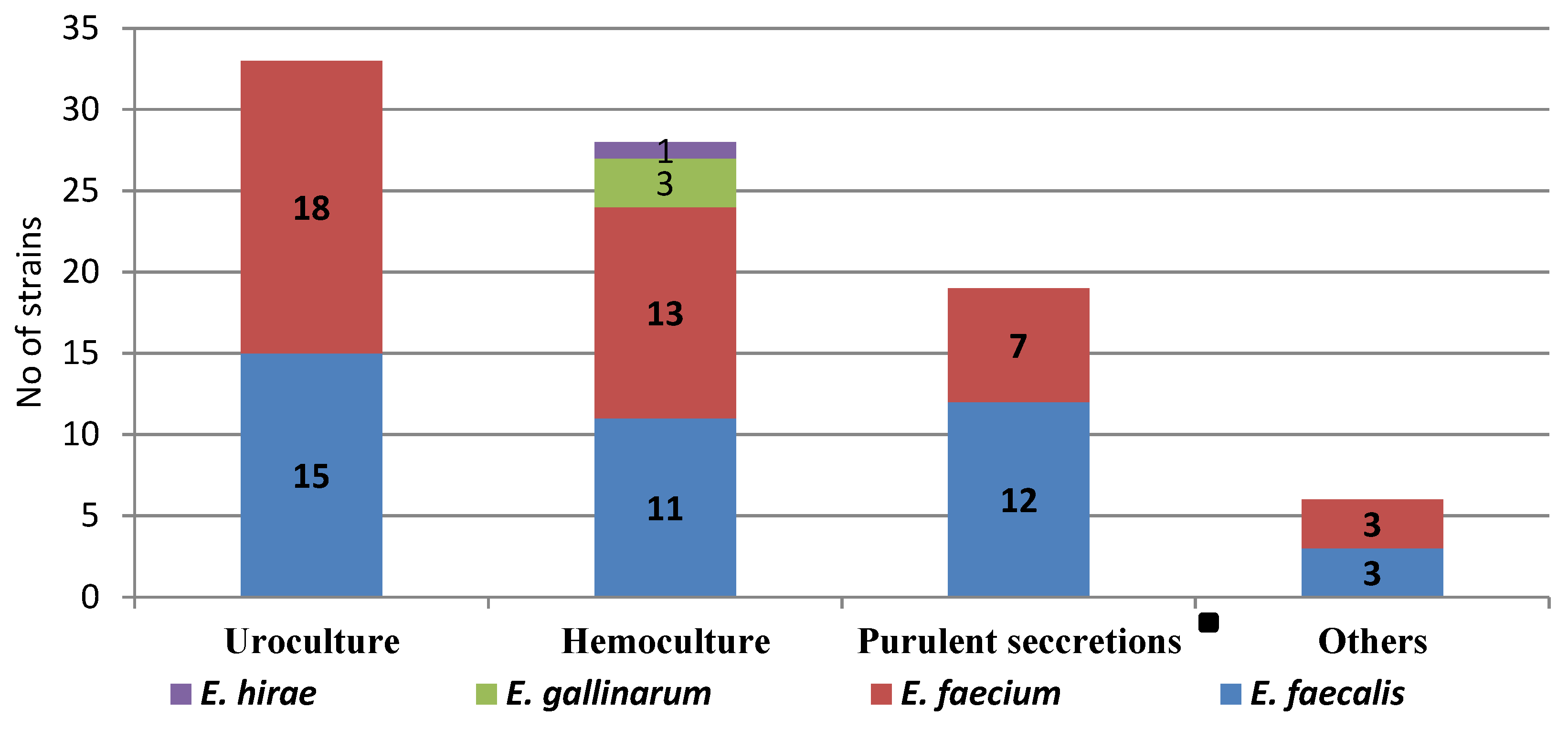
3.3. Comparative of Enterococcus spp. in Three Hospitals of Southeast Romania
4. Discussion
Limits of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

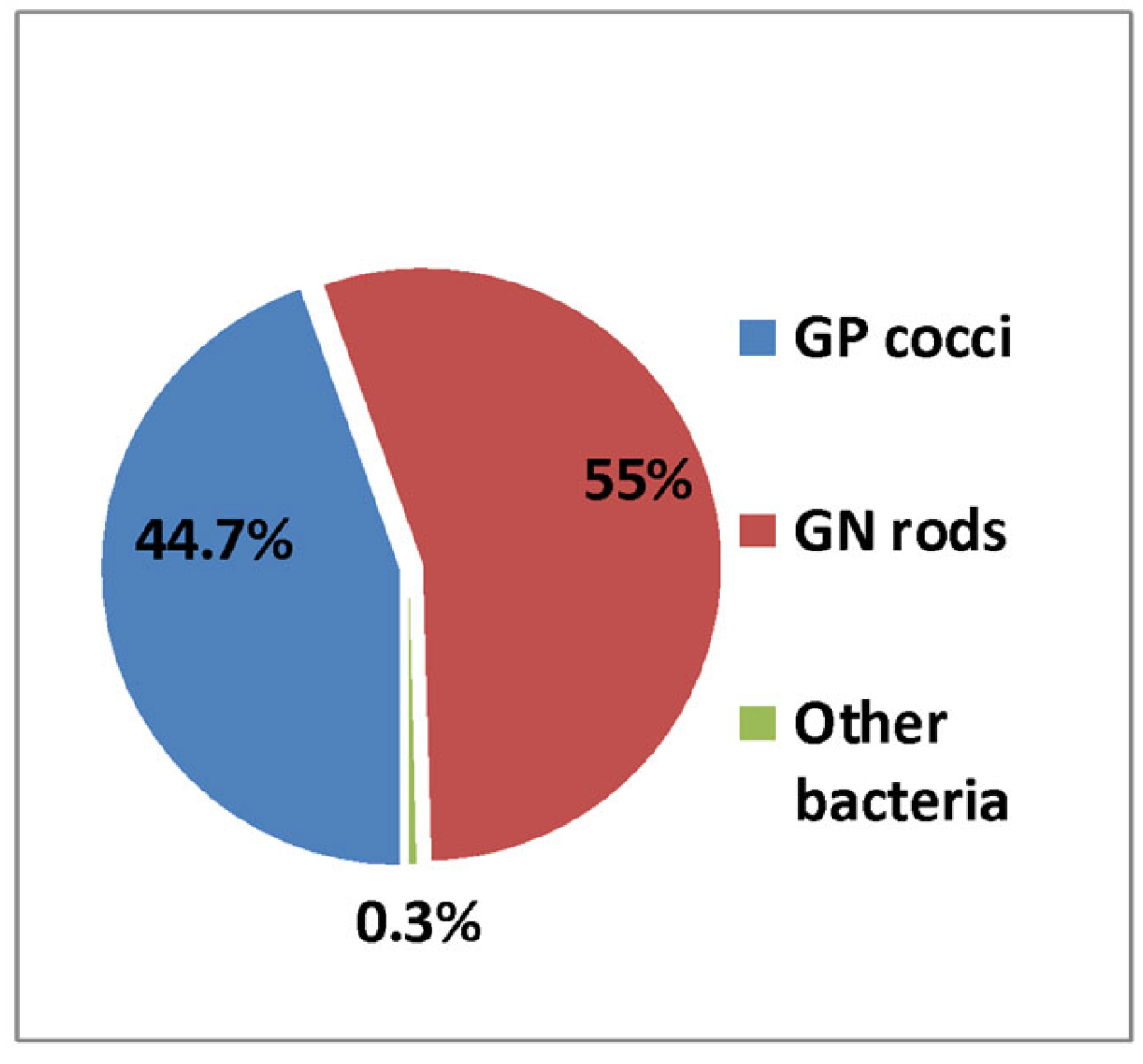
| Genera | 2019 | No—2020 | No—2021 | Total | % | |||
|---|---|---|---|---|---|---|---|---|
| GP Cocci | No | % | No | % | No | % | ||
| Staphylococcus spp. | 3701 | 67.9% | 2128 | 77.6% | 2926 | 80.71% | 8755 | 32.8% |
| Streptococcus spp. β-Hemolytic group | 812 | 14.9% | 330 | 12% | 325 | 8.96% | 1467 | 5.5% |
| Streptococcus pneumoniae | 826 | 15.16% | 225 | 8.2% | 307 | 8.46% | 1358 | 5.08% |
| Enterococcus spp. | 106 | 1.94% | 57 | 2.08% | 67 | 1.84% | 230 | 0.86% |
| Total | 5445 | 100% | 2740 | 100% | 3625 | 100% | 11,810 | 44.24% |
| GN rods | ||||||||
| Escherichia coli | 3325 | 54.6% | 1691 | 46.4% | 2301 | 46.4% | 7317 | 27.4% |
| Klebsiella spp. | 1328 | 21.8% | 1068 | 29.3% | 1526 | 30.8% | 3922 | 14.7% |
| Proteus spp. | 400 | 6.6% | 220 | 6.03% | 278 | 5.6% | 898 | 3.4% |
| Enterobacter spp. | 210 | 3.45% | 80 | 2.19% | 78 | 1.57% | 368 | 1.37% |
| Salmonella spp. | 91 | 1.5% | 44 | 1.2% | 74 | 1.5% | 209 | 0.8% |
| Alte Enterobacterales | 20 | 0.32% | 9 | 0.24% | 14 | 0.28% | 43 | 0.16% |
| Pseudomonas spp. | 624 | 10.2% | 437 | 9.52% | 549 | 11% | 1610 | 6.03% |
| Acinetobacter spp. | 88 | 1.4% | 95 | 2.6% | 139 | 2.8% | 322 | 1.2% |
| Total | 6086 | 100% | 3644 | 100% | 4959 | 100% | 14,689 | 55.1% |
| Other bacteria | 63 | 78 | 48 | 189 | 0.7% | |||
| Total | 11,594 | 6473 | 8632 | 26,699 | 100% | |||
References
- Rice, L.B. Federal Funding for the Study of Antimicrobial Resistance in Nosocomial Pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef] [PubMed]
- García-Solache, M.; Rice, L.B. The Enterococcus: A Model of Adaptability to Its Environment. Clin. Microbiol. Rev. 2019, 32, e00058-18. [Google Scholar] [CrossRef] [PubMed]
- Monticelli, J.; Di Bella, S.; Giacobbe, D.R.; Amato, G.; Antonello, R.M.; Barone, E.; Brachelente, G.; Busetti, M.; Carcione, D.; Carretto, E.; et al. Trends in the Incidence and Antibiotic Resistance of Enterococcal Bloodstream Isolates: A 7-Year Retrospective Multicenter Epidemiological Study in Italy. Microb. Drug Resist. 2021, 27, 529–535. [Google Scholar] [CrossRef]
- Monticelli, J.; Di Bella, S.; Giacobbe, D.R.; Amato, G.; Antonello, R.M.; Barone, E.; Brachelente, G.; Busetti, M.; Carcione, D.; Carretto, E.; et al. Enterococci enhance Clostridioides difficile pathogenesis. Nature 2022, 611, 780–786. [Google Scholar] [CrossRef]
- Stevens, V.W.; Khader, K.; Echevarria, K.; E Nelson, R.; Zhang, Y.; Jones, M.; Timbrook, T.T.; Samore, M.H.; A Rubin, M. Use of Oral Vancomycin for Clostridioides difficile Infection and the Risk of Vancomycin-Resistant Enterococci. Clin. Infect. Dis. 2020, 71, 645–651. [Google Scholar] [CrossRef]
- Tokars, J.I.; Satake, S.; Rimland, D.; Carson, L.; Miller, E.R.; Killum, E.; Sinkowitz-Cochran, R.L.; Arduino, M.J.; Tenover, F.C.; Marston, B.; et al. The prevalence of colonization with vancomycin-resistant Enterococcus at a Veterans’ Affairs institution. Infect. Control Hosp. Epidemiol. 1999, 20, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Harbarth, S.; Samore, M.H.; Lichtenberg, D.; Carmeli, Y. Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on surgical site infections and antimicrobial resistance. Circulation 2000, 101, 2916–2921. [Google Scholar] [CrossRef]
- DeLisle, S.; Perl, T.M. Vancomycin-resistant enterococci: A road map on how to prevent the emergence and transmission of antimicrobial resistance. Circulation 2003, 123, 504S–518S. [Google Scholar]
- Padiglione, A.A.; Wolfe, R.; Grabsch, E.A.; Olden, D.; Pearson, S.H.; Franklin, C.; Spelman, D.; Mayall, B.; Johnson, P.D.R.; Grayson, M.L. Risk factors for new detection of vancomycin-resistant enterococci in acute-care hospitals that employ strict infection control procedures. Antimicrob. Agents Chemother. 2000, 47, 2492–2498. [Google Scholar] [CrossRef]
- Edmond, M.B.; Wallace, S.E.; McClish, D.K.; Pfaller, M.A.; Jones, R.N.; Wenzel, R.P. Nosocomial bloodstream infections in United States hospitals: A three-year analysis. Clin. Infect. Dis. 1999, 29, 239–244. [Google Scholar] [CrossRef]
- Růžičková, M.; Vítězová, M.; Kushkevych, I. The characterization of Enterococcus genus: Resistance mechanisms and inflammatory bowel disease. Open Med. 2020, 15, 211–224. [Google Scholar] [CrossRef]
- Hollenbeck, B.L.; Rice, L.B. Intrinsic and acquired resistance mechanisms in Enterococcus. Virulence 2012, 3, 421–433. [Google Scholar] [CrossRef]
- Guffey, A.A.; Loll, P.J. Regulation of Resistance in Vancomycin-Resistant Enterococci: The VanRS Two-Component System. Microorganisms 2021, 9, 2026. [Google Scholar] [CrossRef]
- Aqib, A.I.; Alsayeqh, A.F. Vancomycin drug resistance, an emerging threat to animal and public health. Front. Vet. Sci. 2022, 28, 1010728. [Google Scholar] [CrossRef]
- Cooper, E.; Paull, A.; O’Reilly, M. Characteristics of a large cluster of vancomycin-resistant enterococci in an Australian hospital. Infect. Control. Hosp. Epidemiol. 2002, 23, 151–153. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. National nosocomial infections surveillance (NNIS) system report, data summary from January 1992–April 2000. Am. J. Infect. Control 2000, 28, 429–448. [Google Scholar] [CrossRef]
- Murray, B.E. Vancomycin-resistant enterococcal infections. N. Engl. J. Med. 2000, 342, 710–721. [Google Scholar] [CrossRef]
- Brinkwirth, S.; Ayobami, O.; Eckmanns, T.; Markwart, R. Hospital-acquired infections caused by enterococci: A systematic review and meta-analysis, WHO European Region, 1 January 2010 to 4 February 2020. Euro. Surveill. 2021, 26, 2001628. [Google Scholar] [CrossRef] [PubMed]
- Hemapanpairoa, J.; Changpradub, D.; Santimaleeworagun, W. Clinical Impact of Vancomycin Treatment in Ampicillin-Susceptible Enterococci Bloodstream Infections. Antibiotics 2022, 11, 1698. [Google Scholar] [CrossRef] [PubMed]
- Janjusevic, A.; Cirkovic, I.; Minic, R.; Stevanovic, G.; Soldatovic, I.; Mihaljevic, B.; Vidovic, A.; Markovic Denic, L. Predictors of Vancomycin-Resistant Enterococcus spp. Intestinal Carriage among High-Risk Patients in University Hospitals in Serbia. Antibiotics 2022, 11, 1228. [Google Scholar] [CrossRef] [PubMed]
- Büchler, A.C.; Ragozzino, S.; Wicki, M.; Spaniol, V.; Jäger, S.; Seth-Smith, H.M.B.; Goldenberger, D.; Hinic, V.; Egli, A.; Frei, R.; et al. Patients exposed to vancomycin-resistant enterococci during in-hospital outbreaks in a low endemic setting: A proposal for risk-based screening. Antimicrob. Resist. Infect. Control 2022, 11, 60. [Google Scholar] [CrossRef]
- Beale, J.W.; Durward-Diioia, M. Clinical Considerations in the Approach to Vancomycin-Resistant Enterococci: A Narrative Review. Int. J. Med. Students 2022, 10, 202–209. [Google Scholar] [CrossRef]
- Todorić, Z.; Majdandžić, I.; Kregar, T.K.; Herljević, Z.; Ćorić, M.; Lešin, J.; Kuliš, T. Increasing trend in enterococcal bacteraemia and vancomycin resistance in a tertiary care hospital in Croatia, 2017–2021. Infect. Dis. 2022, 55, 9–16. [Google Scholar] [CrossRef] [PubMed]
- DiazGranados, C.A.; Zimmer, S.M.; Klein, M.; Jernigan, J.A. Comparison of mortality associated with vancomycin-resistant and vancomycin-susceptible enterococcal bloodstream infections: A meta-analysis. Clin. Infect. Dis. 2005, 41, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Correa-Martinez, C.L.; Tönnies, H.; Froböse, N.J.; Mellmann, A.; Kampmeier, S. Transmission of Vancomycin-Resistant Enterococci in the Hospital Setting: Uncovering the Patient-Environment Interplay. Microorganisms 2020, 8, 203. [Google Scholar] [CrossRef]
- Ramos, S.; Silva, V.; Dapkevicius, M.d.L.E.; Igrejas, G.; Poeta, P. Enterococci, from Harmless Bacteria to a Pathogen. Microorganisms 2020, 8, 1118. [Google Scholar] [CrossRef]
- Zaheer, R.; Cook, S.R.; Barbieri, R.; Goji, N.; Cameron, A.; Petkau, A.; Polo, R.O.; Tymensen, L.; Stamm, C.; Song, J.; et al. Surveillance of Enterococcus spp. reveals distinct species and antimicrobial resistance diversity across a One-Health continuum. Sci. Rep. 2020, 10, 3937. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Antimicrobial Resistance Surveillance in Europe 2022. Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report for 2020. Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-eueea-ears-net-annual-epidemiological-report-2020 (accessed on 10 September 2022).
- Procop, G.W.; Church, D.L.; Hall, G.S.; Janda, W.M. Koneman’s Color Atlas and Textbook of Diagnostic Microbiology, 7th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2017. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: 29th Informational Supplement, CLSI Document M100-S29; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019; Available online: https://clsi.org/media/2663/m100ed29_sample.pdf (accessed on 12 January 2019).
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: 30th Informational Supplement, CLSI Document M100-S30; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020; Available online: https://clsi.org/media/3481/m100ed30_sample.pdf (accessed on 25 January 2020).
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: 31th Informational Supplement, CLSI Document M100-S31; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021; Available online: https://clsi.org/media/z2uhcbmv/m100ed31_sample.pdf (accessed on 8 January 2021).
- Gajic, I.; Kabic, J.; Kekic, D.; Jovicevic, M.; Milenkovic, M.; Mitic Culafic, D.; Trudic, A.; Ranin, L.; Opavski, N. Antimicrobial Susceptibility Testing: A Comprehensive Review of Currently Used Methods. Antibiotics 2022, 11, 427. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drugresistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Popescu, G.A.; Șerban, R.; Niculcea, A.; Centrul National de Supraveghere si Control al Bolilor Transmisibile. CARMIN-ROM 2017 (Consumul de Antibiotice, Rezistența Microbiană și Infecții Asociate Asistenței Medicale în România—2017). Available online: https://www.cnscbt.ro/index.php/analiza-date-supraveghere/infectii-nosocomiale-1/1309-consumul-de-antibiotice-rezistenta-microbiana-si-infectii-asociate-asistentei-medicale-nosocomiale-in-romania-2017/file (accessed on 29 September 2022).
- Rogers, L.A.; Strong, K.; Cork, S.C.; McAllister, T.A.; Liljebjelke, K.; Zaheer, R.; Checkley, S.L. The Role of Whole Genome Sequencing in the Surveillance of Antimicrobial Resistant Enterococcus spp.: A Scoping Review. Front. Public Health 2021, 9, 599285. [Google Scholar] [CrossRef] [PubMed]
- Arbune, M.; Gurau, G.; Niculet, E.; Iancu, A.V.; Lupasteanu, G.; Fotea, S.; Vasile, M.C.; Tatu, A.L. Prevalence of Antibiotic Resistance of ESKAPE Pathogens Over Five Years in an Infectious Diseases Hospital from South-East of Romania. Infect. Drug Resist. 2021, 14, 2369–2378. [Google Scholar] [CrossRef] [PubMed]
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial bloodstream infections in U.S. hospitals: Analysis of 24,000 cases from a prospective national wide surveillance study. Clin.Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef]
- El-Sokkary, R.; Erdem, H.; Kullar, R.; Pekok, A.U.; Amer, F.; Grgić, S.; Carevic, B.; El-Kholy, A.; Liskova, A.; Özdemir, M.; et al. Self-reported antibiotic stewardship and infection control measures from 57 intensive care units: An international ID-IRI survey. J. Infect. Public Health. 2022, 15, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Boccella, M.; Santella, B.; Pagliano, P.; De Filippis, A.; Casolaro, V.; Galdiero, M.; Borrelli, A.; Capunzo, M.; Boccia, G.; Franci, G. Prevalence and Antimicrobial Resistance of Enterococcus Species: A Retrospective Cohort Study in Italy. Antibiotics 2021, 10, 1552. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, M.; Chang, Y.; Chen, L.A.; Zhang, Q. Incidence, clinical characteristics, and outcomes of nosocomial Enterococcus spp. bloodstream infections in a tertiary-care hospital in Beijing, China: A four-year retrospective study. Antimicrob. Resist. Infect. Control 2017, 6, 73. [Google Scholar] [CrossRef]
- Pinholt, M.; Ostergaard, C.; Arpi, M.; Bruun, N.E.; Schønheyder, H.C.; Gradel, K.O.; Søgaard, M.; Knudsen, J.D. Incidence, clinical characteristics and 30-day mortality of enterococcal bacteraemia in Denmark 2006-2009: A population-based cohort study. Clin. Microbiol. Infect. 2014, 20, 145–151. [Google Scholar] [CrossRef]
- Santella, B.; Folliero, V.; Pirofalo, G.M.; Serretiello, E.; Zannella, C.; Moccia, G.; Santoro, E.; Sanna, G.; Motta, O.; De Caro, F.; et al. Sepsis-A Retrospective Cohort Study of Bloodstream Infections. Antibiotics 2020, 9, 851. [Google Scholar] [CrossRef]
- O’Driscoll, T.; Crank, C.W. Vancomycin-resistant enterococcal infections: Epidemiology, clinical manifestations, and optimal management. Infect. Drug Resist. 2015, 8, 217–230. [Google Scholar] [CrossRef]
- Salem-Bekhit, M.; Moussa, I.; Muharram, M.; Alanazy, F.; Hefni, H. Prevalence and antimicrobial resistance pattern of multidrug resistant enterococci isolated from clinical specimens. Indian J. Med. Microbiol. 2012, 30, 44–51. [Google Scholar] [CrossRef]
- Galvan, E.M.; Mateyca, C.; Ielpi, L. Role of interspecies interactions in dual-species biofilms developed in vitro by uropathogens isolated from polymicrobial urinary catheter-associated bacteriuria. Biofouling 2016, 32, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Akhter, J.; Ahmed, S.; Saleh, A.A.; Anwar, S. Antimicrobial resistance and in vitro biofilm-forming ability of Enterococci spp. isolated from urinary tract infection in a tertiary care hospital in Dhaka. Bangladesh Med. Res. Counc. Bull. 2014, 40, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Miftode, I.-L.; Pasare, M.-A.; Miftode, R.-S.; Nastase, E.; Plesca, C.E.; Lunca, C.; Miftode, E.-G.; Timpau, A.-S.; Iancu, L.S.; Dorneanu, O.S. What Doesn’t Kill Them Makes Them Stronger: The Impact of the Resistance Patterns of Urinary Enterobacterales Isolates in Patients from a Tertiary Hospital in Eastern Europe. Antibiotics 2022, 11, 548. [Google Scholar] [CrossRef] [PubMed]

| Enterococcus Species | N | 1R (n) | 1R (%) | 2R (n) | 2R (%) | MDR (n) | MDR (%) |
|---|---|---|---|---|---|---|---|
| E. faecalis | 41 | 3 | 7.3 | 7 | 17.1 | 9 | 21.9 |
| E. faecium | 41 | 2 | 4.9 | 4 | 9.8 | 31 | 75.6 |
| E. gallinarum | 3 | 0 | - | 0 | - | 3 | 100 |
| E. hirae | 1 | 0 | - | 0 | - | 0 | - |
| Total | 86 | 5 | 5.8 | 11 | 12.8 | 43 | 50 |
| n | ECH (N1 = 48) | IDH (N2 = 20) | ECHC (N3 = 18) | Chi-Square Test (p) | ||
|---|---|---|---|---|---|---|
| Gender | Male | 44 | 22 | 11 | 11 | 0.502 |
| Female | 42 | 26 | 9 | 7 | ||
| Living area | Urban | 50 | 30 | 14 | 6 | 0.047 |
| Rural | 36 | 18 | 6 | 12 | ||
| Year of isolation | 2019 | 25 | 5 | 8 | 12 | <0.001 |
| 2020 | 20 | 12 | 5 | 3 | ||
| 2021 | 41 | 31 | 7 | 3 | ||
| COVID-19 | Positive | 8 | 5 | 3 | 0 | 0.261 |
| Negative | 78 | 43 | 17 | 18 | ||
| Biological sample | Hemoculture | 29 | 26 | 2 | 1 | 0.005 |
| Uroculture | 33 | 11 | 11 | 11 | ||
| SSTI | 18 | 8 | 6 | 4 | ||
| Other | 6 | 3 | 1 | 2 | ||
| Enterococcus spp. | E. faecium | 41 | 28 | 3 | 10 | 0.018 |
| E. faecalis | 41 | 17 | 17 | 7 | ||
| Other | 4 | 3 | 0 | 1 | ||
| MDR | Yes | 43 | 34 | 2 | 7 | <0.001 |
| No | 43 | 14 | 18 | 11 | ||
| Van-R | Yes | 20 | 16 | 1 | 3 | 0.031 |
| No | 66 | 32 | 19 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iancu, A.-V.; Arbune, M.; Zaharia, E.-A.; Tutunaru, D.; Maftei, N.-M.; Peptine, L.-D.; Țocu, G.; Gurău, G. Prevalence and Antibiotic Resistance of Enterococcus spp.: A Retrospective Study in Hospitals of Southeast Romania. Appl. Sci. 2023, 13, 3866. https://doi.org/10.3390/app13063866
Iancu A-V, Arbune M, Zaharia E-A, Tutunaru D, Maftei N-M, Peptine L-D, Țocu G, Gurău G. Prevalence and Antibiotic Resistance of Enterococcus spp.: A Retrospective Study in Hospitals of Southeast Romania. Applied Sciences. 2023; 13(6):3866. https://doi.org/10.3390/app13063866
Chicago/Turabian StyleIancu, Alina-Viorica, Manuela Arbune, Eliza-Andreea Zaharia, Dana Tutunaru, Nicoleta-Maricica Maftei, Lucian-Daniel Peptine, George Țocu, and Gabriela Gurău. 2023. "Prevalence and Antibiotic Resistance of Enterococcus spp.: A Retrospective Study in Hospitals of Southeast Romania" Applied Sciences 13, no. 6: 3866. https://doi.org/10.3390/app13063866
APA StyleIancu, A.-V., Arbune, M., Zaharia, E.-A., Tutunaru, D., Maftei, N.-M., Peptine, L.-D., Țocu, G., & Gurău, G. (2023). Prevalence and Antibiotic Resistance of Enterococcus spp.: A Retrospective Study in Hospitals of Southeast Romania. Applied Sciences, 13(6), 3866. https://doi.org/10.3390/app13063866






