Nitrogenous Bases in Relation to the Colloidal Silver Phase: Adsorption Kinetic, and Morphology Investigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Colloidal Silver Solution
2.3. Preparation of Anb/AgNP/C Composite
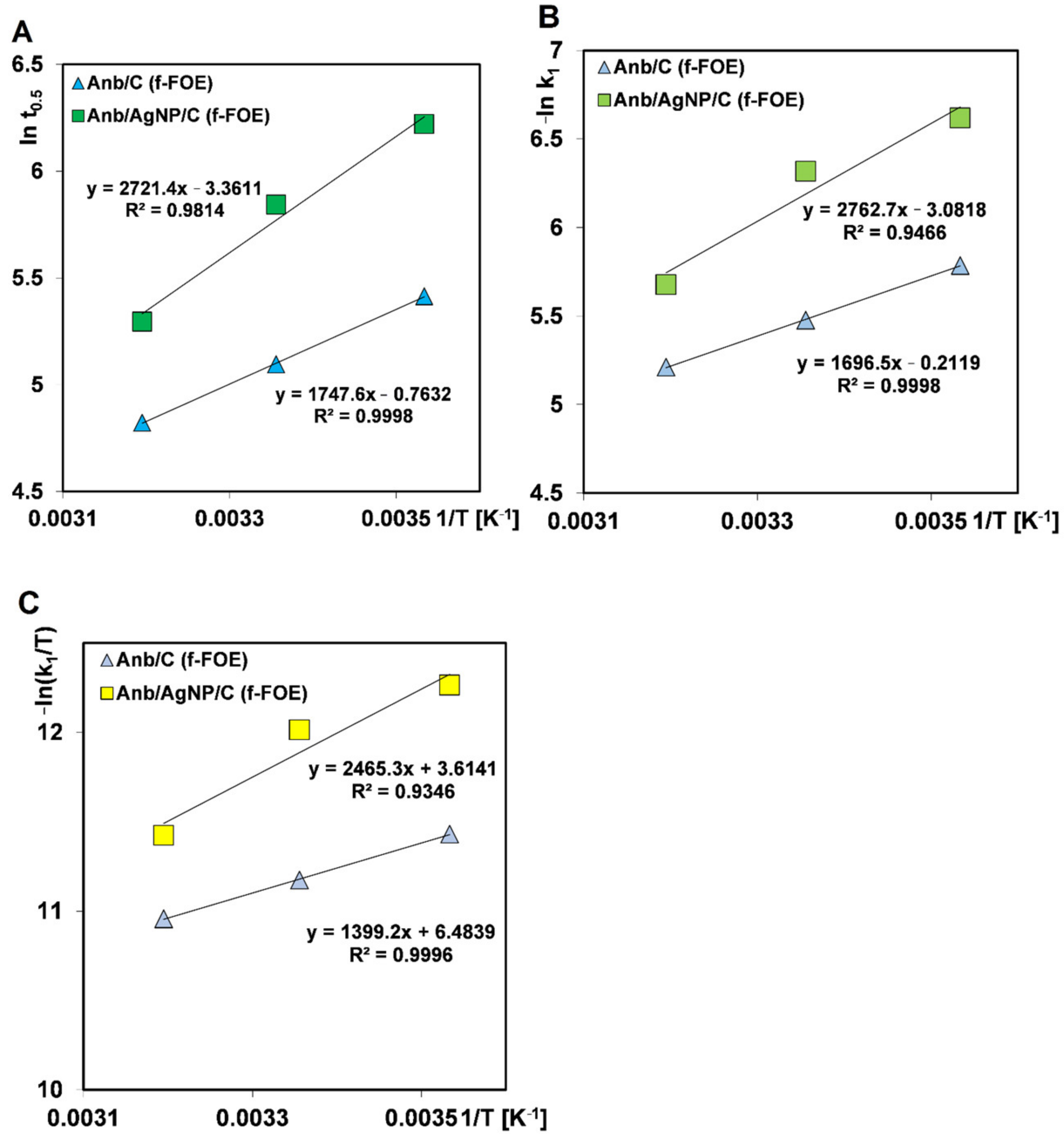
2.4. Methods and Calculations
2.4.1. Porosity Evaluation by Low-Temperature Nitrogen Sorption
2.4.2. Adsorption Studies by Kinetic Approach
2.4.3. Other Techniques and Calculations
3. Results and Discussion
3.1. Characteristics of Silver Colloidal Solution
3.2. Characteristics of Anb/C and Anb/AgNP/C Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, Y.; Halas, N.J. Shape-Controlled Synthesis and Surface Plasmonic Properties of Metallic Nanostructures. MRS Bull. 2005, 30, 338–348. [Google Scholar] [CrossRef]
- Sharma, V.; Park, K.; Srinivasarao, M. Colloidal dispersion of gold nanorods: Historical background, optical properties, seed-mediated synthesis, shape separation and self-assembly. Mater. Sci. Eng. R Rep. 2009, 65, 1–38. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Malakoff, D. Congress Wants Studies of Nanotech’s ‘Dark Side’. Science 2003, 301, 27. [Google Scholar] [CrossRef]
- Service, R.F. American Chemical Society meeting. Nanomaterials show signs of toxicity. Science 2003, 300, 243. [Google Scholar] [CrossRef]
- Steel, A.B.; Herne, T.M.; Tarlov, M.J. Electrochemical Quantitation of DNA Immobilized on Gold. Anal. Chem. 1998, 70, 4670–4677. [Google Scholar] [CrossRef]
- Tao, N.J.; DeRose, J.A.; Lindsay, S.M. Self-assembly of molecular superstructures studied by in situ scanning tunneling microscopy: DNA bases on gold (111). J. Phys. Chem. 1993, 97, 910–919. [Google Scholar] [CrossRef]
- Thomas Boland and Buddy, D.R. Two-Dimensional Assembly of Purines and Pyrimidines on Au(111). Langmuir 1994, 10, 3845–3852. [Google Scholar]
- Hill, H.A.O. Metal ions in genetic information transfer. Volume 3 of advances in inorganic biochemistry: Edited by G. L. Eichhorn and L. G. Marzilli, Elsevier/North-Holland, 1981. $47.95. Trends Biochem. Sci. 1982, 7, 417–418. [Google Scholar] [CrossRef]
- Kholodar, S.A.; Allen, C.L.; Gulick, A.M.; Murkin, A.S. The Role of Phosphate in a Multistep Enzymatic Reaction: Reactions of the Substrate and Intermediate in Pieces. J. Am. Chem. Soc. 2015, 137, 2748–2756. [Google Scholar] [CrossRef]
- Simulescu, V.; Crasmareanu, E.; Ilia, G. Synthesis, Properties and Structures of Phosphorus-Nitrogen Heterocycles. Heterocycles 2011, 83, 275–291. [Google Scholar] [CrossRef]
- Gheonea, R.; Mak, C.; Crasmareanu, E.C.; Simulescu, V.; Plesu, N.; Ilia, G. Surface Modification of SnO2 with Phosphonic Acids. J. Chem. 2017, 2017, 2105938. [Google Scholar] [CrossRef]
- Lovio-Fragoso, J.P.; de Jesús-Campos, D.; López-Elías, J.A.; Medina-Juárez, L.Á.; Fimbres-Olivarría, D.; Hayano-Kanashiro, C. Biochemical and Molecular Aspects of Phosphorus Limitation in Diatoms and Their Relationship with Biomolecule Accumulation. Biology 2021, 10. [Google Scholar] [CrossRef]
- Lee, W.; Abdillah, A.; Palma, J.; Churchill, D. Phosphorus-Nature’s Versatile Pentavalent and Tetrahedral Covalent Building Block and Agent for Energy, Disease and Health. 2020. Available online: www.intechopen.com/chapters/72693URL (accessed on 9 March 2023).
- Sridharan, S.; Kurzawa, N.; Werner, T.; Günthner, I.; Helm, D.; Huber, W.; Bantscheff, M.; Savitski, M.M. Proteome-wide solubility and thermal stability profiling reveals distinct regulatory roles for ATP. Nat. Commun. 2019, 10, 1155. [Google Scholar] [CrossRef]
- Dumaz, N.; Hayward, R.; Martin, J.; Ogilvie, L.; Hedley, D.; Curtin, J.A.; Bastian, B.C.; Springer, C.; Marais, R. In Melanoma, RAS Mutations Are Accompanied by Switching Signaling from BRAF to CRAF and Disrupted Cyclic AMP Signaling. Cancer Res. 2006, 66, 9483–9491. [Google Scholar] [CrossRef]
- Goldford, J.E.; Hartman, H.; Smith, T.F.; Segrè, D. Remnants of an Ancient Metabolism without Phosphate. Cell 2017, 168, 1126–1134. [Google Scholar] [CrossRef]
- Grządka, E.; Matusiak, J. The effect of ionic and non-ionic surfactants and pH on the stability, adsorption and electrokinetic properties of the alginic acid/alumina system. Carbohydr. Polym. 2017, 175, 192–198. [Google Scholar] [CrossRef]
- Hansson, P. Self-Assembly of Ionic Surfactants in Polyelectrolyte Solutions: A Model for Mixtures of Opposite Charge. Langmuir 2001, 17, 4167–4180. [Google Scholar] [CrossRef]
- Langevin, D. Complexation of oppositely charged polyelectrolytes and surfactants in aqueous solutions. A review. Adv. Colloid Interface Sci. 2009, 147–148, 170–177. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase Liquid–Liquid system. J. Chem. Soc. Chem. Commun. 1994, 801–802. [Google Scholar] [CrossRef]
- Nath, S.; Ghosh, S.K.; Kundu, S.; Praharaj, S.; Panigrahi, S.; Pal, T. Is Gold Really Softer than Silver? HSAB Principle Revisited. J. Nanoparticle Res. 2006, 8, 111–116. [Google Scholar] [CrossRef]
- Basu, S.; Jana, S.; Pande, S.; Pal, T. Interaction of DNA bases with silver nanoparticles: Assembly quantified through SPRS and SERS. J. Colloid Interface Sci. 2008, 321, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, O. Optical Properties of Noble Metal Clusters in Ultrathin Solid Films. J. Clust. Sci. 1999, 10, 169–193. [Google Scholar] [CrossRef]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated Absorption and Scattering Properties of Gold Nanoparticles of Different Size, Shape, and Composition: Applications in Biological Imaging and Biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef]
- El-Sayed, M.A. Some Interesting Properties of Metals Confined in Time and Nanometer Space of Different Shapes. Acc. Chem. Res. 2001, 34, 257–264. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Shape and size dependence of radiative, non-radiative and photothermal properties of gold nanocrystals. Int. Rev. Phys. Chem. 2000, 19, 409–453. [Google Scholar] [CrossRef]
- Takenaka, S.; Yamashita, K.; Takagi, M.; Uto, Y.; Kondo, H. DNA Sensing on a DNA Probe-Modified Electrode Using Ferrocenylnaphthalene Diimide as the Electrochemically Active Ligand. Anal. Chem. 2000, 72, 1334–1341. [Google Scholar] [CrossRef]
- Azek, F.; Grossiord, C.; Joannes, M.; Limoges, B.; Brossier, P. Hybridization Assay at a Disposable Electrochemical Biosensor for the Attomole Detection of Amplified Human Cytomegalovirus DNA. Anal. Biochem. 2000, 284, 107–113. [Google Scholar] [CrossRef]
- Drummond, T.G.; Hill, M.G.; Barton, J.K. Electrochemical DNA sensors. Nat. Biotechnol. 2003, 21, 1192–1199. [Google Scholar] [CrossRef]
- Graham, D.; Smith, W.E.; Linacre, A.M.T.; Munro, C.H.; Watson, N.D.; White, P.C. Selective Detection of Deoxyribonucleic Acid at Ultralow Concentrations by SERRS. Anal. Chem. 1997, 69, 4703–4707. [Google Scholar] [CrossRef]
- Peterlinz, K.A.; Georgiadis, R.M.; Herne, T.M.; Tarlov, M.J. Observation of Hybridization and Dehybridization of Thiol-Tethered DNA Using Two-Color Surface Plasmon Resonance Spectroscopy. J. Am. Chem. Soc. 1997, 119, 3401–3402. [Google Scholar] [CrossRef]
- Demers, L.M.; Mirkin, C.A.; Mucic, R.C.; Reynolds, R.A.; Letsinger, R.L.; Elghanian, R.; Viswanadham, G. A Fluorescence-Based Method for Determining the Surface Coverage and Hybridization Efficiency of Thiol-Capped Oligonucleotides Bound to Gold Thin Films and Nanoparticles. Anal. Chem. 2000, 72, 5535–5541. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.W. Chemistry of Surfaces. Nature 1967, 215, 215–216. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Choma, J. Critical discussion of simple adsorption methods used to evaluate the micropore size distribution. Adsorption 1997, 3, 209–219. [Google Scholar] [CrossRef]
- Cheng, L.S.; Yang, R.T. Predicting isotherms in micropores for different molecules and temperatures from a known isotherm by improved Horvath-Kawazoe equations. Adsorption 1995, 1, 187–196. [Google Scholar] [CrossRef]
- Lastoskie, C.; Gubbins, K.E.; Quirke, N. Pore size distribution analysis of microporous carbons: A density functional theory approach. J. Phys. Chem. 1993, 97, 4786–4796. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Azizian, S. Kinetic models of sorption: A theoretical analysis. J. Colloid Interface Sci. 2004, 276, 47–52. [Google Scholar] [CrossRef]
- Marczewski, A.W. Kinetics and equilibrium of adsorption of organic solutes on mesoporous carbons. Appl. Surf. Sci. 2007, 253, 5818–5826. [Google Scholar] [CrossRef]
- Lagergren, S. Zur Theorie der sogenannten Adsorption gelöster Stoffe. Z. Für Chem. Und Ind. Der Kolloide 1907, 2, 15. [Google Scholar]
- Blachnio, M.; Derylo-Marczewska, A.; Charmas, B.; Zienkiewicz-Strzalka, M.; Bogatyrov, V.; Galaburda, M. Activated Carbon from Agricultural Wastes for Adsorption of Organic Pollutants. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Kinetic Models for the Sorption of Dye from Aqueous Solution by Wood. Process Saf. Environ. Prot. 1998, 76, 183–191. [Google Scholar] [CrossRef]
- Marczewski, A.W. Application of mixed order rate equations to adsorption of methylene blue on mesoporous carbons. Appl. Surf. Sci. 2010, 256, 5145–5152. [Google Scholar] [CrossRef]
- Marczewski, A.W. Analysis of Kinetic Langmuir Model. Part I: Integrated Kinetic Langmuir Equation (IKL): A New Complete Analytical Solution of the Langmuir Rate Equation. Langmuir 2010, 26, 15229–15238. [Google Scholar] [CrossRef]
- Marczewski, A.W.; Deryło-Marczewska, A.; Słota, A. Adsorption and desorption kinetics of benzene derivatives on mesoporous carbons. Adsorption 2013, 19, 391–406. [Google Scholar] [CrossRef]
- Haerifar, M.; Azizian, S. Fractal-Like Adsorption Kinetics at the Solid/Solution Interface. J. Phys. Chem. C 2012, 116, 13111–13119. [Google Scholar] [CrossRef]
- Derylo-Marczewska, A.; Blachnio, M.; Marczewski, A.W.; Seczkowska, M.; Tarasiuk, B. Phenoxyacid pesticide adsorption on activated carbon – Equilibrium and kinetics. Chemosphere 2019, 214, 349–360. [Google Scholar] [CrossRef]
- Derylo-Marczewska, A.; Blachnio, M.; Marczewski, A.W.; Swiatkowski, A.; Buczek, B. Adsorption of chlorophenoxy pesticides on activated carbon with gradually removed external particle layers. Chem. Eng. J. 2017, 308, 408–418. [Google Scholar] [CrossRef]
- Bosacka, A.; Zienkiewicz-Strzałka, M.; Deryło-Marczewska, A.; Chrzanowska, A.; Wasilewska, M.; Sternik, D. Physicochemical, structural, and adsorption properties of chemically and thermally modified activated carbons. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129130. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Oxford University Press: Oxford, UK, 1975. [Google Scholar]
- McKay, G.; El Geundi, M.; Nassar, M.M. Pore Diffusion During the Adsorption of Dyes Onto Bagasse Pith. Process Saf. Environ. Prot. 1996, 74, 277–288. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Balsamo, M.; Montagnaro, F. Liquid–Solid Mass Transfer in Adsorption Systems—An Overlooked Resistance? Ind. Eng. Chem. Res. 2020, 59, 22007–22016. [Google Scholar] [CrossRef]
- Jensen, T.; Kelly, L.; Lazarides, A.; Schatz, G.C. Electrodynamics of Noble Metal Nanoparticles and Nanoparticle Clusters. J. Clust. Sci. 1999, 10, 295–317. [Google Scholar] [CrossRef]
- Marczewski, A.W.; Seczkowska, M.; Derylo-Marczewska, A.; Blachnio, M. Adsorption equilibrium and kinetics of selected phenoxyacid pesticides on activated carbon: Effect of temperature. Adsorption 2016, 22, 777–790. [Google Scholar] [CrossRef]
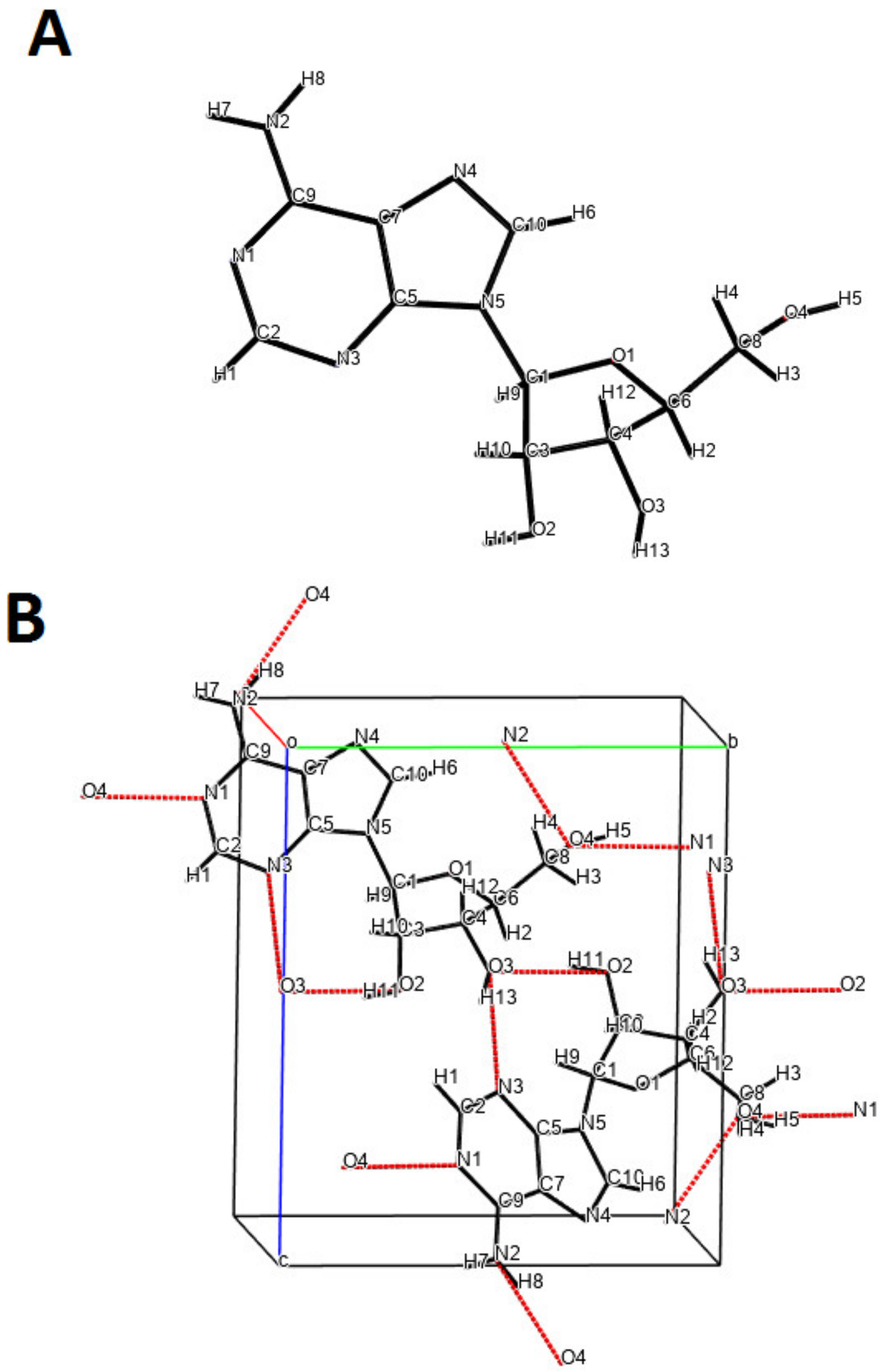

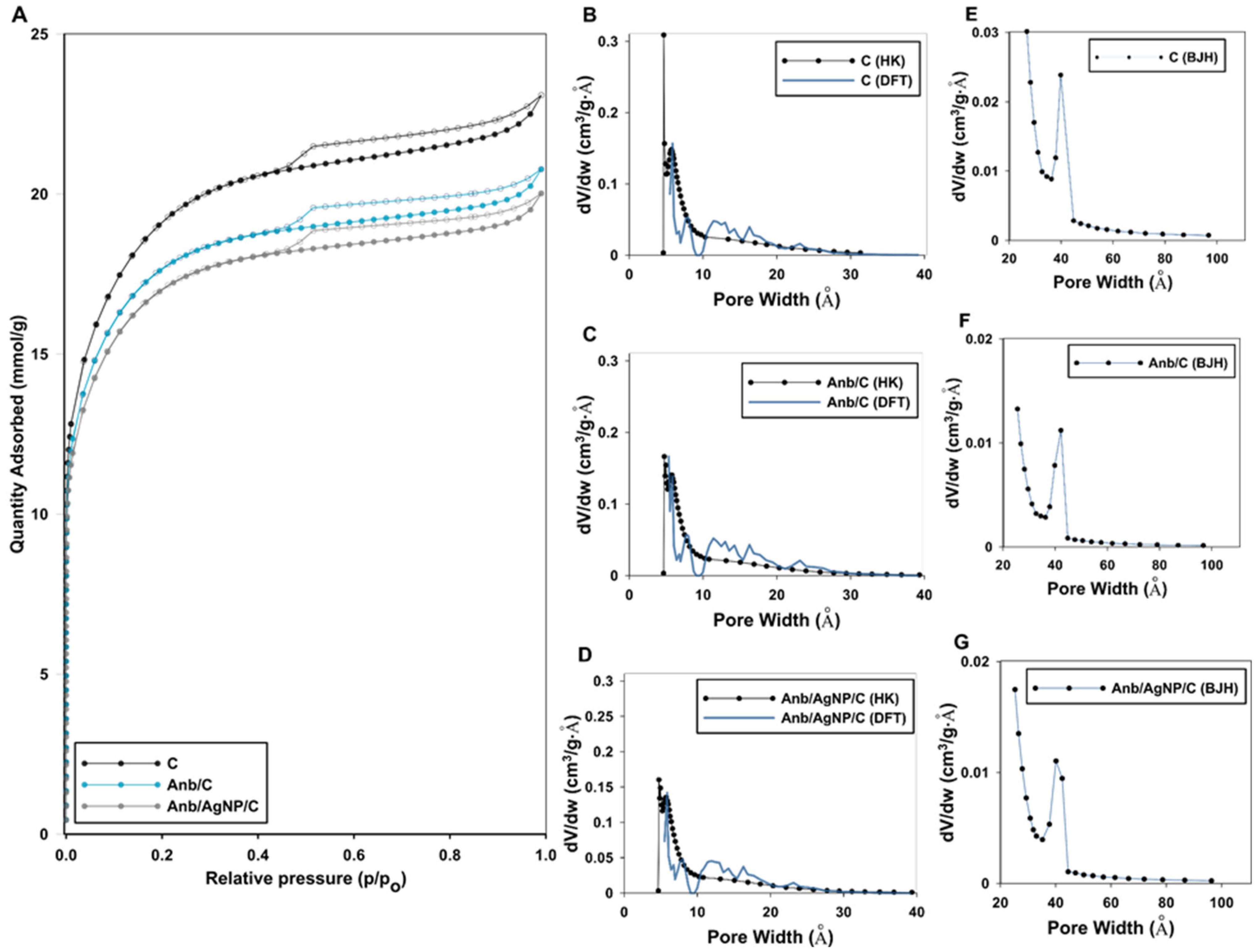

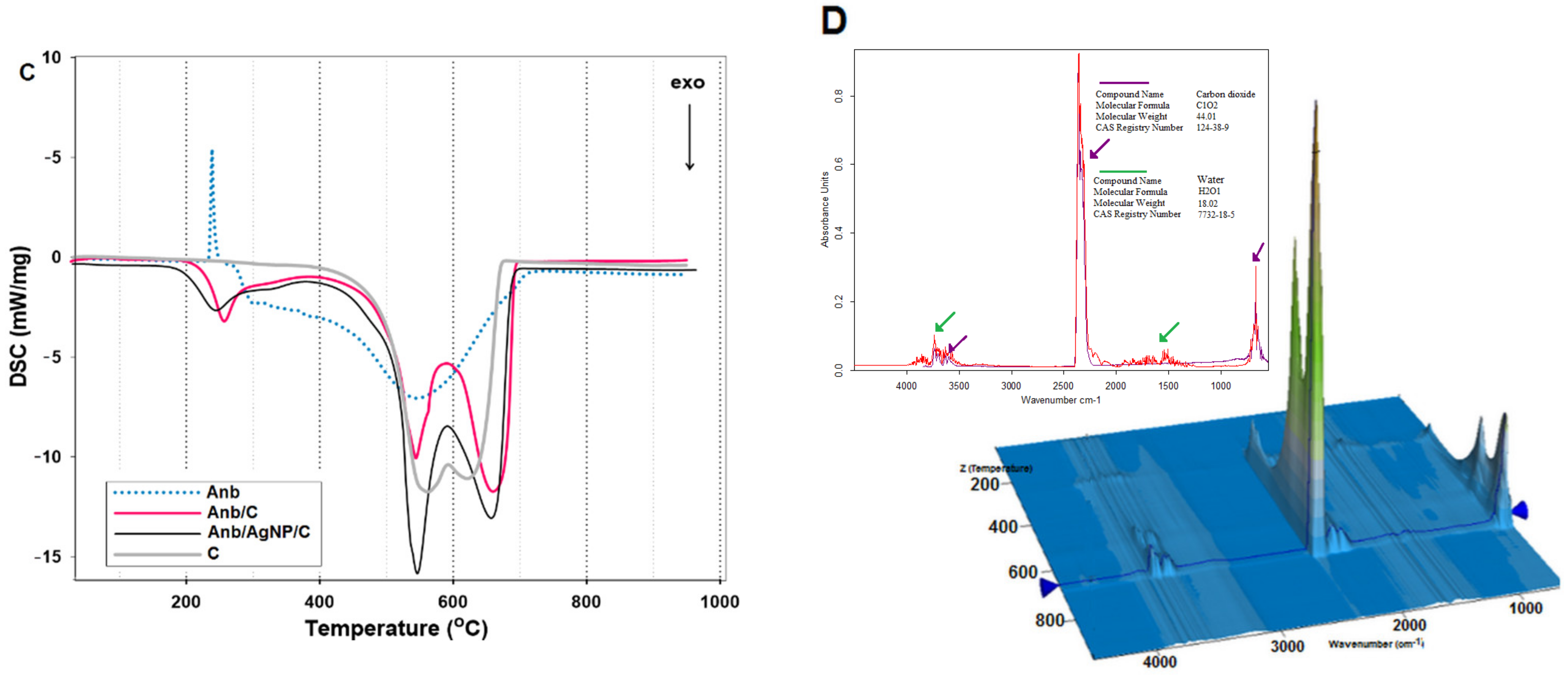
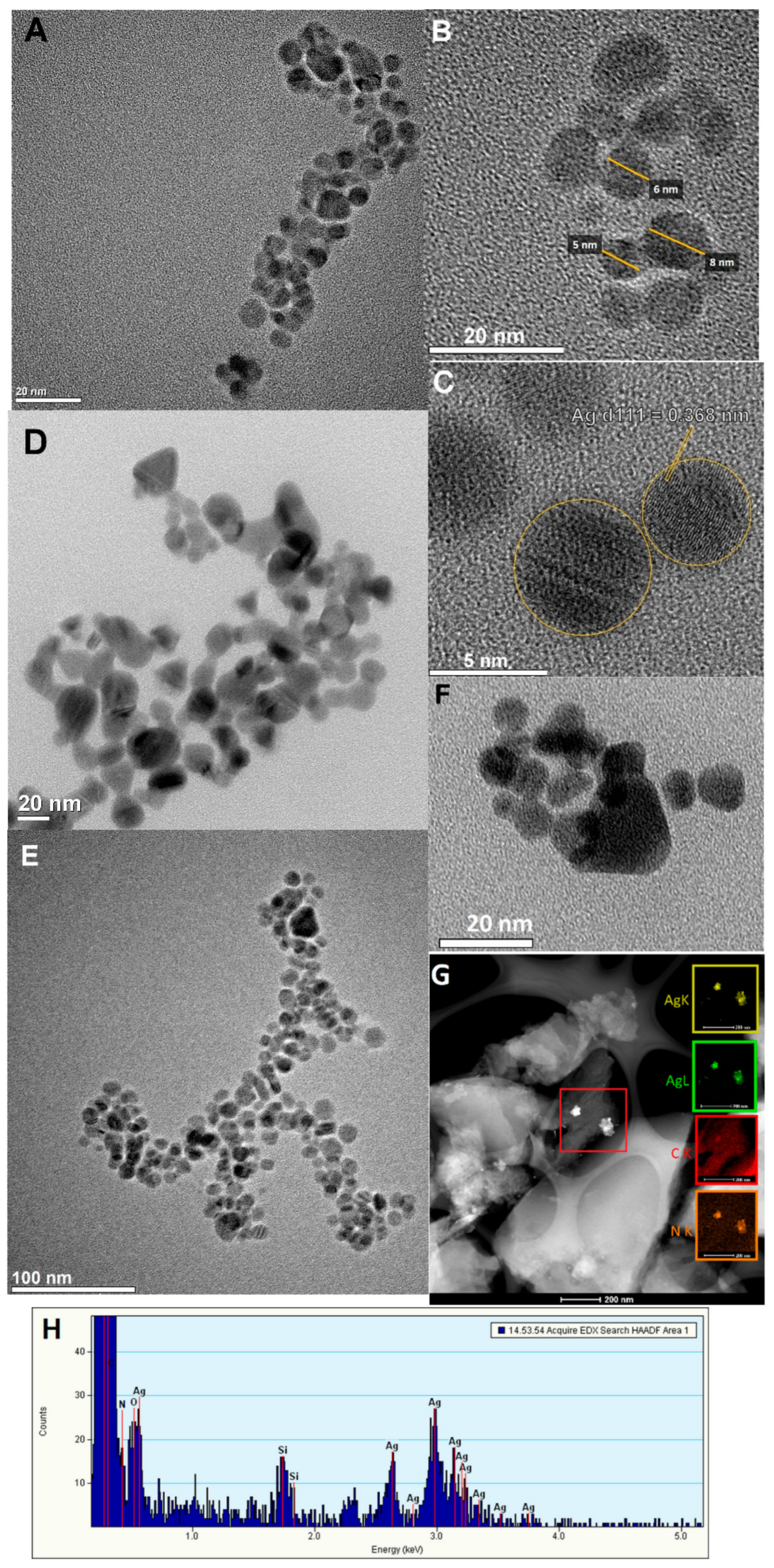





| Kinetic Equation (Abbreviation) | Short Description | Kinetic Equations |
|---|---|---|
| First-order reaction (FOE) | Adsorption kinetics in the systems with a standard concentration gradient and processes based on diffusion, (without intraparticle diffusion) [39]. | ; (1) |
| where c is the temporary concentration, the o and eq are initial and equilibrium states respectively, and k1 is the adsorption rate coefficient [40,41]. | ||
| Pseudo-first order (PFOE) | Adsorption kinetics is dependent on adsorption value instead concentration [42]. | (2) |
| Linear form: | ||
| is the actual adsorbed amount. | ||
| Second-order equation and pseudo-second-order equation (SOE and PSOE) | Suitable when concentration changes proceed rapidly and the rate is proportional to (aeq−a)2 [43,44]. | (3) |
| and | ||
| where k2 = k2aaeq and k2a are the rate coefficients for pseudo-second-order kinetics [43]. Pseudo-second-order equations may be used for energetically heterogeneous surfaces. | ||
| 1,2-mixed-order equation (MOE) | Generalization of the first- and second-order kinetic equations. Relevant for the systems which behave in the middle of these two [45]. | or (4) |
| where F is the relative adsorption progress in time, f2 < 1 is the normalized share of the second-order process in the kinetics. In special cases, the MOE equation may be degraded to the simple kinetic equations of the first (f2 = 0) and the second order (f2 = 1) type [46,47]. | ||
| Fractal-like MOE equation (f-MOE) | Includes probably distribution of rate coefficients. Adequate description of the non-ideality effects [48]. | (5) |
| where p is the fractal coefficient. | ||
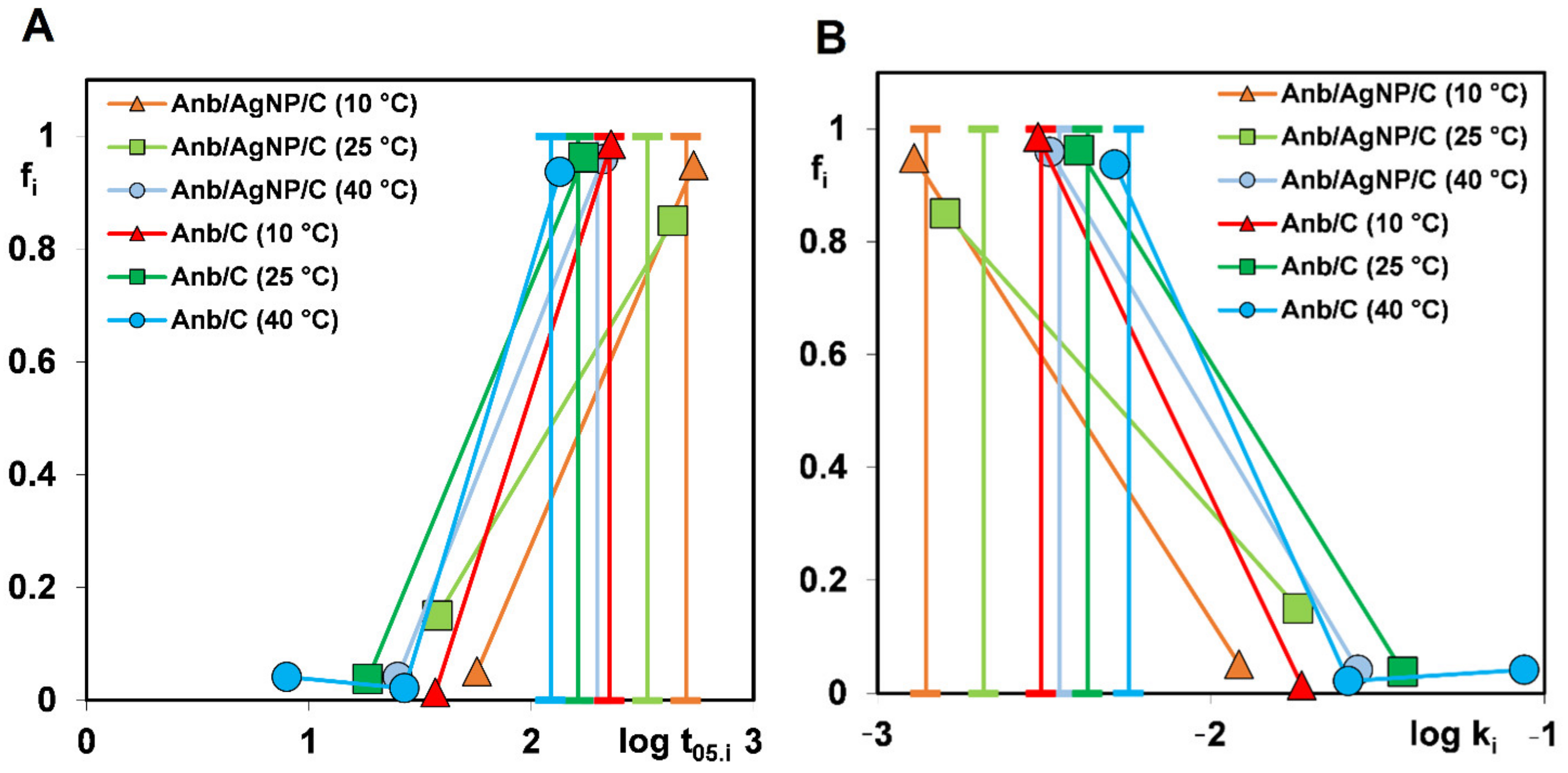
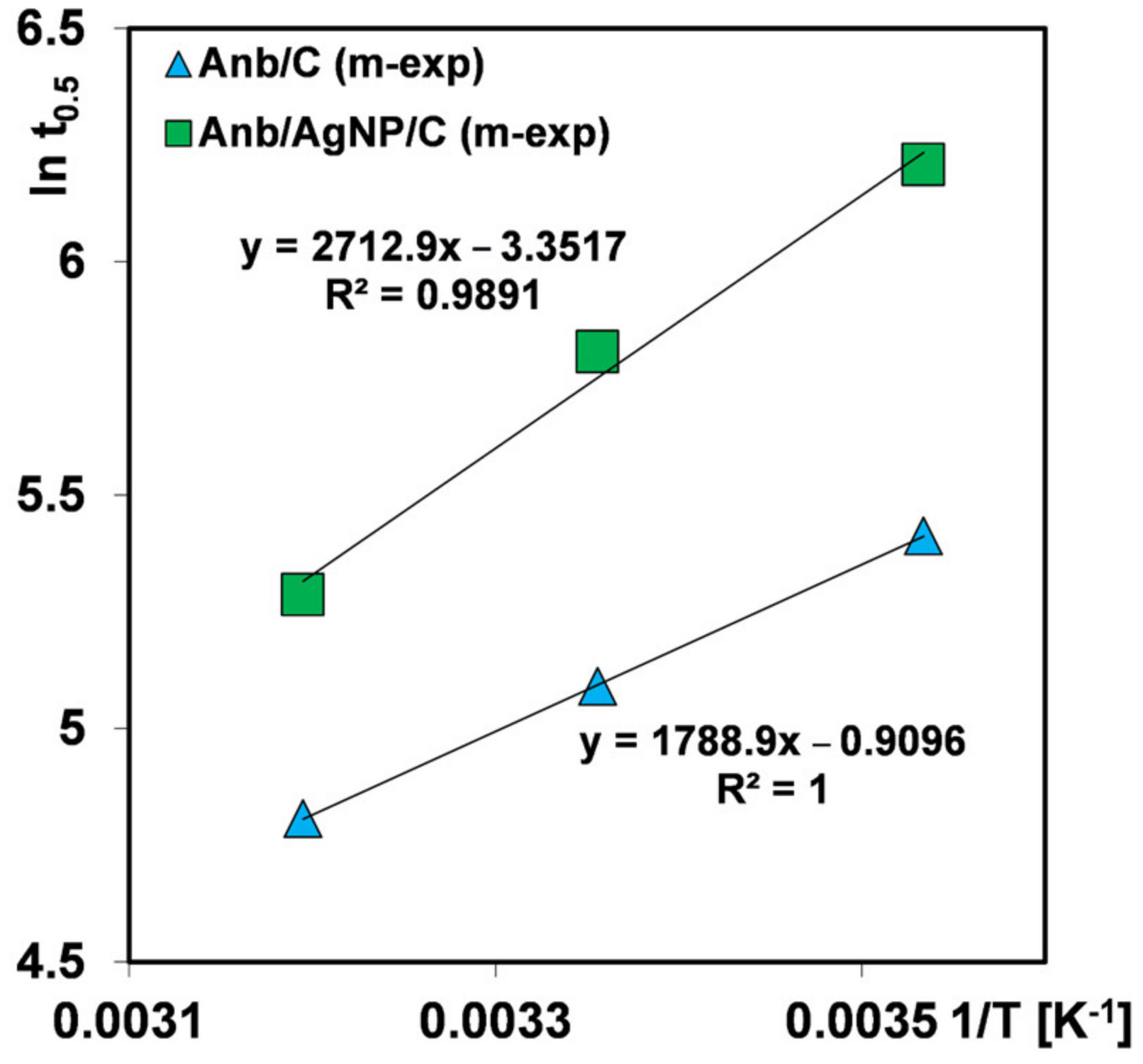
| Multiexponential equation (m-exp) | Generalization of Lagergren equation to a series of parallel first-order processes [49,50]. Right for a description of the adsorption kinetics on energetically heterogeneous solids that could not be expressed by the FOE/SOE/MOE equations [51]. | (6) |
| or | ||
| where “i” is the term of m-exp equation, ki is the rate coefficient and ueq = 1 − ceq/co is the relative loss of adsorbate from the solution. | ||
| Intraparticle Diffusion Model (IDM, Crank) | Adsorption on the spherical adsorbent grains [52]. | (7) |
| where r is the radius of the adsorbent particle, Da is the effective diffusion coefficient: | ||
| (8) | ||
| where D is the molecular diffusion coefficient, τp–the dimensionless pore tortuosity factor, ρ is the particle density, p is the particle porosity, KH–the Henry adsorption constant. | ||
| Pore Diffusion Model (PDM, McKay) | Also called as shrinking core approach. Adsorption on porous solids (additional resistance connected to the transition through the solution/particle interfacial layer) [53]. | (9) |
| where ueq is the relative adsorbate loss, the parameter B = 1 − 1/Bi, where Bi = Kf/Dp is the Biot number, Dp is the pore diffusion coefficient, Kf is the external mass transfer coefficient, τs is the undersized model time: | ||
| (10) | ||
| where (11) |
| Sample | Surface Area (SBET) (m2/g) | Pore Volume (cm3/g) | Pore Size Distribution (nm) | ||||
|---|---|---|---|---|---|---|---|
| STotal 1 | Smic 2 | VTotal 3 | Vmic 4 | Dh 5 | Dmo(NLDFT) 6 | Dmo(HK) 7 | |
| AC | 1441 | 1071 | 0.80 | 0.49 | 2.22 | 0.54 | 0.52 |
| Anb/C | 1234 | 666 | 0.72 | 0.35 | 2.33 | 0.61 | 0.55 |
| Anb/AgNP/C | 1207 | 535 | 0.69 | 0.29 | 2.29 | 0.71 | 0.56 |
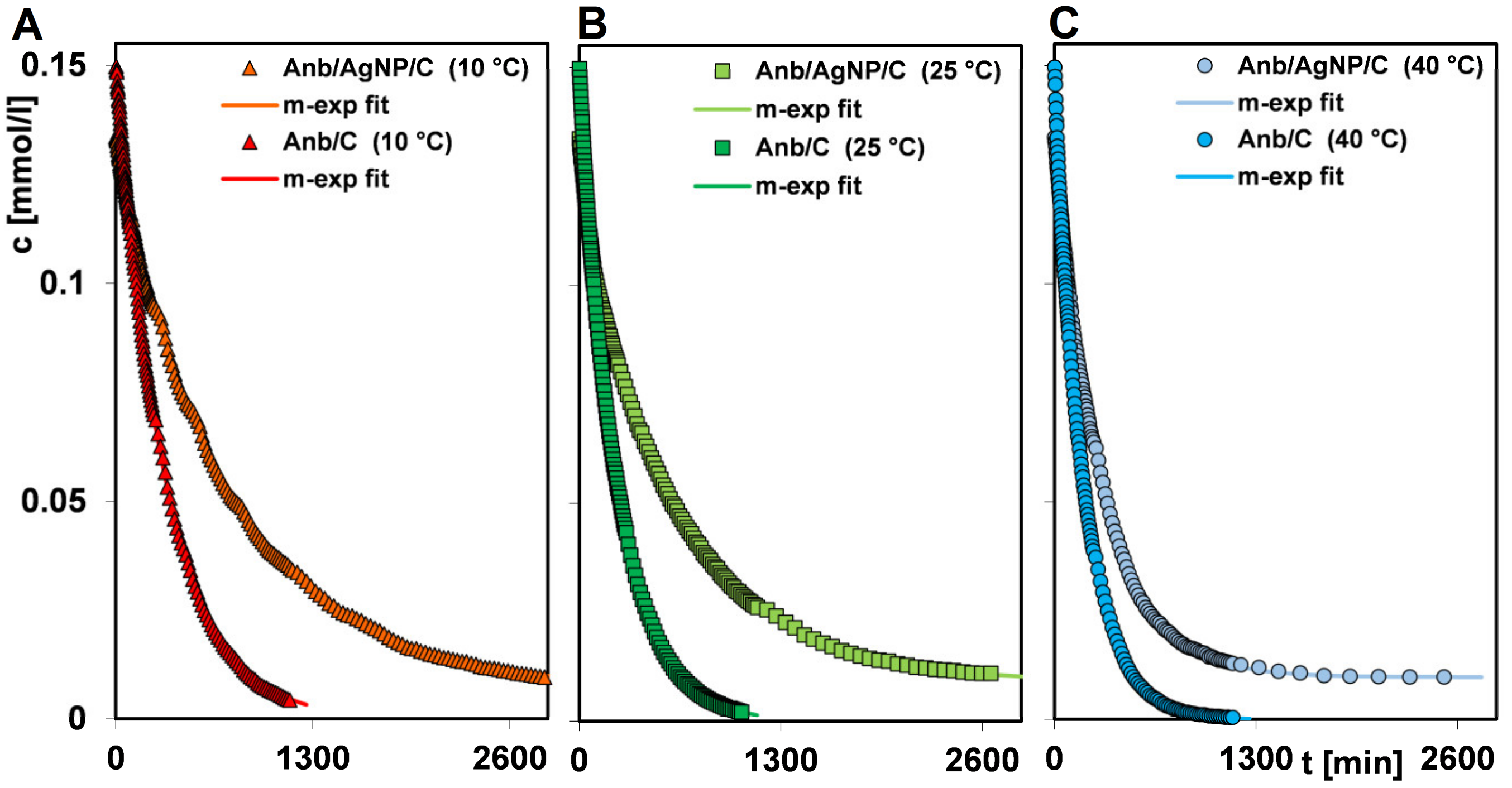
| Efficiency/Temperature | Time (min) | |||||
|---|---|---|---|---|---|---|
| 10 °C | 25 °C | 40 °C | ||||
| Anb/C | Anb/AgNP/C | Anb/C | Anb/AgNP/C | Anb/C | Anb/AgNP/C | |
| 25% | 93 | 197 | 55 | 110 | 45 | 84 |
| 50% | 222 | 555 | 138 | 387 | 121 | 228 |
| 75% | 470 | 1200 | 296 | 920 | 258 | 478 |
| 95% | 970 | - | 666 | - | 560 | - |
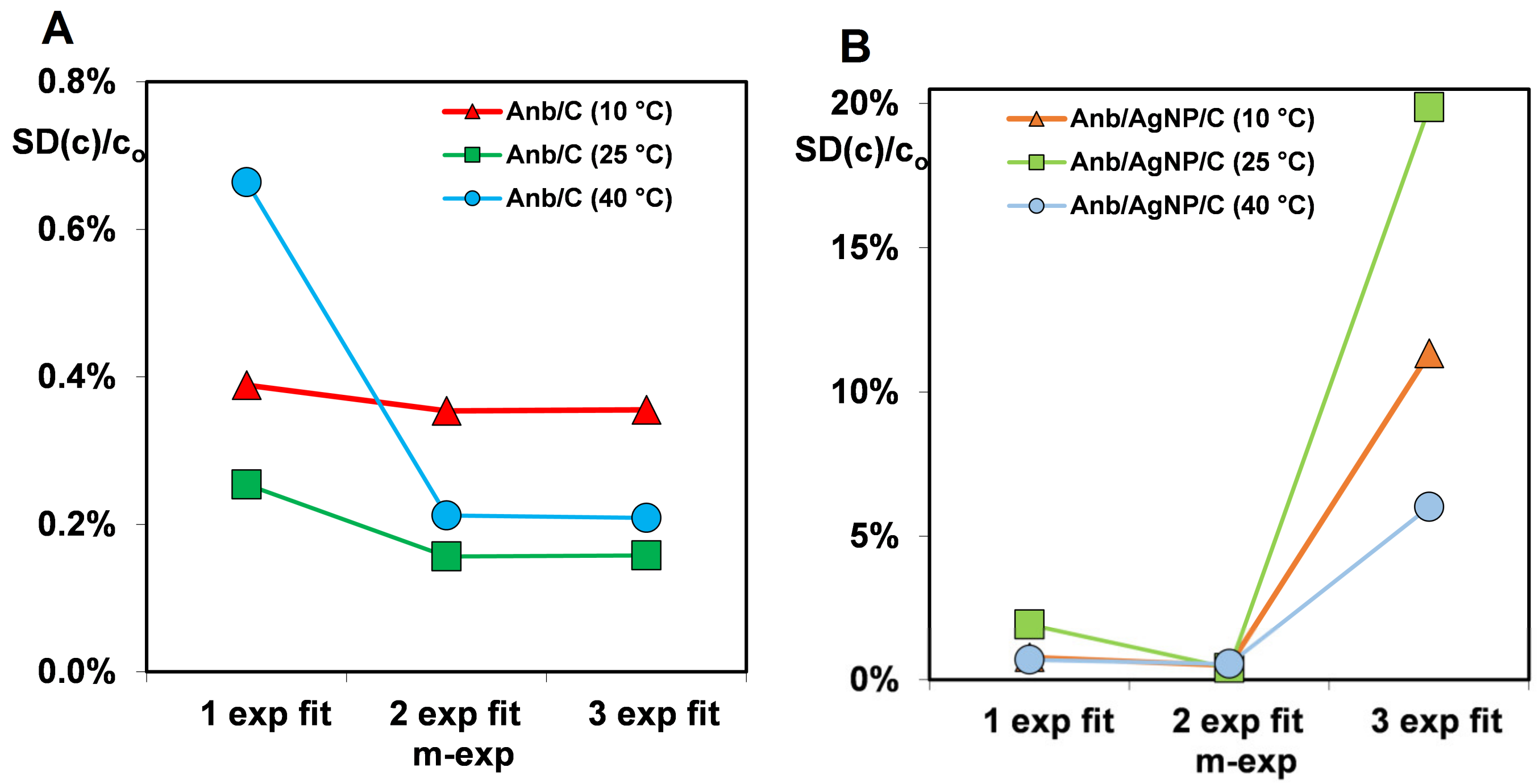
| System | m-exp | FOE | SOE | MOE | f-FOE | PDM (a) | PDM (b) |
|---|---|---|---|---|---|---|---|
| Anb/C (10 °C) | 0.356% | 0.385% | 6.58% | 0.369% | 0.366% | 2.90% | 0.501% |
| Anb/C (25 °C) | 0.157% | 0.464% | 6.30% | 0.344% | 0.287% | 2.27% | 0.269% |
| Anb/C (40 °C) | 0.209% | 0.661% | 6.47% | 0.483% | 0.403% | 2.11% | 0.358% |
| Anb/AgNP/C (10 °C) | 0.500% | 0.778% | 4.17% | 0.559% | 0.505% | 0.680% | 0.552% |
| Anb/AgNP/C (25 °C) | 0.415% | 1.93% | 2.42% | 1.18% | 0.797% | 1.11% | 0.674% |
| Anb/AgNP/C (40 °C) | 0.556% | 0.699% | 3.94% | 0.656% | 0.623% | 1.17% | 0.754% |
| average | 0.366% | 0.820% | 4.98% | 0.599% | 0.497% | 1.71% | 0.518% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zienkiewicz-Strzalka, M.; Blachnio, M. Nitrogenous Bases in Relation to the Colloidal Silver Phase: Adsorption Kinetic, and Morphology Investigation. Appl. Sci. 2023, 13, 3696. https://doi.org/10.3390/app13063696
Zienkiewicz-Strzalka M, Blachnio M. Nitrogenous Bases in Relation to the Colloidal Silver Phase: Adsorption Kinetic, and Morphology Investigation. Applied Sciences. 2023; 13(6):3696. https://doi.org/10.3390/app13063696
Chicago/Turabian StyleZienkiewicz-Strzalka, Malgorzata, and Magdalena Blachnio. 2023. "Nitrogenous Bases in Relation to the Colloidal Silver Phase: Adsorption Kinetic, and Morphology Investigation" Applied Sciences 13, no. 6: 3696. https://doi.org/10.3390/app13063696
APA StyleZienkiewicz-Strzalka, M., & Blachnio, M. (2023). Nitrogenous Bases in Relation to the Colloidal Silver Phase: Adsorption Kinetic, and Morphology Investigation. Applied Sciences, 13(6), 3696. https://doi.org/10.3390/app13063696







