Comparative Study on the Total Phenolics, Total Flavonoids, and Biological Activities of Papaver rhoeas L. Extracts from Different Geographical Regions of Morocco
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of Extracts and Extraction Yield
2.3. Determination of Phenolic, Flavonoid, and Anthocyanin Content
2.4. Antioxidant Activity of P. rhoeas Extracts
2.4.1. DPPH Free Radical Scavenging Test
2.4.2. Total Antioxidant Capacity Test (TAC)
2.5. Antimicrobial Activity of P. rhoeas Extracts
2.5.1. Disc Diffusion Technique
2.5.2. Determination of the Minimum Inhibitory Concentration (MIC)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Extract Yields
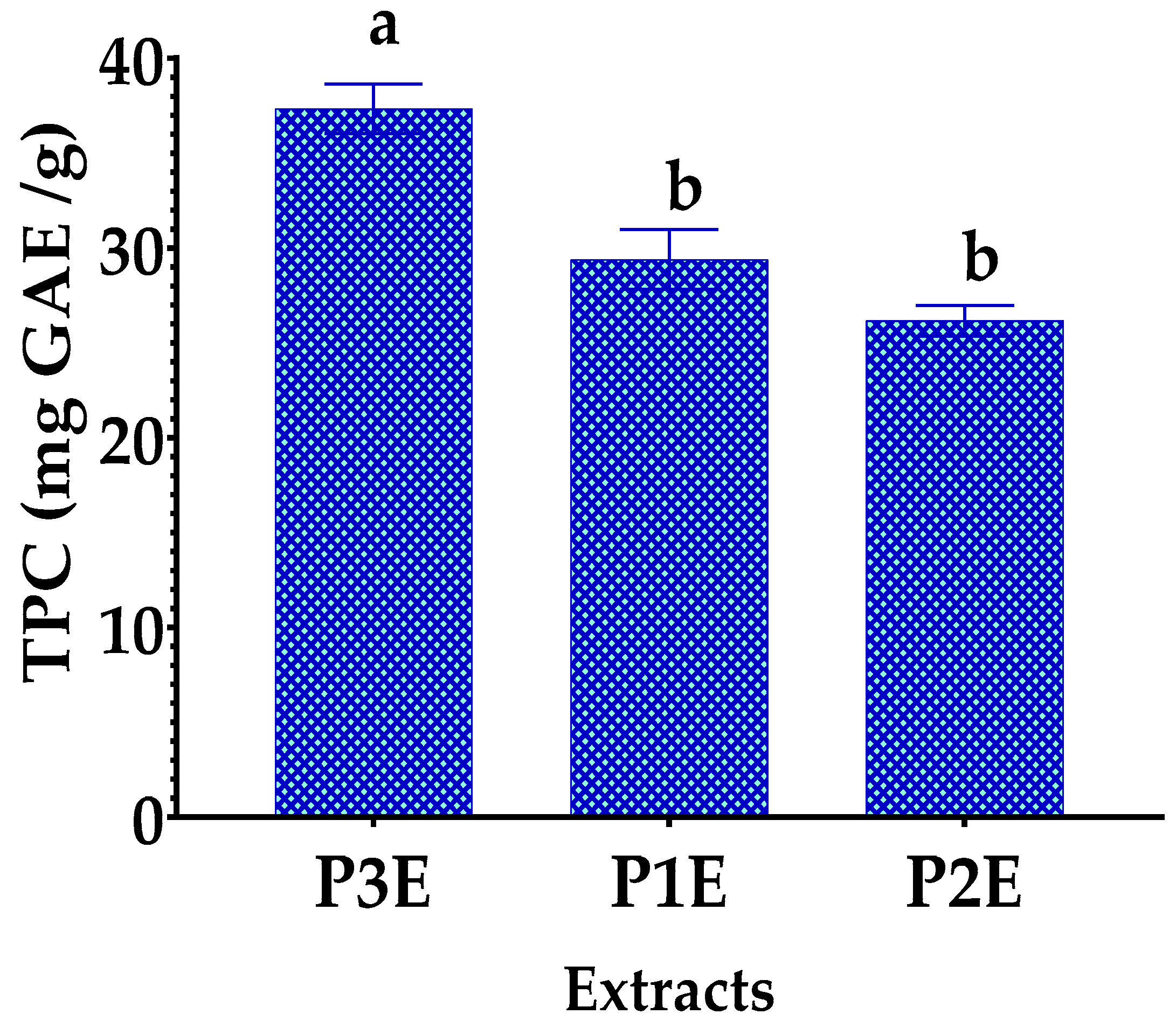
3.2. Determination of Total Polyphenol Content (TPC)
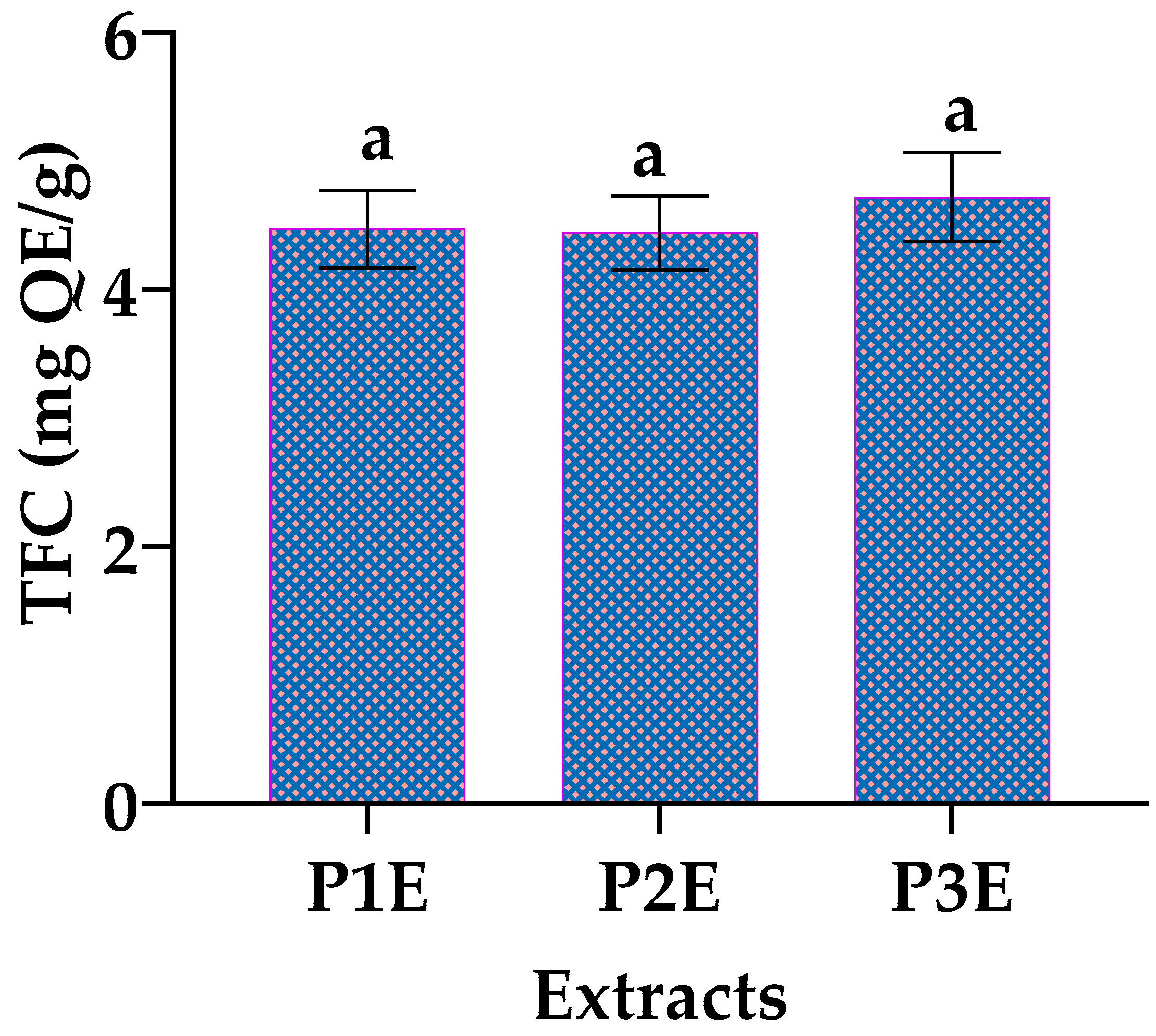
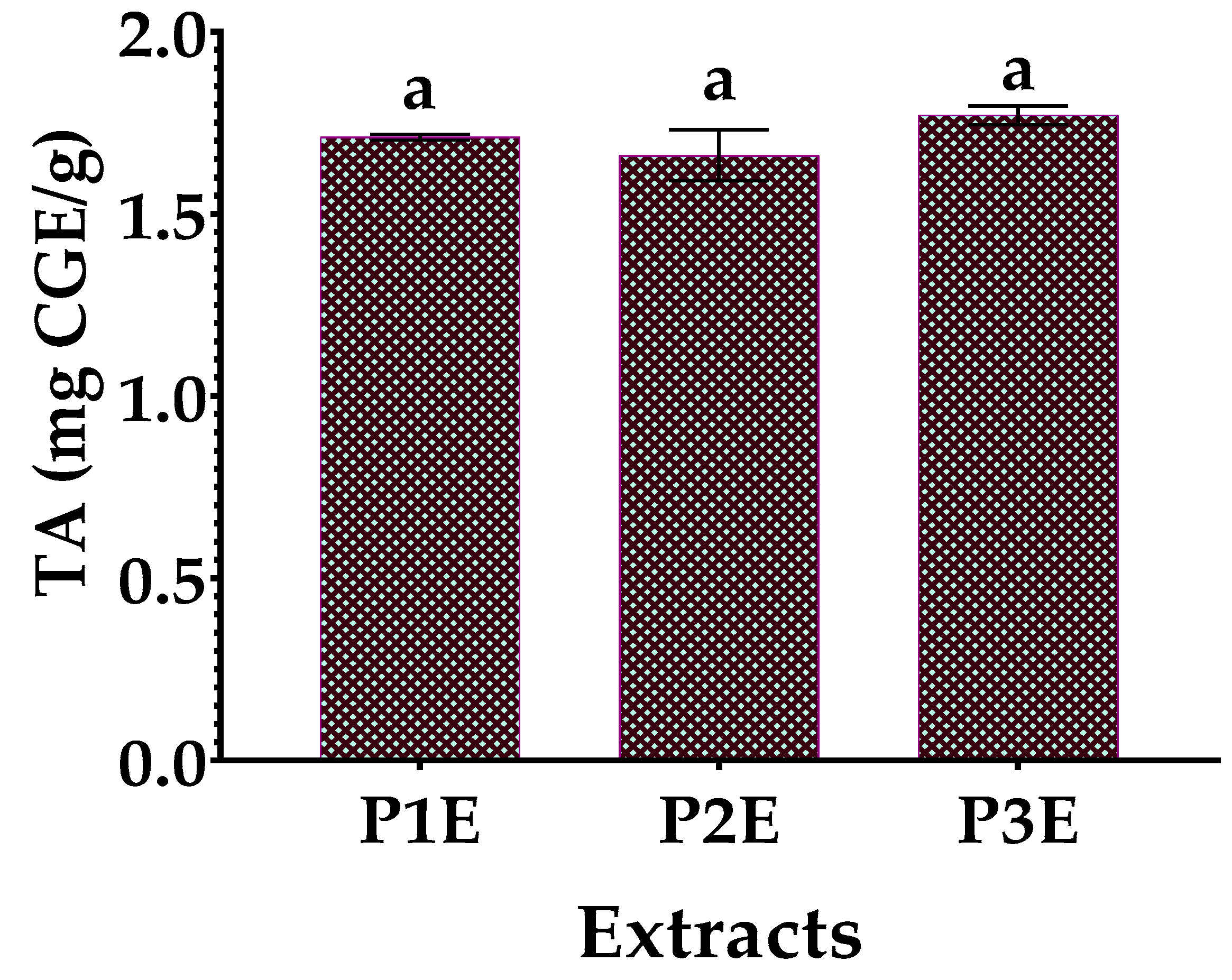
3.3. Determination of Total Flavonoid (TFC) and Total Anthocyanin Content (TA)
3.4. Antioxidant Activity
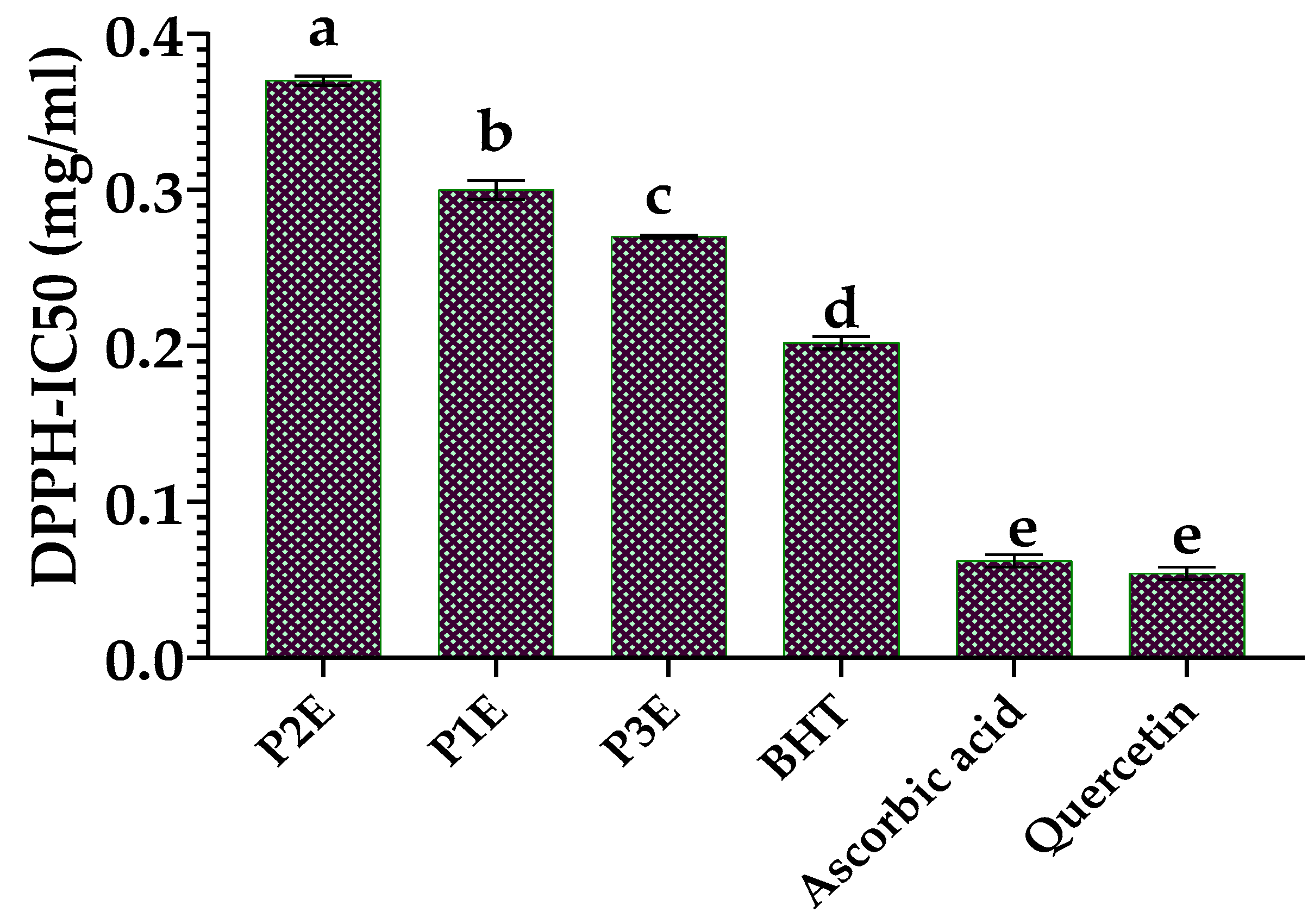
3.4.1. Scavenging of the Free Radical DPPH
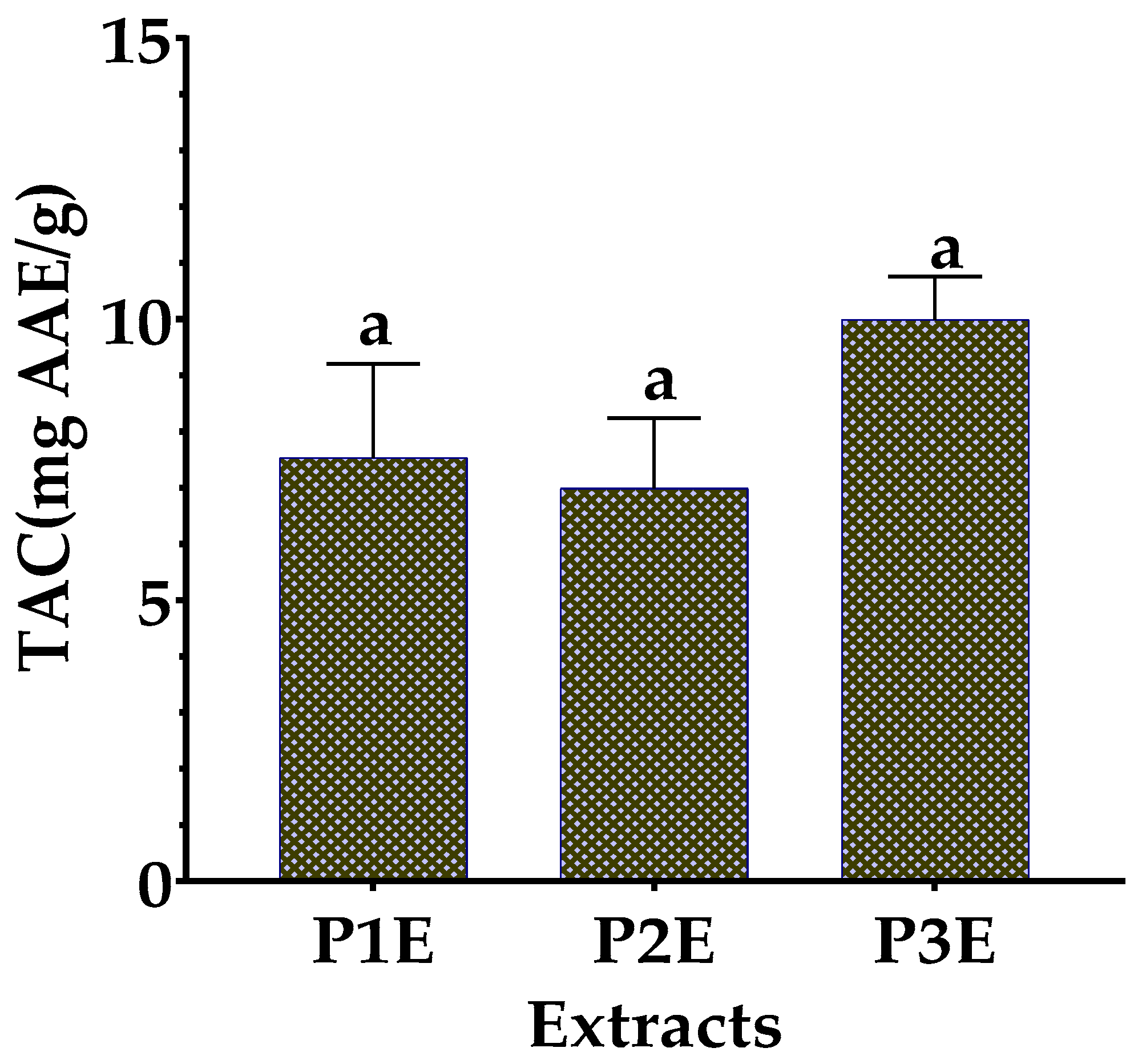
3.4.2. Total Antioxidant Capacity
3.5. Antimicrobial Activity
3.5.1. Disc Diffusion Test
3.5.2. Determination of the Minimum Inhibitory Concentration (MIC) of P. rhoeas Extracts
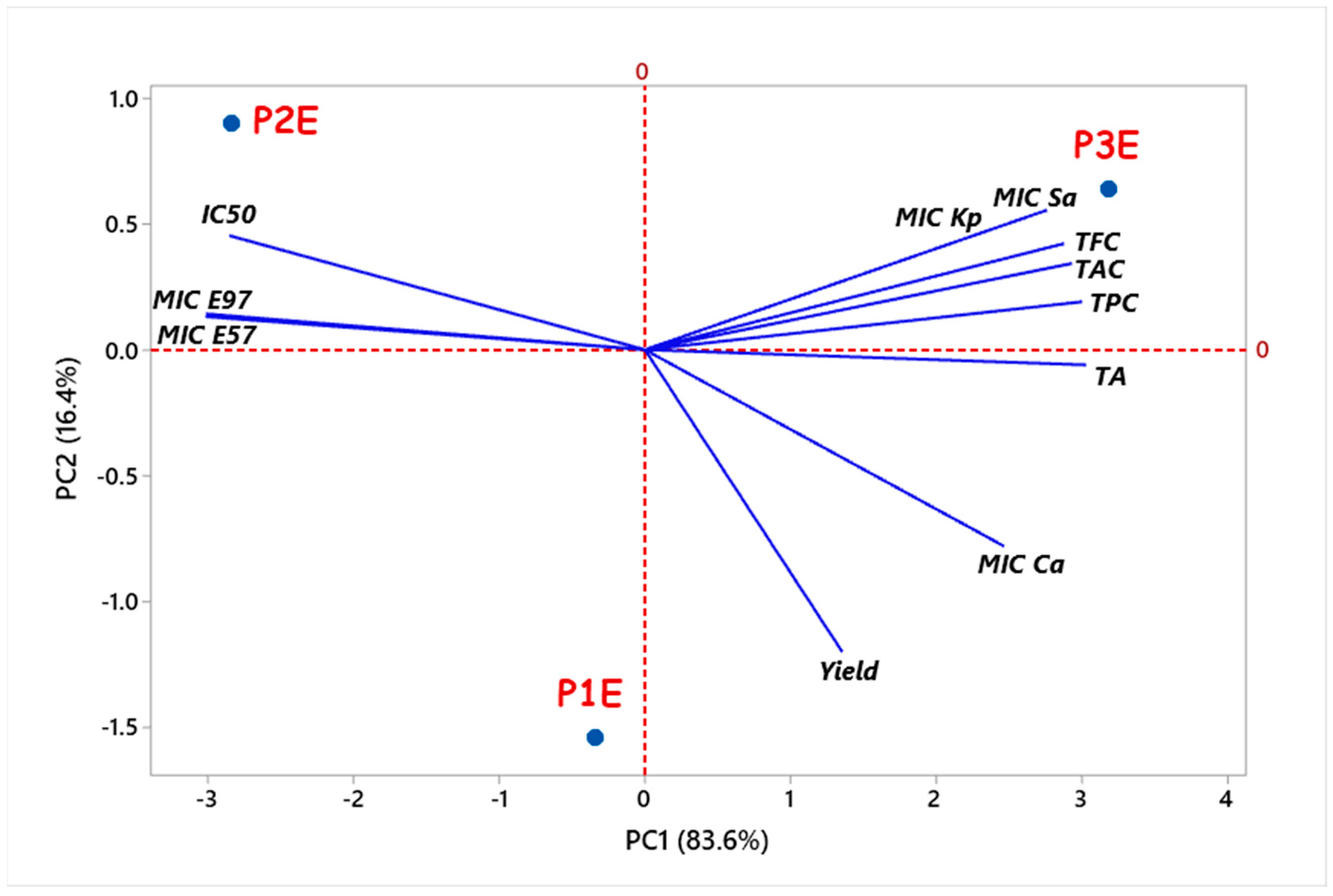
3.6. Relationships between Studied Parameters of P. rhoeas Extracts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Assri, E.; Barnossi, A.E.; Chebaibi, M.; Hmamou, A.; Asmi, H.E.; Bouia, A.; Eloutassi, N. Ethnobotanical Survey of Medicinal and Aromatic Plants in Taounate, Pre-Rif of Morocco. Ethnobot. Res. Appl. 2021, 22, 1–23. [Google Scholar]
- Khomsi, M.E.; Imtara, H.; Kara, M.; Hmamou, A.; Assouguem, A.; Bourkhiss, B.; Tarayrah, M.; AlZain, M.N.; Alzamel, N.M.; Noman, O.; et al. Antimicrobial and Antioxidant Properties of Total Polyphenols of Anchusa Italica Retz. Molecules 2022, 27, 416. [Google Scholar] [CrossRef]
- Tlemcani, S.; Lahkimi, A.; Eloutassi, N.; Bendaoud, A.; Hmamou, A.; Bekkari, H. Ethnobotanical Study of Medicinal Plants in the Fez-Meknes Region of Morocco. J. Pharm. Pharm. Res. 2023, 11, 137–159. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A Historical Overview of Natural Products in Drug Discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- El-Assri, E.-M.; Eloutassi, N.; Azeddin, E.B.; Bakkari, F.; Hmamou, A.; Bouia, A. Wild Chamomile (Matricaria recutita L) from the Taounate Province, Morocco: Extraction and Valorisation of the Antibacterial Activity of Its Essential Oils. TJNPR 2021, 5, 883–888. [Google Scholar] [CrossRef]
- Lahkimi, A.; Nechad, I.; Chaouch, M.; Eloutassi, N. Antibacterial, Antifungal and Antioxidant Activity of Lavandula Angustifolia of the Middle Atlas Central (Morocco). Moroc. J. Chem. 2020, 8, 905–918. [Google Scholar]
- Mushtaq, S.; Abbasi, B.H.; Uzair, B.; Abbasi, R. Natural Products as Reservoirs of Novel Therapeutic Agents. EXCLI J. 2018, 17, 420–451. [Google Scholar] [CrossRef]
- El oumari, F.E.; Mammate, N.; Imtara, H.; Lahrichi, A.; Elhabbani, R.; El mouhri, G.; Alqahtani, A.S.; Noman, O.M.; Ibrahim, M.N.; Grafov, A.; et al. Chemical Composition, Antioxidant Potentials, and Calcium Oxalate Anticrystallization Activity of Polyphenol and Saponin Fractions from Argania spinosa L. Press Cake. Plants 2022, 11, 1852. [Google Scholar] [CrossRef]
- El oumari, F.E.; Bousta, D.; Imtara, H.; Lahrichi, A.; Elhabbani, R.; El mouhri, G.; Al kamaly, O.; Saleh, A.; Parvez, M.K.; Grafov, A.; et al. Chemical Composition and Anti-Urolithiatic Activity of Extracts from Argania spinosa (L.) Skeels Press-Cake and Acacia senegal (L.) Willd. Molecules 2022, 27, 3973. [Google Scholar] [CrossRef]
- Cole, L.; Kramer, P.R. Human Physiology, Biochemistry and Basic Medicine; Academic Press: Boston, MA, USA, 2016; Chapter 6.2; pp. 193–196. ISBN 978-0-12-803699-0. [Google Scholar]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Selen Isbilir, S.; Sagiroglu, A. An Assessment of In Vitro Antioxidant Activities of Different Extracts from Papaver rhoeas L. Leaves. Int. J. Food Prop. 2012, 15, 1300–1308. [Google Scholar] [CrossRef]
- Scherrer, A.M.; Motti, R.; Weckerle, C.S. Traditional Plant Use in the Areas of Monte Vesole and Ascea, Cilento National Park (Campania, Southern Italy). J. Ethnopharmacol. 2005, 97, 129–143. [Google Scholar] [CrossRef]
- Soulimani, R.; Younos, C.; Jarmouni-Idrissi, S.; Bousta, D.; Khalouki, F.; Laila, A. Behavioral and Pharmaco-Toxicological Study of Papaver rhoeas L. in Mice. J. Ethnopharmacol. 2001, 74, 265–274. [Google Scholar] [CrossRef]
- Grauso, L.; Emrick, S.; Bonanomi, G.; Lanzotti, V. Metabolomics of the Alimurgic Plants Taraxacum Officinale, Papaver rhoeas and Urtica Dioica by Combined NMR and GC–MS Analysis. Phytochem. Anal. 2019, 30, 535–546. [Google Scholar] [CrossRef]
- Hakim, S.a.E.; Mijović, V.; Walker, J. Distribution of Certain Poppy-Fumaria Alkaloids and a Possible Link with the Incidence of Glaucoma. Nature 1961, 189, 198–201. [Google Scholar] [CrossRef]
- Hijazi, M.A.; Aboul-Ela, M.; Bouhadir, K.; Fatfat, M.; Khalife, H.; Ellakany, A.; Gali-Muhtasib, H. Cytotoxic Activity of Alkaloids from Papaver Rhoeas Growing in Lebanon. Rec. Nat. Prod. 2017, 11, 211–216. [Google Scholar]
- Hillenbrand, M.; Zapp, J.; Becker, H. Depsides from the Petals of Papaver rhoeas. Planta Med. 2004, 70, 380–382. [Google Scholar] [CrossRef]
- Saleh, N.A.M.; Maksoud, S.A.; El-hadidi, M.N.; Amer, W.M.M. A Comparative Study of Flavonoids in Some Members of the Papaveraceae. Biochem. Syst. Ecol. 1987, 15, 673–675. [Google Scholar] [CrossRef]
- Çoban, İ.; Toplan, G.G.; Özbek, B.; Gürer, Ç.U.; Sarıyar, G. Variation of Alkaloid Contents and Antimicrobial Activities of Papaver rhoeas L. Growing in Turkey and Northern Cyprus. Pharm. Biol. 2017, 55, 1894–1898. [Google Scholar] [CrossRef]
- Gürbüz, I.; Üstün, O.; Yesilada, E.; Sezik, E.; Kutsal, O. Anti-Ulcerogenic Activity of Some Plants Used as Folk Remedy in Turkey. J. Ethnopharmacol. 2003, 88, 93–97. [Google Scholar] [CrossRef]
- Hasplova, K.; Hudecova, A.; Miadokova, E.; Magdolenova, Z.; Galova, E.; Vaculcikova, L.; Gregan, F.; Dusinska, M. Biological Activity of Plant Extract Isolated from Papaver rhoeas on Human Lymfoblastoid Cell Line. Neoplasma 2011, 58, 386–391. [Google Scholar] [CrossRef]
- Kim, H.; Han, S.; Song, K.; Lee, M.Y.; Park, B.; Ha, I.J.; Lee, S.-G. Ethyl Acetate Fractions of Papaver rhoeas L. and Papaver nudicaule L. Exert Antioxidant and Anti-Inflammatory Activities. Antioxidants 2021, 10, 1895. [Google Scholar] [CrossRef]
- Maurizi, A.; De Michele, A.; Ranfa, A.; Ricci, A.; Roscini, V.; Coli, R.; Bodesmo, M.; Burini, G. Bioactive Compounds and Antioxidant Characterization of Three Edible Wild Plants Traditionally Consumed in the Umbria Region (Central Italy): Bunias erucago L.(Corn Rocket), Lactuca perennis L.(Mountain Lettuce) and Papaver rhoeas L.(Poppy). J. Appl. Bot. Food Qual. 2015, 88, 109–114. [Google Scholar]
- Osanloo, N.; Najafi-Abedi, A.; Jafari, F.; Javid, F.; Pirpiran, M.; Memar Jafari, M.-R.; Mousavi Khosravi, S.A.; Rahimzadeh Behzadi, M.; Ranjbaran, M.; Sahraei, H. Papaver rhoeas L. Hydroalcoholic Extract Exacerbates Forced Swimming Test-Induced Depression in Mice. Basic Clin. Neurosci. 2016, 7, 195–202. [Google Scholar] [CrossRef]
- Sahraei, H.; Fatemi, S.M.; Pashaei-Rad, S.; Faghih-Monzavi, Z.; Salimi, S.H.; Kamalinegad, M. Effects of Papaver rhoeas Extract on the Acquisition and Expression of Morphine-Induced Conditioned Place Preference in Mice. J. Ethnopharmacol. 2006, 103, 420–424. [Google Scholar] [CrossRef]
- Ünsal, Ç.; Özbek, B.; Sarıyar, G.; Mat, A. Antimicrobial Activity of Four Annual Papaver Species Growing in Turkey. Pharm. Biol. 2009, 47, 4–6. [Google Scholar] [CrossRef]
- Bai, Z.-Z.; Yu, R.; Tang, J.-M.; Zhou, Y.; Zheng, T.-T.; Ni, J.; Sun, D.-Y.; Liu, P.; Niu, L.-X.; Zhang, Y.-L. Comparative Investigation on Metabolites and Biological Activities of Paeonia Ostii Stamens from Different Geographical Regions of China. Ind. Crops Prod. 2021, 172, 114038. [Google Scholar] [CrossRef]
- Direction Générale des Collectivités Locales Monographie Générale de La Région de Fès – Meknès. Available online: https://collectivites-territoriales.gov.ma/fr/node/738 (accessed on 16 February 2023).
- Lawag, I.L.; Yoo, O.; Lim, L.Y.; Hammer, K.; Locher, C. Optimisation of Bee Pollen Extraction to Maximise Extractable Antioxidant Constituents. Antioxidants 2021, 10, 1113. [Google Scholar] [CrossRef]
- Oh, H.-T.; Kim, S.-H.; Choi, H.-J.; Chung, M.J.; Ham, S.-S. Antioxidative and Antimutagenic Activities of 70% Ethanol Extract from Masou Salmon (Oncorhynchus masou). Toxicol. Vitr. 2008, 22, 1484–1488. [Google Scholar] [CrossRef]
- Falleh, H.; Ksouri, R.; Chaieb, K.; Karray-Bouraoui, N.; Trabelsi, N.; Boulaaba, M.; Abdelly, C. Phenolic Composition of Cynara cardunculus L. Organs, and Their Biological Activities. Comptes Rendus Biol. 2008, 331, 372–379. [Google Scholar] [CrossRef]
- Hmamou, A.; Eloutassi, N.; Alshawwa, S.Z.; Al kamaly, O.; Kara, M.; Bendaoud, A.; El-Assri, E.-M.; Tlemcani, S.; El Khomsi, M.; Lahkimi, A. Total Phenolic Content and Antioxidant and Antimicrobial Activities of Papaver rhoeas L. Organ Extracts Growing in Taounate Region, Morocco. Molecules 2022, 27, 854. [Google Scholar] [CrossRef]
- Dong, T.; Han, R.; Yu, J.; Zhu, M.; Zhang, Y.; Gong, Y.; Li, Z. Anthocyanins Accumulation and Molecular Analysis of Correlated Genes by Metabolome and Transcriptome in Green and Purple Asparaguses (Asparagus officinalis, L.). Food Chem. 2019, 271, 18–28. [Google Scholar] [CrossRef]
- Tepe, B.; Daferera, D.; Sokmen, A.; Sokmen, M.; Polissiou, M. Antimicrobial and Antioxidant Activities of the Essential Oil and Various Extracts of Salvia Tomentosa Miller (Lamiaceae). Food Chem. 2005, 90, 333–340. [Google Scholar] [CrossRef]
- Kara, M.; Assouguem, A.; Fadili, M.E.; Benmessaoud, S.; Alshawwa, S.Z.; Kamaly, O.A.; Saghrouchni, H.; Zerhouni, A.R.; Bahhou, J. Contribution to the Evaluation of Physicochemical Properties, Total Phenolic Content, Antioxidant Potential, and Antimicrobial Activity of Vinegar Commercialized in Morocco. Molecules 2022, 27, 770. [Google Scholar] [CrossRef]
- Balouiri, M.; Bouhdid, S.; Harki, E.H.; Sadiki, M.; Ouedrhiri, W.; Ibnsouda, S.K. Antifungal activity of Bacillus spp. isolated from calotropis procera ait. rhizosphere against candida albicans. Asian J. Pharm. Clin. Res. 2015, 8, 6. [Google Scholar]
- Kara, M.; Assouguem, A.; Kamaly, O.M.A.; Benmessaoud, S.; Imtara, H.; Mechchate, H.; Hano, C.; Zerhouni, A.R.; Bahhou, J. The Impact of Apple Variety and the Production Methods on the Antibacterial Activity of Vinegar Samples. Molecules 2021, 26, 5437. [Google Scholar] [CrossRef]
- Marsoul, A.; Ijjaali, M.; Oumous, I.; Bennani, B.; Boukir, A. Determination of Polyphenol Contents in Papaver rhoeas L. Flowers Extracts (Soxhlet, Maceration), Antioxidant and Antibacterial Evaluation. Mater. Today Proc. 2020, 31, S183–S189. [Google Scholar] [CrossRef]
- Bouslamti, M.; El Barnossi, A.; Kara, M.; Alotaibi, B.S.; Al Kamaly, O.; Assouguem, A.; Lyoussi, B.; Benjelloun, A.S. Total Polyphenols Content, Antioxidant and Antimicrobial Activities of Leaves of Solanum elaeagnifolium Cav. from Morocco. Molecules 2022, 27, 4322. [Google Scholar] [CrossRef]
- Kostic, D.A.; Mitic, S.S.; Mitic, M.N.; Zarubica, A.R.; Velickovic, J.M.; Dordevic, A.S.; Randelovic, S.S. Phenolic Contents, Antioxidant and Antimicrobial Activity of Papaver rhoeas L. Extracts from Southeast Serbia. JMPR 2010, 4, 1727–1732. [Google Scholar] [CrossRef]
- Morales, P.; Ferreira, I.; Carvalho, A.; Sánchez-Mata, M.; Cámara, M.; Fernández-Ruiz, V.; Pardo de Santayana, M.; Tardío, J. Mediterranean Non-Cultivated Vegetables as Dietary Sources of Compounds with Antioxidant and Biological Activity. Lebensm.-Wiss. Technol. 2014, 55, 389–396. [Google Scholar] [CrossRef]
- Bakour, M.; Campos, M.d.G.; Imtara, H.; Lyoussi, B. Antioxidant Content and Identification of Phenolic/Flavonoid Compounds in the Pollen of Fourteen Plants Using HPLC-DAD. J. Apic. Res. 2020, 59, 35–41. [Google Scholar] [CrossRef]
- Grauso, L.; de Falco, B.; Motti, R.; Lanzotti, V. Corn Poppy, Papaver rhoeas L.: A Critical Review of Its Botany, Phytochemistry and Pharmacology. Phytochem. Rev. 2021, 20, 227–248. [Google Scholar] [CrossRef]
- Nepali, K.; Sharma, S.; Sharma, M.; Bedi, P.M.S.; Dhar, K.L. Rational Approaches, Design Strategies, Structure Activity Relationship and Mechanistic Insights for Anticancer Hybrids. Eur. J. Med. Chem. 2014, 77, 422–487. [Google Scholar] [CrossRef]
- Bournine, L.; Bensalem, S.; Wauters, J.-N.; Iguer-Ouada, M.; Maiza-Benabdesselam, F.; Bedjou, F.; Castronovo, V.; Bellahcène, A.; Tits, M.; Frédérich, M. Identification and Quantification of the Main Active Anticancer Alkaloids from the Root of Glaucium Flavum. Int. J. Mol. Sci. 2013, 14, 23533–23544. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Hibiya, Y.; Mutoh, M.; Koshiji, M.; Akao, S.; Fujiwara, H. Inhibition by Berberine of Cyclooxygenase-2 Transcriptional Activity in Human Colon Cancer Cells. J. Ethnopharmacol. 1999, 66, 227–233. [Google Scholar] [CrossRef]
- Todorova, T.; Pesheva, M.; Gregan, F.; Chankova, S. Antioxidant, Antimutagenic, and Anticarcinogenic Effects of Papaver rhoeas L. Extract on Saccharomyces Cerevisiae. J. Med. Food 2015, 18, 460–467. [Google Scholar] [CrossRef]
- Mazzini, S.; Bellucci, M.C.; Mondelli, R. Mode of Binding of the Cytotoxic Alkaloid Berberine with the Double Helix Oligonucleotide d(AAGAATTCTT)(2). Bioorg. Med. Chem. 2003, 11, 505–514. [Google Scholar] [CrossRef]
- Qi, H.; Xiao, Z.; Ma, J.; Duan, S.; Wang, Y. Comparative Study of Antimicrobial Effects of Three Chinese Medicinal Herbs Containing Protoberberine Alkaloids. Lat. Am. J. Pharm. 2013, 32, 335–339. [Google Scholar]
- El Khomsi, M.; Kara, M.; Hmamou, A.; Assouguem, A.; Al Kamaly, O.; Saleh, A.; Ercisli, S.; Fidan, H.; Hmouni, D. In Vitro Studies on the Antimicrobial and Antioxidant Activities of Total Polyphenol Content of Cynara humilis from Moulay Yacoub Area (Morocco). Plants 2022, 11, 1200. [Google Scholar] [CrossRef]
- Miliauskas, G.; Venskutonis, P.R.; Van Beek, T.A. Screening of Radical Scavenging Activity of Some Medicinal and Aromatic Plant Extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Rota, M.C.; Herrera, A.; Martínez, R.M.; Sotomayor, J.A.; Jordán, M.J. Antimicrobial Activity and Chemical Composition of Thymus Vulgaris, Thymus Zygis and Thymus Hyemalis Essential Oils. Food Control 2008, 19, 681–687. [Google Scholar] [CrossRef]
- Helal, G.A.; Sarhan, M.M.; Abu Shahla, A.N.K.; Abou El-Khair, E.K. Effects Of Cymbopogon citratus L. Essential Oil on the Growth, Morphogenesis and Aflatoxin Production OfAspergillus Flavus ML2-Strain. J. Basic Microbiol. 2007, 47, 5–15. [Google Scholar] [CrossRef]
- Prashar, A.; Hili, P.; Veness, R.G.; Evans, C.S. Antimicrobial Action of Palmarosa Oil (Cymbopogon martinii) on Saccharomyces Cerevisiae. Phytochemistry 2003, 63, 569–575. [Google Scholar] [CrossRef]
- Elyemni, M.; Ouadrhiri, F.E.; Lahkimi, A.; Elkamli, T.; Bouia, A.; Eloutassi, N. Chemical Composition and Antimicrobial Activity of Essential Oil of Wild and Cultivated Rosmarinus Officinalis from Two Moroccan Localities. J. Ecol. Eng. 2022, 23, 214–222. [Google Scholar] [CrossRef]
- Ahmed, S.; Jubair, A.; Hossain, M.A.; Hossain, M.M.; Azam, M.S.; Biswas, M. Free Radical-Scavenging Capacity and HPLC-DAD Screening of Phenolic Compounds from Pulp and Seed of Syzygium Claviflorum Fruit. J. Agric. Food Res. 2021, 6, 100203. [Google Scholar] [CrossRef]
- Kiselova, Y.; Ivanova, D.; Chervenkov, T.; Gerova, D.; Galunska, B.; Yankova, T. Correlation between the In Vitro Antioxidant Activity and Polyphenol Content of Aqueous Extracts from Bulgarian Herbs. Phytother. Res. 2006, 20, 961–965. [Google Scholar] [CrossRef]
- Maisuthisakul, P.; Suttajit, M.; Pongsawatmanit, R. Assessment of Phenolic Content and Free Radical-Scavenging Capacity of Some Thai Indigenous Plants. Food Chem. 2007, 100, 1409–1418. [Google Scholar] [CrossRef]
- Imtara, H.; Elamine, Y.; Lyoussi, B. Physicochemical Characterization and Antioxidant Activity of Palestinian Honey Samples. Food Sci. Nutr. 2018, 6, 2056–2065. [Google Scholar] [CrossRef]
| Sample | Gram − Bacteria | Gram + Bacteria | Yeast | ||
|---|---|---|---|---|---|
| Escherichia coli ATCC | Escherichia coli CIP | Klebsiella pneumoniae | Staphylococcus aureus | Candida albicans | |
| P1E | 12.66 ± 0.57 b | 15.00 ± 1.00 b | 13.33 ± 1.52 ab | 13.66 ± 0.57 b | R |
| P2E | 13.00 ± 0.00 b | 12.33 ± 0.57 c | 10.66 ± 1.15 b | 14.00 ± 1.00 b | 10.00 ± 2.00 b |
| P3E | 13.00 ± 0.00 b | 11.66 ± 0.57 c | R | 12.33 ± 0.57 b | R |
| Tetracycline | 19.00 ± 1.00 a | 18.10 ± 0.70 a | 14.90 ± 0.60 a | 16.70 ± 0.90 a | — |
| Streptomycin | R | R | R | 09.61 ± 0.200 c | — |
| Fluconazole | — | — | — | — | 21.20 ± 04.20 a |
| Sample | Gram − Bacteria | Gram + Bacteria | Yeast | ||
|---|---|---|---|---|---|
| Escherichia coli ATCC | Escherichia coli CIP | Klebsiella pneumoniae | Staphylococcus aureus | Candida albicans | |
| P1E | 25 | 25 | 25 | 25 | 50 |
| P2E | 50 | 50 | 25 | 25 | 25 |
| P3E | 0.78 | 0.78 | ND | 50 | ND |
| Streptomycin | 0.25 | 0.50 | 0.003 | 0.062 | — |
| Tetracycline | 0.25 | 0.25 | 0.062 | 0.003 | — |
| Fluconazole | — | — | — | — | 0.40 |
| Yield | TPC | TFC | TA | IC50 | TAC | MIC E. coli ATCC | MIC E. coli CIP | MIC K. pneumoniae | MIC S. aureus | |
|---|---|---|---|---|---|---|---|---|---|---|
| TPC | 0.315 | |||||||||
| TFC | 0.143 | 0.984 | ||||||||
| TA | 0.487 | 0.983 | 0.934 | |||||||
| IC50 | −0.723 | −0.884 | −0.787 | −0.955 | ||||||
| TAC | 0.203 | 0.993 | 0.998 | 0.954 | −0.824 | |||||
| MIC E. coli ATCC | −0.539 | −0.969 | −0.911 | −0.998 | 0.972 | −0.934 | ||||
| MIC E. coli CIP | −0.532 | −0.971 | −0.914 | −0.999 | 0.970 | −0.937 | 1.000 | |||
| MIC K. pneumoniae | 0.037 | 0.960 | 0.994 | 0.891 | −0.717 | 0.986 | −0.861 | −0.866 | ||
| MIC S. aureus | 0.037 | 0.960 | 0.994 | 0.891 | −0.717 | 0.986 | −0.861 | −0.866 | 1.000 | |
| MIC C. albicans | 0.884 | 0.723 | 0.589 | 0.839 | −0.962 | 0.638 | −0.871 | −0.866 | 0.500 | 0.500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hmamou, A.; Kara, M.; Khomsi, M.E.; Saleh, A.; Al Kamaly, O.; Bendaoud, A.; El Ouadrhiri, F.; Adachi, A.; Tlemcani, S.; Eloutassi, N.; et al. Comparative Study on the Total Phenolics, Total Flavonoids, and Biological Activities of Papaver rhoeas L. Extracts from Different Geographical Regions of Morocco. Appl. Sci. 2023, 13, 2695. https://doi.org/10.3390/app13042695
Hmamou A, Kara M, Khomsi ME, Saleh A, Al Kamaly O, Bendaoud A, El Ouadrhiri F, Adachi A, Tlemcani S, Eloutassi N, et al. Comparative Study on the Total Phenolics, Total Flavonoids, and Biological Activities of Papaver rhoeas L. Extracts from Different Geographical Regions of Morocco. Applied Sciences. 2023; 13(4):2695. https://doi.org/10.3390/app13042695
Chicago/Turabian StyleHmamou, Anouar, Mohammed Kara, Mostafa El Khomsi, Asmaa Saleh, Omkulthom Al Kamaly, Ahmed Bendaoud, Faiçal El Ouadrhiri, Abderrazzak Adachi, Sara Tlemcani, Noureddine Eloutassi, and et al. 2023. "Comparative Study on the Total Phenolics, Total Flavonoids, and Biological Activities of Papaver rhoeas L. Extracts from Different Geographical Regions of Morocco" Applied Sciences 13, no. 4: 2695. https://doi.org/10.3390/app13042695
APA StyleHmamou, A., Kara, M., Khomsi, M. E., Saleh, A., Al Kamaly, O., Bendaoud, A., El Ouadrhiri, F., Adachi, A., Tlemcani, S., Eloutassi, N., & Lahkimi, A. (2023). Comparative Study on the Total Phenolics, Total Flavonoids, and Biological Activities of Papaver rhoeas L. Extracts from Different Geographical Regions of Morocco. Applied Sciences, 13(4), 2695. https://doi.org/10.3390/app13042695








