Abstract
Lignocellulosic biomass dissolution in an inorganic salt hydrate (ZnCl2·4H2O) and its subsequent precipitation with water for the separation of its main compounds were investigated. For this purpose, different dissolution times and temperatures were studied, where 24 h and 70 °C were found to be the optimal choice. Three solids were obtained, which were analyzed and identified by XRD, SEM, NMR, and FTIR spectroscopy. Solid I is the undissolved part of the starting material, and it consists of lignin, which does not react with the inorganic salt hydrate and the unreacted cellulose. Solid II is a cellulose-rich solid with a low portion of hemicellulose and lignin, and Solid III is mainly pure lignin as the characterization results showed. Hemicellulose is mainly dissolved and hydrolyzed in the dissolution treatment and the amount present in all solids was very small. The reactivity of Solid I and Solid II in a hydrolysis reaction was tested (0.2 M/L H2SO4, 5 h, and 140 °C), where a significant improvement in the conversion and the yield of sugars was obtained with respect to the untreated samples in both cases. Solid II yields a large amount of total reducing sugars, with a % selectivity of 78–88%, depending on the starting biomass.
1. Introduction
It is essential to find new alternatives to the current energy model based on the use of fossil resources to avoid the environmental problems that the use of these fossil resources entails. In a global scenario characterized by the scarcity of raw materials, lignocellulosic biomass residues are considered a sustainable natural resource for the efficient production of high-value-added products [1,2] and biofuels [3] and are sustainable within the framework of biorefineries. Integration into a biorefinery strategy will enable an evolution towards an economy based on natural resources, which will replace the current economy based on raw fossil materials [4,5]. In fact, in the near future, the chemical industry could rely solely on biomass as an alternative source [6,7], and biorefineries will then be able to compete with fossil fuel resources [4,8,9]. However, for effective use of these biomass residues, pretreatments are key to alter the inherent recalcitrance of these residues and improve the reactivity of the biomass for further transformations and to obtain high-value-added products [9,10,11,12,13]. The composition of the raw materials varies according to the different biomasses presenting different proportions of components. Due to their complex structure, fragmentation is required to achieve a better utilization of the biomass and to improve the porosity of the cellulosic matrix that facilitates chemical hydrolysis [14,15,16,17,18,19].
In previous studies, the dissolution of cellulose and barley straw in ionic liquids (ILs) has been investigated [10,14,20,21,22,23]. However, despite being effective in dissolving lignocellulosic biomass and fractionating it into cellulose, hemicellulose, and lignin using selective antisolvents, if a low-priced ionic liquid is not used as solvent [20,24] or the expensive ionic liquid cannot be recovered, it is essential to find another solvent as an alternative [25,26,27,28].
Inorganic salt hydrates are an attractive alternative to ILs because they are cheaper, recyclable, non-toxic, and usually require lower temperatures for lignocellulosic biomass dissolution. Hydrated inorganic salt is inserted between the fibers of the lignocellulosic residues, breaking the hydrogen bonds and allowing their dissolution [29,30,31,32]. In a previous study, an inorganic salt hydrate (ZnCl2·4H2O) was subsequently precipitated by adding water [18]. Salt hydrates can dissolve structural carbohydrates (cellulose and hemicellulose), leaving lignin in the solid [33], so the pretreatment of biomass will consist of partial dissolution of the polymeric carbohydrates of the biomass and its regeneration with water.
In the present work, three different types of lignocellulosic biomass residues (LB) will be studied, barley straw, pruning waste, and vine shoots, and treated with the inorganic salt hydrate ZnCl2·4H2O. The treatment yields the fractionation of the biomass. The different solids obtained were analyzed by different characterization techniques and those with structural carbohydrates were tested in acid hydrolysis. The obtained results are compared with the results for untreated lignocellulosic biomass to check the effectiveness of the treatment. To the best of our knowledge, an inorganic salt has never before been used for fractionation of full lignocellulosic biomass.
2. Materials and Methods
Materials and Chemicals. All chemicals were acquired from Merck, excluding xylan (from beechwood) and lignin (alkaline), which were acquired from TCI-Europe; zinc chloride anhydrous (98+%) acquired from Alfa Aesar; acetone (synthesis grade) and sulfuric acid (1 M), acquired from Scharlab; hydrochloric acid (37%, pure pharma grade) acquired from AppliChem Panreac; and sulfuric acid (0.01 N), acquired from VWR. LB (barley straw, pruning waste, and vine shoot) was used as well as the compositional analysis was provided by the BIOCAR Unit of CIEMAT.
Analysis of lignocellulosic biomass. Lignocellulosic biomasses were analyzed by standard methods adapted for biomass analysis from the National Renewable Energy Laboratory (NREL; Denver, CO, USA) [34]. The data obtained on a dry basis are shown in Table 1.

Table 1.
Composition results (wt.%) of the different lignocellulosic biomasses dried at 105 °C.
Treatment of lignocellulosic biomass. The scheme of the dissolution process of LB using inorganic salt hydrate as a solvent is shown in Figure 1. A total of 8 g of lignocellulosic biomass was placed on slowly into 224.2 g of ZnCl2·4H2O at the selected temperature in a flask equipped with magnetic stirring, this proportion was selected on the basis of previous works where the dissolution of cellulose was fast and very efficient [10,35,36]. After dissolution, the mixture was centrifuged, and a dark viscous liquid was separated from a black solid:
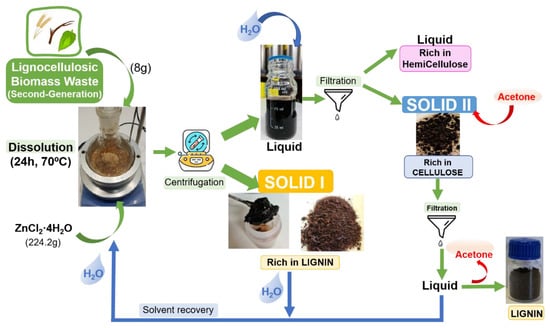
Figure 1.
Scheme of the process of the fragmentation of lignocellulosic biomass using ZnCl2·4H2O.
- The black solid obtained (Solid I) was washed with 5 × 20 mL of 0.1 N HCl and 5 × 20 mL of distilled water to eliminate residues of inorganic salt hydrate [18] or until the color of the solution became transparent.
- The dark viscous liquid obtained in the centrifugation was mixed with twice the water weight of the liquid, and then precipitation occurred. The solid, labeled Solid II, was filtered, and the mother liquor, which contain the dissolved hemicellulose, was separated. Solid II was isolated and washed with 5 × 20 mL of 0.1 N HCl and 5 × 20 mL of distilled water to remove the inorganic salt hydrate traces. Once the solution became clear, the solid was washed with acetone, and the clean solid was labeled Solid II.
- The dark acetone solution [10,17,37] was recovered, and a solid was recovered by solvent evaporation in a rotary evaporator (Solid III).
Hydrolysis. Hydrolysis reactions were carried out in a thermostatic Teflon-lined steel stirred reactor equipped with a stirred magnet and a pressure addition funnel (BR-100, Berghof Intruments) [14,16,38]. A total of 0.5 g of material was added to the reactor with 40 mL of water and heated to 140 °C. Subsequently, 10 mL of H2SO4 (0.2 M) was added and from this moment, the “reaction time” was measured.
The original biomass samples and all solids obtained were identified by different characterization techniques (scanning electron microscopy (SEM), Fourier transform infrared (FTIR) spectroscopy, X-ray diffraction (XRD), and nuclear magnetic resonance (NMR)) [14,16,38].
Detailed information about the characterization methods and hydrolysis reaction conditions are included in the Supplementary Information.
3. Results
3.1. Treatment Using ZnCl2·4H2O
Inorganic salt hydrates (ISHs) can be dissolved depending on the amount of water in their structure, the state of dissolution, and the variety of salts used [18,39]. The cellulose dissolution capacity of ISHs depends on the type of salt used, the water content in the preparation of the salt, the temperature, and the dissolution time, as already demonstrated in previous work [18]. The amount of water added revealed that the use of a ratio R = 4 is the optimum option for biomass dissolution [18,31]. Therefore, the use of different salt hydrates with different cations for the dissolution of pure cellulose was studied, and we established that ZnCl2·4H2O was an excellent solvent to study due to its chemical characteristics and low price [18]. ISHs are able to dissolve the structural carbohydrates of the lignocellulosic biomass, leaving the lignin undissolved as a solid [33].
The dissolution of cellulose has been studied in several previous reports, where cellulose (1.5g) was dissolved in ZnCl2·4H2O (28.5 g) at 70 °C in half an hour [10,35]. However, reports about the dissolution of whole biomass in salt hydrates are scarce; for this reason, we started with the study of pruning waste dissolution at different times: 30 min, 1 h, 3 h, 4.5 h, and 24 h at 70 °C. The use of short dissolution times (30 min, 1 h, 3 h, and 4.5 h) produces a very small biomass dissolution, with only a slight increase with time; in all cases, the recovery of Solid II was very small. However, after 24 h of dissolution at 70 °C, we detected an evident partial dissolution of the biomass (approximately 40 wt.%) with a significant recovery of Solid II. For this reason, we decided to continue the study using 24 h of dissolution time with different dissolution temperatures (60, 70, 80, and 90 °C).
The results obtained after dissolution and precipitation at different temperatures are shown in Table 2. The recovery data for solid I (mostly lignin) and solid II (mostly structural carbohydrates) are shown along with total solid recovery data. We calculated the degree of biomass recovered for each dissolution temperature. These data were obtained by the difference between the weight of the isolated solids recovered and the amount of the main components in each original biomass according to the NREL analyses shown in Table 1. The loss of biomass can be related to the dissolution and partial hydrolysis of more reactive carbohydrates, mainly hemicellulose, which remains dissolved in the Solid II mother liquor. The results obtained show the following: The dissolution of biomass is very small at 60 °C, with a high recovery of Solid I and a small recovery of Solid II. The recovery of Solid I is higher at 60 °C and decreases for higher dissolution temperatures, probably due to the dissolution and partial hydrolysis of soluble oligomers. An increase in temperature increases the dissolution of biomass, with similar values for 70 °C to 90 °C. In view of the results (Table 2), at high temperatures (90 °C) we detected a slight increase in Solid I recovery and total recovered biomass, possibly due to the occurrence of insoluble byproducts such as humins that remained undissolved and were retained in Solid I. At low temperatures (60 °C), a great separation is not achieved. Therefore, it was decided to use a temperature of 70 °C because it demonstrated the greatest separation of Solid I and Solid II.

Table 2.
Mass balance results of pruning waste after treatment with ISH between 60 and 90 °C after 24 h of reaction (solids dried at 105 °C).
A summary of the mass recovered is shown in Table 3, where a third solid (Solid III) is included. After cleaning Solid II with HCl and water, acetone was added assist in drying and we discovered that the mother liquors were colored. Therefore, they were taken to a rotary evaporator where a third solid was obtained, recovered, and characterized. The recovery was similar in all cases (between 60 and 70%) with respect to the cellulose, hemicellulose, and lignin composition of the starting material. It is observed that the amount of recovered biomass is inversely proportional to the amount of hemicellulose present in the original biomass. The amount of Solid I recovered was similar in all cases (between 35 and 50%) but was much higher than expected if the recovered Solid I contained only non-solubilized lignin. This was especially the case for barley straw, which is the biomass with the lowest lignin (Table 1). These data indicate that in addition to lignin, part of the structural carbohydrates remained insoluble in Solid I.

Table 3.
Mass balance results of pruning waste after treatment with ISH at 70 °C after 24 h of reaction (solids dried at 105 °C).
Characterization of solids. The objective of carrying out an exhaustive characterization of the solids obtained by this treatment was to study the composition, structure, and morphology of each solid and to observe the efficiency of the process. X-ray diffraction allows an analysis of the crystallinity of the materials obtained. The profiles of the original biomasses were compared with the respective solids obtained (Figure 2). The different peaks [16,40,41] are described in the XRD part of Characterization Section in the Supporting Information.
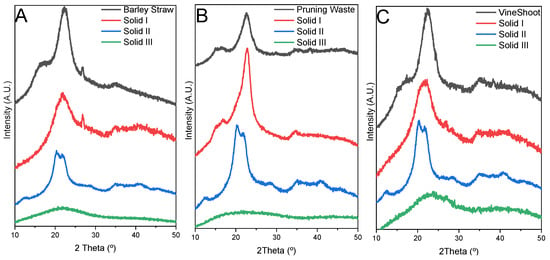
Figure 2.
XRD profiles of the original biomass, Solid I, Solid II, and Solid III. ((A) Barley straw; (B) pruning waste; (C) vine shoots).
In summary, in all three cases, morphological changes compatible with those observed in X-ray diffraction were observed. Samples of Solid I indicated a partial dissolution of the starting material but still contain part of the original structure; this structure seems more accessible for hydrolysis reagents. The structures of Solid II and Solid III are completely different from the structure of the original biomass [10,17] because the process of dissolution and recovery produces amorphous solids that favor greater accessibility to the components of the biomass, which is expected to result in the higher reactivity of this material.
FTIR spectroscopy was used to study the functional groups of the samples. The region of 3800–2600 cm−1 for the original biomass and the obtained solids from the dissolution/recovery after treatment is shown in Figure 3. The spectra showed an intense and broad band of OH groups and interactions with water molecules through hydrogen bridging bonds [42]. There are overlapping peaks between 3000 and 2800 cm−1 that can be assigned to the stretching vibrational modes of C-H bonds. The intensity of these peaks increased in Solid I and Solid III with respect to the spectra of the corresponding lignocellulosic biomass and Solid II, indicating a higher proportion of C-H bonds than in other samples. This fact could be attributed to an enrichment in lignin, the component that contains more C-H bonds. In contrast, Solid II showed a different intensity in all cases, indicating enrichment of cellulose and hemicellulose. For this reason, the comparison of both Solid I and Solid III with a commercial lignin reference and Solid II with commercial cellulose and hemicellulose references will be carried out.
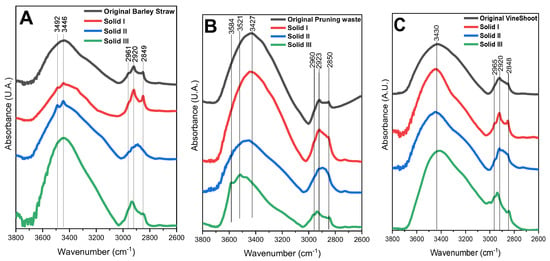
Figure 3.
FTIR spectra of Solid I, Solid II, and Solid III and their corresponding lignocellulosic biomasses ((A): Barley straw; (B): pruning waste; (C): vine shoots).
Figure 4 and Figure 5 show the spectra of the different solids obtained after treatment compared to the lignin, cellulose, and xylan references in the “fingerprint” region of lignocellulosic biomass, i.e., between 1800 and 800 cm−1. Figure 4 shows the spectrum of commercial alkaline lignin compared with Solid I (the supposedly lignin-enriched solid) and Solid III (obtained after washing Solid II with acetone).
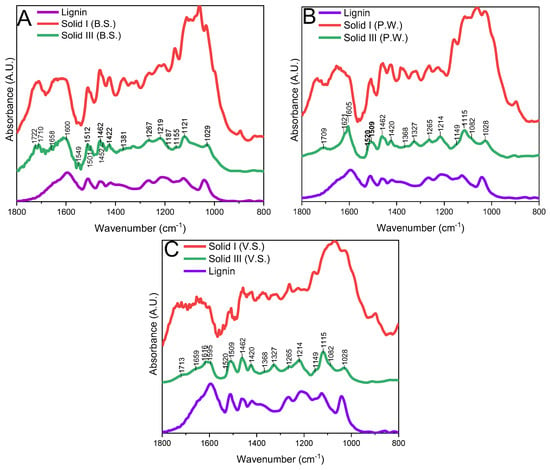
Figure 4.
FTIR spectra of Solid I, Solid II, Solid III, and lignin ((A) B.S.: barley straw; (B) P.W.: pruning waste; (C) V.S.: vine shoot).
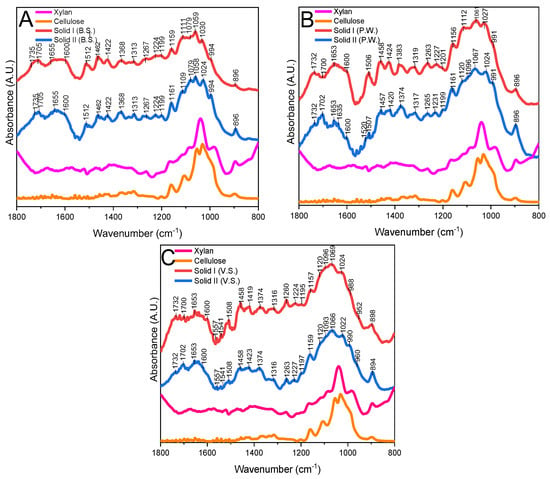
Figure 5.
FTIR spectra of Solid I, Solid II, xylan, and cellulose ((A) B.S.: barley straw; (B) P.W.: pruning waste; (C) V.S. vine shoot).
When comparing Solid I and Solid III with the alkaline commercial lignin, we can see that Solid I differs from the commercial lignin profile; however, Solid III has many similarities for all biomasses used. The most significant peaks found of lignin are shown in Figure 4 [43,44,45,46] and are described in the FTIR part of the Characterization Section in the Supporting Information. Solid I shows bands typical of lignin, but it also shows very intense bands between 900 and 1200 cm−1, attributed to polysaccharides from cellulose and hemicellulose, especially in barley straw (Figure 4), confirming the results obtained in the mass balance data (Table 3). However, no polysaccharide peaks are found in the spectrum of Solid III, but lignin bands are found for all three biomasses used, this indicates a selective separation of Solid III.
Figure 5 shows the spectrum of commercial xylans and cellulose compared with Solid I and Solid II, because Solid I is not pure lignin, as has been verified in Figure 4. In Figure 5, different peaks are assigned, these peaks are described [43,44,45,46] in the FTIR part of the Characterization Section in the Supporting Information.
In summary, Solid I and Solid II have a similar infrared spectrum profile, showing typical polysaccharide bands in all three biomass samples; however, in Solid I, we observed that the characteristic bands of lignin are intense and defined, and this observation in Solid II is not as evident.
A 1H NMR analysis was performed for all samples obtained after treatment and was compared with the reference materials [10] using DMSO-d6 solution. We describe the results for all the solids obtained with the different biomasses [10,47,48,49,50] after the treatment in NMR part of Characterization Section in the Supporting Information.
The compositions of Solids I, II, and III of the three biomass samples were determined using NREL standard methods [34]. The results confirmed the characterization data (Figure 4). Full fractionation was not achieved, but we observed partial fractionation of the biomass.
Solid I is a solid enriched in lignin (>39 wt.%) and cellulose (>45 wt.%). Solid II is highly enriched in cellulose (>80 wt.%) and Solid III is pure lignin. The amount of hemicellulose is very small in all samples, probably due to the slight acidic character of the ZnCl2·4H2O liquid that hydrolyzes the hemicellulose and leaves it dissolved in the reaction medium. In summary, we showed a method for obtaining a cellulose-rich solid with a very low amount of hemicellulose and lignin (Solid II) and mainly pure lignin (Solid III), as shown by the characterization results.
Based on the composition analysis (Table 4) and the solids recovered from the three biomass materials (Table 3), the amount of cellulose, lignin, and hemicellulose recovered can be calculated. For all three biomasses studied, almost 100% of cellulose and lignin were recovered, but the recovery of hemicellulose was very low (from 6% for the pruning waste to 1.5% for the barley straw), because hemicellulose was dissolved/hydrolyzed in the treatment of the biomass remaining in the liquid phase.

Table 4.
Composition analysis (wt.%) of recovered solids. Samples dried at 105 °C.
3.2. Catalytic Actvity
The obtained solids were subjected to a hydrolysis procedure in aqueous media at a temperature of 140 °C using H2SO4 (0.2M/L) as a catalyst, in order to evaluate their reactivity after the treatment. Solid III, which actually was lignin, did not form any sugar; in contrast, Solid I and Solid II formed sugar. The hydrolyses of the original biomass sources were measured as a reference.
The conversion results of Solids I and II and the untreated biomass (Table 5) were different among the samples studied. The conversion data were obtained by measuring the difference in dry weight between the initial sample and the one obtained after the hydrolysis reaction using the following formula:

Table 5.
Results of hydrolysis conversion for original biomass and fractioned biomass (Solid I and Solid II). The reaction conditions were 140 °C and 5 h, and H2SO4 (0.2 mol/L) was used as a catalyst.
The lowest conversion values were for the original biomass and the highest were for the fractionated samples. The conversion of Solid II, higher than 90% in all three cases, demonstrated that is was the most reactive among the samples.
Due to side reactions of cellulose and hemicellulose, giving rise to by-products and humins, the conversion data obtained are inconclusive. Therefore, levulinic acid (a by-product of cellulose hydrolysis) and furfural (a by-product of hemicellulose hydrolysis) were also analyzed. The conversion of glucose to HMF and the subsequent formation of levulinic acid and formic acid was so rapid that no HMF was detected in the samples.
The yield of glucose and levulinic acid for the original biomass and Solid I and Solid II obtained after the treatment are compiled in Figure 6, Figure 7 and Figure 8. In all cases, the yield of glucose was relatively low for the original biomasses studied. The yield of glucose was approximately 20–30% after 5 h of reaction depending on the biomass sample. The fractionated samples Solid I and Solid II clearly presented higher glucose yields. The increase in the glucose yield can be attributed to the change in the biomass structure in the fractionated samples, with a loss of crystallinity with respect to the original biomass, as shown in the XRD studies, and a more porous structure, confirmed by SEM, which implies higher accessibility to the ß(1→4)-d-glucosidic bonds and therefore favors a hydrolysis reaction, in agreement with previous works [18,38]. The yield of levulinic acid was similar to that of glucose (Figure 6B, Figure 7B and Figure 8B), and since it is a reaction by-product, the higher the concentration of glucose, the higher the concentration of levulinic acid. However, the yield was always lower than glucose.
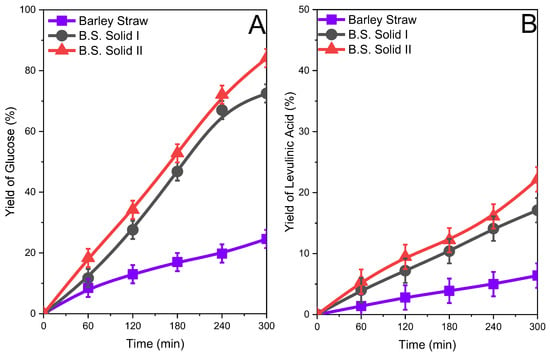
Figure 6.
Glucose (A) and levulinic acid (B) yields after the hydrolysis of the original barley straw and barley straw solids obtained (Solid I and Solid II) after treatment with ISH.
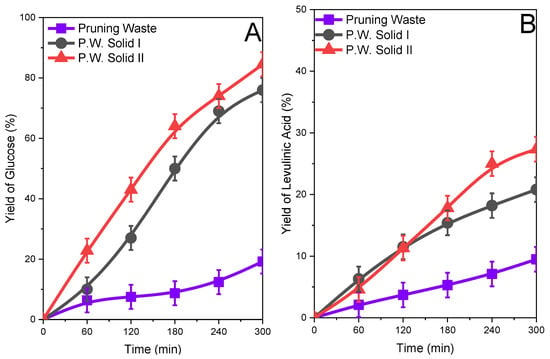
Figure 7.
Glucose (A) and levulinic acid (B) yields after the hydrolysis of the original pruning waste and pruning waste solids obtained (Solid I and Solid II) after treatment with ISH.
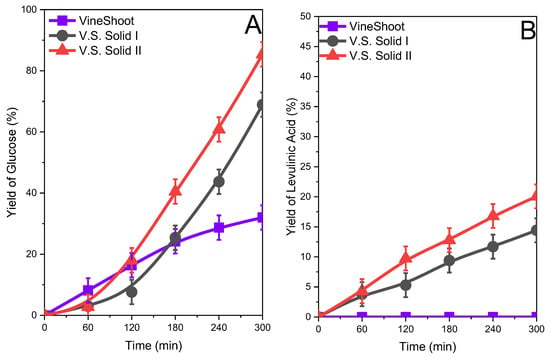
Figure 8.
Glucose (A) and levulinic acid (B) yields after the hydrolysis of the original vine shoots and vine shoot solids obtained (Solid I and Solid II) after treatment with ISH.
Glucose production depended on the cellulose contents in the solid used in the reaction, and similar yields from solids with different starting cellulose contents implied that there were different glucose concentrations in the reaction media (see Figures S7, S9, and S11 in the Supplementary Information). The amount of glucose produced from Solid II was higher than that obtained from other samples.
In summary, in all studied biomasses, an improvement in the reactivity in hydrolysis reactions was observed by obtaining glucose and levulinic acid when treated with the inorganic salt hydrate. Solid II exhibits the highest yield of glucose, and therefore levulinic acid, because it has a higher content of structural carbohydrates compared to Solid I and the starting biomass.
The main proportion of hemicellulose was dissolved in the pretreatment and remains in the liquid, as depicted previously. However, a very small amount of hemicellulose is present in the solids, so we have detected some products from hemicellulose hydrolysis such as xylose and furfural. In all cases, the amount was very small, with similar profiles (Figures S8, S10, and S12). The xylose profile shows that all samples reach a maximum and then decrease for longer reaction times. This behavior is typical of intermediate behavior in consecutive reactions. Furfural is obtained after dehydration of xylose upon contact with the acid catalyst in the medium. The furfural concentration profiles increase with increasing reaction time. The increase in the reactivity of the treated samples is very small with respect to the original samples, because the main part of the hemicellulose is dissolved in the treatment and remains unreacted.
In addition to glucose and xylose, in the hydrolysis process, oligomers with soluble units are obtained due to the breaking of the polymer chains of cellulose and hemicellulose into smaller fragments. For this reason, the final reaction liquid was analyzed (after 5 h of reaction) and the content of total reducing sugars (TRS) was calculated. The obtained results are shown in Table 6, where Solid I and Solid II are compared with their corresponding untreated biomasses. The TRS values are higher than the TRS values obtained after the sum of monomeric sugars provided by the HPLC analysis, showing that there were some oligomers in the reaction medium. This result disagrees with the data from the untreated biomass samples in all cases.

Table 6.
Results of TRS measurements for the samples obtained after the acid hydrolysis reaction.
The TRS is greater when the sample has been treated because the chains are more accessible after treatment and it is easier for the acid catalyst to act on them. The yield of sugars, which has been calculated considering the TRS obtained and the maximum total monomeric sugars that can be obtained according to the composition of the corresponding sample, decreases according to the following order: Solid II (rich in structural carbohydrates > Solid I (rich in lignin but with traces of structural carbohydrates) > original biomass, which coincides with the conversion table shown above (Table 5). The selectivity of reducing sugars is higher in Solid II (between 78 and 88% depending on the biomass), which has a greater amount of structural carbohydrates than Solid I and is consistent with data obtained in previous studies with another ionic liquid treatment [10], which were the highest described in the literature [51,52,53,54,55,56,57].
4. Conclusions
Using inorganic salt hydrate and water as an antisolvent for dissolving biomass can be used to fragment and obtain cellulose, hemicellulose, and lignin. Carrying out dissolution with ZnCl2·4H2O for 24 h at 70 °C is the appropriate time and temperature to obtain a favorable separation. In this procedure, we obtained three solids: (a) a first solid obtained from direct filtration after the dissolution treatment (Solid I); (b) a solid obtained after precipitation with the antisolvent (water) (Solid II); and (c) a solid obtained by washing Solid II with acetone and evaporating the acetone (Solid III).
Characterization by different techniques (FTIR, XRD, SEM, and NMR) and analysis by NREL show that Solid I, the undissolved part of the starting material, is a solid rich in lignin and undissolved cellulose with changes in its structure and its morphology with respect to the original biomass. Solid II is a solid rich in cellulose (<80% in all cases) with a low presence of lignin and hemicellulose, where cellulose type II (FTIR) is present. Finally, Solid III is pure lignin, as shown by the complete loss of crystallinity and morphology in XRD and SEM and the great similarity with the original lignin in FTIR or NMR. An HPLC analysis showed that hemicellulose is mainly dissolved and hydrolyzed in the dissolution treatment, and the amount present in all solids is very small.
An acid hydrolysis carried out on Solid I and Solid II demonstrated the effectiveness of the treatment with the inorganic salt hydrate with respect to the original biomass. In all cases, the conversion and glucose yield decreased in the order Solid II > Solid I > untreated biomass. Solid II presented the best results due to the higher concentration of structural carbohydrates; barley straw had a conversion of 91% and a glucose yield of 83%, the pruning waste had a conversion of 94.6% and a glucose yield of 84%, and finally, the vine shoots had a conversion of 95.4% and a glucose yield of 84%. These results are similar, and show an improvement on in all cases, to the data obtained for the solid obtained after treated biomass with ionic liquid [10] with a conversion of 93% and a glucose yield of 52%.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app13052953/s1, Detailed Experimental Methods, Characterization Section, and Figures S1–S12.
Author Contributions
Experimental work: M.L.-S. and D.M.S.; supervision: S.M.-d. and J.M.C.-M.; writing—original draft preparation: M.L.-S. and S.M.-d.; writing—review and editing: J.M.C.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Comunidad de Madrid (Spain) and the ERDF (European Regional Development Fund), grant number S2018/EMT-4344 (BIOTRES-CM), and by MCIN/AEI/10.13039/501100011033 and by “ERDF A way of making Europe” Grant PID2020-112594RB-C33.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.
Acknowledgments
M.L.-S. and D.M.S., due to the CSIC-UAM framework agreement, thank Ph.D. Program in Applied Chemistry, Doctoral School of Autonomous University of Madrid.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Preethi; Gunasekaran, M.; Kumar, G.; Karthikeyan, O.P.; Varjani, S.; Rajesh Banu, J. Lignocellulosic Biomass as an Optimistic Feedstock for the Production of Biofuels as Valuable Energy Source: Techno-Economic Analysis, Environmental Impact Analysis, Breakthrough and Perspectives. Environ. Technol. Innov. 2021, 24, 102080. [Google Scholar] [CrossRef]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent Trends in the Pretreatment of Lignocellulosic Biomass for Value-Added Products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Saravanan, A.; Senthil Kumar, P.; Jeevanantham, S.; Karishma, S.; Vo, D.V.N. Recent Advances and Sustainable Development of Biofuels Production from Lignocellulosic Biomass. Bioresour. Technol. 2022, 344, 126203. [Google Scholar] [CrossRef]
- Manzanares, P. The Role of Biorefinering Research in the Development of a Modern Bioeconomy. Acta Innov. 2020, 37, 47–56. [Google Scholar] [CrossRef]
- Morone, P.; Yilan, G. A Paradigm Shift in Sustainability: From Lines to Circles. Acta Innov. 2020, 36, 5–16. [Google Scholar] [CrossRef]
- Amjith, L.R.; Bavanish, B. A Review on Biomass and Wind as Renewable Energy for Sustainable Environment. Chemosphere 2022, 293, 133579. [Google Scholar] [CrossRef]
- Rahman, A.; Farrok, O.; Haque, M.M. Environmental Impact of Renewable Energy Source Based Electrical Power Plants: Solar, Wind, Hydroelectric, Biomass, Geothermal, Tidal, Ocean, and Osmotic. Renew. Sustain. Energy Rev. 2022, 161, 112279. [Google Scholar] [CrossRef]
- Davis, R.; Bhatt, A.H.; Zhang, Y.; Tan, E.C.D.; Ravi, V.; Heath, G. Biorefinery Upgrading of Herbaceous Biomass to Renewable Hydrocarbon Fuels, Part 1: Process Modeling and Mass Balance Analysis. J. Clean. Prod. 2022, 362, 132439. [Google Scholar] [CrossRef]
- Velvizhi, G.; Balakumar, K.; Shetti, N.P.; Ahmad, E.; Kishore Pant, K.; Aminabhavi, T.M. Integrated Biorefinery Processes for Conversion of Lignocellulosic Biomass to Value Added Materials: Paving a Path towards Circular Economy. Bioresour. Technol. 2022, 343, 126151. [Google Scholar] [CrossRef]
- Lara-Serrano, M.; Morales-delaRosa, S.; Campos-Martín, J.M.; Fierro, J.L.G. Fractionation of Lignocellulosic Biomass by Selective Precipitation from Ionic Liquid Dissolution. Appl. Sci. 2019, 9, 1862. [Google Scholar] [CrossRef]
- Galbe, M.; Wallberg, O. Pretreatment for Biorefineries: A Review of Common Methods for Efficient Utilisation of Lignocellulosic Materials. Biotechnol. Biofuels 2019, 12, 294. [Google Scholar] [CrossRef]
- Zhang, M.; Bobokalonov, J.; Dzhonmurodov, A.; Xiang, Z. Optimizing Yield and Chemical Compositions of Dimethylsulfoxide-Extracted Birchwood Xylan. J. Bioresour. Bioprod. 2022, 7, 211–219. [Google Scholar] [CrossRef]
- Linan, L.Z.; Cidreira, A.C.M.; da Rocha, C.Q.; de Menezes, F.F.; Rocha, G.J.d.M.; Paiva, A.E.M. Utilization of Acai Berry Residual Biomass for Extraction of Lignocellulosic Byproducts. J. Bioresour. Bioprod. 2021, 6, 323–337. [Google Scholar] [CrossRef]
- Morales-delaRosa, S.; Campos-Martin, J.M.; Fierro, J.L.G. Chemical Hydrolysis of Cellulose into Fermentable Sugars through Ionic Liquids and Antisolvent Pretreatments Using Heterogeneous Catalysts. Catal. Today 2018, 302, 87–93. [Google Scholar] [CrossRef]
- Morales-de la Rosa, S.; García Fierro, J.L.; Campos Martín, J.M. Procedimiento de Hidrólisis de Biomasa Lignocelulósica. WIPO Patent WO2015004296, 15 January 2015. [Google Scholar]
- Morales-delaRosa, S.; Campos-Martin, J.M.; Fierro, J.L.G. Optimization of the Process of Chemical Hydrolysis of Cellulose to Glucose. Cellulose 2014, 21, 2397–2407. [Google Scholar] [CrossRef]
- Lara-Serrano, M.; Sáez Angulo, F.; Negro, M.J.; Morales-Delarosa, S.; Campos-Martin, J.M.; Fierro, J.L.G. Second-Generation Bioethanol Production Combining Simultaneous Fermentation and Saccharification of IL-Pretreated Barley Straw. ACS Sustain. Chem. Eng. 2018, 6, 7086–7095. [Google Scholar] [CrossRef]
- Lara-Serrano, M.; Fierro, J.L.G.; Morales-Delarosa, S.; Campos-Martín, J.M. High Enhancement of the Hydrolysis Rate of Cellulose after Pretreatment with Inorganic Salt Hydrates. Green Chem. 2020, 22, 3860–3866. [Google Scholar] [CrossRef]
- Padrino, B.; Lara-Serrano, M.; Morales-delaRosa, S.; Campos-Martín, J.M.; García Fierro, J.L.; Martínez, F.; Melero, J.A.; Puyol, D. Resource Recovery Potential from Lignocellulosic Feedstock upon Lysis with Ionic Liquids. Front. Bioeng. Biotechnol. 2018, 6, 119. [Google Scholar] [CrossRef]
- Brandt-Talbot, A.; Gschwend, F.J.V.; Fennell, P.S.; Lammens, T.M.; Tan, B.; Weale, J.; Hallett, J.P. An Economically Viable Ionic Liquid for the Fractionation of Lignocellulosic Biomass. Green Chem. 2017, 19, 3078–3102. [Google Scholar] [CrossRef]
- Rodríguez, H. Ionic Liquids in the Pretreatment of Lignocellulosic Biomass. Acta Innov. 2021, 38, 23–36. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Gupta, P.; Karpichev, Y.; Gathergood, N.; Bhat, R.; Gupta, V.K. Ionic Liquid Based Pretreatment of Lignocellulosic Biomass for Enhanced Bioconversion. Bioresour. Technol. 2020, 304, 123003. [Google Scholar] [CrossRef]
- Varanasi, P.; Singh, P.; Auer, M.; Adams, P.D.; Simmons, B.A.; Singh, S. Survey of Renewable Chemicals Produced from Lignocellulosic Biomass during Ionic Liquid Pretreatment. Biotechnol. Biofuels 2013, 6, 14. [Google Scholar] [CrossRef]
- Gschwend, F.J.V.; Brandt, A.; Chambon, C.L.; Tu, W.C.; Weigand, L.; Hallett, J.P. Pretreatment of Lignocellulosic Biomass with Low-Cost Lonic Liquids. J. Vis. Exp. 2016, 2016, e54246. [Google Scholar] [CrossRef]
- Morais, A.R.C.; Pinto, J.V.; Nunes, D.; Roseiro, L.B.; Oliveira, M.C.; Fortunato, E.; Bogel-Łukasik, R. Imidazole: Prospect Solvent for Lignocellulosic Biomass Fractionation and Delignification. ACS Sustain. Chem. Eng. 2016, 4, 1643–1652. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Zetzl, C.; Gairola, K.; Kirsch, C.; Perez-Cantu, L.; Smirnova, I. High Pressure Processes in Biorefineries. Chem.-Ing.-Tech. 2011, 83, 1016–1025. [Google Scholar] [CrossRef]
- Morais, A.R.C.; Da Costa Lopes, A.M.; Bogel-Łukasik, R. Carbon Dioxide in Biomass Processing: Contributions to the Green Biorefinery Concept. Chem. Rev. 2015, 115, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Farma, R.; Julita, R.I.; Apriyani, I.; Awitdrus, A.; Taer, E. ZnCl2-Assisted Synthesis of Coffee Bean Bagasse-Based Activated Carbon as a Stable Material for High-Performance Supercapacitors. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Jiang, H.; Chu, Q.; Ma, J.; Wu, S.; Shao, L.; Zhou, X. Dissolution of Lignocellulose with High Lignin Content in AlCl3/ZnCl2 Aqueous System and Properties of the Regenerated Cellulose Film. Int. J. Biol. Macromol. 2023, 234, 123590. [Google Scholar] [CrossRef]
- Awosusi, A.A.; Ayeni, A.; Adeleke, R.; Daramola, M.O. Effect of Water of Crystallization on the Dissolution Efficiency of Molten Zinc Chloride Hydrate Salts during the Pre-Treatment of Corncob Biomass. J. Chem. Technol. Biotechnol. 2017, 92, 2468–2476. [Google Scholar] [CrossRef]
- Duan, G.; Zhao, L.; Chen, L.; Wang, F.; He, S.; Jiang, S.; Zhang, Q. ZnCl2 Regulated Flax-Based Porous Carbon Fibers for Supercapacitors with Good Cycling Stability. N. J. Chem. 2021, 45, 22602–22609. [Google Scholar] [CrossRef]
- Bi, Z.; Lai, B.; Zhao, Y.; Yan, L. Fast Disassembly of Lignocellulosic Biomass to Lignin and Sugars by Molten Salt Hydrate at Low Temperature for Overall Biorefinery. ACS Omega 2018, 3, 2984–2993. [Google Scholar] [CrossRef]
- Sluiter, J.B.; Ruiz, R.O.; Scarlata, C.J.; Sluiter, A.D.; Templeton, D.W. Compositional Analysis of Lignocellulosic Feedstocks. 1. Review and Description of Methods. J. Agric. Food Chem. 2010, 58, 9043–9053. [Google Scholar] [CrossRef]
- Lindman, B.; Karlström, G.; Stigsson, L. On the Mechanism of Dissolution of Cellulose. J. Mol. Liq. 2010, 156, 76–81. [Google Scholar] [CrossRef]
- Sen, S.; Losey, B.P.; Gordon, E.E.; Argyropoulos, D.S.; Martin, J.D. Ionic Liquid Character of Zinc Chloride Hydrates Define Solvent Characteristics That Afford the Solubility of Cellulose. J. Phys. Chem. B 2016, 120, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Lavarda, G.; Morales-Delarosa, S.; Centomo, P.; Campos-Martin, J.M.; Zecca, M.; Fierro, J.L.G. Gel-Type and Macroporous Cross-Linked Copolymers Functionalized with Acid Groups for the Hydrolysis of Wheat Straw Pretreated with an Ionic Liquid. Catalysts 2019, 9, 675. [Google Scholar] [CrossRef]
- Morales-delaRosa, S.; Campos-Martin, J.M.; Fierro, J.L.G. Complete Chemical Hydrolysis of Cellulose into Fermentable Sugars through Ionic Liquids and Antisolvent Pretreatments. ChemSusChem 2014, 7, 3467–3475. [Google Scholar] [CrossRef]
- Cao, Y.; Li, H.; Zhang, Y.; Zhang, J.; He, J. Structure and Properties of Novel Regenerated Cellulose Films Prepared from Cornhusk Cellulose in Room Temperature Ionic Liquids. J. Appl. Polym. Sci. 2010, 116, 547–554. [Google Scholar] [CrossRef]
- French, A.D.; Santiago Cintrón, M. Cellulose Polymorphy, Crystallite Size, and the Segal Crystallinity Index. Cellulose 2013, 20, 583–588. [Google Scholar] [CrossRef]
- French, A.D. Idealized Powder Diffraction Patterns for Cellulose Polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Capel-Sanchez, M.C.; Barrio, L.; Campos-Martin, J.M.; Fierro, J.L.G. Silylation and Surface Properties of Chemically Grafted Hydrophobic Silica. J. Colloid Interface Sci. 2004, 277, 146–153. [Google Scholar] [CrossRef]
- Pandey, K.K.; Pitman, A.J. FTIR Studies of the Changes in Wood Chemistry Following Decay by Brown-Rot and White-Rot Fungi. Int. Biodeterior. Biodegrad. 2003, 52, 151–160. [Google Scholar] [CrossRef]
- Hergert, H.L. Infrared Spectra of Lignin and Related Compounds. II. Conifer Lignin and Model Compounds1,2. J. Org. Chem. 1960, 25, 405–413. [Google Scholar] [CrossRef]
- Sun, R.; Hughes, S. Fractional Extraction and Physico-Chemical Characterization of Hemicelluloses and Cellulose from Sugar Beet Pulp. Carbohydr. Polym. 1998, 36, 293–299. [Google Scholar] [CrossRef]
- Sun, X.F.; Xu, F.; Sun, R.C.; Geng, Z.C.; Fowler, P.; Baird, M.S. Characteristics of Degraded Hemicellulosic Polymers Obtained from Steam Exploded Wheat Straw. Carbohydr. Polym. 2005, 60, 15–26. [Google Scholar] [CrossRef]
- Toledano, A.; Serrano, L.; Garcia, A.; Mondragon, I.; Labidi, J. Comparative Study of Lignin Fractionation by Ultrafiltration and Selective Precipitation. Chem. Eng. J. 2010, 157, 93–99. [Google Scholar] [CrossRef]
- Korbag, I.; Mohamed Saleh, S. Studies on the Formation of Intermolecular Interactions and Structural Characterization of Polyvinyl Alcohol/Lignin Film. Int. J. Environ. Stud. 2016, 73, 226–235. [Google Scholar] [CrossRef]
- Kakuchi, R.; Yamaguchi, M.; Endo, T.; Shibata, Y.; Ninomiya, K.; Ikai, T.; Maeda, K.; Takahashi, K. Correction: Efficient and Rapid Direct Transesterification Reactions of Cellulose with Isopropenyl Acetate in Ionic Liquids. RSC Adv. 2017, 7, 14321. [Google Scholar] [CrossRef]
- Wan Daud, W.R.; Djuned, F.M. Cellulose Acetate from Oil Palm Empty Fruit Bunch via a One Step Heterogeneous Acetylation. Carbohydr. Polym. 2015, 132, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Vanoye, L.; Fanselow, M.; Holbrey, J.D.; Atkins, M.P.; Seddon, K.R. Kinetic Model for the Hydrolysis of Lignocellulosic Biomass in the Ionic Liquid, 1-Ethyl-3-Methyl-Imidazolium Chloride. Green Chem. 2009, 11, 390–396. [Google Scholar] [CrossRef]
- Schneider, L.; Haverinen, J.; Jaakkola, M.; Lassi, U. Solid Acid-Catalyzed Depolymerization of Barley Straw Driven by Ball Milling. Bioresour. Technol. 2016, 206, 204–210. [Google Scholar] [CrossRef]
- Bai, C.; Zhu, L.; Shen, F.; Qi, X. Black Liquor-Derived Carbonaceous Solid Acid Catalyst for the Hydrolysis of Pretreated Rice Straw in Ionic Liquid. Bioresour. Technol. 2016, 220, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Du, H.; Qian, X.; Chen, E.Y.X. Ionic Liquid-Water Mixtures: Enhanced Kw for Efficient Cellulosic Biomass Conversion. Energy Fuels 2010, 24, 2410–2417. [Google Scholar] [CrossRef]
- Si, W.; Li, Y.; Zheng, J.; Wei, S.; Wang, D. Enhanced Hydrolysis of Bamboo Biomass by Chitosan Based Solid Acid Catalyst with Surfactant Addition in Ionic Liquid. Carbohydr. Polym. 2017, 174, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Kassaye, S.; Pant, K.K.; Jain, S. Hydrolysis of Cellulosic Bamboo Biomass into Reducing Sugars via a Combined Alkaline Solution and Ionic Liquid Pretreament Steps. Renew. Energy 2017, 104, 177–184. [Google Scholar] [CrossRef]
- Liang, J.; Chen, X.; Wang, L.; Wei, X.; Wang, H.; Lu, S.; Li, Y. Subcritical Carbon Dioxide-Water Hydrolysis of Sugarcane Bagasse Pith for Reducing Sugars Production. Bioresour. Technol. 2017, 228, 147–155. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).