Sediment Contamination and Toxicity in the Guadalquivir River (Southwest, Spain)
Abstract
1. Introduction
2. Materials and Methods
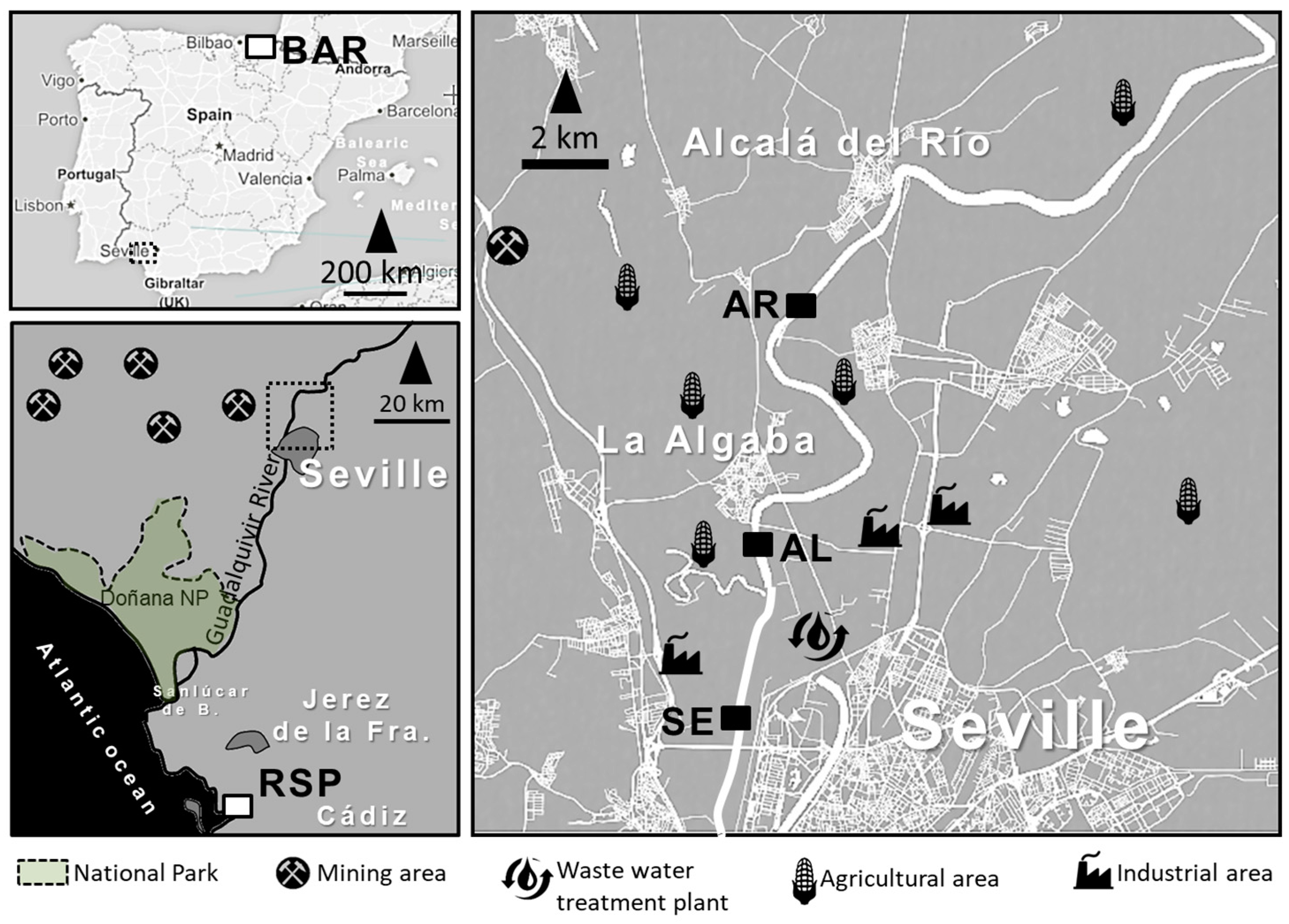
2.1. Study Area
2.2. Sediment Sampling
2.3. Chemical Analyses
2.4. Toxicity Tests in Laboratory
2.4.1. Amphipod Toxicity Test
2.4.2. Bacterial Toxicity Test
2.4.3. Sea Urchin Toxicity Tests
2.4.4. Oligochaete Chronic Toxicity Test
2.5. Data Treatment
3. Results and Discussion
3.1. Sediment Metals Concentration
3.2. Toxicity Tests in Laboratory
3.2.1. Amphipod Laboratory Bioassay
3.2.2. Microtox® Bioassay
3.2.3. Paracentrotus lividus Toxicity Tests
P. lividus Fertilization Bioassay
P. lividus Embryo Development Test
Tubifex tubifex Chronic Bioassay
3.3. Linking Contamination and Toxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cabrera, F.; Toca, C.; Diaz, E.; Arambarri, P. Acid mine-water and agricultural pollution in a river skirting the Doñana National Park (Guadiamar river, southwest Spain). Water Res. 1987, 18, 1469–1482. [Google Scholar] [CrossRef]
- DelValls, T.A.; Conradi, M. Advances in marine ecotoxicology: Laboratory tests versus field assessments data on sediment quality studies. Cienc. Mar. 2000, 26, 39–64. [Google Scholar] [CrossRef]
- Chapman, P.M.; Ho, K.T.; Munns, W.R.; Solomon, K.; Weistein, M.P. Issues in sediment toxicity and ecological risk assessment. Mar. Pollut. Bull. 2002, 44, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Gómez, J.; Martín-Díaz, M.L.; Rodríguez, A.; Riba, I.; DelValls, T.A. In Situ Evaluation of Sediment Toxicity in Guadalete Estuary (SW Spain) After Exposure of Caged Arenicola marina. Environ. Toxicol. 2008, 23, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Förstner, U.; Wittman, G.T.W. (Eds.) Metal Pollution in Aquatic Environment; Springer: Berlin/Heidelberg, Germany, 1979; 485p. [Google Scholar]
- Riba, I.; Conradi, M.; Forja, J.M.; DelValls, T.A. Sediment quality in the Guadalquivir estuary: Lethal effects associated with the Aznalcóllar mining spill. Mar. Pollut. Bull. 2004, 48, 144–152. [Google Scholar] [CrossRef]
- USEPA/USACE, United States Environmental Protection Agency/United States Army Corps of Engineers. Evaluation of Dredged Material Proposed for Ocean Disposal-Testing Manual, EPA-503-8-91/001; Environmental Protection Agency Office of Marine and Estuarine Protection: Washington, DC, USA, 1991. [Google Scholar]
- Petrovic, M.; Barceló, D. Seeking harmonisation in assessing sediments and dredged materials, Meeting Report. Trends Anal. Chem. 2004, 23, 10–12. [Google Scholar] [CrossRef]
- Cesar, A.; Choueri, R.B.; Riba, I.; Moralles-Caselles, C.; Pereira, C.D.S.; Santos, A.R.; Abessa, D.M.S.; DelValls, T.A. Comparative sediment quality assessment in different littoral ecosystems from Spain (Gulf of Cadiz) and Brazil (Santos and São Vicente Estuarine System). Environ. Int. 2007, 33, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Casado-Martínez, M.C.; Beiras, R.; Belzunce, M.J.; González-Castromil, M.A.; Marín-Guirao, L.; Postma, J.F.; Riba, I.; DelValls, T.A. Interlaboratory assessment of marine bioassays to evaluate the environmental quality of coastal sediments in Spain. IV. Whole sediment toxicity test using crustacean amphipods. Cienc. Mar. 2006, 32, 149–157. [Google Scholar] [CrossRef]
- Morales-Caselles, C.; Kalman, J.; Riba, I.; DelValls, T.A. Comparing sediment quality in Spanish littoral areas affected by acute (Prestige, 2002) and chronic (Bay of Algeciras) oil spills. Environ. Pollut. 2007, 146, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Morales-Caselles, C.; Riba, I.; Sarasquete, C.; DelValls, T.A. Using a classical weight-of-evidence approach for 4-years monitoring of the impact of an accidental oil spill on sediment quality. Environ. Int. 2007, 34, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Gómez, J.; Martín-Díaz, M.L.; DelValls, T.A. Acute toxicity measured in the amphipod Ampelisca brevicornis after exposure to contaminated sediments from Spanish littoral. Ecotoxicology 2009, 18, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Riba, I.; DelValls, T.A.; Forja, J.M.; Gómez-Parra, A. Comparative toxicity of contaminated sediment from a mining spill using two amphipods species: Corophium volutator and Ampelisca brevicornis. Bull. Environ. Contam. Toxicol. 2003, 71, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Mowat, F.S.; Bundy, K.J. Correlation of field-measured toxicity with chemical concentration and pollutant availability. Environ. Int. 2001, 27, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Stronkhorst, J.; Schipper, C.; Brils, J.; Dubbeldam, M.; Postman, J.; Van De Hoeven, N. Using marine bioassays to classify the toxicity of Dutch Harbor sediments. Environ. Toxicol. Chem. 2003, 22, 1535–1547. [Google Scholar] [CrossRef] [PubMed]
- Brohon, B.; Delolme, C.; Gourdon, R. Complementarity of bioassays and microbial activity measurements for the evaluation of hydrocarbon-contaminated soils quality. Soil Biol. Biochem. 2001, 33, 883–891. [Google Scholar] [CrossRef]
- Pelletier, E.; Delille, D.; Delille, B. Crude oil bioremediation in sub-Antarctic intertidal sediments: Chemistry and toxicity of oiled residues. Mar. Environ. Res. 2004, 57, 311–327. [Google Scholar] [CrossRef]
- Beiras, R.; Fernández, N.; Bellas, J.; Besada, V.; González-Quijano, A.; Nunes, T. Integrative assessment of marine pollution in Galician estuaries using sediment chemistry, mussel bioaccumulation, and embryo-larval toxicity bioassays. Chemosphere 2003, 52, 1209–1224. [Google Scholar] [CrossRef]
- Cesar, A.; Marín, A.; Marín-Guirao, L.; Vita, R. Amphipod and sea urchin tests to assess the toxicity of Mediterranean sediments: The case of Portman Bay. Sci. Mar. 2004, 68, 205–213. [Google Scholar] [CrossRef]
- Silva, C.; Yáñez, E.; Martín-Díaz, M.L.; Riba, I.; DelValls, T.A. Integrated ecotoxicological assessment of marine sediments affected by land-based marine fish farm effluents: Physicochemical, acute toxicity and benthic community analyses. Ecotoxicology 2013, 22, 996–1011. [Google Scholar] [CrossRef]
- USEPA, United States Environmental Protection Agency. Short-Term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Water to West Coast Marine and Estuarine Organisms, 3rd ed.; USEPA: Cincinnati, OH, USA, 2002; p. 370. [Google Scholar]
- Environment Canada. Biological Test Method: Reference Method for Determining the Toxicity of Sediment Using Luminescent Bacteria in a Solid-Phase Test; Report EPS 1/RM/42; Environmental Protection Service: Ottawa, ON, USA, 2002; p. 83. [Google Scholar]
- Bailey, R.; Day, K.E.; Norris, R.H.; Reynoldson, T.B. Macroinvertebrate community structure and sediment bioassay results from areas of North American Great Lakes. J. Great Lakes Res. 1995, 21, 42–52. [Google Scholar] [CrossRef]
- Reynoldson, T.B.; Bailey, R.C.; Day, K.E.; Norris, R.H. Biological guidelines for freshwater sediment based on benthic of sediment (THE BEAST) using a multivariate approach for predicting biological state. Aust. J. Ecol. 1995, 20, 198–219. [Google Scholar] [CrossRef]
- Martinez-Madrid, M.; Rodriguez, P.; Pérez-Iglesias, J.I.; Navarro, E. Sediment toxicity bioassays for assessment of contaminated sites in the Nervion River (Northern Spain). 2. Tubifex tubifex reproduction sediment bioassay. Ecotoxicology 1999, 8, 111–124. [Google Scholar] [CrossRef]
- Pasteris, A.; Vecchi, M.; Reynoldson, T.; Bonomi, G. Toxicity of copper-spiked sediments to Tubifex tubifex (Oligochaeta, Tubificide): A comparison of the 28-day reproductive bioassay with a 6-month cohort experiment. Aquat. Toxicol. 2003, 65, 253–265. [Google Scholar] [CrossRef]
- Bettinetti, R.; Provini, A. Toxicity of 4-nonylphenol to Tubifex tubifex and Chironomous riparius in 28-days whole sediments tests. Ecotoxicol. Environ. Saf. 2002, 53, 113–121. [Google Scholar] [CrossRef]
- Mosleh, Y.Y.; Paris-Palacios, S.; Biagianti-Risbourg, S. Metallothioneins induction and antioxidative response in aquatic worms Tubifex tubifex (Oligochaeta, Tubificidae) exposed to cooper. Chemosphere 2006, 64, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Mosleh, Y.Y.; Paris-Palacios, S.; Couderchet, M.; Biagianti-Risboug, S.; Vernet, G. Metallothionein induction, antioxidative responses, glycogen and growth changes in Tubifex tubifex (Oligochaeta, Tubificidae) exposed to the fungicide, fenhexamid. Environ. Pollut. 2006, 135, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Egeler, P.; Rombke, J.; Meller, M.; Knacker, T.; Franke, C.; Studinger, G.; Nagel, R. Bioaccumulation of lindane and hexachlorobenzene by tubificid sludgeworms (Oligochaeta) under standardized laboratory conditions. Chemosphere 1997, 35, 835–852. [Google Scholar] [CrossRef]
- Egeler, P.; Rombke, J.; Meller, M.; Knacker, T.; Nagel, R. Bioaccumulation test with Tubificid Sludgeworms in artificial media—Development of a standardisable method. Hydrobiologia 1999, 406, 271–280. [Google Scholar] [CrossRef]
- American Society for Testing Materials (ASTM). Standard Test Method for Measuring the Toxicity of Sediments-Associated Contaminants with Freshwater Invertebrates; American Society for Testing and Materials E1706-05; American Society for Testing and Materials: Philadelphia, PA, USA, 2005. [Google Scholar]
- Chapman, P.M.; Farrell, M.O.; Brinkhurst, R.O. Relative Tolerances of Selected Aquatic Oligochaetes to Combinations of Pollutants and Environmental Factors. Aquat. Toxicol. 1982, 2, 69–78. [Google Scholar] [CrossRef]
- Bailey, H.C.; Lui, D.H.W. Lumbriculus variegatus, a Benthic Oligochaete, as a Bioassay Organism. In Aquatic Toxicology, Third Symposium; Eaton, J.C., Parrish, P.R., Hendricks, A.C., Eds.; American Society for Testing and Materials: Philadelphia, PA, USA, 1980; pp. 205–215. [Google Scholar]
- McMurtry, M.J. Avoidance of sublethal doses of copper and zinc by tubificid oligochaetes. J. Great Lakes Res. 1984, 10, 267–272. [Google Scholar] [CrossRef]
- Confederación Hidrográfica del Guadalquivir. Memoria Ambiental; Confederación Hidrográfica del Guadalquivir: Seville, Spain, 2008; p. 41. [Google Scholar]
- Blasco, J.; Gomes, T.; García-Barrera, T.; Rodríguez-Romero, A.; Gonzalez-Rey, M.; Morán-Roldán, F.; Tromibini, C.; Miotk, M.; Gómez-Ariza, J.L.; Bebianno, M.J. Trace metal concentrations in sediments from the southwest of the Iberian Peninsula. Sci. Mar. 2010, 74, 99–106. [Google Scholar] [CrossRef]
- Silva, C.; Mattioli, M.; Fabbri, E.; Yáñez, E.; DelValls, T.A.; Martín-Díaz, M.L. Benthic community structure and biomarker responses of the clam Scrobicularia plana in a shallow tidal creek affected by fish farm effluents (Rio San Pedro, SW Spain). Environ. Int. 2012, 47, 86–98. [Google Scholar] [CrossRef]
- Maestre, Z.; Martínez-Madrid, M.; Rodriguez, P.; Reynoldson, T. Ecotoxicity assessment of river sediments and a critical evaluation of some of the procedures used in the aquatic oligochaete Tubifex tubifex chronic bioassay. Arch. Environ. Contam. Toxicol. 2007, 53, 559–570. [Google Scholar] [CrossRef]
- El Rayis, O.A. Re-assessment of the titration method for the determination of organic carbon in recent sediments. Rapp. Com. Int. Mer. Medit. 1985, 29, 45–47. [Google Scholar]
- Casado-Martinez, C.; Forja, J.M.; DelValls, T.A. Direct comparison of amphipod sensitivities to dredged sediments from Spanish ports. Chemosphere 2007, 68, 677–685. [Google Scholar] [CrossRef] [PubMed]
- ASTM, American Society for Testing and Materials. Standard Guide for Conducting 10-Day Static Sediment Toxicity Tests with Marine and Estuarine Amphipods; ASTM: Philadelphia, PA, USA, 1993; p. 26. [Google Scholar]
- Azur Environmental. Microtox Acute Toxicity Basic Test Procedures; Azur Environmental: Carlsbad, CA, USA, 1998. [Google Scholar]
- Campisi, T.; Abbondanzi, F.; Casado-Martínez, C.; DelValls, T.A.; Guerra, R.; Iacondini, A. Effect of sediment turbidity and color on light output measurement or Microtox Basic Solid-Phase Test. Chemosphere 2005, 60, 9–15. [Google Scholar] [CrossRef] [PubMed]
- USEPA/USACE. Evaluation of Dredged Material Proposed for Discharge in Waters of the U.S. Washington DC-Testing Manual; EPA-823-B-98-004; USEPA/USACE: Washington, DC, USA, 1998. [Google Scholar]
- Carballeira, C.; Martín-Díaz, L.; DelValls, T.A. Influence of salinity on fertilization and larval development toxicity tests with two species of sea urchin. Mar. Environ. Res. 2011, 72, 196–203. [Google Scholar] [CrossRef]
- Ghirardini, A.V.; Novelli, A.A. A sperm cell toxicity test procedure for the Mediterranean species Paracentrotus lividus (Echinodermata: Echinoidea). Environ. Technol. 2001, 22, 439–445. [Google Scholar] [CrossRef]
- Reynoldson, T.B.; Thompson, S.P.; Bampsey, J.L. A sediment bioassay using the tubificid oligochaete worm Tubifex tubifex. Environ. Toxicol. Chem. 1991, 10, 1061–1072. [Google Scholar] [CrossRef]
- Chapman, P.M.; Benton, M.J.; Brinkhurst, R.O.; Scheuerman, P.R. Use of the aquatic oligochaetes Lumbriculus variegatus and Tubifex tubifex for assessing the toxicity of copper and cadmium in a spiked-artificial-sediment toxicity test. Environ. Toxicol. 1999, 14, 271–278. [Google Scholar] [CrossRef]
- DelValls, T.A.; Chapman, P.M. Site-specific sediment quality values for the Gulf of Cadiz (Spain) and San Francisco Bay (USA), using the sediment quality triad and multivariate analysis. Cienc. Mar. 1998, 24, 313–336. [Google Scholar] [CrossRef]
- DelValls, T.A.; Forja, J.M.; González-Mazo, E.; Gómez-Parra, A. Determining contamination sources in marine sediments using multivariate analysis. TrAC Trends Anal. Chem. 1998, 17, 181–192. [Google Scholar] [CrossRef]
- Tabachnic, B.G.; Fidell, L.S. Using Multivariate Statistics; Harper Collins, College Publishers: New York, NY, USA, 1996. [Google Scholar]
- Choueri, R.B.; Cesar, A.; Abessa, D.M.S.; Torres, R.J.; Morais, R.D.; Riba, I.; Pereira, C.D.S.; Nascimento, M.R.L.; Mozeto, A.A.; DelValls, T.A. Development of site-specific sediment quality guidelines for North and South Atlantic littoral zones: Comparison against national and international sediment quality benchmarks. J. Hazard. Mater. 2009, 170, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Bonnail, E.; Riba, I.; de Seabra, A.A.; DelValls, T.Á. Sediment quality assessment in the Guadalquivir River (SW, Spain) using caged Asian clams: A biomarker field approach. Sci. Total Environ. 2019, 650, 1996–2003. [Google Scholar] [CrossRef]
- Linde, A.R.; Arribas, P.; Sánchez-Galán, S.; Marañón, E.; Izquierdo, J.L.; García-Vázquez, F. Preliminary assessment impact of trace metal pollution in Piles river (Spain). In Water Pollution III: Modelling, Measurement and Prediction; Wrobel, L.C., Latinopoulos, P., Eds.; Computation Mechanics Publication Ashurst: Southampton, UK, 1995; pp. 407–414. [Google Scholar]
- CCME Protocol for the Derivation of Canadian Sediment Quality Guidelines for the Protection of Aquatic Life. CCME, EPC-98E. 1995. Available online: https://www.elaw.org/system/files/sediment_summary_table.pdf (accessed on 12 March 2021).
- CEDEX. Directrices Para la Caracterización del Material Dragado y su Reubicación en Aguas del Dominio Público Marítimo-Terrestre; Comisión Interministerial De Estrategias Marinas: Madrid, Spain, 2014. [Google Scholar]



| Element | AR | AL | SE | RSP | |
|---|---|---|---|---|---|
| As | µg/g | 10.22 | 9.77 | 9.84 | 7.14 |
| Cd | µg/g | 0.27 | 0.26 | 0.26 | 0.13 |
| Co | µg/g | 8.63 | 7.73 | 7.70 | 5.82 |
| Cu | µg/g | 37.42 | 35.37 | 35.51 | 18.00 |
| Fe | µg/g | 23,990 | 21,920 | 22,140 | 15,239 |
| Ni | µg/g | 25.42 | 23.39 | 23.56 | 14.00 |
| Pb | µg/g | 29.65 | 27.30 | 31.73 | 22.30 |
| Zn | µg/g | 75.89 | 66.79 | 69.65 | 49.00 |
| OC | % | 1.18 | 1.06 | 1.16 | 0.83 |
| Variance % | Factor 1 78.93 | Factor 2 14.31 | Factor 3 6.76 |
|---|---|---|---|
| A. brevicornis mortality | 0.764 | 0.555 | −0.327 |
| V. fischeri bioluminescence inhibition | 0.954 | 0.301 | |
| P. lividus fertilization | 0.881 | 0.436 | |
| P. lividus embriogenesis | 0.917 | 0.322 | |
| T. tubifex mortality | 0.410 | 0.346 | 0.843 |
| T. tubifex TYG | 0.874 | −0.413 | 0.378 |
| T. tubifex TGR | 0.982 | ||
| Cu | 0.948 | 0.309 | |
| Zn | 0.974 | ||
| Fe | 0.960 | ||
| Co | 0.939 | 0.332 | |
| Ni | 0.956 | ||
| Cd | 0.948 | 0.301 | |
| Pb | 0.985 | ||
| As | 0.955 | 0.344 | |
| OC | 0.998 |
| Contaminant | Sediment Quality Guidelines (SQGs) | ||
|---|---|---|---|
| Not Polluted | Moderately Polluted | Highly Polluted | |
| Cu | ≤35.37 | 35.37–35.51 | ≥35.51 |
| Zn | ≤66.79 | 66.79–69.65 | ≥69.65 |
| Fe | ≤21,920 | 21,920–22,140 | ≥22,140 |
| Co | ≤5.82 | 5.82–7.70 | ≥7.70 |
| Ni | ≤23.39 | 23.39–23.56 | ≥23.56 |
| Cd | ≤0.13 | 0.13–0.26 | ≥0.26 |
| Pb | ≤27.29 | 27.29–28.40 | ≥28.40 |
| As | ≤9.77 | 9.77–9.84 | ≥9.84 |
| CCME 1 | Riba [6] | CEDEX 2 | Present Study | |||||
|---|---|---|---|---|---|---|---|---|
| ISQG | PEL | V1 | V2 | AL1 | AL2 | AL3 | ALR | |
| As | 7.24 | 41.6 | 27.4 | 213 | 35 | 70 | 280 | 9.77–9.84 |
| Cd | 0.7 | 4.2 | 0.51 | 0.96 | 1.20 | 2.40 | 9.60 | 0.13–0.26 |
| Cu | 18.7 | 108 | 209 | 979 | 70 | 168 | 675 | 35.37–35.51 |
| Ni | - | - | 30 | 63 | 234 | 23.39–23.56 | ||
| Pb | 30.2 | 112 | 260 | 270 | 80 | 219 | 600 | 27.29–28.4 |
| Zn | 124 | 271 | 513 | 1310 | 205 | 410 | 1640 | 66.79–69.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riba, I.; Luque-Escalona, A.; Costa, M.H. Sediment Contamination and Toxicity in the Guadalquivir River (Southwest, Spain). Appl. Sci. 2023, 13, 3585. https://doi.org/10.3390/app13063585
Riba I, Luque-Escalona A, Costa MH. Sediment Contamination and Toxicity in the Guadalquivir River (Southwest, Spain). Applied Sciences. 2023; 13(6):3585. https://doi.org/10.3390/app13063585
Chicago/Turabian StyleRiba, Inmaculada, Angel Luque-Escalona, and Maria Helena Costa. 2023. "Sediment Contamination and Toxicity in the Guadalquivir River (Southwest, Spain)" Applied Sciences 13, no. 6: 3585. https://doi.org/10.3390/app13063585
APA StyleRiba, I., Luque-Escalona, A., & Costa, M. H. (2023). Sediment Contamination and Toxicity in the Guadalquivir River (Southwest, Spain). Applied Sciences, 13(6), 3585. https://doi.org/10.3390/app13063585





