Abstract
The Weight-of-Evidence (WOE) approach uses multiple lines of evidence to analyze the adverse effects associated with CO2 enrichment in two stations from the Gulf of Cádiz (Spain) with different contamination degrees. Sediment contamination and metal (loid) mobility, toxicity, ecological integrity, and bioaccumulation from the samples exposed to different acidification scenarios (pH gradient from 8.0 to 6.0) were used in the WOE. The experiments were conducted under laboratory conditions using a CO2-bubbling system. Different integration approaches such as multivariate analyses were used to evaluate the results. The results indicated that the adverse biological effects under pH 6.5 were related to the mobility of dissolved elements (As, Fe, Cu, Ni, and Zn). Furthermore, the pH reduction was correlated to the increase of bioaccumulation of As, Cr, Cu, Fe, and Ni in the tissues of mussels at pH 7.0. The noncontaminated sediment showed environmental degradation related to the acidification at pH values of 7.0; whereas the sediment moderately contaminated showed both environmental risks, caused by acidification and the presence and the increase of the bioavailability of contaminants. The WOE approach supposes an effective tool to identify and distinguish the causes of adverse effects related to the enrichment of CO2 in marine environments.
1. Introduction
Understanding the impacts of climate change and global warming is one of the greatest challenges faced by the scientific community, as are promoting mitigation and adaptation strategies [1,2]. The release of carbon dioxide (CO2) from human activities is considered a significant contributor to observed changes in global climate patterns and the acidification of the oceans [3]. In addition, ocean acidification associated with global warming is projected to amplify the adverse effects of warming (further at 2 °C), provoking impacts on the growth, development, survivorship, calcification, and richness/abundance of species [4]. Therefore, in the last years, many studies analyzed the potential effects of acidification and CO2 enrichment in marine environments [5,6,7,8,9] among natural processes [10]. They demonstrated a relationship between adverse effects on marine organisms promoted by acidification in seawater and the increase in the bioavailable forms of toxic elements. However, not many of them were designed or executed from an integrative point of view integrating multiple lines of evidence (LOEs): contamination, toxicity, and bioavailability.
The integrative methods based on the weight-of-evidence (WOE) were applied to assess the environmental quality of aquatic ecosystems in the last decades [11,12,13,14,15]. This approach uses a different set of data obtained within the application of multiple lines of evidence (LOEs) that usually implies (1) sediment physical characteristics (grain-size distribution); (2) chemical factors (contaminants in specific phases); (3) ecotoxicologically based on the use of acute and chronic toxicity tests and biomarker responses in key organisms; (4) ecological factors through the analysis of the structure and function of benthic communities; and (5) the bioaccumulation assessment of contaminants in living organisms.
The risk assessment based on the WOE approach to assessing the pollution derived from CO2 enrichment in aquatic environments was one of the adopted methods. It integrates the different responses, whilst it reduces the uncertainty associated with the complexity [10].
A recent study used the WOE to assess the environmental risks related to the enrichment of CO2 in the coastal zones in Brazil [9]. Results showed that the elemental bioavailability increased from pH 7.0, being responsible for the adverse effects caused in marine organisms. In this sense, the current study uses the WOE integrating LOEs to understand the potential adverse effects associated with the enrichment of CO2 in marine ecosystems, and to identify the source of contamination responsible for the pollution.
The main goal of the current study was to determine the environmental risk of CO2 enrichment in marine ecosystems using the WOE approach. Therefore, different LOEs from two sampling sites with different contamination degrees were employed under the influence of acidification (pH gradient from 8.0 to 6.0).
2. Materials and Methods
2.1. Study Area
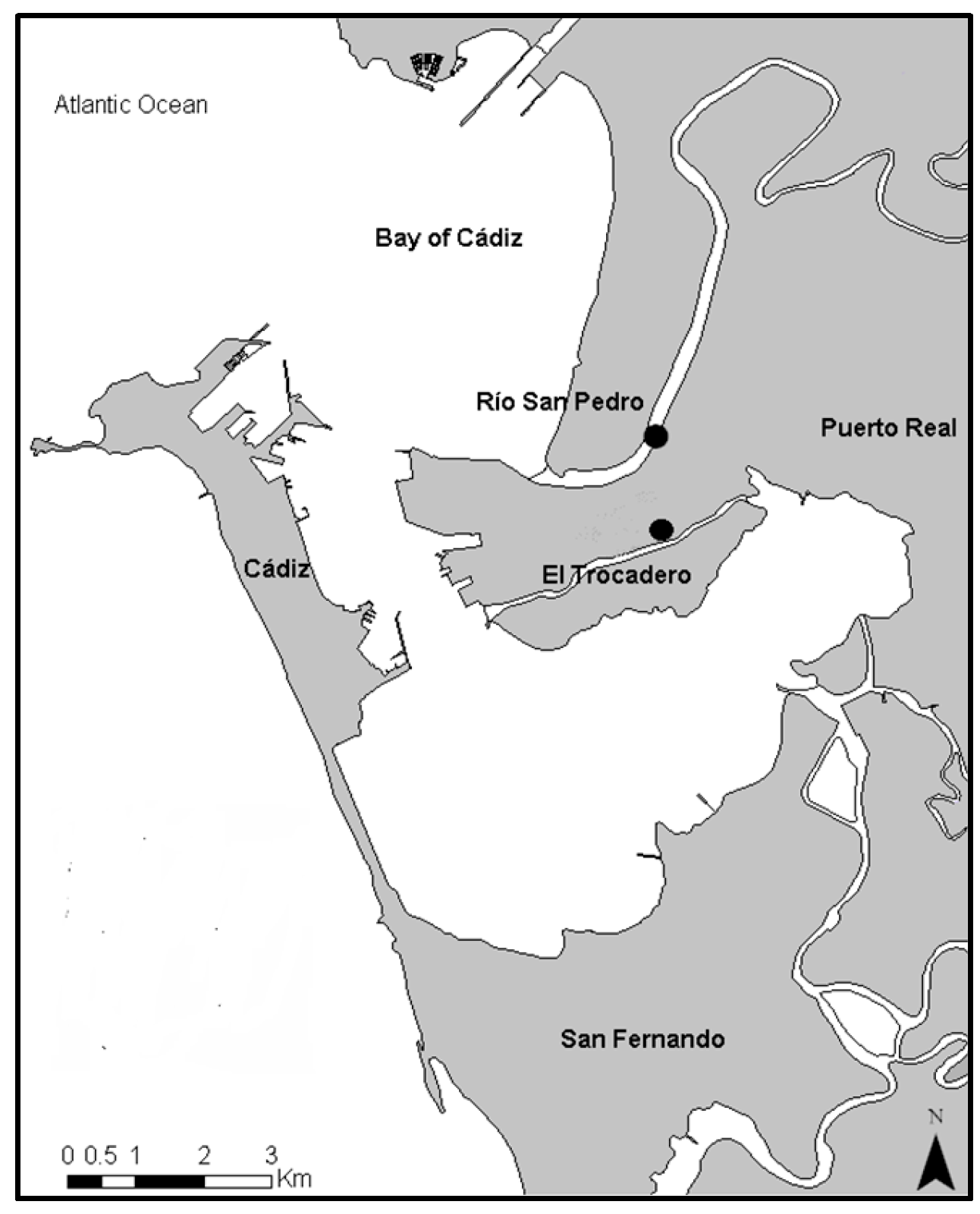
Two sediment samples were collected from the Bay of Cádiz (Figure 1). The selected areas were previously studied and were classified according to the metal contamination levels [5,16,17]. The Rio San Pedro (36°31′52.90″ N; 6°12′48.43″ W) sampling site is classified as low metal concentration (control site), while the Trocadero (36°31′15.5″ N; 6°12′25.1″ W) is considered a sampling site with a moderate metal concentration (contaminated site) [16,18].
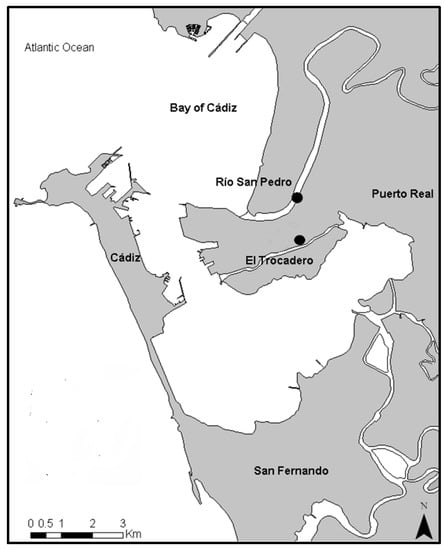
Figure 1.
Sediment sampling sites located in the Bay of Cádiz (SW, Spain): Río San Pedro (RSP) and El Trocadero (TRO).
Water used in toxicity tests was collected from the surface (1 m depth) of the Rio San Pedro (RSP), during high tide, transported to the laboratory using 50 L gallons, and placed in a 400 L tank (pH 7.9 ± 1; Salinity 34 psu, dissolved oxygen >81%, and temperature 18 ± 1 °C). As previously mentioned, the RSP has been used as a reference point in other works since no contaminants are present.
2.2. Experimental Design
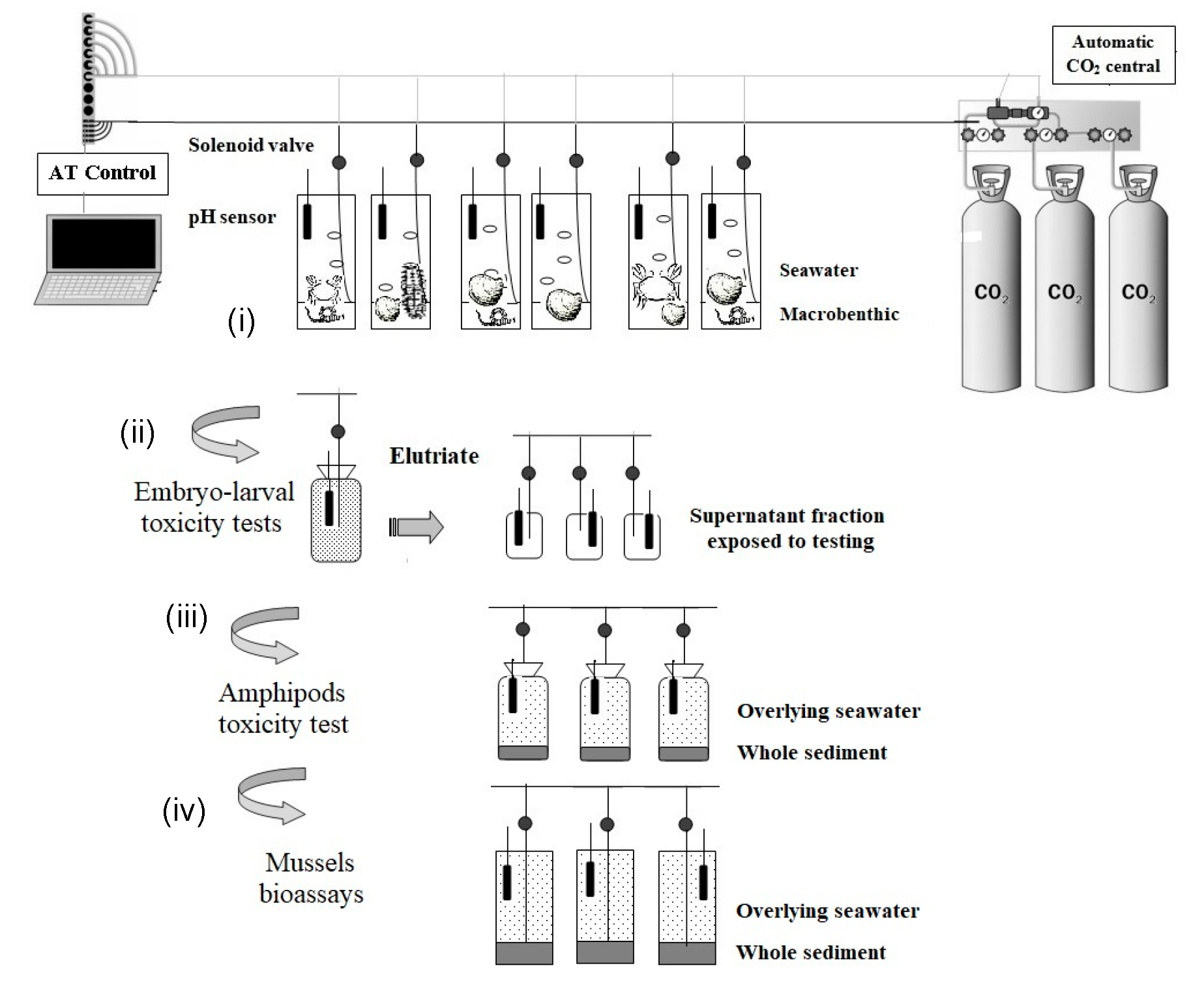
Figure 2 shows, in detail, the schematic design used for each experiment included in the WOE: (i) design to assess the benthic integrity; (ii) design used to elutriate assays employed as samples prior to acidification; (iii), and (iv) acute toxicity tests with amphipods and mussels design, respectively. In addition, it was adapted (iv) to be used for the bioaccumulation and NRRT assays.
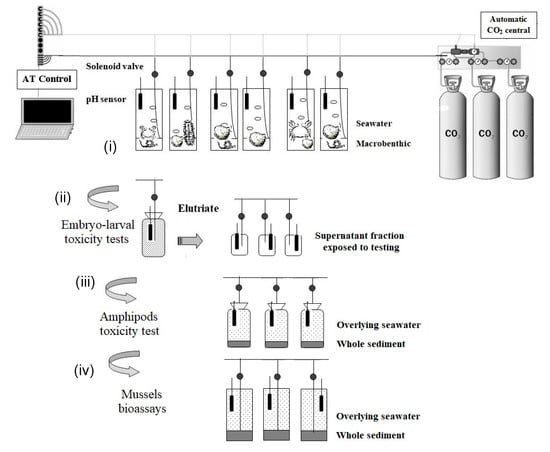
Figure 2.
Schematic design of the CO2 injection system used in this study for the different LOEs: (i) the benthic integrity; (ii) the elutriate assays for chronic toxicity; (iii,iv) acute toxicity tests with amphipods and mussels, respectively, and (iv) it was also applied for bioaccumulation and NRRT assays.
The laboratory-scale injection systems employed (Figure 2) for ecotoxicological and ecological integrity assessments were described by [16] and chemical analyses, acute and chronic toxicity tests, ecological integrity, and metal bioaccumulation are further described in [18,19]. The CO2 injection system proposed mimic acidification by CO2 enrichment in marine ecosystems.
2.3. Chemical Analyses
Sediment and water samples were collected from each station (RSP and TRO, Figure 1) at the beginning and the end of the toxicity and mesocosms tests. The metal and metalloid concentrations (Al, As, Cr, Cu, Fe, Ni, and Zn) in the sediment were analysed in the total fraction. The dissolved metal concentration in overlying and elutriate seawater were determined in filtered (0.45 mm) and acidified (pH < 2 with ultrapure HNO3) subsamples for metal(loid) analyses.
2.4. Toxicity Tests
Different biological responses were selected: (i) acute toxicity tests: amphipod and mussel mortalities; (ii) chronic toxicity tests: embryo–larval development success of sea urchins and mussels; (iii) metal bioaccumulation in the soft bodies of mussels; (iv) macrobenthic community alteration and; (v) biomarker response of cellular stress: neutral red retention time (NRRT) in the hemolymph of mussels.
The pH values of the treatments ranged between 8.0 and 6.0 (8.0, 7.5, 7.0, 6.5±, and 6.0) and were conducted in replicates. Table 1 summarises the description of the different toxicity tests performed in the study using different species and conditions and the ecological integrity assessment conditions.

Table 1.
Summarized description of the biological tests and the ecological integrity assessment tested in this study.
2.4.1. Acute-Toxicity Tests
Individuals of amphipods Ampelisca brevicornis were collected from a noncontaminated area at the Bay of Cádiz. These are used as environmental quality indicators and are recommended by the specific protocol for dredged-sediment characterization [20]. Individuals of mussels Mytilus galloprovincialis were obtained from an aquaculture farm (Cetária, Cádiz, Spain). Both organisms were acclimated for a week before testing. Parameters were adjusted according to the optimal conditions established for each species employed in this study and kept until the end of the experiments (Table 1). The experiments were adapted from [21,22].
2.4.2. Chronic Toxicity Using Elutriates
Specimens of sea urchin Paracentrotus lividus were collected from a rocky intertidal platform from a protected area in the Gulf of Cádiz (Southwestern Spain); whereas the specimens of mussels M. galloprovincialis were obtained from an aquaculture farm (Cetária, Cádiz, Spain).
Sea urchin gametes were obtained from direct extraction from the gonads. On the other hand, mussels were induced to spawn by thermal stimulation according to the protocol recommended by [23,24] and exposed to acidified sediment elutriate following [22].
2.4.3. Metal Bioaccumulation
M. galloprovincialis mussels were exposed to acidified sediments for 14 days. Assays were performed using whole sediment with a sediment-to-water ratio of 1:2 v/v as reported by [25]. The concentrations of metals Zn, Cu, Al, Fe, Mn, Ni, Cr, and the metalloid As in the soft tissue were determined using an inductively coupled plasma mass spectrometer (ICP-MS) (Thermo Elemental Series-X, Santa Clara, CA, USA).
2.4.4. Ecological Integrity
After the acclimation time, mesocosms were maintained for 21 days, natural photoperiod and constant aeration and temperature. Then, the macrobenthic community structure (total abundance, richness, and diversity) was determined using Margalef’s and Shannon’s methods, respectively.
2.4.5. Neutral Red Retention Time Assay
The NRRT assay was based on the principle that only lysosomes in healthy cells take up and retain the neutral red vital stain [26]. Lysosomal membrane damage caused by xenobiotics can decrease the NRRTs [27]. The experiments were performed following the method described by [28].
2.5. Statistical Analysis (WOE Approach)
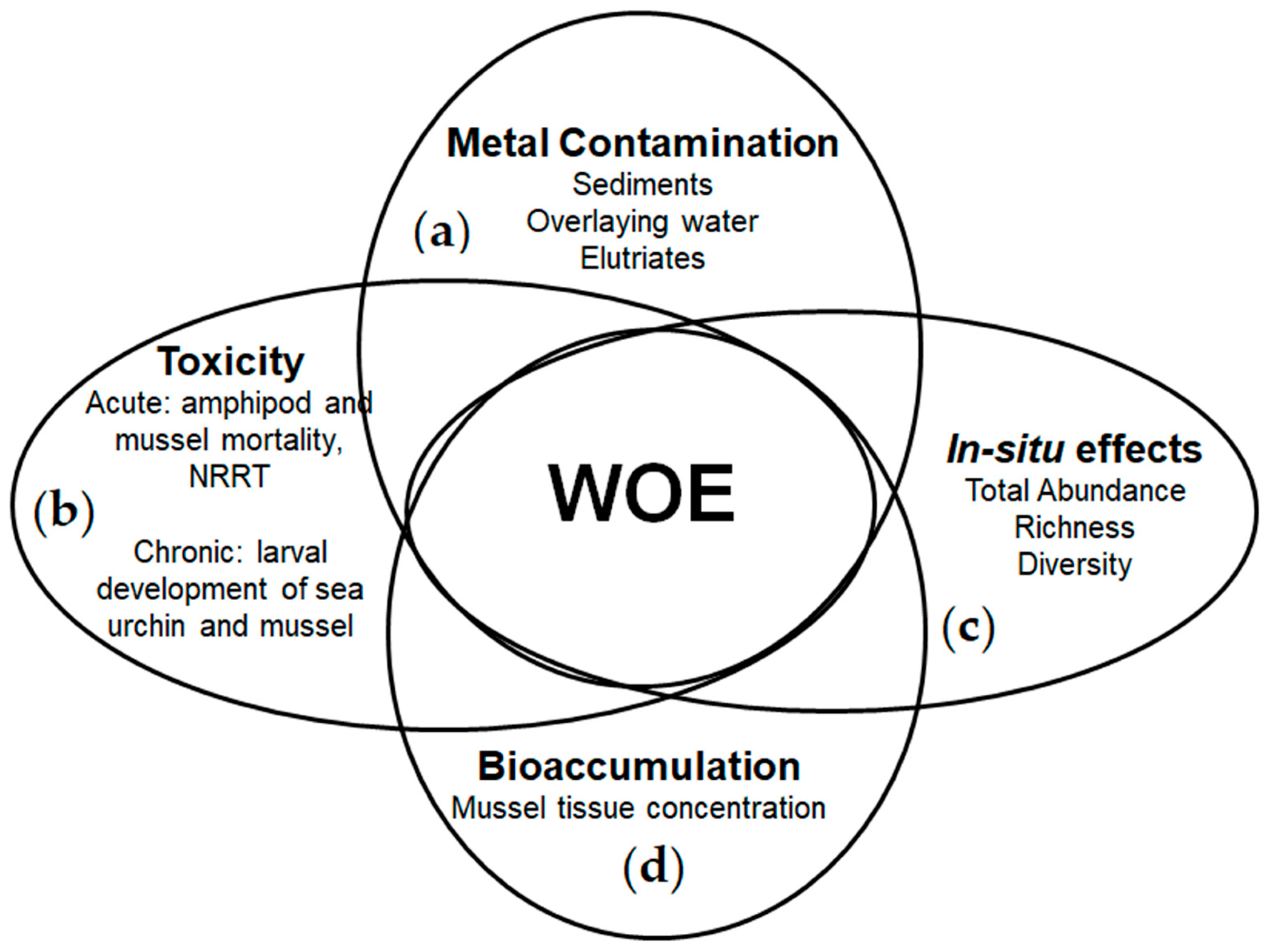
Figure 3 represents the four LOEs integration used to characterize and distinguish the impacts related to the contamination levels and acidification degrees.
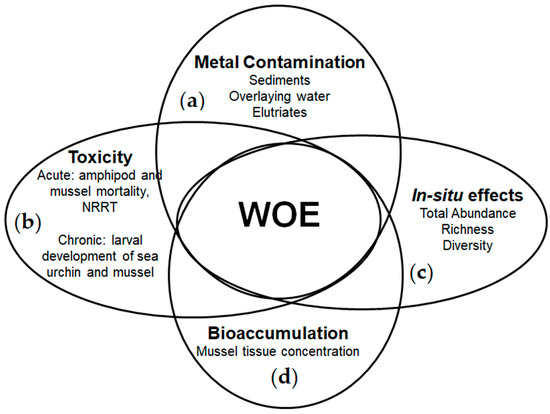
Figure 3.
Integration of the LOEs used to assess the environmental risks in CO2-enriched aquatic environments: (a) contamination type and degree; (b) laboratory toxicity tests; (c) in situ conditions alterations; (d) metal bioaccumulation.
The obtained results from the application of each LOE were linked for each component assessed using three different methods (WOE): (a) tabular matrix based on statistical significance (p < 0.05) compared to the reference case (RSP sediment, pH 8.0); (b) pie charts, showing statistical differences (p < 0.05 significantly different and positive effect; 0.01 ≥ p > 0.05 significantly different but moderate effect; and p > 0.01 not significantly different) among the samples sites and pH values compared to the reference case (RSP sediment, pH 8.0); and (c) linking the results using a multivariate analysis using the factor analysis principal component as extraction method [29].
Significant differences found between the reference site and the pH treatments were determined by an ANOVA and Dunnet’s tests (TOXSTAT software, version 3.5) to develop the pie charts and tabular matrix.
The indexes of contamination, toxicity, bioaccumulation, and ecological integrity were calculated as outlined by [11], considering the ratio-to-reference (RTR) values. Results from each LOE and each pH treatment were referred to the reference case and then derived as the minimum value. The RTRs were averaged for contamination in sediments and mobility, acute and chronic toxicity, bioaccumulation, and ecological integrity.
The contamination index was obtained regarding the metal concentration in sediments (RTRsediment), and concentration in seawater and elutriates (RTRmobility). The elements As, Cr, Cu, Ni, and Zn were considered to determine the indexes. The acute toxicity index was derived from the average of RTR calculated for amphipod and mussel mortalities and NRRT assays. The values of NRRT were transformed as their inverse to be treated with equal importance. The chronic toxicity index was estimated from the average RTR for larval development inhibition results of mussels and sea urchins. Regarding the index of macrobenthic community structure (ecological integrity), the values were also inverted to be treated with equal importance, and the average of RTR was calculated based on the results of total abundance, diversity, and richness. The bioaccumulation index was derived from the average RTR estimated for the metals bioaccumulated in the tissues of mussels.
Furthermore, to develop an integrated and more precise interpretation of the risks associated with CO2 enrichment in the marine environment, a multivariate analysis (principal-component analysis, PCA-with-varimax rotation) was carried out. Therefore, the correlation between increasing pollution, pH reduction, and biological effects measured in the laboratory was further explained. Factor scores for each sampling station were calculated following the protocols outlined by [11,30] and using the STATISTICA® software package (version 13.2). The principal factors were extracted and the eigenvalues above 1.0 were taken into account. Variables having loadings ≥0.40 to a particular factor were considered associated with the respective factor, following [31].
3. Results and Discussion
The results obtained for each of the LOEs used in this study are summarized in Table 2. The concentrations of most of the studied metal(loid)s in the TRO sediment sample were higher than in RSP sediment samples. Concentrations of Al, Cr, and Fe showed little variation among the pH values for both sediment samples (RSP and TRO). The Cu concentration in sediment decreased with pH for both sampling sites, whereas an increase in Zn concentration was observed in the TRO sediment at pH 6.0. However, greater variability in the concentration of metals was observed in the samples of overlying water and elutriates when compared with the sediment samples. Overall, the metal concentrations were higher in the elutriate treatment than in the overlying seawater. The concentrations of the studied elements were higher in sediment from TRO elutriates, while RSP sediments exhibited higher concentrations for most of the metals than for the overlying seawater samples.

Table 2.
Summarized results for the different lines of evidence (LOEs) used in this study: a) chemical concentrations in sediment (sed, in µg g−1 dry weight), overlying water (sw, in mg L−1), and elutriate (elu, in mg L−1), and the bioaccumulation of elements in the soft tissue of mussels (bio, in µg g−1 dry weight); b) acute and chronic toxicity tests and neutral red assays (mortality of amphipods (Mort_A), and mortality of mussels (Mort_M); inhibition of larval development of mussels (Inh_M) and sea urchins (Inh_SU); neutral red retention time (NRRT) for 24 h, 96 h, and 10 d of exposure are expressed in minutes); and d) ecological integrity (total abundance, richness, and diversity) exposed to the pH treatments (8.0, 7.5, 7.0, 6.5, and 6.0) using the RSP and TRO sample sites.
Regarding the toxicity tests, the mortality of amphipods and mussels were 14% and 20%, respectively, for the control (not CO2 injection, RSP sediment). Significant mortality (p < 0.05) of amphipods was found at pH 7.0, and for mussels at pH 6.0 when exposed to RSP sediment. However, exposure to TRO sediment control displayed a mortality of 37% for amphipods and 35% for mussels. The absence of acidification treatment with TRO sediments implies that contamination strongly influenced the obtained results [18].
However, for the embryo–larval development test, a percentage of inhibition of less than 20% was observed for both species (sea urchin and mussel) and both sediment and elutriates samples (RSP and TRO) for the control treatment (not CO2 injection). The mussel–larval development inhibition differed significantly between the control and pH 6.5 and 6.0 for RSP elutriates; though it was statistically significant (p < 0.05) for pH 7.5 and below for TRO samples. On the other hand, the sea urchin larval-development inhibition presented significant differences (p < 0.05) at pH 7.0 for both sediment samples.
In general, a different bioaccumulation trend for each metal(loid) was shown by M. galloprovincialis with acidification. Organisms exposed to RSP samples accumulated greater concentrations of elements than those exposed to TRO samples for all the studied elements, except for Zn and Cr. Results are further detailed in [32].
Results using the ecological integrity showed a decrease in the total abundance, richness, and diversity of benthic fauna for pH 6.0 when compared to the control for both sediment samples tested (Table 2), in agreement with [33].
3.1. Tabular Matrix
Statistically significant differences (p < 0.05) between the reference treatment (RSP sediment, pH 8.0—no CO2 added) and the other treatments and stations are presented as showing a positive response in contamination, toxicity, integrity, or bioaccumulation (Table 3).

Table 3.
Matrix results for the different pH treatments used and sediment samples, representing significant differences (p < 0.05) were found when compared to the reference case (RSP, pH 8.0). Responses are shown as either positive (+), negative (−), and (+/−) when the result was close to being positive, but not, showed a significant difference.
In general, the metal concentration in sediment was not significantly affected by acidification for RSP sediment. Nevertheless, significant differences (p < 0.05) were found between the metal concentration in the TRO and the control. These results were expected since the metal contamination in the TRO station was higher than in the RSP station. These results agreed with those reported by [16], which characterized the RSP as a low metal-concentration station and, the TRO as a station with moderate metal concentration. On the other hand, the results obtained in the mobility index showed significant differences (p < 0.05) between the reference case and pH 6.5 and 6.0 for the RSP and starting at pH 7.5 for the TRO. These findings indicated that acidification could increase the mobility of metals from the sediment samples to the water column, which might cause toxic effects on marine organisms. Previous studies reported the pH as a variable that mostly might influence the availability of elements from sediments [17]. Furthermore, it can be suggested that the significant difference found in the toxicity indexes at pH 7.0, using the RSP sediment, could also be related to the increase in metal mobility. Nevertheless, a significant toxic effect at pH 7.5 was observed, and, according with previous results, showed significant effects on the larval development inhibition of mussels and amphipods, and clam mortalities associated with acidification [7,22,34,35].
The results of the integrity of the benthic community showed significant (p < 0.05) differences for all treatments tested using the TRO sediment. These results were expected since the TRO sediment sample presented higher contamination than the RSP sample and the lowest value of total abundance of macrobenthic found in the study. On the other hand, the results of the bioaccumulation of metals in the tissues of mussels showed a significant (p < 0.05) increase in the concentration of metals at pH 6.5 for both sediment samples.
3.2. Sector Charts
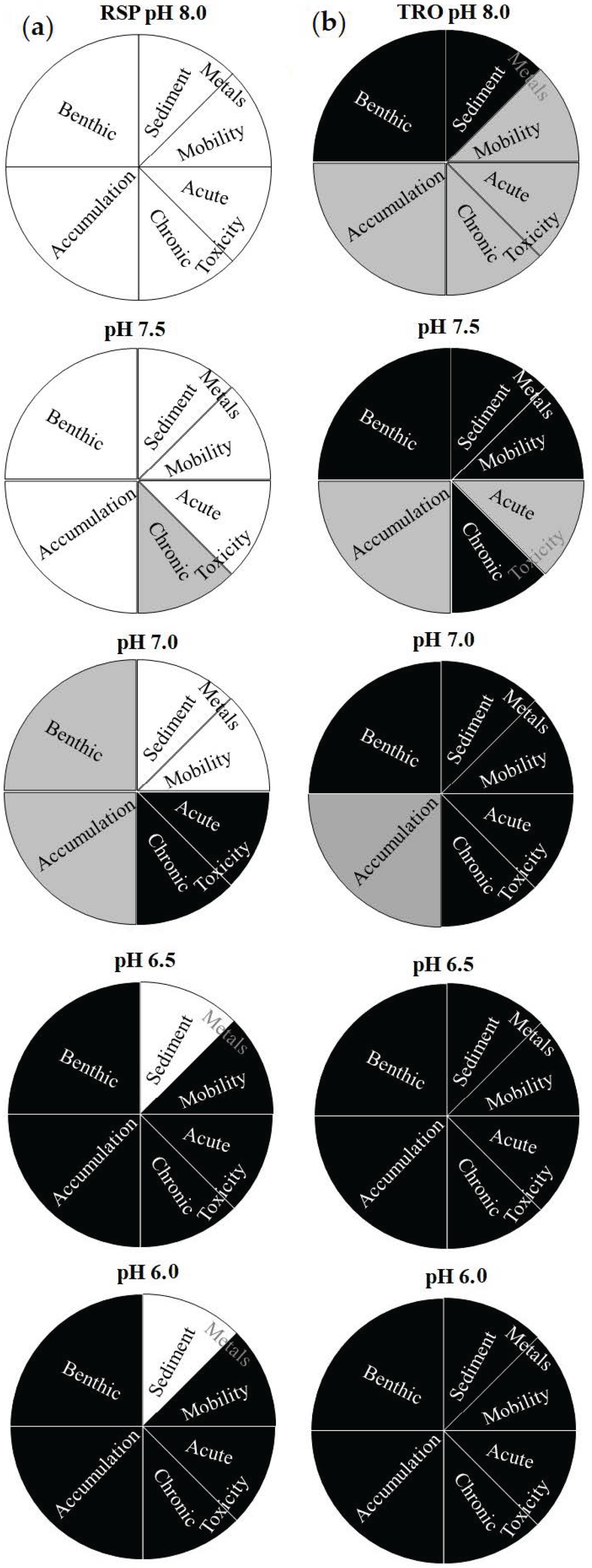
The obtained results of the present study were also expressed in sector charts that used different levels of the statistical p-value (Figure 4) to assess the influence of ocean acidification promoted by CO2 enrichment.
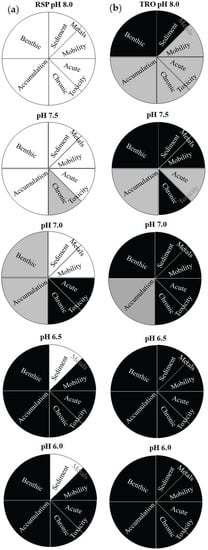
Figure 4.
Statistical differences of the LOEs among the pH levels are shown in sector charts: (a) RSP treatments and (b) TRO treatments. Black colour represents a significant difference (p < 0.05) against the control, grey colour represents differences using different p-values (0.01 ≥ p > 0.05), and white colour represents no significant difference concerning the reference case.
Significant differences were represented with different colours and weights for the LOEs. The RSP site was selected as the reference site at the control pH (not CO2, pH 8.0 ± 0.1). Then, each case (pH treatment in the sampling site) was compared to this reference case and the significant difference, with respect to the reference case, is represented as black segments when p < 0.05, whereas when 0.01 ≥ p > 0.05, they were represented by grey segments, and no significant difference (p > 0.01) was represented by white segments (Figure 4).
This approach allows clear visualization of the influence of CO2 enrichment on marine organisms concerning the pH tested and the contamination levels in each site used. According to [36], pie charts represent the strength of the statistical tool and the simplicity of interpretation, though without quantification. Results showed that both sediment contamination and contamination caused by acidification were associated with the biological responses in this study. Significant results (black area) were observed at pH 7.0 when using noncontaminated sediment. On the other hand, the results obtained using the TRO sediments showed significant effects even in the control treatment (pH 8.0, not CO2 added) for macrobenthic and contamination by metals in sediment. These results demonstrated that the sediment sampling sites presented the initial differences in their adverse effects associated with the contamination levels being not related to the acidification. Grey areas (moderate effects) indicate the beginning of the adverse effects and are useful for classifying cases in which additional attention should be taken, especially for potential scenarios of acidification.
3.3. Multivariate Analysis
The principal-component analysis (PCA) identified the correlations between sediment contamination, biological responses, and the pH decrease in seawater caused by CO2 enrichment in the ocean (Table 4). A total of 30 variables were used, including dissolved metals in overlying seawater (As, Cr, Cu, Fe, Ni, and Zn) and elutriate treatment (Cu, Fe, and Zn), toxicity to marine organisms (mortalities, embryo–larval development inhibition, and NRRT time after 24 h, 96 h, and 10 d of exposure), sediment characteristics (concentrations of organic matter, fines, and metals), concentrations of metals bioaccumulated in the whole body of mussels (As, Cr, Cu, Fe, Ni, and Zn) and benthic integrity (total abundance, diversity, and richness). The two main factors explained almost 75% of the original variance in the original data set. The criterion selected to interpret a variable associated with a particular factor was a loading of 0.4 or higher. Each factor is described according to the dominant group of variables.

Table 4.
The sorted rotated factor of the original variables on the two principal factors. Just loadings >0.4 are shown.
The first factor (F1, 48.41% of variance) linked all dissolved metals (except Fe) in seawater and elutriates, the metal concentration in mussels (except Zn), mortalities of amphipods and mussels, embryo–larval development inhibition of sea urchins and mussels and the increasing proton (H+) concentration, in the other words, the CO2 acidification. Furthermore, the F1 also linked negative values to the results of NRRT at 24 h, 96 h, and 10 d, and the richness of the macrobenthic community. The second factor (24.93% of variance) linked all metals in sediment, dissolved Ni in the seawater and Cu in the elutriate, As and Zn bioaccumulated in the mussels, mortality of mussels, sea urchin larval development inhibition, and the values of total abundance and diversity of the macrobenthic community. Moreover, negative values were linked in factor two for Cr and Fe in seawater.
In general, the multivariate analysis showed that both causes can influence the adverse biological effect measured: acidification by enrichment of CO2 and the metal concentration analyzed in the sediments.
The ecological integrity of the macrobenthic community related to the total abundance and diversity showed to be associated with the sediment contamination of the sampling sites, though not with the pH reduction. However, previous studies showed significant changes in the structure and reductions in diversity, abundance, and biomass of the benthic communities exposed to acidification scenarios [8,37,38]. These results confirm the obtained results (Table 3 and Figure 4) that showed a significant (p < 0.05) difference in the TRO benthic community at pH 8.0 when compared to the reference case. Therefore, the pH level probably was not correlated with these results, and the macrobenthic community could be affected by the CO2 enrichment in the ocean only at highly-acidified conditions.
It is known that the forms in which metals are bound in the sediments and the sediment porosity stability and grain size may influence their bioavailability and toxicity [39,40,41,42,43]. Factor one suggests that the majority of metals can increase their mobility in acidified conditions, and might cause adverse effects on marine organisms. These results were also observed in the tabular matrix and pie charts (Table 3 and Figure 4, respectively) that showed a significant difference (p < 0.05) in the mobility of metals starting at pH 6.5 for the RSP sediment and at pH 7.5 for the TRO sediment.
Furthermore, acute and chronic toxicity were linked in factor one with the metal’s mobility and the increase in the concentrations of H+ (enrichment of CO2). These results were indicated in the tabular matrix and pie charts as significantly differenced starting at pH 7.5 and 7.0 for TRO and RSP sediment, respectively, and corroborate with the results found by [9], which showed significant effects starting at pH 7.0 in no contaminated sediment sample. On the other hand, the multivariate analysis identified which metals were correlated with their concentration increase and the toxicity caused to the organisms (As, Ni, and Zn in seawater and Cu, Fe, and Zn in elutriate sediments). These results corroborate previous studies which showed adverse effects on marine organisms associated with increases in the metal concentrations and the pH reduction [10,16,22,34,35] reported with the mortality of amphipod Hyale youngi with seawater acidification due to an increase in Ni and As concentrations. In addition, an increase in the mobility of dissolved Zn promoted mortality in bivalves (adults and juveniles) [22,25] and amphipods [16].
Furthermore, it was reported that there was a correlation between the increase in the mobility of Cu, Zn, and As with the mortality of Hediste diversicolor polychaete [44]. Furthermore, it has been observed that there is variability in the feeding behaviour of marine amphipods (O. tuberculata) in response to ocean acidification [45].
Regarding the results of bioaccumulation of metals, it was correlated that factor one increases in the concentrations of As, Cr, Cu, Fe, and Ni in the tissues of mussels, increases in the mobility of the metals mentioned above, and increases in the concentrations of H+ protons. These results were described in Table 3 and Figure 4, presenting significant differences from pH 6.5 for both sediment sampling sites. These findings are similar to [44], a study that showed a correlation between the increase of Al, Fe, Mn, Cu, Zn, and As accumulated in the tissues of H. diversicolor with the pH reduction. Furthermore, the bioaccumulation of As and Zn was shown in factor two which was correlated with the sediment contamination. These results are expected since mussels exposed to trace elements in seawater can accumulate high amounts in their tissue [6]. In addition, a previous study reported that the bioaccumulation in Tellina deltoidalis showed that the bioavailability of metals was likely to be a result of the continued cycling between the pore water and the surface sediments due to the bioturbation process [42].
According to [45], the bioaccumulation of metals may be influenced by some factors, such as metal specificity, environmental influences, exposure routes, and species-specific characterization. Results of metal bioaccumulation in mussels may be associated with different parameters such as CO2 enrichment in seawater, sediment contamination, metals behaviour, etc.
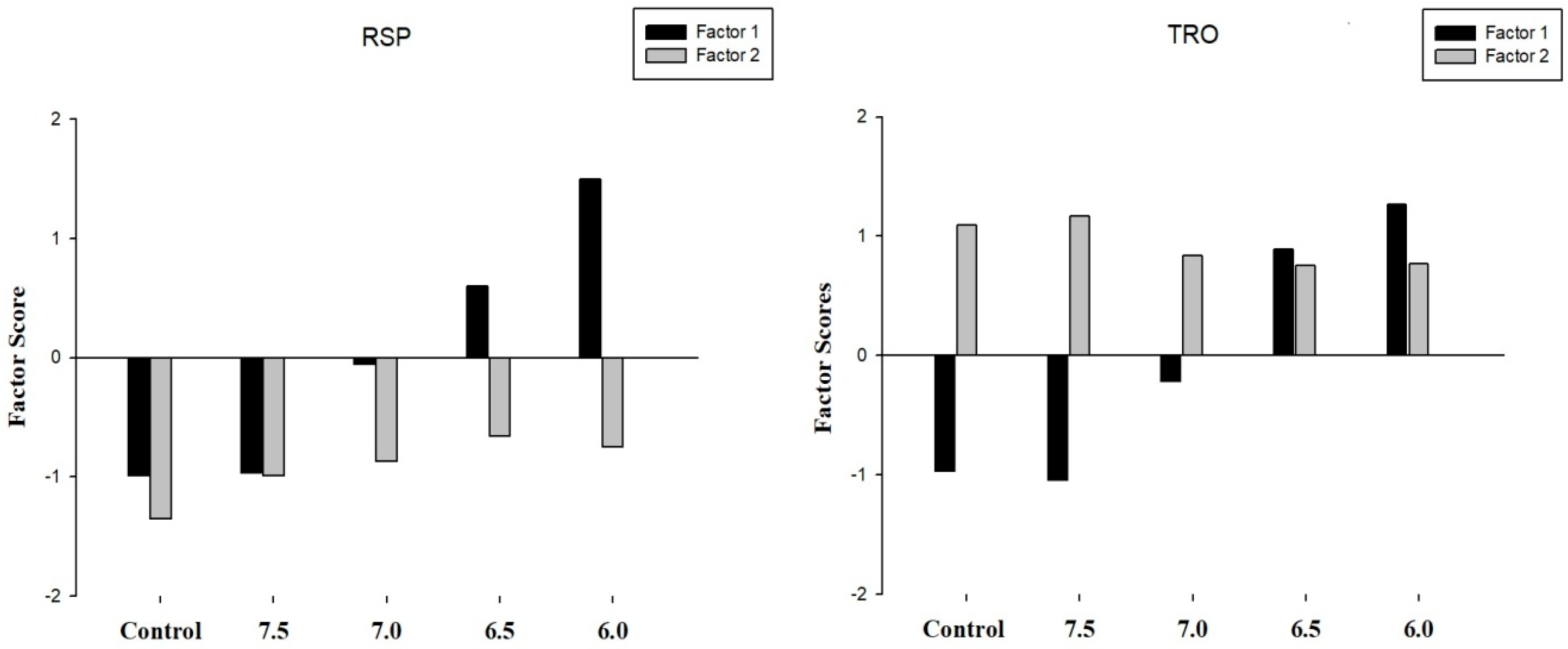
To confirm the factors’ descriptions and to establish the relationship between components associated with contamination, biological effects and acidification at each studied site, it was proposed as a representation of estimated factor scores per case (Figure 5).
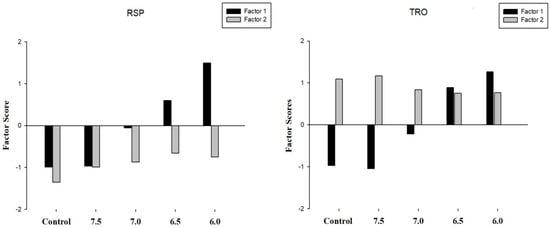
Figure 5.
Representation of the estimated factor scores for each sediment sampling site (RSP and TRO) and treatment (control, 7.5, 7.0., 6.5, and 6.0).
The factor scores have demonstrated that the contamination levels of sediment influence the obtained results. Overall, the RSP sediment that is considered a noncontaminated sediment site did not correlate with the contamination levels and biological responses (factor one) for the higher pH values. Moreover, the results of factor scores confirm those previously reported (Table 2) with lower levels of metal contamination in sediments (factor two).
Furthermore, as expected, the results of factor two were correlated even at the control pH values using the TRO which also confirms the results obtained using the tabular matrix and pie charts. Furthermore, it was noted that factor two decreases with the concentration of protons increase (pH reduction) while the opposite results were demonstrated for factor one, which increases its value as the pH values decrease.
In this sense, the pH value of 6.5 was correlated to provoke adverse effects on marine organisms that could also be associated with the increase in the mobility of metals. Moreover, it is important to perform a complete risk assessment in areas affected by CO2 enrichment considering different LOEs associated with biological responses and sediment characterization for a proper interpretation of the impacts caused by the acidification in a marine environment.
4. Conclusions
The present study applied for the first time a complete and specific design of a weight-of-evidence approach (WOE) to address the potential impacts of CO2 enrichment in the marine environment using different lines of evidence. It has been demonstrated from the different lines of evidence (LOEs) employed in this study using a WOE that the acidification by enrichment of CO2 provokes adverse effects on the marine organisms, starting at pH 7.0, which considerably enhances at pH 6.5.
Metal contamination in sediments seems to play a very important role in the environmental risk assessment under the enrichment of CO2 scenarios. The results showed that dissolved elements such as As, Fe, Cu, Ni, and Zn increased their concentrations and contributed to causing toxic effects on marine organisms under acidified conditions.
The WOE distinguished between contamination sources that are causing biological adverse effects in marine organisms. It differs between the extension of the effects produced by the acidification by enrichment of CO2, the extension of the effects associated with the levels of contaminants (mainly metals), and the combination of both causes of adverse effects, acidification, and metal contamination at the same time.
Author Contributions
M.C.P. and I.R. contributed to all aspects of the article from the conceptualization to the funding acquisition including methodology software, validation, formal analysis, investigation, resources, data curation, and writing and editing the paper from the start to the final version. E.B. contributes in most of the aspects including conceptualization, investigation, resources, data curation, writing—original draft preparation, writing—review, and editing and visualization. A.C. contributed to most of the aspects including methodology, validation, formal analysis, writing—review and editing, visualization, supervision, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Ministry of Education of Spain (PRX14/00134) and partially by FAPESP (#2017/25936-0). This study was also partially funded by FAPESP through grants #2015/17329-0 and #2018/18456-4.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors thank Fabio H. Pusceddu for the support in the data analysis and Eric Russell for the English revision. A.C. thanks CNPq (PQ 305417/2019-3) for productivity fellowships, and FAPESP (Process #2017/07353-7). I.R. thanks FAPESP (#2017/25936-0) for her Visiting Researcher Program at Universidade Federal do Sao Paulo. The first author would like to thank the Erasmus Mundus Program for the doctoral fellowship. E.B. thanks to ANID/FONDECYT 11180015.
Conflicts of Interest
The authors declare no conflict of interest.
References
- United Nations Framework Convention on Climate Change (UNFCCC). Adoptions of the Paris Agreement. 2015. Available online: http://unfccc.int/files/essential_background/convention/application/pdf/english_paris_agreement.pdf (accessed on 2 May 2017).
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2014: Mitigation of Climate Change; Edenhofer, O., Pichs-Madruga, R., Sokona, Y., Minx, J.C., Farahani, E., Kadner, S., Sevboth, K., Minx, J.C., Eds.; Working Group III Technical Support Unit, Cambridge University Press: Cambridge, UK, 2014; p. 1454. [Google Scholar]
- IPCC; Global CCS Institute. The Global Status of CCS 2016: Summary Report. 2016. Available online: https://www.globalccsinstitute.com/publications/global-status-ccs-2016-summary-report (accessed on 2 May 2017).
- IPCC. Summary for Policymakers. In Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Pörtner, H.-O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; World Meteorological Organization: Geneva, Switzerland, 2018; 32p. [Google Scholar]
- De Orte, M.R.; Lombardi, A.T.; Sarmiento, A.M.; Basallote, M.; Rodriguez-Romero, A.; Riba, I.; Del Valls, A. Metal mobility and toxicity to microalgae associated with acidification of sediments: CO2 and acid comparison. Mar. Environ. Res. 2014, 96, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Cao, R.; Ning, X.; You, L. Effects of ocean acidification on immune responses of the Pacific oyster Crassostrea gigas. Fish Shellfish Immunol. 2016, 49, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Conradi, M.; Sánchez-Moyano, J.E.; Galotti, A.; Jiménez-Gómez, F.; Jiménez-Melero, R.; Guerrero, F.; Parra, G.; Bonnail, E.; DelValls, T. CO2 leakage simulation: Effects of the decreasing pH to the survival and reproduction of two crustacean species. Mar. Pollut. Bull. 2019, 143, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Borrero-Santiago, A.R.; Ribicic, D.; Bonnail, E.; Netzer, R.; Koseto, D.; Ardelan, M. Response of bacterial communities in Barents Sea sediments in case of a potential CO2 leakage from carbon reservoirs. Mar. Environ. Res. 2020, 160, 105050. [Google Scholar] [CrossRef] [PubMed]
- Passarelli, M.; Bonnail, E.; Cesar, A.; DelValls, T.A.; Riba, I. Integrative assessment of sediments affected by CO2 enrichment: A case study in the Bay of Santos—SP, Brazil. Appl. Sci. 2021, 11, 11603. [Google Scholar] [CrossRef]
- DelValls, T.A.; Souza, L.S.; Seabra, A.A.; Pereira, C.D.S.; Bonnail, E.; Riba, I. Integrative assessment of sediment quality in acidification scenarios associated with carbon capture and storage operations. Environ. Rev. 2019, 27, 333–345. [Google Scholar] [CrossRef]
- DelValls, T.Á.; Forja, J.M.; Gómez-Parra, A. Integrative assessment of sediment quality in two littoral ecosystems from the Gulf of Cadiz, Spain. Environ. Toxicol. Chem. 1998, 17, 1073–1084. [Google Scholar] [CrossRef]
- Abessa, D.M.S.; Carr, R.S.; Sousa, E.C.P.M.; Rachid, B.R.; Zaroni, L.P.; Pinto, Y.A.; Gasparro, M.R.; Bícego, M.C.; Hortellani, M.A.; Sarkis, J.E.S.; et al. Integrative Ecotoxicological Assessment of a Complex Tropical Estuarine System; Hoffer, T.N., Ed.; Nova Science Publishers: New York, NY, USA, 2008; pp. 125–159. [Google Scholar]
- Choueri, R.B.; Cesar, D.M.S.; Torres, R.J.; Morais, R.; Riba, I.; Pereira, C.; Nascimento, M.; Mozeto, A.; DelValls, T. Development of site-specific sediment quality guidelines for North and South Atlantic littoral zones: Comparison against national and international sediment quality benchmarks. J. Hazard. Mat. 2009, 170, 320–331. [Google Scholar] [CrossRef]
- Torres, R.J.; Cesar, A.; Pastor, V.A.; Pereira, C.D.S.; Choueri, R.B.; Cortez, F.S.; Morais, R.D.; Abessa, D.M.S.; Nascimento, M.R.L.D.; Morais, C.R.; et al. A critical comparison of different approaches to sediment- Quality Assessments in the Santos Estuarine System in Brazil. Arch. Environ. Contam. Toxicol. 2015, 68, 132–147. [Google Scholar] [CrossRef]
- Bonnail, E.; Riba, I.; Seabra, A.; DelValls, T.A. Sediment quality assessment in the Guadalquivir River (SW, Spain) using caged Asian clams: A biomarker field approach. Sci. Total Environ. 2019, 650, 1996–2003. [Google Scholar] [CrossRef]
- Basallote, M.D.; DelValls, T.Á.; Riba, I. Studying the effect of CO2-Induced acidification on sediment toxicity using acute amphipod toxicity test. Environ. Sci. Technol. 2014, 48, 8864–8872. [Google Scholar] [CrossRef] [PubMed]
- De Orte, M.R.; Bonnail, E.; Sarmiento, A.M.; Bautista-Chamizo, E.; Basallote, M.D.; Riba, I.; DelValls, Á.; Nieto, J.M. Metal fractionation in marine sediments acidified by enrichment of CO2: A risk assessment. Mar. Pollut. Bull. 2018, 131, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Passarelli, M.C.; Riba, I.; Cesar, A.; Serrano-Bernando, F.; DelValls, T.A. Assessing the influence of ocean acidification to marine amphipods: A comparative study. Sci. Total Environ. 2017, 595, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Passarelli, M.C.; Cesar, A.; Riba, I.; DelValls, T.A. Comparative evaluation of sea-urchin larval stage sensitivity to ocean acidification. Chemosphere 2017, 184, 224–234. [Google Scholar] [CrossRef]
- CEDEX. Directrices Para la Caracterización del Material Dragado y su Reubicación en Aguas del Dominio Público Marítimo-Terrestre. Comisión Interministerial de Estrategias Marina. 2015. Available online: http://www.mapama.gob.es/es/costas/temas/proteccion-medio-marino/directrices2015_tcm7-325119.pdf (accessed on 2 May 2017).
- Basallote, M.; Rodríguez-Romero, A.; Blasco, J.; DelValls, A.; Riba, I. Lethal effects on different marine organisms, associated with sediment–seawater acidification deriving from CO2 leakage. Environ. Sci. Pollut. Res. 2012, 19, 2550–2560. [Google Scholar] [CrossRef]
- Basallote, M.; Rodríguez-Romero, A.; De Orte, M.R.; DelValls, A.; Riba, I. Evaluation of the threat of marine CO2 leakage-associated acidification on the toxicity of sediment metals to juvenile bivalves. Aquat. Toxicol. 2015, 166, 63–71. [Google Scholar] [CrossRef]
- American Society of Testing and Materials (ASTM). Standard Guide for Conducting Static Toxicity Tests Starting with Embryos of Four Species of Saltwater Bivalve Mollusks. In Annual Book of ASTM Standards: Water and Environmental Technology; ASTM: Philadelphia, PA, USA, 1992; Volume 11, pp. 377–394. [Google Scholar]
- Cortez, F.S.; Pereira, C.D.S.; Santos, A.R.; Cesar, A.; Choueri, R.; Martini, G.D.A.; Bohrer-Morel, M.B. Biological effects of environmentally relevant concentrations of the pharmaceutical Triclosan in the marine mussel Perna perna (Linnaeus, 1758). Environ. Pollut. 2012, 168, 145–150. [Google Scholar] [CrossRef]
- Riba, I.; Kalman, J.; Vale, C.; Blasco, J. Influence of sediment acidification on the bioaccumulation of metals in Ruditapes philippinarum. Environ. Sci. Pollut. Res. 2010, 17, 1519–1528. [Google Scholar] [CrossRef]
- Moore, M.N.; Lowe, D.; Allen, I.; McVeigh, A.; Somerfield, P. Predicting health of environment—Lysosomal biomarker responses in mussels. In Proceedings of the Workshop on the Med Pol Biological Effects Programme: Acheivements and Future Orientations, Alessandria, Italy, 20–21 December 2006; p. 245. [Google Scholar]
- Dailianis, S.; Domouhtsidou, G.P.; Raftopoulou, E.; Kaloyianni, M.; Dimitriadis, V. Evaluation of neutral red retention assay, micronucleus test, acetylcho-linesterase activity and a signal transduction molecule (cAMP) in tissues of Mytilus galloprovincialis (L.), in pollution monitoring. Mar. Environ. Res. 2003, 56, 443–470. [Google Scholar] [CrossRef]
- Lowe, D.M.; Fossato, V.U.; Depledge, M.H. Contaminant-induced lysosomal membrane damage in blood cells of mussels Mytilus galloprovincialis from the Venice Lagoon: An in vitro study. Mar. Ecol. Prog. Ser. 1995, 129, 189–196. [Google Scholar] [CrossRef]
- Morales-Caselles, C.; Riba, I.; Sarasquete, C.; DelValls, T.A. Using a classical weight of evidence approach for 4 years monitoring of the impact of an accidental oil spill on sediment quality. Environ. Int. 2008, 34, 514–523. [Google Scholar] [CrossRef] [PubMed]
- DelValls, T.A.; Chapman, P.M. The use of multivariate analysis to link the sediment quality triad components defining site-specific sediment quality values in the Gulf of Cádiz and in San Francísco Bay. In Proceedings of the Second Symposium on the Atlantic Iberian Continental Margin, Cádiz, Spain, 17–20 September 1997. [Google Scholar]
- Tabachinic, B.G.; Fidell, L.S. Using Multivariate Statistics; Harper Collins, College Publishers: New York, NY, USA, 1996. [Google Scholar]
- Passarelli, M.C.; Ray, S.; Cesar, A.; DelValls, T.A.; Riba, I. Effects of CO2 enrichment on metal bioavailability and bioaccumulation using Mytilus galloprovincialis. Mar. Pollut. Bull. 2018, 133, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Riba, I.; Passarelli, M.C.; Cesar, A. Chapter 3: Risk assessment of CO2 acidification in aquatic ecosystems: A weight-of evidence approach. In CO2 Acidification in Aquatic Ecosystems; Riba, D.V., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 978-0-12-823552-2. [Google Scholar]
- Szalaj, D.; De Orte, M.R.; Goulding, T.A.; Medeiros, I.D.; DelValls, T.A.; Cesar, A. The effects of ocean acidification and a carbon dioxide capture and storage leak on the early life stages of the marine mussel Perna perna (Linneaus, 1758) and metal bioavailability. Environ. Sci. Pollut. Res. 2016, 24, 765–781. [Google Scholar] [CrossRef]
- Goulding, T.; De Orte, M.R.; Szalaj, D.; Basallote, M.D.; DelValls, T.A.; Cesar, A. Assessment of the environmental impacts of Ocean acidification (OA) and Carbon Capture and Storage (CCS) leaks using the amphipod Hayle young. Ecotoxicology 2017, 26, 521–533. [Google Scholar] [CrossRef]
- Riba, I.; Casado-Martínez, C.; Forja, J.M.; DelValls, T.Á. Sediment quality in littoral regions of the Gulf of Cádiz: A triad approach to address the influence of mining activities. Environ. Pollut. 2004, 132, 341–353. [Google Scholar] [CrossRef]
- Christen, N.; Calosi, P.; McNeill, C.L. Structural and functional vulnerability to elevated pCO2 in marine benthic communities. Mar. Biol. 2013, 160, 2113–2128. [Google Scholar] [CrossRef]
- Almagro-Pastor, V.; Conradi, M.; DelValls, T.A.; Riba, I. Alterations in the macrobenthic fauna from Guadarranque River (Southern Spain) associated with sediment–seawater acidification deriving from CO2 leakage. Mar. Pollut. Bull. 2015, 96, 65–75. [Google Scholar] [CrossRef]
- Chapman, P.M.; Wang, F.; Janssen, C.R.; Goulet, R.R.; Kamunde, C.N. Conducting ecological risk assessments of inorganic metals and metalloids: Current status. Hum. Ecol. Risk Assess. 2003, 9, 641–697. [Google Scholar] [CrossRef]
- Eggleton, J.; Thomas, K.V. A review of factors affecting the release and bioavailability of contaminants during sediment disturbance events. Environ. Int. 2004, 30, 973–980. [Google Scholar] [CrossRef]
- Atkinson, C.A.; Jolley, D.F.; Simpson, S.L. Effect of overlying water pH, dissolved oxygen, salinity and sediment disturbances on metal release and sequestration from metal contaminated marine sediments. Chemosphere 2007, 69, 1428–1437. [Google Scholar] [CrossRef]
- Maar, M.; Larsen, M.M.; Torring, D.; Petersen, J.K. Bioaccumulation of metals (Cd, Cu, Ni, Pb and Zn) in suspended cultures of blue mussels exposed to different environmental conditions. Estuar. Coast. Shelf Sci. 2018, 201, 185–197. [Google Scholar] [CrossRef]
- Rodríguez-Romero, A.; Basallote, M.D.; Manoela, R.; DelValls, T.Á.; Riba, I.; Blasco, J. Simulation of CO2 leakages during injection and storage in sub-seabed geological formations: Metal mobilization and biota effects. Environ. Int. 2014, 68, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Benítez, S.; Duarte, C.; López, J.; Manríquez, P.H.; Navarro, J.M.; Bonta, C.C.; Torres, R.; Quijón, P.A. Ontogenetic variability in the feeding behaviour of a marine amphipod in response to ocean acidification. Mar. Pollut. Bull. 2016, 112, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Luoma, S.N.; Rainbow, P.S. Why is metal bioaccumulation so variable? Biodynamics as a unifying concept. Environ. Sci. Technol. 2005, 39, 1921–1931. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).