Chemoprotective Effect of Plantago sempervirens Crantz Extract on Ovarian Structure and Folliculogenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Extract Preparation
2.2. Chemicals
2.3. Animals
2.4. Study Design
2.5. Measurement of Nitro-Oxidative Stress
2.6. Measurement of Sex Hormones
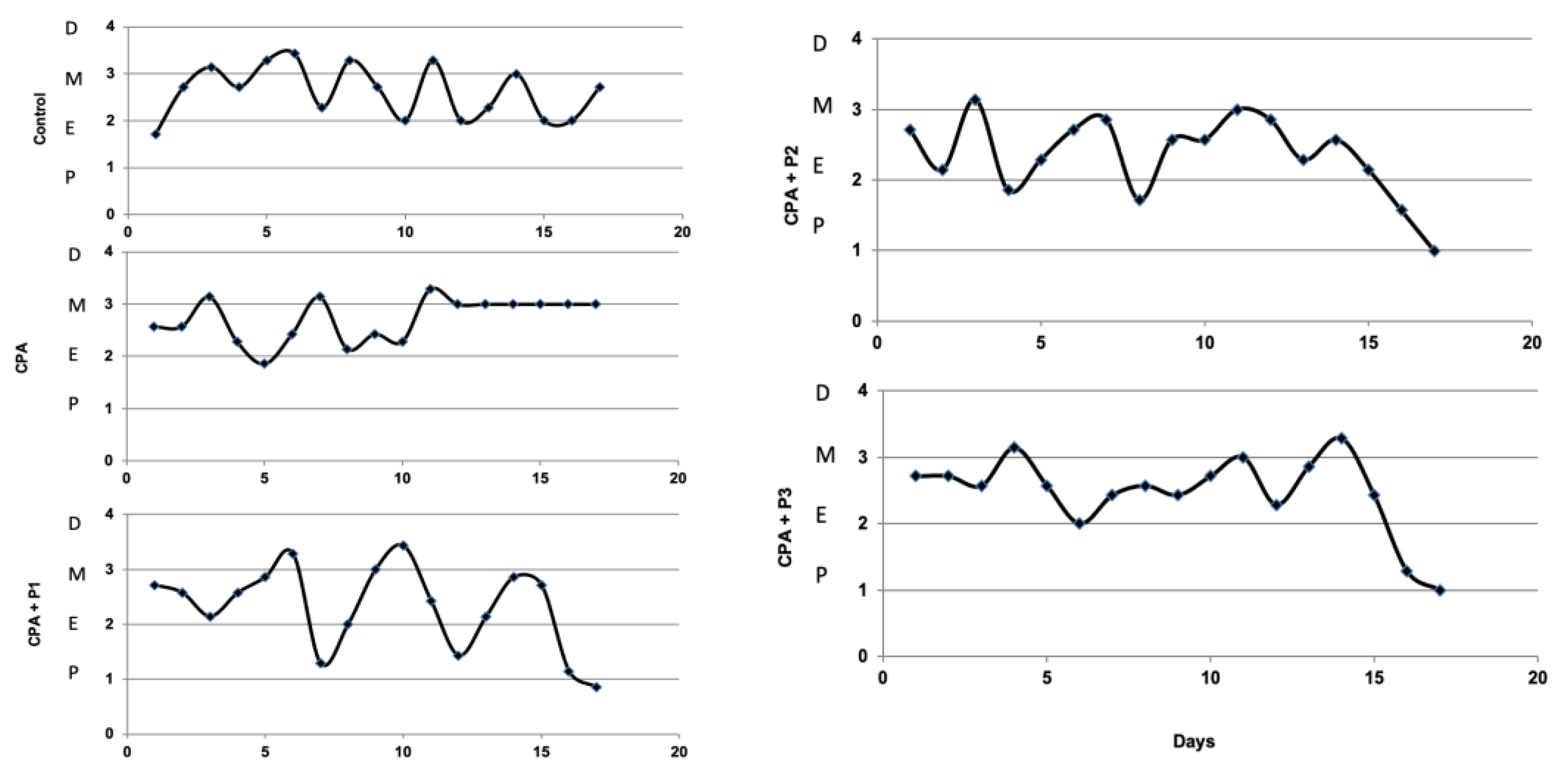
2.7. Oestrus Cycles Monitoring
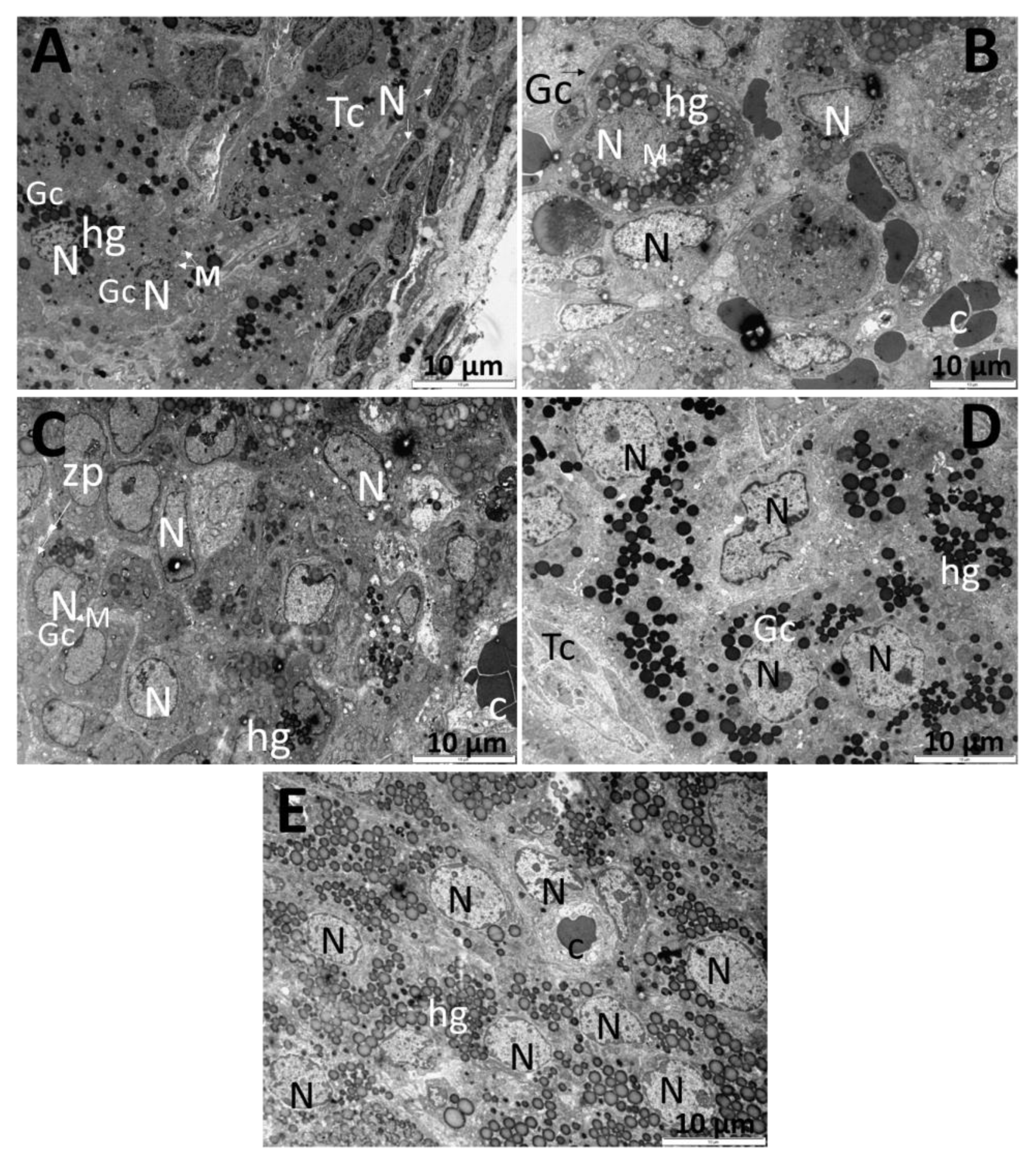
2.8. Transmission Electron Microscopy of the Ovarian Tissue
2.9. Statistical Analysis
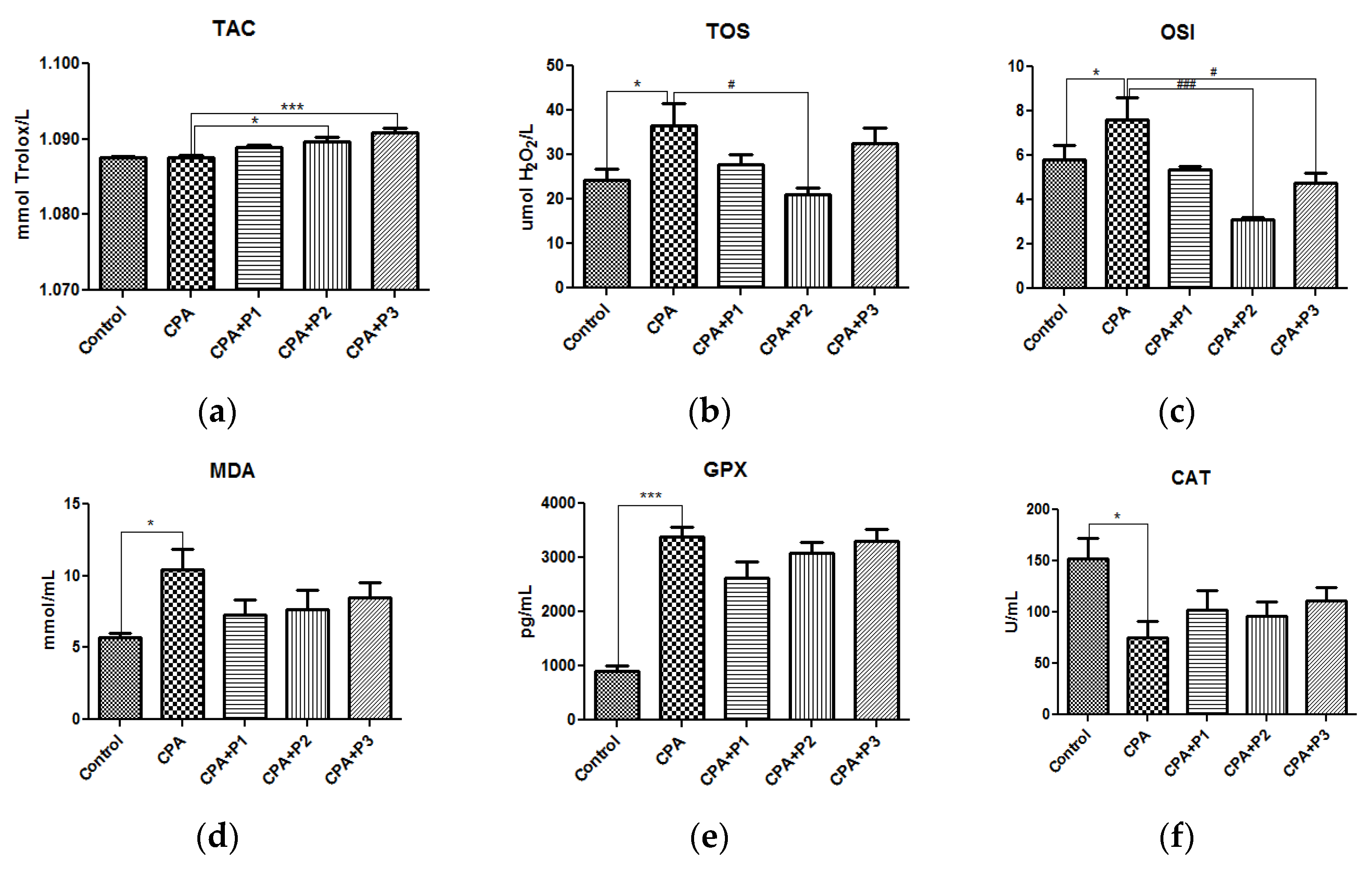
3. Results
3.1. Nitro-Oxidative Stress Status
3.2. Sex Hormone Levels
3.3. Ultrastructure of the Ovarian Cell
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chi, Y.-N.; Yang, J.-M.; Liu, N.; Cui, Y.-H.; Ma, L.; Lan, X.-B.; Ma, W.-Q.; Liu, Y.-J.; Yu, J.-Q.; Du, J. Development of Protective Agents against Ovarian Injury Caused by Chemotherapeutic Drugs. Biomed. Pharmacother. 2022, 155, 113731. [Google Scholar] [CrossRef]
- Behranvand, N.; Nasri, F.; Zolfaghari Emameh, R.; Khani, P.; Hosseini, A.; Garssen, J.; Falak, R. Chemotherapy: A Double-Edged Sword in Cancer Treatment. Cancer Immunol. Immunother. 2022, 71, 507–526. [Google Scholar] [CrossRef]
- Li, G.; Zhou, J.; Sun, M.; Cen, J.; Xu, J. Role of Luteolin Extracted from Clerodendrum Cyrtophyllum Turcz Leaves in Protecting HepG2 Cells from TBHP-Induced Oxidative Stress and Its Cytotoxicity, Genotoxicity. J. Funct. Foods 2020, 74, 104196. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, Z.; Li, X.; Du, D.; Wu, T.; Zhou, S.; Yan, W.; Wu, M.; Jin, Y.; Zhang, J.; et al. Epigallocatechin Gallate and Theaflavins Independently Alleviate Cyclophosphamide-Induced Ovarian Damage by Inhibiting the Overactivation of Primordial Follicles and Follicular Atresia. Phytomedicine 2021, 92, 153752. [Google Scholar] [CrossRef]
- Lins, T.L.B.G.; Gouveia, B.B.; Barberino, R.S.; Silva, R.L.S.; Monte, A.P.O.; Pinto, J.G.C.; Campinho, D.S.P.; Palheta, R.C., Jr.; Matos, M.H.T. Rutin Prevents Cisplatin-Induced Ovarian Damage via Antioxidant Activity and Regulation of PTEN and FOXO3a Phosphorylation in Mouse Model. Reprod. Toxicol. 2020, 98, 209–217. [Google Scholar] [CrossRef]
- Liu, X.; Song, Y.; Zhou, F.; Zhang, C.; Li, F.; Hu, R.; Ma, W.; Song, K.; Tang, Z.; Zhang, M. Network and Experimental Pharmacology on Mechanism of Si-Wu-Tang Improving Ovarian Function in a Mouse Model of Premature Ovarian Failure Induced by Cyclophosphamide. J. Ethnopharmacol. 2023, 301, 115842. [Google Scholar] [CrossRef]
- Barberino, R.S.; Lins, T.L.B.G.; Monte, A.P.O.; Silva, R.L.S.; Andrade, K.O.; Campinho, D.S.P.; Palheta Junior, R.C.; Smitz, J.E.J.; Matos, M.H.T. Epigallocatechin-3-Gallate Attenuates Cyclophosphamide-Induced Damage in Mouse Ovarian Tissue via Suppressing Inflammation, Apoptosis, and Expression of Phosphorylated Akt, FOXO3a and RpS6. Reprod. Toxicol. 2022, 113, 42–51. [Google Scholar] [CrossRef]
- Masciangelo, R.; Hossay, C.; Chiti, M.C.; Manavella, D.D.; Amorim, C.A.; Donnez, J.; Dolmans, M.-M. Role of the PI3K and Hippo Pathways in Follicle Activation after Grafting of Human Ovarian Tissue. J. Assist. Reprod. Genet. 2020, 37, 101–108. [Google Scholar] [CrossRef]
- Horicks, F.; Van Den Steen, G.; Houben, S.; Englert, Y.; Demeestere, I. Folliculogenesis Is Not Fully Inhibited during GnRH Analogues Treatment in Mice Challenging Their Efficiency to Preserve the Ovarian Reserve during Chemotherapy in This Model. PLoS ONE 2015, 10, e0137164. [Google Scholar] [CrossRef]
- Rampogu, S.; Balasubramaniyam, T.; Lee, J.-H. Phytotherapeutic Applications of Alkaloids in Treating Breast Cancer. Biomed. Pharmacother. 2022, 155, 113760. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Wang, X.-L.; He, D.-H.; Cheng, Y.-X. Protection against Chemotherapy- and Radiotherapy-Induced Side Effects: A Review Based on the Mechanisms and Therapeutic Opportunities of Phytochemicals. Phytomedicine 2021, 80, 153402. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Nybom, H.; Lindholm, C.; Rumpunen, K. Major Polyphenols in Aerial Organs of Greater Plantain (Plantago major L.), and Effects of Drying Temperature on Polyphenol Contents in the Leaves. Sci. Hortic. 2011, 128, 523–529. [Google Scholar] [CrossRef]
- Beara, I.N.; Lesjak, M.M.; Orčić, D.Z.; Simin, N.Đ.; Četojević-Simin, D.D.; Božin, B.N.; Mimica-Dukić, N.M. Comparative Analysis of Phenolic Profile, Antioxidant, Anti-Inflammatory and Cytotoxic Activity of Two Closely-Related Plantain Species: Plantago altissima L. and Plantago lanceolata L. LWT—Food Sci. Technol. 2012, 47, 64–70. [Google Scholar] [CrossRef]
- Gonçalves, S.; Romano, A. The Medicinal Potential of Plants from the Genus Plantago (Plantaginaceae). Ind. Crops Prod. 2016, 83, 213–226. [Google Scholar] [CrossRef]
- Farcaș, A.D.; Moț, A.C.; Pârvu, A.E.; Toma, V.A.; Popa, M.A.; Mihai, M.C.; Sevastre, B.; Roman, I.; Vlase, L.; Pârvu, M. In Vivo Pharmacological and Anti-Inflammatory Evaluation of Xerophyte Plantago sempervirens Crantz. Oxid. Med. Cell Longev. 2019, 2019, 5049643. [Google Scholar] [CrossRef]
- Farcaș, A.D.; Zăgrean-Tuza, C.; Vlase, L.; Gheldiu, A.-M.; Pârvu, M.; Moț, A.C. EPR Fingerprinting and Antioxidant Response of Four Selected Plantago Species. Stud. Univ. Babeș-Bolyai Chem. 2020, 65, 209–220. [Google Scholar] [CrossRef]
- Harma, M.; Harma, M.; Erel, O. Increased Oxidative Stress in Patients with Hydatidiform Mole. Swiss Med. Wkly. 2003, 133, 563–566. [Google Scholar] [CrossRef]
- Erel, O. A Novel Automated Method to Measure Total Antioxidant Response against Potent Free Radical Reactions. Clin. Biochem. 2004, 37, 112–119. [Google Scholar] [CrossRef]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A Rapid, Simple Spectrophotometric Method for Simultaneous Detection of Nitrate and Nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef]
- Mitev, D.; Gradeva, H.; Stoyanova, Z.; Petrova, N.; Karova, N.; Dimov, D.; Iliev, V.; Koychev, A.; Prakova, G.; Vlaykova, T. Evaluation of Thiol Compounds and Lipid Peroxidative Products in Plasma of Patients With COPD. Trakia J. Sci. 2010, 8, 306–314. [Google Scholar]
- Li, Y.; Schellhorn, H.E. Rapid Kinetic Microassay for Catalase Activity. J. Biomol. Tech. JBT 2007, 18, 185. [Google Scholar] [PubMed]
- Byers, S.L.; Wiles, M.V.; Dunn, S.L.; Taft, R.A. Mouse Estrous Cycle Identification Tool and Images. PLoS ONE 2012, 7, e35538. [Google Scholar] [CrossRef] [PubMed]
- Suciu, M.; Mirescu, C.; Crăciunescu, I.; Macavei, S.G.; Leoștean, C.; Ştefan, R.; Olar, L.E.; Tripon, S.-C.; Ciorîță, A.; Barbu-Tudoran, L. In Vivo Distribution of Poly(Ethylene Glycol) Functionalized Iron Oxide Nanoclusters: An Ultrastructural Study. Nanomaterials 2021, 11, 2184. [Google Scholar] [CrossRef] [PubMed]
- Toiu, A.; Mocan, A.; Vlase, L.; Pârvu, A.E.; Vodnar, D.C.; Gheldiu, A.-M.; Moldovan, C.; Oniga, I. Comparative Phytochemical Profile, Antioxidant, Antimicrobial and In Vivo Anti-Inflammatory Activity of Different Extracts of Traditionally Used Romanian Ajuga genevensis L. and A. Reptans L. (Lamiaceae). Molecules 2019, 24, 1597. [Google Scholar] [CrossRef]
- Roness, H.; Kashi, O.; Meirow, D. Prevention of Chemotherapy-Induced Ovarian Damage. Fertil. Steril. 2016, 105, 20–29. [Google Scholar] [CrossRef]
- Melekoglu, R.; Ciftci, O.; Eraslan, S.; Cetin, A.; Basak, N. Beneficial Effects of Curcumin and Capsaicin on Cyclophosphamide-Induced Premature Ovarian Failure in a Rat Model. J. Ovarian Res. 2018, 11, 33. [Google Scholar] [CrossRef]
- Yang, H.; Xie, Y.; Yang, D.; Ren, D. Oxidative Stress-Induced Apoptosis in Granulosa Cells Involves JNK, P53 and Puma. Oncotarget 2017, 8, 25310–25322. [Google Scholar] [CrossRef]
- Shen, M.; Lin, F.; Zhang, J.; Tang, Y.; Chen, W.-K.; Liu, H. Involvement of the Up-Regulated FoxO1 Expression in Follicular Granulosa Cell Apoptosis Induced by Oxidative Stress. J. Biol. Chem. 2012, 287, 25727–25740. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Y.; Liu, Y.; Xing, Y.; Miao, C.; Zhao, Y.; Chang, X.; Zhang, Q. The Role of Oxidative Stress and Natural Antioxidants in Ovarian Aging. Front. Pharmacol. 2021, 11, 617843. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Meldrum, D.R.; Katz-Jaffe, M.G.; Krisher, R.L.; Schoolcraft, W.B. Oocyte Environment: Follicular Fluid and Cumulus Cells Are Critical for Oocyte Health. Fertil. Steril. 2015, 103, 303–316. [Google Scholar] [CrossRef]
- Moţ, A.C.; Coman, C.; Miron, C.; Damian, G.; Sarbu, C.; Silaghi-Dumitrescu, R. An Assay for Pro-Oxidant Reactivity Based on Phenoxyl Radicals Generated by Laccase. Food Chem. 2014, 143, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Are Polyphenols Antioxidants or Pro-Oxidants? What Do We Learn from Cell Culture and in Vivo Studies? Arch. Biochem. Biophys. 2008, 476, 107–112. [Google Scholar] [CrossRef] [PubMed]
- De Torre, M.P.; Cavero, R.Y.; Calvo, M.I.; Vizmanos, J.L. A Simple and a Reliable Method to Quantify Antioxidant Activity In Vivo. Antioxidants 2019, 8, 142. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.; Shikha, D.; Thakur, M.; Aneja, A. Functionality of Apigenin as a Potent Antioxidant with Emphasis on Bioavailability, Metabolism, Action Mechanism and in Vitro and in Vivo Studies: A Review. J. Food Biochem. 2022, 46, e13950. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Almeida, A.; López-Yerena, A.; Pinto, S.; Sarmento, B.; Lamuela-Raventós, R.; Vallverdú-Queralt, A.; Delerue-Matos, C.; Rodrigues, F. Appraisal of a New Potential Antioxidants-Rich Nutraceutical Ingredient from Chestnut Shells through in-Vivo Assays—A Targeted Metabolomic Approach in Phenolic Compounds. Food Chem. 2023, 404, 134546. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Pan, J.-H.; Huang, X.-F.; Liao, Y.-Q.; Ling, Y.-J.; Luo, J.-Y. Effect of Melatonin on Oxidative Stress Indicators in Animal Models of Fibrosis: A Systematic Review and Meta-Analysis. Free Radic. Biol. Med. 2023, 195, 158–177. [Google Scholar] [CrossRef] [PubMed]
- Issac, P.K.; Guru, A.; Velayutham, M.; Pachaiappan, R.; Arasu, M.V.; Al-Dhabi, N.A.; Choi, K.C.; Harikrishnan, R.; Arockiaraj, J. Oxidative Stress Induced Antioxidant and Neurotoxicity Demonstrated in Vivo Zebrafish Embryo or Larval Model and Their Normalization Due to Morin Showing Therapeutic Implications. Life Sci. 2021, 283, 119864. [Google Scholar] [CrossRef]
- Beken, A.T.; Saka, Ş.; Aydın, İ.; Fırat, K.; Suzer, C.; Benzer, F.; Erişir, M.; Özden, O.; Hekimoğlu, M.A.; Engin, S.; et al. In Vivo and in Vitro Evolution of the Effects of Cypermethrin on Turbot (Scophthalmus maximus, Linnaeus, 1758) Spermatozoa. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 256, 109298. [Google Scholar] [CrossRef]
- Rashid, S.; Sameti, M.; Alqarni, M.H.; Abdel Bar, F.M. In Vivo Investigation of the Inhibitory Effect of Peganum Harmala L. and Its Major Alkaloids on Ethylene Glycol-Induced Urolithiasis in Rats. J. Ethnopharmacol. 2023, 300, 115752. [Google Scholar] [CrossRef]
- Grover, P.; Khanna, K.; Bhatnagar, A.; Purkayastha, J. In Vivo-Wound Healing Studies of Sodium Thiosulfate Gel in Rats. Biomed. Pharmacother. 2021, 140, 111797. [Google Scholar] [CrossRef]
- Baud, O. Glutathione Peroxidase-Catalase Cooperativity Is Required for Resistance to Hydrogen Peroxide by Mature Rat Oligodendrocytes. J. Neurosci. 2004, 24, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Erdal, N.; Gürgül, S.; Tamer, L.; Ayaz, L. Effects of Long-Term Exposure of Extremely Low Frequency Magnetic Field on Oxidative/Nitrosative Stress in Rat Liver. J. Radiat. Res. 2008, 49, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Ricciardolo, F.L.M.; Sorbello, V.; Benedetto, S.; Paleari, D. Effect of Ambroxol and Beclomethasone on Lipopolysaccharide-Induced Nitrosative Stress in Bronchial Epithelial Cells. Respiration 2015, 89, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Spears, N.; Lopes, F.; Stefansdottir, A.; Rossi, V.; De Felici, M.; Anderson, R.A.; Klinger, F.G. Ovarian Damage from Chemotherapy and Current Approaches to Its Protection. Hum. Reprod. Update 2019, 25, 673–693. [Google Scholar] [CrossRef]
- Yuksel, A.; Bildik, G.; Senbabaoglu, F.; Akin, N.; Arvas, M.; Unal, F.; Kilic, Y.; Karanfil, I.; Eryılmaz, B.; Yilmaz, P.; et al. The Magnitude of Gonadotoxicity of Chemotherapy Drugs on Ovarian Follicles and Granulosa Cells Varies Depending upon the Category of the Drugs and the Type of Granulosa Cells. Hum. Reprod. 2015, 30, 2926–2935. [Google Scholar] [CrossRef]
- Fu, X.; He, Y.; Xie, C.; Liu, W. Bone Marrow Mesenchymal Stem Cell Transplantation Improves Ovarian Function and Structure in Rats with Chemotherapy-Induced Ovarian Damage. Cytotherapy 2008, 10, 353–363. [Google Scholar] [CrossRef]
- Isachenko, V.; Isachenko, E.; Michelmann, H.W.; Alabart, J.L.; Vazquez, I.; Bezugly, N.; Nawroth, F. Lipolysis and Ultrastructural Changes of Intracellular Lipid Vesicles after Cooling of Bovine and Porcine GV-Oocytes. Anat. Histol. Embryol. J. Vet. Med. Ser. C 2001, 30, 333–338. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, Y.; Yu, Y.; Xin, X. GnRH Antagonist Cetrorelix Inhibits Mitochondria-Dependent Apoptosis Triggered by Chemotherapy in Granulosa Cells of Rats. Gynecol. Oncol. 2010, 118, 69–75. [Google Scholar] [CrossRef]
- Sonigo, C.; Beau, I.; Binart, N.; Grynberg, M. The Impact of Chemotherapy on the Ovaries: Molecular Aspects and the Prevention of Ovarian Damage. Int. J. Mol. Sci. 2019, 20, 5342. [Google Scholar] [CrossRef]
- Xiong, J.; Xue, L.; Li, Y.; Tang, W.; Chen, D.; Zhang, J.; Dai, J.; Zhou, S.; Lu, Z.; Wu, M.; et al. Therapy of Endocrine Disease: Novel Protection and Treatment Strategies for Chemotherapy-Associated Ovarian Damage. Eur. J. Endocrinol. 2021, 184, R177–R192. [Google Scholar] [CrossRef]
- De Assis, E.I.T.; Azevedo, V.A.N.; De Lima Neto, M.F.; Costa, F.C.; Paulino, L.R.F.M.; Barroso, P.A.A.; Donato, M.A.M.; Peixoto, C.A.; Do Monte, A.P.O.; Matos, M.H.T.; et al. Protective Effect of Cimicifuga racemosa (L.) Nutt Extract on Oocyte and Follicle Toxicity Induced by Doxorubicin during In Vitro Culture of Mice Ovaries. Animals 2022, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Tanihara, F.; Hirata, M.; Nhien, N.T.; Hirano, T.; Kunihara, T.; Otoi, T. Effect of Ferulic Acid Supplementation on the Developmental Competence of Porcine Embryos during in Vitro Maturation. J. Vet. Med. Sci. 2018, 80, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Abdel Fattah, S.M.; Mohmed, H.K.; Mohamed, M.A.E.H. The Potential Protective Effect of Ferulic Acid against Gamma Irradiation Induced Ovarian Failure in Rats. Egypt. J. Radiat. Sci. Appl. 2019, 32, 1–12. [Google Scholar] [CrossRef]
- Mun, G.-I.; Kim, S.; Choi, E.; Kim, C.S.; Lee, Y.-S. Pharmacology of Natural Radioprotectors. Arch. Pharm. Res. 2018, 41, 1033–1050. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoica, A.D.; Sevastre, B.; Suciu, M.; Pârvu, A.E.; Pârvu, M.; Toma, V.A.; Roman, I.; Dobre, C. Chemoprotective Effect of Plantago sempervirens Crantz Extract on Ovarian Structure and Folliculogenesis. Appl. Sci. 2023, 13, 3134. https://doi.org/10.3390/app13053134
Stoica AD, Sevastre B, Suciu M, Pârvu AE, Pârvu M, Toma VA, Roman I, Dobre C. Chemoprotective Effect of Plantago sempervirens Crantz Extract on Ovarian Structure and Folliculogenesis. Applied Sciences. 2023; 13(5):3134. https://doi.org/10.3390/app13053134
Chicago/Turabian StyleStoica, Anca D., Bogdan Sevastre, Maria Suciu, Alina Elena Pârvu, Marcel Pârvu, Vlad Alexandru Toma, Ioana Roman, and Camelia Dobre. 2023. "Chemoprotective Effect of Plantago sempervirens Crantz Extract on Ovarian Structure and Folliculogenesis" Applied Sciences 13, no. 5: 3134. https://doi.org/10.3390/app13053134
APA StyleStoica, A. D., Sevastre, B., Suciu, M., Pârvu, A. E., Pârvu, M., Toma, V. A., Roman, I., & Dobre, C. (2023). Chemoprotective Effect of Plantago sempervirens Crantz Extract on Ovarian Structure and Folliculogenesis. Applied Sciences, 13(5), 3134. https://doi.org/10.3390/app13053134







