Computer Percolation Models for Espresso Coffee: State of the Art, Results and Future Perspectives
Abstract
1. Introduction
2. Materials and Methods
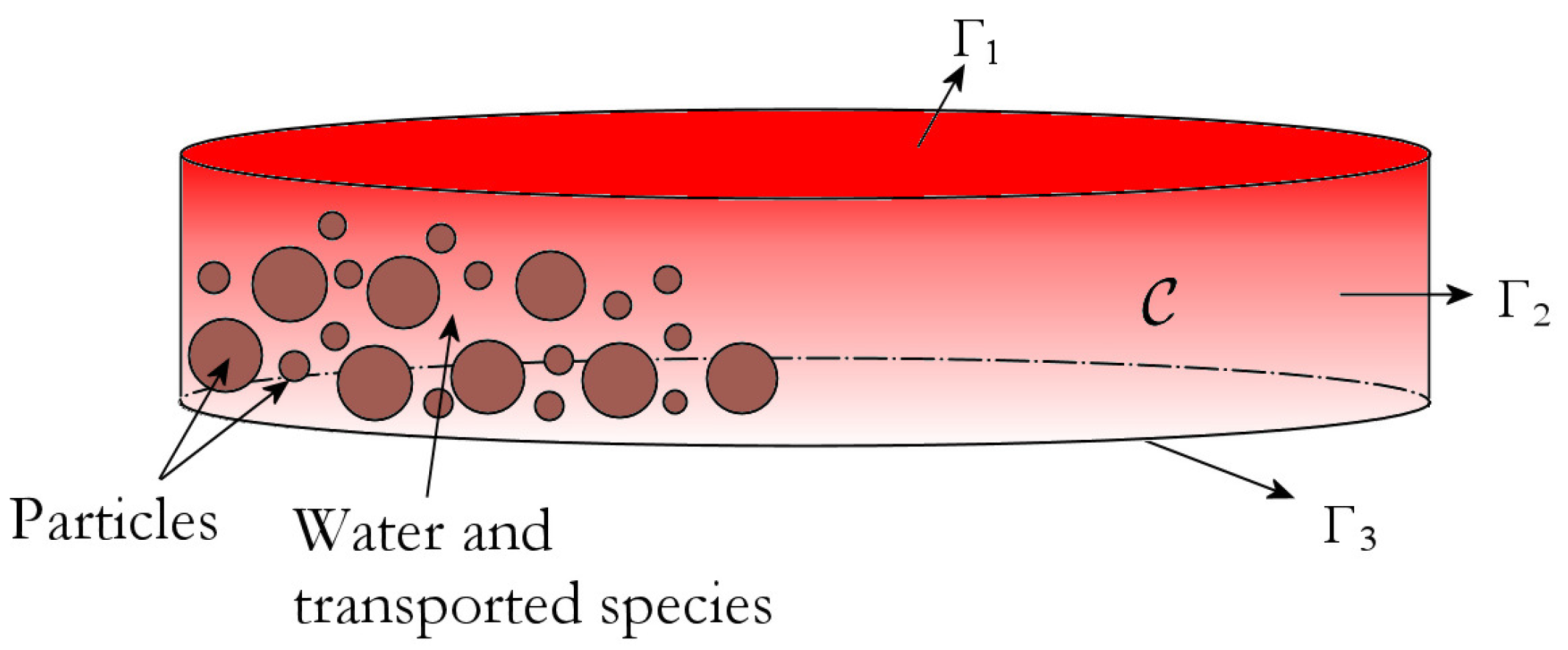
2.1. The Percolation Model
- The porous medium is isotropic and homogeneous.
- A chemical species is called liquid if it undergoes the transport process; in contrast, a chemical species is called solid if it is not involved in the transport process so it is bound to the porous medium. Thus, being liquid or solid is independent of the actual physical phase.
- The first 5 s of the real extraction process are called imbibition, when the porous medium gets wet. After imbibition, there is no gaseous phase in the porous medium, the porous medium is saturated and a local thermal balance occurs between coffee powder and water. The percolation process is investigated after imbibition.
2.2. Chemical Analyses
2.2.1. Chemicals and Reagents
2.2.2. Total Solids and Total Lipids
2.2.3. Bioactive Compounds Analysis by HPLC-DAD
3. Results and Discussion
3.1. Results of Chemical Analyses
3.1.1. Total Solids and Total Lipids: Results
3.1.2. Bioactive Compounds Analysis by HPLC-DAD: Results
3.2. Numerical Results
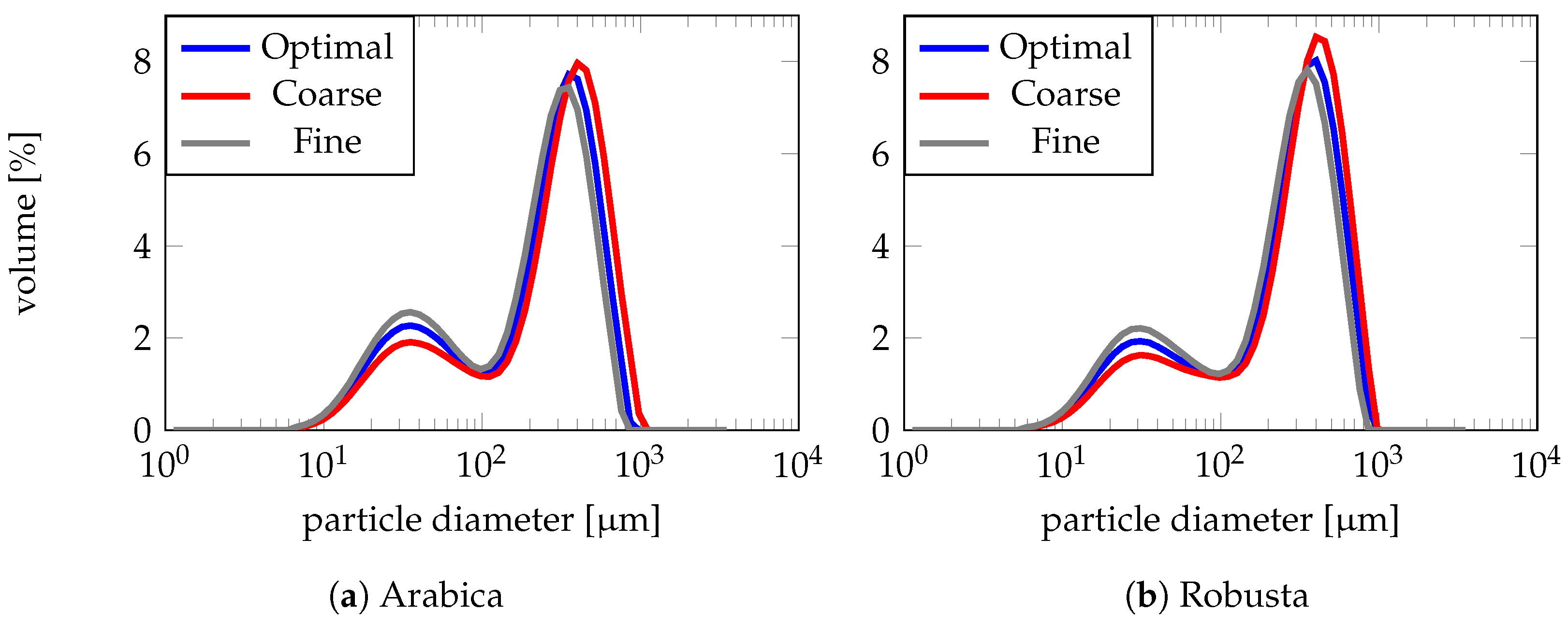
3.2.1. Numerical Approximation
3.2.2. Numerical Simulation Settings
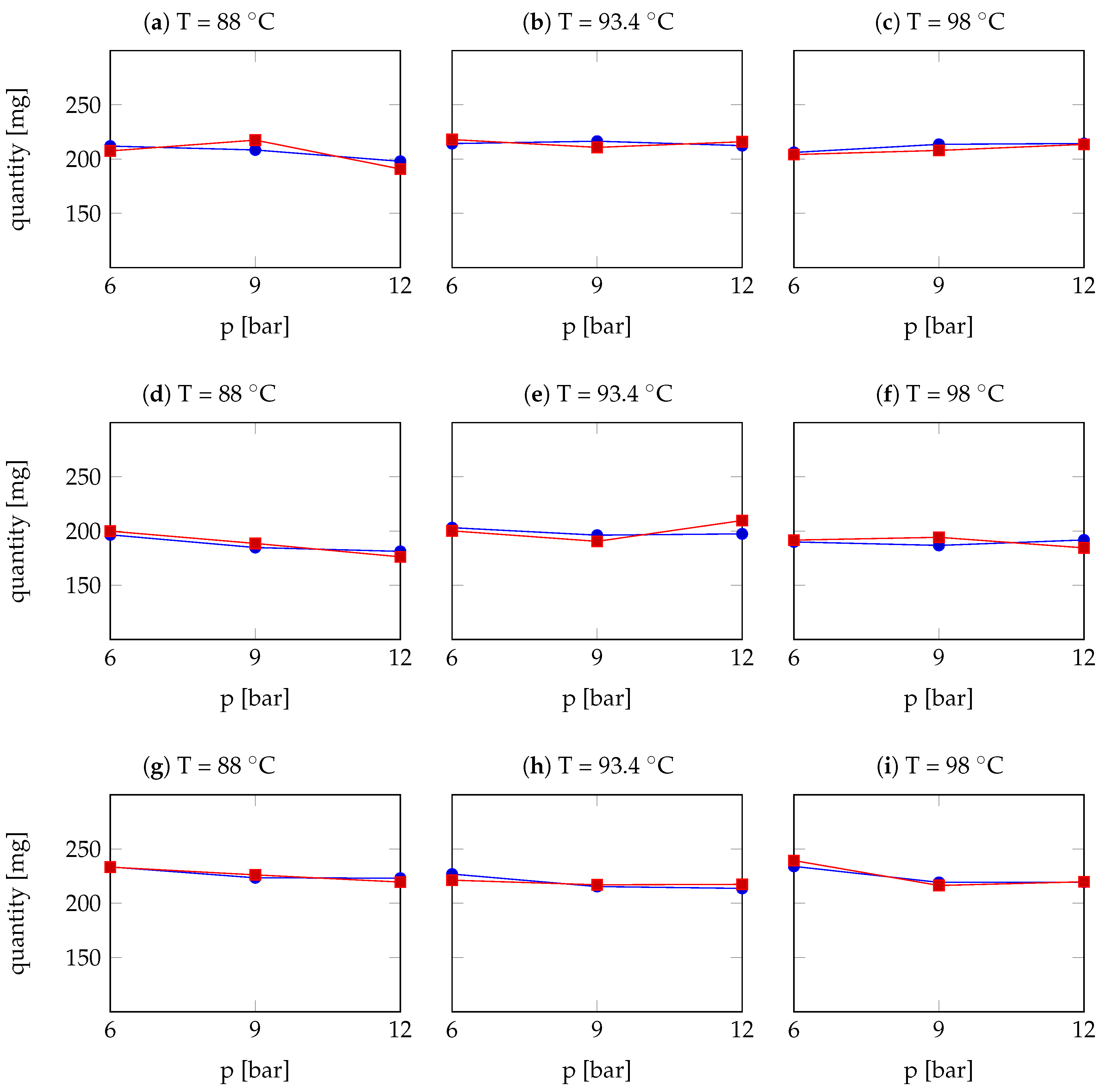
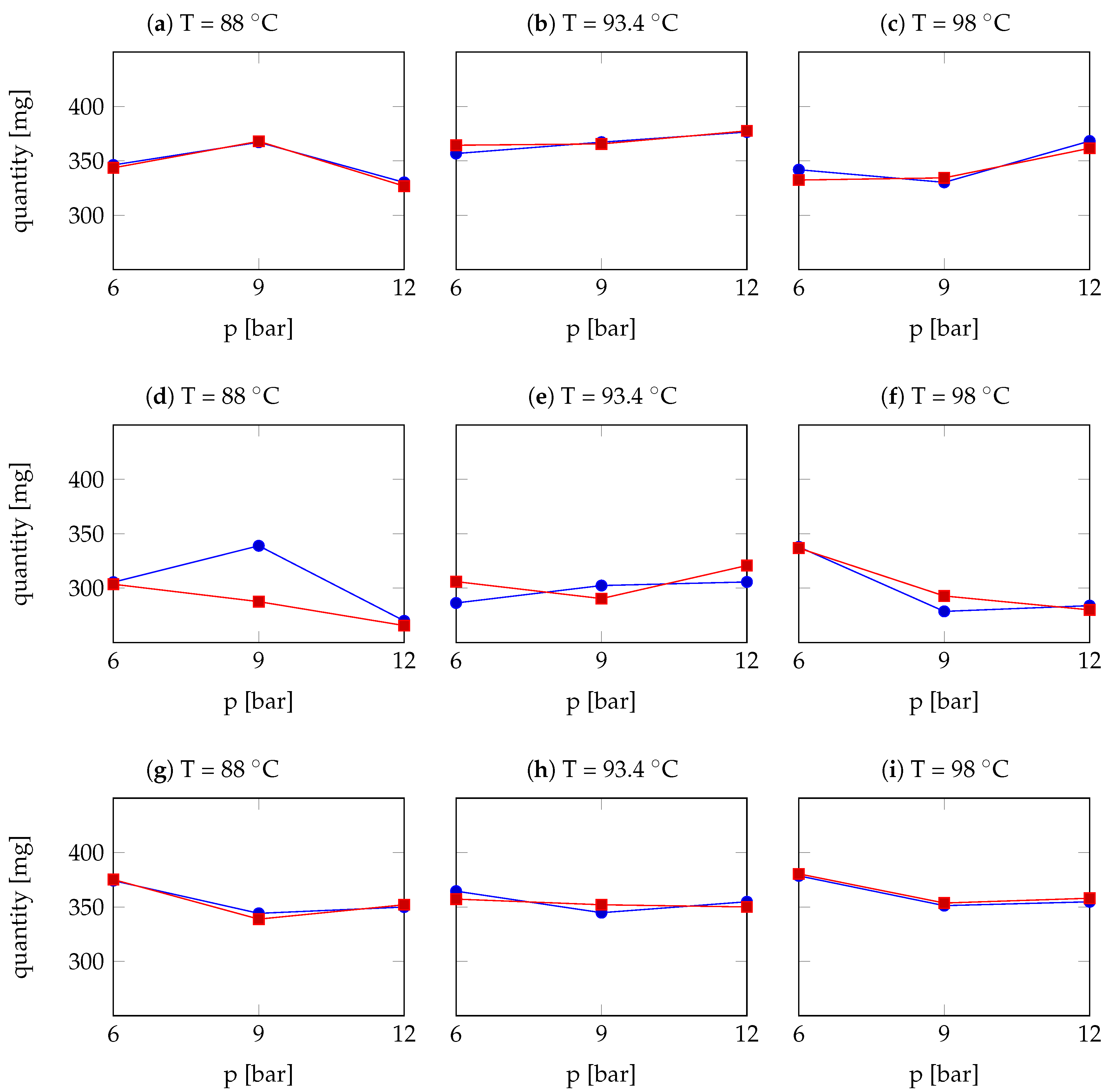
3.2.3. Results: Comparison and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Data for the Approximation of Reaction Terms
| CF | CQA | TR | CA | AA | TA | FA | LP | |
|---|---|---|---|---|---|---|---|---|
| −6.0 × | −5.8 × | 3.8 × | −4.4 × | −8.2 × | −2.7 × | 1.5 × | −1.2 × | |
| a | 1.5 × | 5.9 × | −5.7 × | 9.4 × | 1.9 × | 8.4 × | −2.8 × | 2.6 × |
| b | −1.0 × | 2.3 × | −1.6 × | 1.9 × | −6.3 × | −1.6 × | −2.8 × | 6.6 × |
| c | −8.5 | 7.0 × | 2.6 | −5.0 × | −1.1 × | −5.2 | 1.4 × | −1.4 |
| d | −1.3 × | −1.1 × | 2.6 × | −6.5 | 4.4 × | 7.4 | 5.6 × | −9.3 × |
| f | 1.3 × | 1.4 × | 1.2 × | −1.9 × | 5.4 × | 1.5 × | 2.0 × | −5.1 × |
| l | 0 | −1.8 | 0 | 0 | 0 | 0 | 0 | 0 |
| m | 0 | 1.1 × | 0 | 0 | 0 | 0 | 0 | 0 |
| CF | CQA | TR | CA | AA | TA | FA | LP | |
|---|---|---|---|---|---|---|---|---|
| −1.9 × | 7.6 × | −2.3 × | −2.9 × | −4.7 × | −1.7 × | −1.7 × | 2.9 × | |
| a | 4.3 × | −1.8 × | 5.4 × | 6.1 × | 1.0 × | 4.2 × | 3.8 × | −6.3 × |
| b | −1.9 × | −5.6 × | −2.9 × | 3.4 × | −6.1 × | −2.2 × | −6.4 × | 1.1 × |
| c | −2.4 × | 1.1 × | −3.0 × | −3.2 × | −5.5 × | −2.3 × | −2.1 × | 3.6 |
| d | 2.8 × | −2.4 × | 5.7 × | 3.1 × | 7.3 × | 4.8 × | 9.3 | 1.2 × |
| f | 1.4 × | 1.7 × | 2.0 × | −4.4 × | −8.6 × | 1.4 × | 5.0 | −3.3 |
| l | 0 | −1.2 × | 0 | 0 | 0 | 0 | 0 | 0 |
| m | 0 | 2.5 × | 0 | 0 | 0 | 0 | 0 | 0 |
| CF | CQA | TR | CA | AA | TA | FA | LP | |
|---|---|---|---|---|---|---|---|---|
| 5.5 × | 1.0 × | −2.9 × | 4.0 × | 1.6 × | 8.5 × | 3.2 × | 1.2 × | |
| a | −1.1 × | −2.1 × | 1.2 × | −8.6 × | −3.8 × | −8.8 × | −5.1 × | −2.6 × |
| b | −1.1 × | −1.0 × | −6.0 × | −8.8 × | 2.0 × | −4.4 × | −1.5 × | 1.1 × |
| c | 6.0 | 1.1 × | −5.1 × | 4.6 × | 2.0 × | 4.7 × | 2.4 | 1.4 |
| d | 1.1 × | 7.7 × | 9.3 | 4.4 × | −1.0 × | 1.8 × | 2.8 × | −3.3 |
| f | −1.4 | 2.0 × | −1.4 | 1.1 | −1.1 × | 9.9 × | 1.0 × | −6.1 × |
| l | 0 | −1.0 | 0 | 0 | 0 | 0 | 0 | 0 |
| m | 0 | −6.4 × | 0 | 0 | 0 | 0 | 0 | 0 |
| CF | CQA | TR | CA | AA | TA | FA | LP | |
|---|---|---|---|---|---|---|---|---|
| 6.2 × | 5.2 × | 1.0 × | 2.5 × | −3.1 × | 1.1 × | 1.4 × | −4.6 × | |
| a | −9.2 × | −1.2 × | −1.7 × | −5.4 × | 7.5 × | −1.9 × | −2.8 × | 1.0 × |
| b | −2.3 × | −1.6 × | −3.3 × | 7.8 × | −6.3 × | −3.2 × | 2.0 × | −4.2 × |
| c | 3.2 | 6.6 × | 6.9 | 3.0 × | −4.5 × | 8.5 | 1.5 × | −5.6 × |
| d | −2.1 × | −1.5 × | 8.3 × | 1.3 × | −7.2 × | 1.5 × | −1.9 × | 1.8 |
| f | 2.8 × | 5.7 × | 3.4 × | −1.1 × | 8.0 × | 3.1 × | 6.2 × | 1.2 × |
| l | 0 | −4.3 | 0 | 0 | 0 | 0 | 0 | 0 |
| m | 0 | 1.6 × | 0 | 0 | 0 | 0 | 0 | 0 |
| CF | CQA | TR | CA | AA | TA | FA | LP | |
|---|---|---|---|---|---|---|---|---|
| −2.1 × | −1.5 × | 6.7 × | −5.0 × | −2.7 × | 9.6 × | 4.4 × | −1.2 × | |
| a | 3.8 × | 3.1 × | −1.6 × | 6.0 × | 5.3 × | −2.3 × | −1.0 × | 2.7 × |
| b | 2.2 × | 2.2 × | 4.4 × | 8.8 × | 7.0 × | 4.9 × | 1.9 × | −1.3 × |
| c | −1.0 | −1.6 × | 1.1 × | −1.4 | −2.5 × | 1.4 × | 6.8 | −1.5 |
| d | −1.9 | −3.7 × | 1.1 × | −1.5 × | 6.1 × | 5.6 | 4.4 × | −9.3 × |
| f | −2.4 × | −4.4 × | −4.9 × | −6.4 × | −8.3 × | −5.3 × | −2.9 × | 1.9 × |
| l | 0 | 2.2 | 0 | 0 | 0 | 0 | 0 | 0 |
| m | 0 | 5.1 × | 0 | 0 | 0 | 0 | 0 | 0 |
| CF | CQA | TR | CA | AA | TA | FA | LP | |
|---|---|---|---|---|---|---|---|---|
| −3.9 × | 2.9 × | −1.5 × | 6.2 × | 4.9 × | 6.9 × | −1.8 × | −1.9 × | |
| a | 9.6 × | −6.9 × | 3.6 × | 1.6 × | −9.7 × | −1.2 × | 4.0 × | 4.3 × |
| b | 3.3 × | −3.2 × | −8.6 × | −1.2 × | −6.7 × | −9.0 × | 2.6 × | −1.9 × |
| c | −5.7 | 4.0 × | −2.1 × | −2.4 | 4.8 × | 4.3 | −2.3 × | −2.4 × |
| d | −2.4 × | −1.0 × | −5.2 × | −4.1 × | 8.7 × | −7.0 × | −7.6 × | 6.9 × |
| f | 4.1 | 9.0 × | 1.8 × | 2.1 × | 5.5 × | 2.3 × | 1.2 × | 7.5 × |
| l | 0 | −5.8 | 0 | 0 | 0 | 0 | 0 | 0 |
| m | 0 | 1.1 × | 0 | 0 | 0 | 0 | 0 | 0 |
References
- Coffee Consumption by Country. Available online: https://wisevoter.com/country-rankings/coffee-consumption-by-country/ (accessed on 30 January 2023).
- Angeloni, S.; Mustafa, A.M.; Abouelenein, D.; Alessandroni, L.; Acquaticci, L.; Nzekoue, F.K.; Petrelli, R.; Sagratini, G.; Vittori, S.; Torregiani, E.; et al. Characterization of the aroma profile and main key odorants of espresso coffee. Molecules 2021, 26, 3856. [Google Scholar] [CrossRef]
- Heckman, M.A.; Weil, J.; De Mejia, E.G. Caffeine (1, 3, 7-trimethylxanthine) in foods: A comprehensive review on consumption, functionality, safety, and regulatory matters. J. Food Sci. 2010, 75, R77–R87. [Google Scholar] [CrossRef]
- Kraehenbuehl, K.; Page-Zoerkler, N.; Mauroux, O.; Gartenmann, K.; Blank, I.; Bel-Rhlid, R. Selective enzymatic hydrolysis of chlorogenic acid lactones in a model system and in a coffee extract. Application to reduction of coffee bitterness. Food Chem. 2017, 218, 9–14. [Google Scholar] [CrossRef]
- Baeza, G.; Amigo-Benavent, M.; Sarriá, B.; Goya, L.; Mateos, R.; Bravo, L. Green coffee hydroxycinnamic acids but not caffeine protect human HepG2 cells against oxidative stress. Food Res. Int. 2014, 62, 1038–1046. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef]
- Khamitova, G.; Angeloni, S.; Fioretti, L.; Ricciutelli, M.; Sagratini, G.; Torregiani, E.; Vittori, S.; Caprioli, G. The impact of different filter baskets, heights of perforated disc and amount of ground coffee on the extraction of organics acids and the main bioactive compounds in espresso coffee. Food Res. Int. 2020, 133, 109220. [Google Scholar] [CrossRef]
- Illy, A.; Viani, R. Espresso Coffee: The Science of Quality; Academic Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Folmer, B. The Craft and Science of Coffee; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Moroney, K.M.; Lee, W.T.; O’Brien, S.; Suijver, F.; Marra, J. Asymptotic Analysis of the Dominant Mechanisms in the Coffee Extraction Process. SIAM J. Appl. Math. 2016, 76, 2196–2217. [Google Scholar] [CrossRef]
- Moroney, K.M.; O’Connell, K.; Meikle-Janney, P.; O’Brien, S.B.G.; Walker, G.M.; Lee, W.T. Analysing extraction uniformity from porous coffee beds using mathematical modelling and computational fluid dynamics approaches. PLoS ONE 2019, 14, e0219906. [Google Scholar] [CrossRef]
- Cameron, M.I.; Morisco, D.; Hofstetter, D.; Uman, E.; Wilkinson, J.; Kennedy, Z.C.; Fontenot, S.A.; Lee, W.T.; Hendon, C.H.; Foster, J.M. Systematically improving espresso: Insights from mathematical modeling and experiment. Matter 2020, 2, 631–648. [Google Scholar] [CrossRef]
- Egidi, N.; Giacomini, J.; Maponi, P.; Perticarini, A.; Cognigni, L.; Fioretti, L. An advection–diffusion–reaction model for coffee percolation. Comput. Appl. Math. 2022, 41, 229. [Google Scholar] [CrossRef]
- Giacomini, J.; Maponi, P.; Perticarini, A. CMMSE: A reduced percolation model for espresso coffee. J. Math. Chem. 2022, 61, 520–538. [Google Scholar] [CrossRef]
- Moroney, K.; Lee, W.; O’Brien, S.; Suijver, F.; Marra, J. Modelling of coffee extraction during brewing using multiscale methods: An experimentally validated model. Chem. Eng. Sci. 2015, 137, 216–234. [Google Scholar] [CrossRef]
- Giacomini, J.; Khamitova, G.; Maponi, P.; Vittori, S.; Fioretti, L. Water flow and transport in porous media for in-silico espresso coffee. Int. J. Multiph. Flow 2020, 126, 103252. [Google Scholar] [CrossRef]
- Kuhn, M.; Lang, S.; Bezold, F.; Minceva, M.; Briesen, H. Time-resolved extraction of caffeine and trigonelline from finely-ground espresso coffee with varying particle sizes and tamping pressures. J. Food Eng. 2017, 206, 37–47. [Google Scholar] [CrossRef]
- Cordoba, N.; Fernandez-Alduenda, M.; Moreno, F.L.; Ruiz, Y. Coffee extraction: A review of parameters and their influence on the physicochemical characteristics and flavour of coffee brews. Trends Food Sci. Technol. 2020, 96, 45–60. [Google Scholar] [CrossRef]
- Caprioli, G.; Cortese, M.; Maggi, F.; Minnetti, C.; Odello, L.; Sagratini, G.; Vittori, S. Quantification of caffeine, trigonelline and nicotinic acid in espresso coffee: The influence of espresso machines and coffee cultivars. Int. J. Food Sci. Nutr. 2014, 65, 465–469. [Google Scholar] [CrossRef]
- Nespresso. Available online: https://www.nespresso.com/it/it/vertuo-system (accessed on 30 January 2023).
- Bear, J.; Bachmat, Y. Introduction to Modeling of Transport Phenomena in Porous Media; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 4. [Google Scholar]
- Vafai, K. Handbook of Porous Media; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Egidi, N.; Gioia, E.; Maponi, P.; Spadoni, L. A numerical solution of Richards equation: A simple method adaptable in parallel computing. Int. J. Comput. Math. 2018, 97, 2–17. [Google Scholar] [CrossRef]
- Diersch, H.J.G. FEFLOW: Finite Element Modeling of Flow, Mass and Heat Transport in Porous and Fractured Media; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Mythos 1. Available online: https://www.victoriaarduino.com/mythos-1/ (accessed on 30 January 2023).
- Puq Press. Available online: https://www.puqpress.com/products/puq-m-line/puqpress-m2-for-mythos/ (accessed on 30 January 2023).
- VA388 Black Eagle. Available online: https://www.victoriaarduino.com/wp-content/uploads/VA388.pdf (accessed on 30 January 2023).
- Caprioli, G.; Cortese, M.; Cristalli, G.; Maggi, F.; Odello, L.; Ricciutelli, M.; Sagratini, G.; Sirocchi, V.; Tomassoni, G.; Vittori, S. Optimization of espresso machine parameters through the analysis of coffee odorants by HS-SPME–GC/MS. Food Chem. 2012, 135, 1127–1133. [Google Scholar] [CrossRef]
- Parenti, A.; Guerrini, L.; Masella, P.; Spinelli, S.; Calamai, L.; Spugnoli, P. Comparison of espresso coffee brewing techniques. J. Food Eng. 2014, 121, 112–117. [Google Scholar] [CrossRef]
- Efthymiopoulos, I.; Hellier, P.; Ladommatos, N.; Russo-Profili, A.; Eveleigh, A.; Aliev, A.; Kay, A.; Mills-Lamptey, B. Influence of solvent selection and extraction temperature on yield and composition of lipids extracted from spent coffee grounds. Ind. Crop. Prod. 2018, 119, 49–56. [Google Scholar] [CrossRef]
- Andueza, S.; De Peña, M.P.; Cid, C. Chemical and sensorial characteristics of espresso coffee as affected by grinding and torrefacto roast. J. Agric. Food Chem. 2003, 51, 7034–7039. [Google Scholar] [CrossRef]
- Severini, C.; Ricci, I.; Marone, M.; Derossi, A.; De Pilli, T. Changes in the aromatic profile of espresso coffee as a function of the grinding grade and extraction time: A study by the electronic nose system. J. Agric. Food Chem. 2015, 63, 2321–2327. [Google Scholar] [CrossRef]
- Khamitova, G.; Angeloni, S.; Borsetta, G.; Xiao, J.; Maggi, F.; Sagratini, G.; Vittori, S.; Caprioli, G. Optimization of espresso coffee extraction through variation of particle sizes, perforated disk height and filter basket aimed at lowering the amount of ground coffee used. Food Chem. 2020, 314, 126220. [Google Scholar] [CrossRef]
- Salamanca, C.A.; Fiol, N.; González, C.; Saez, M.; Villaescusa, I. Extraction of espresso coffee by using gradient of temperature. Effect on physicochemical and sensorial characteristics of espresso. Food Chem. 2017, 214, 622–630. [Google Scholar] [CrossRef]
- Rodrigues, C.I.; Marta, L.; Maia, R.; Miranda, M.; Ribeirinho, M.; Máguas, C. Application of solid-phase extraction to brewed coffee caffeine and organic acid determination by UV/HPLC. J. Food Compos. Anal. 2007, 20, 440–448. [Google Scholar] [CrossRef]
- Sunarharum, W.B.; Williams, D.J.; Smyth, H.E. Complexity of coffee flavor: A compositional and sensory perspective. Food Res. Int. 2014, 62, 315–325. [Google Scholar] [CrossRef]
- Severini, C.; Derossi, A.; Ricci, I.; Caporizzi, R.; Fiore, A. Roasting conditions, grinding level and brewing method highly affect the healthy benefits of a coffee cup. Int. J. Clin. Nutr. Diet 2018, 4, 127. [Google Scholar] [CrossRef]
- Andueza, S.; Maeztu, L.; Pascual, L.; Ibáñez, C.; de Peña, M.P.; Cid, C. Influence of extraction temperature on the final quality of espresso coffee. J. Sci. Food Agric. 2003, 83, 240–248. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Pina, G.; Vergara-Castañeda, H.A.; Oomah, B.D. Spent coffee grounds: A review on current research and future prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- FeFlow—DHI. Available online: https://www.mikepoweredbydhi.com/products/feflow (accessed on 30 January 2023).
- Egidi, N.; Giacomini, J.; Maponi, P. Mathematical model to analyze the flow and heat transfer problem in U-shaped geothermal exchangers. Appl. Math. Model. 2018, 61, 83–106. [Google Scholar] [CrossRef]
- Giacomini, J.; Invernizzi, M.C.; Maponi, P.; Verdoya, M. Testing a model of flow and heat transfer for u-shaped geothermal exchangers. Adv. Model. Anal. A 2018, 55, 151–157. [Google Scholar]
- Bear, J.; Verruijt, A. Modeling Groundwater Flow and Pollution; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1987; Volume 2. [Google Scholar]
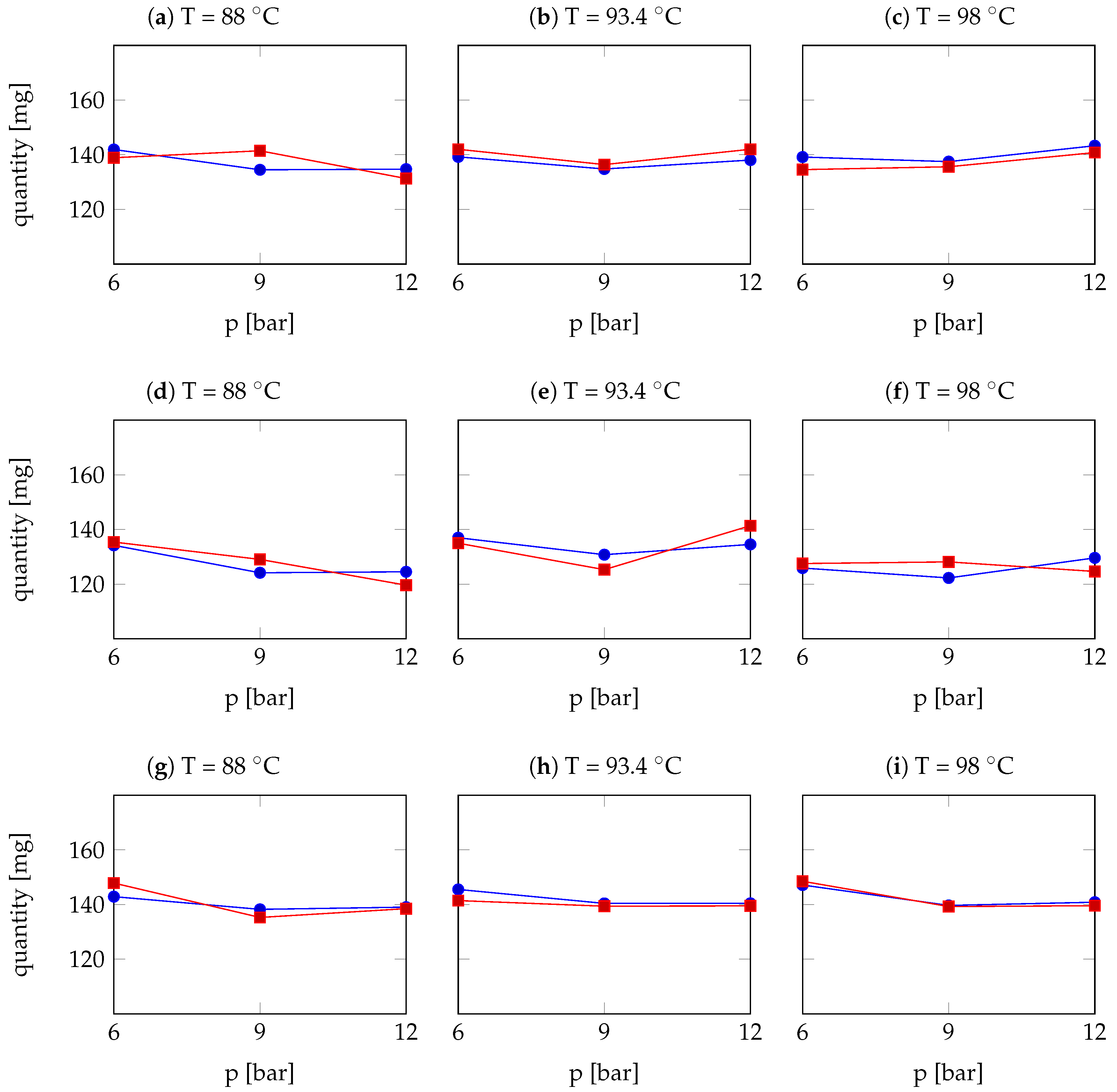
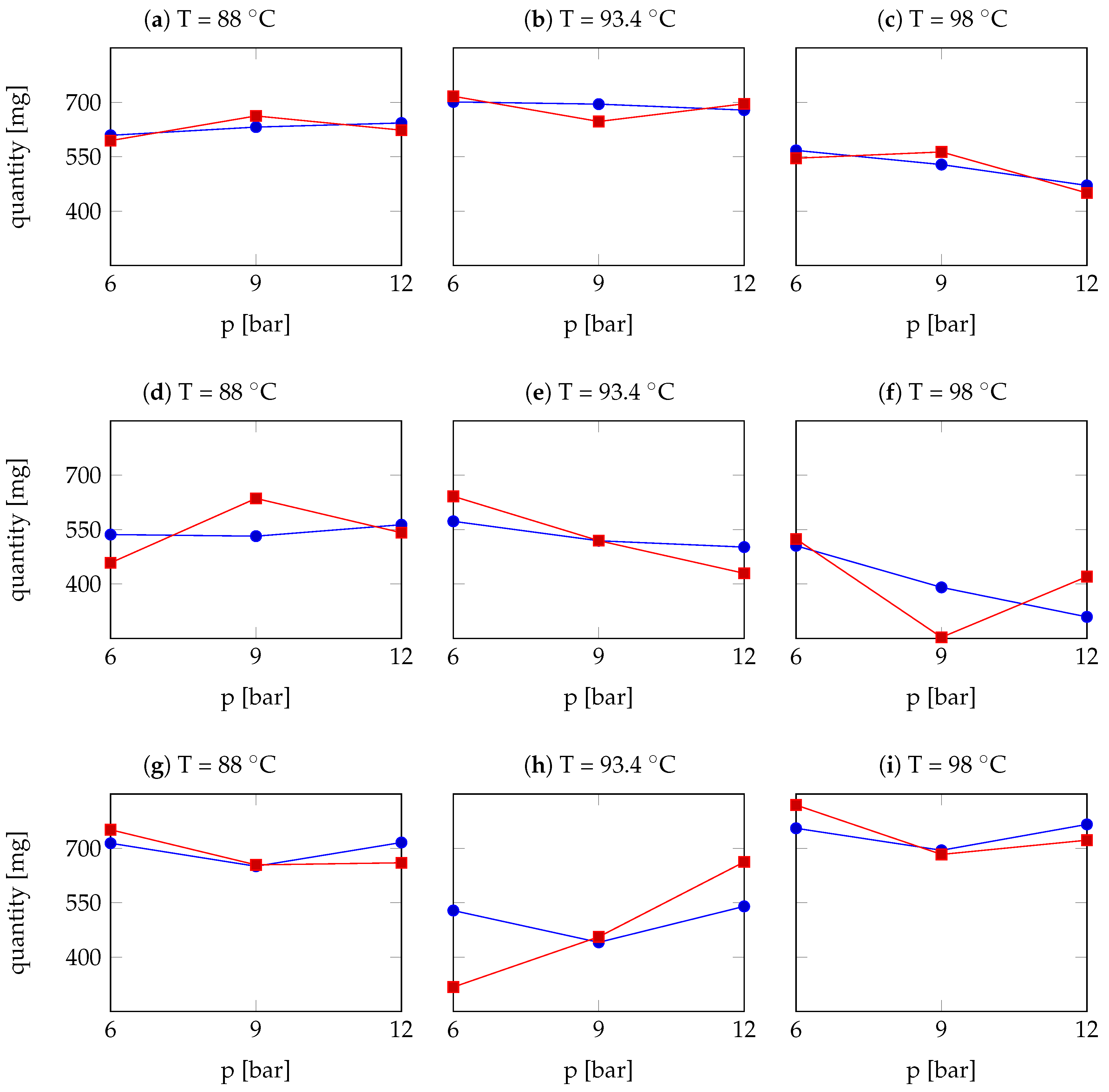
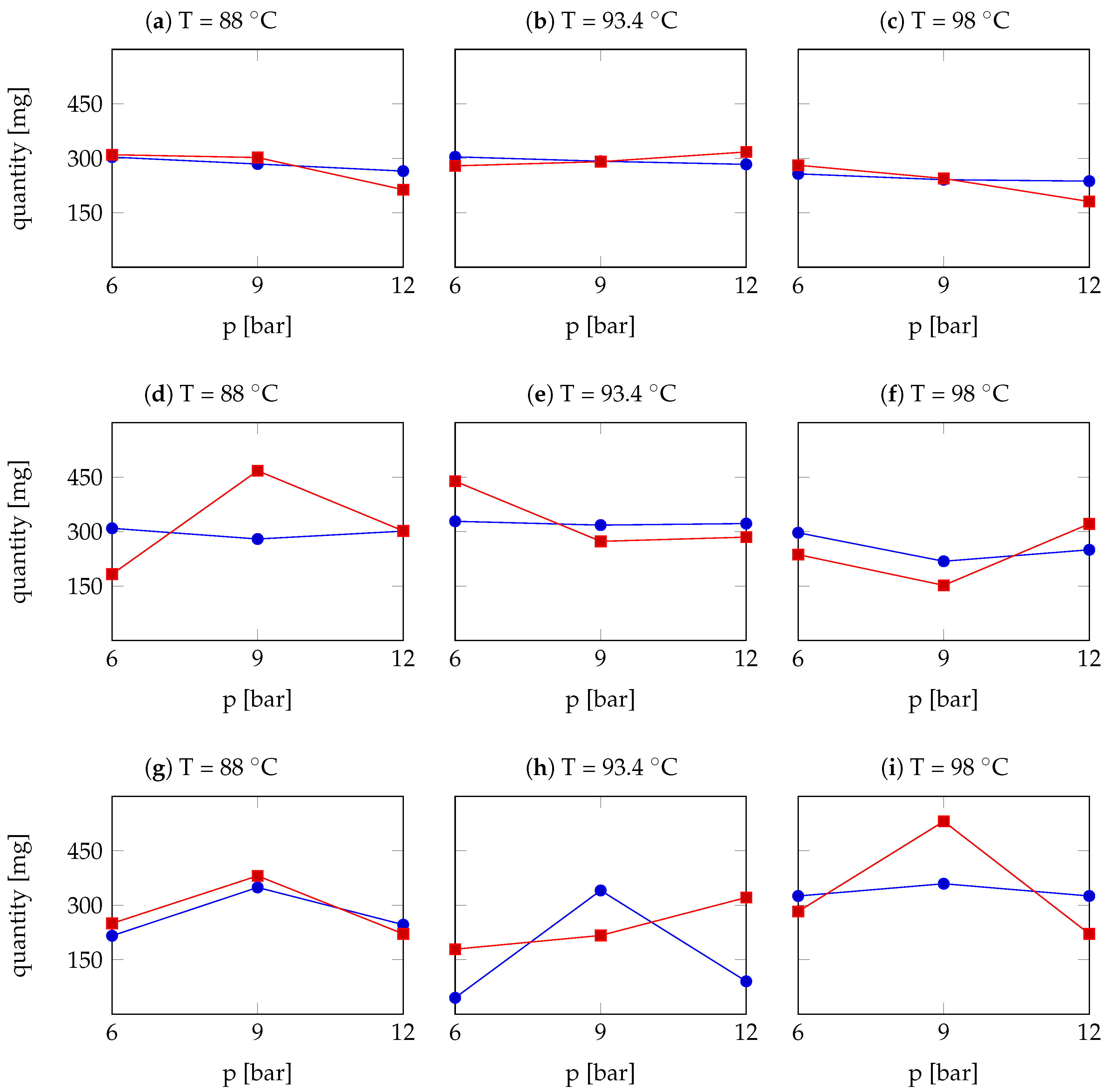
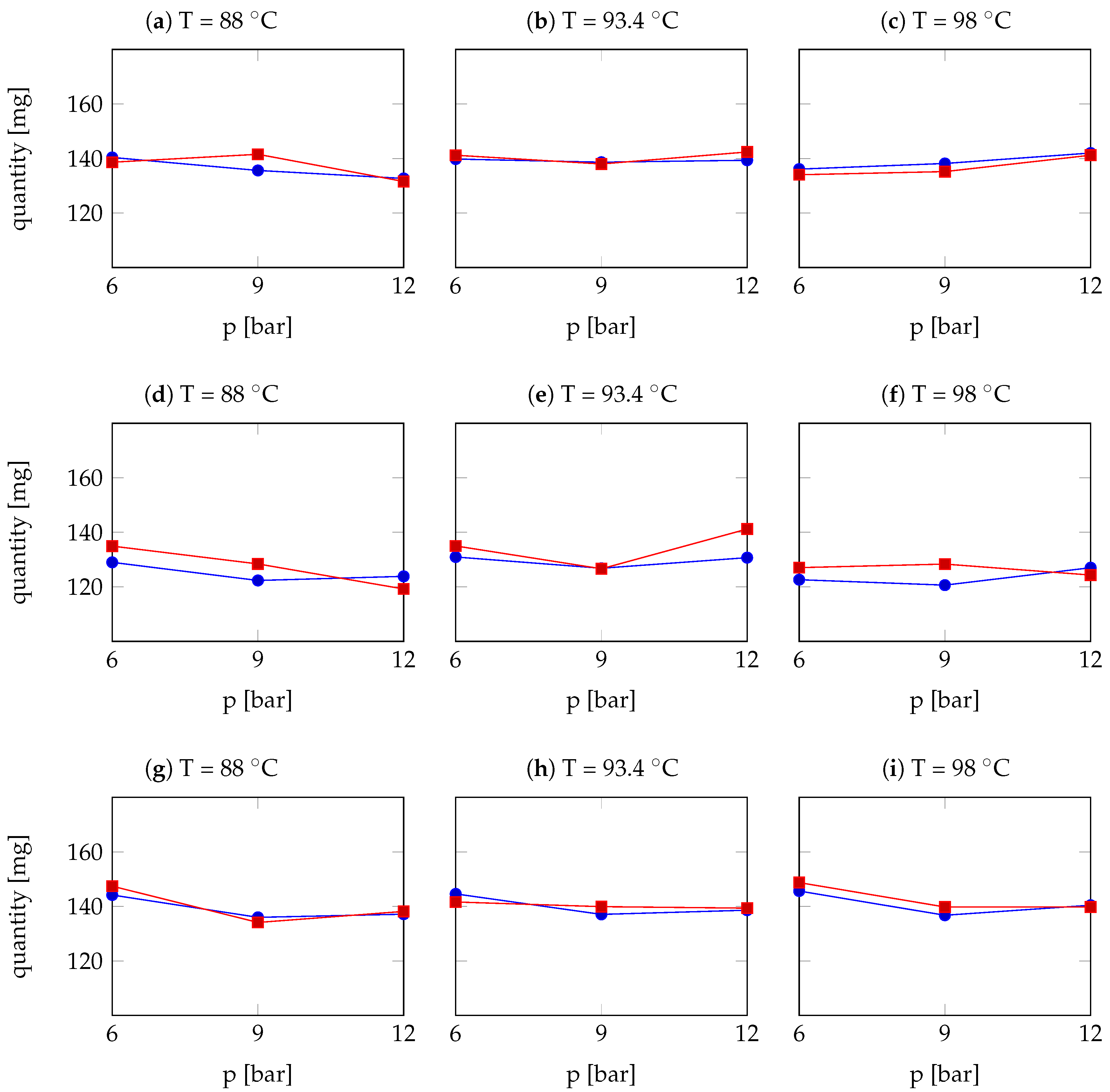

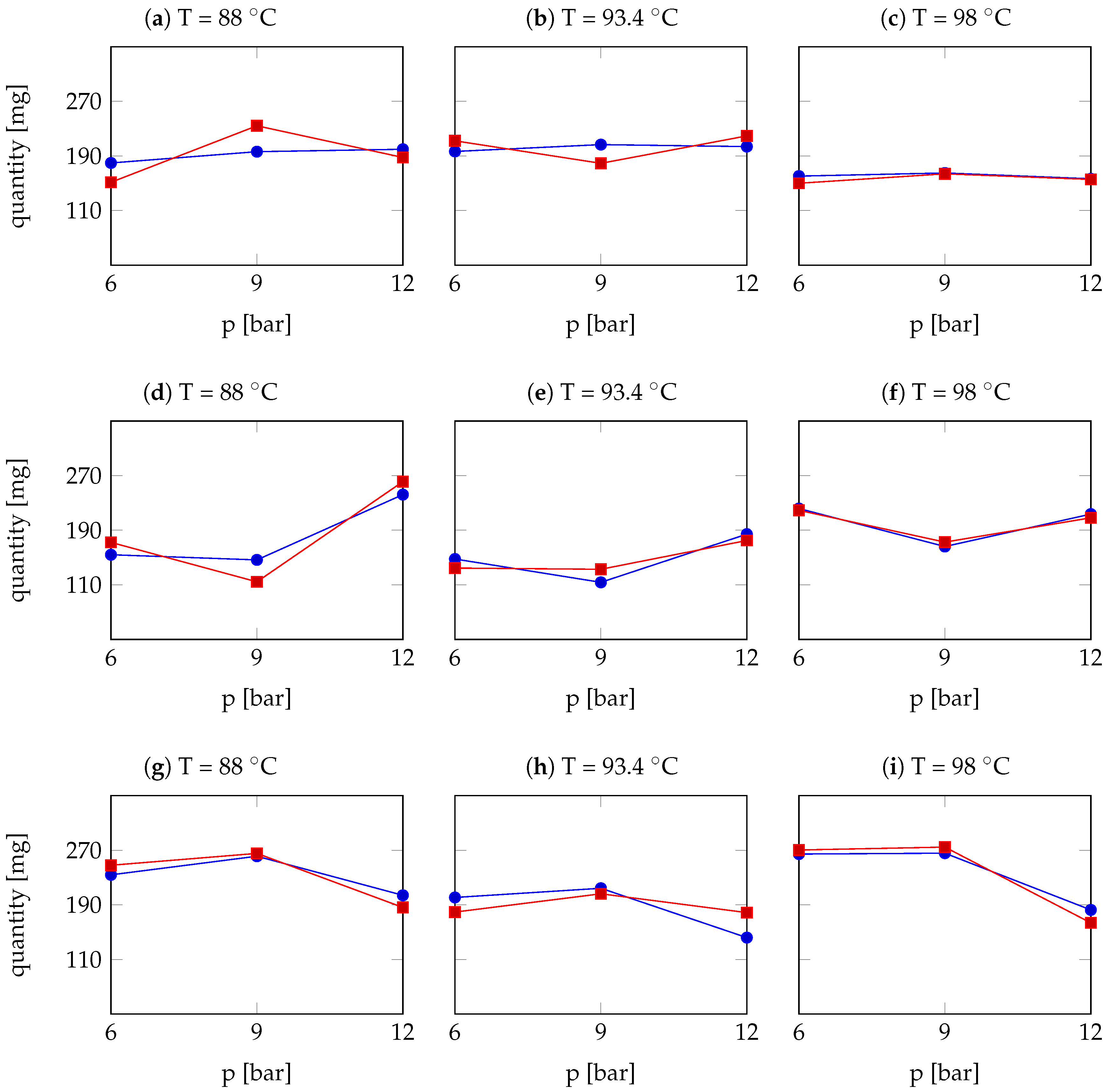
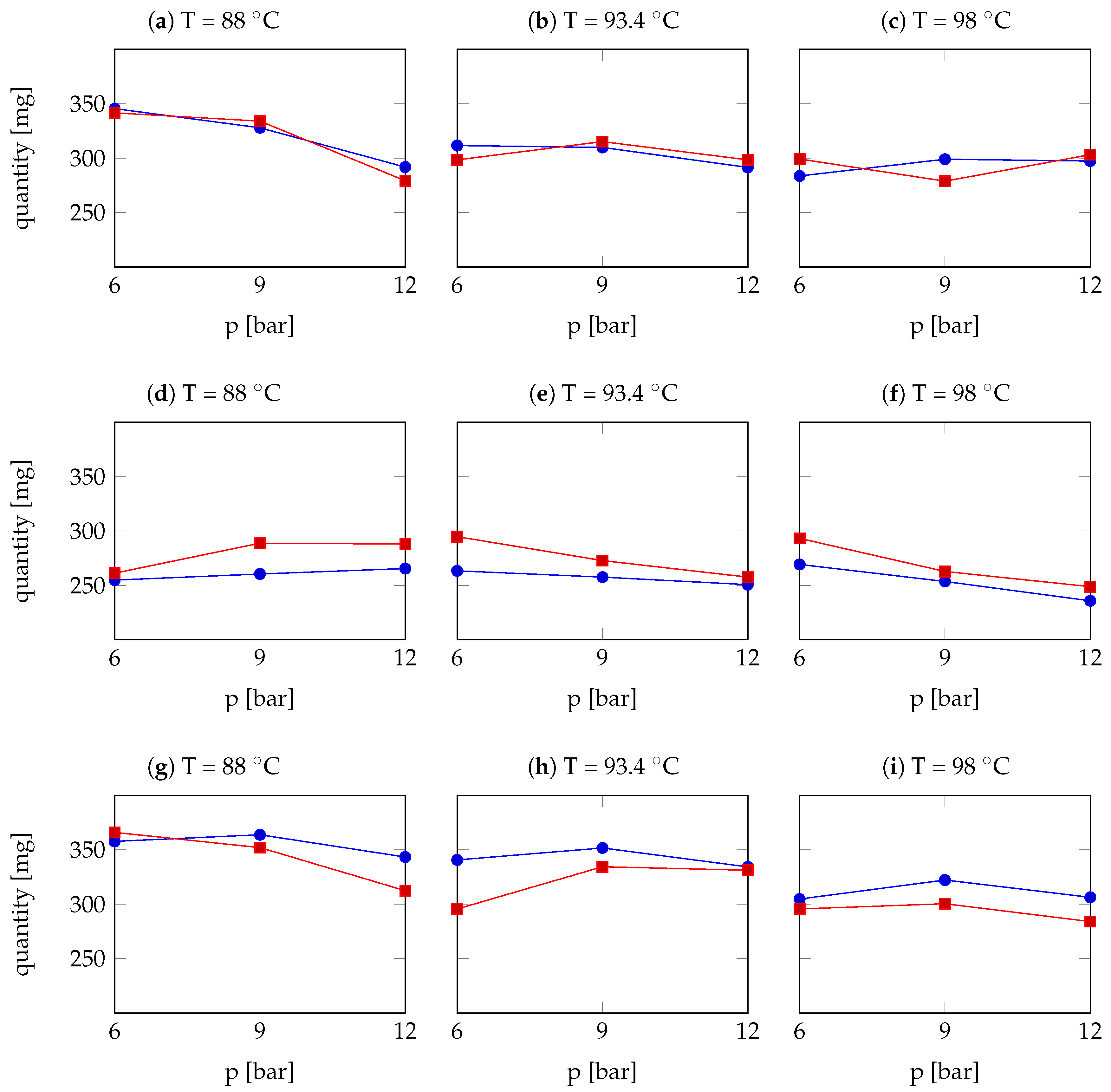
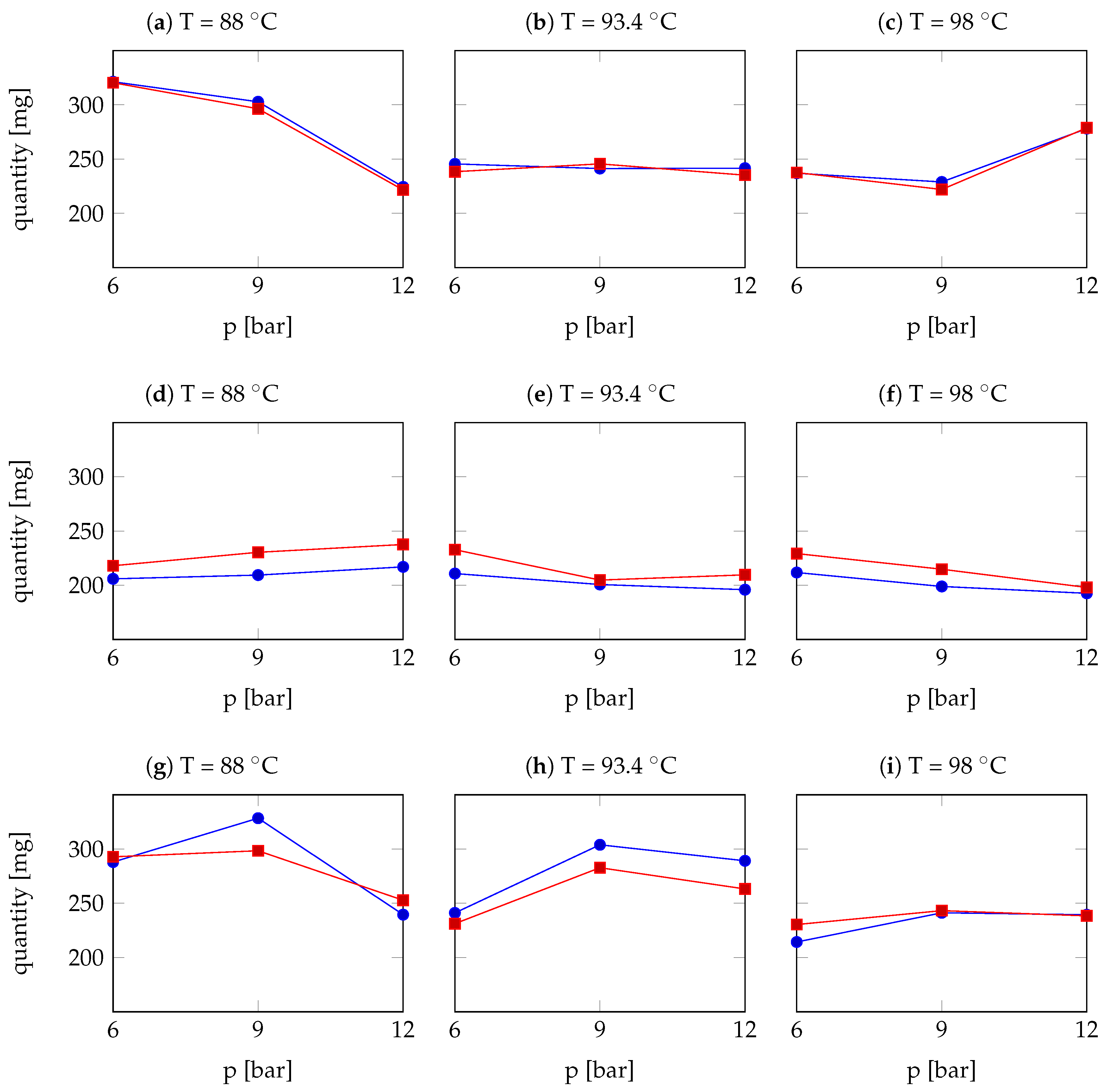
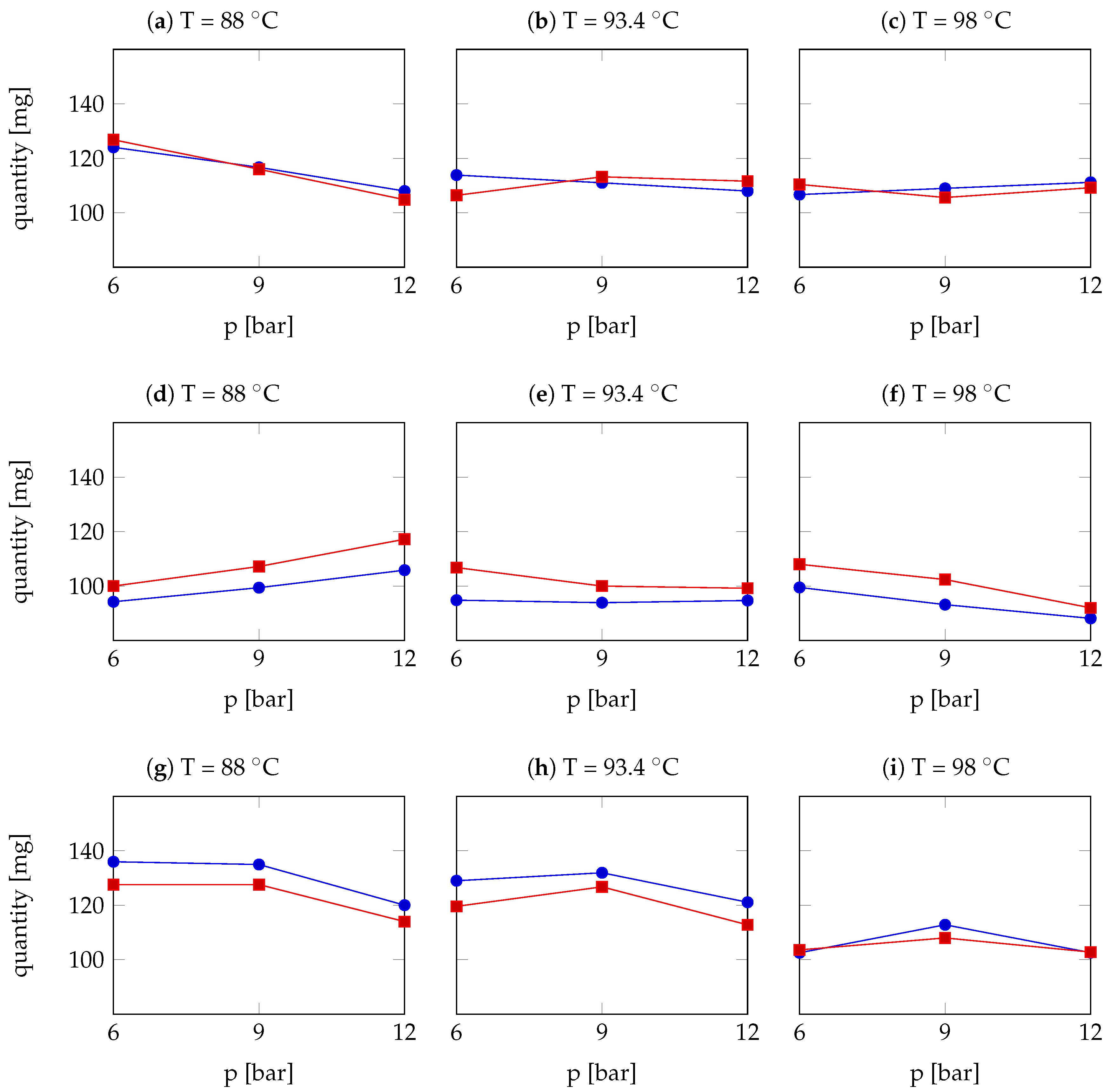

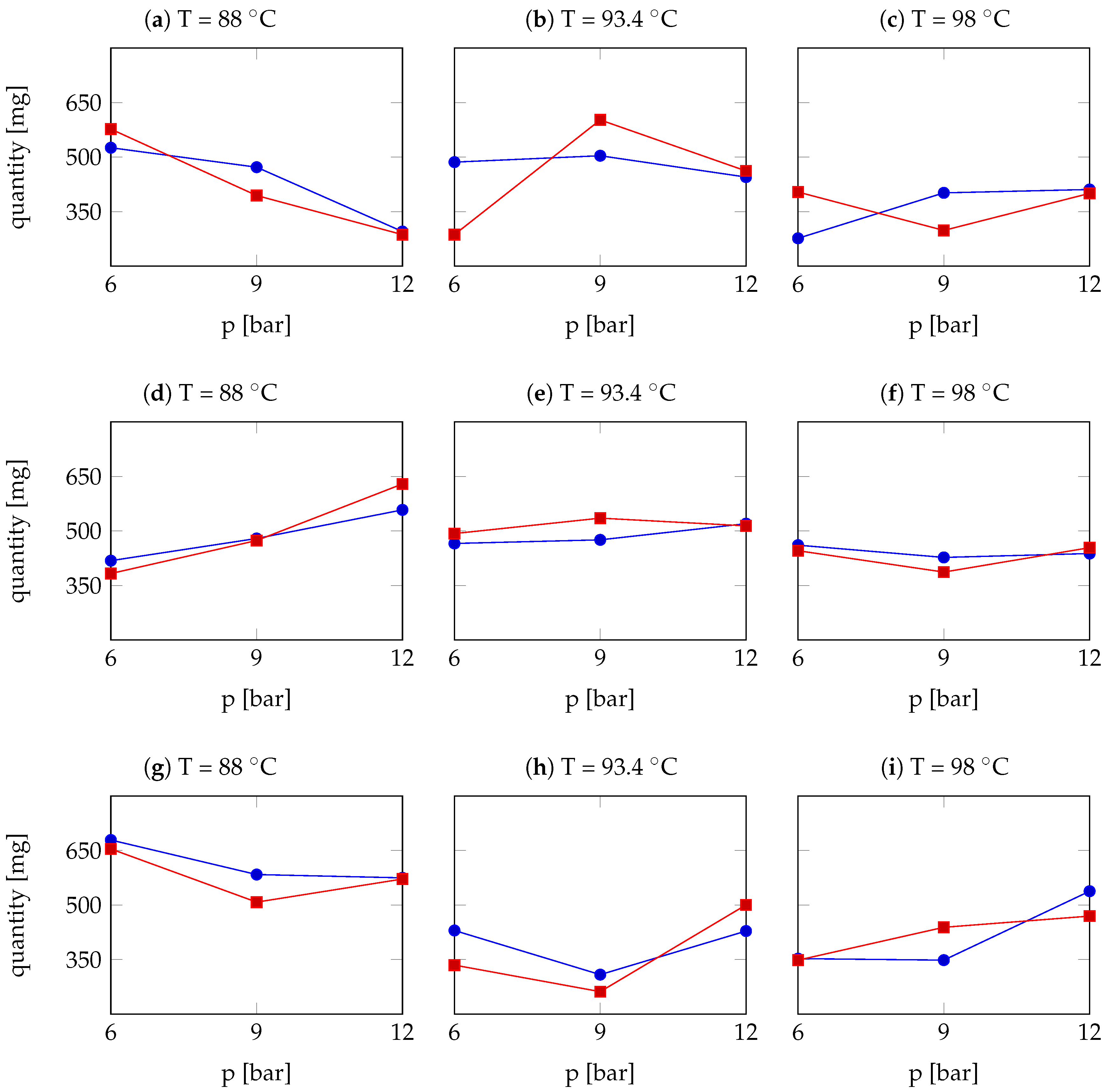

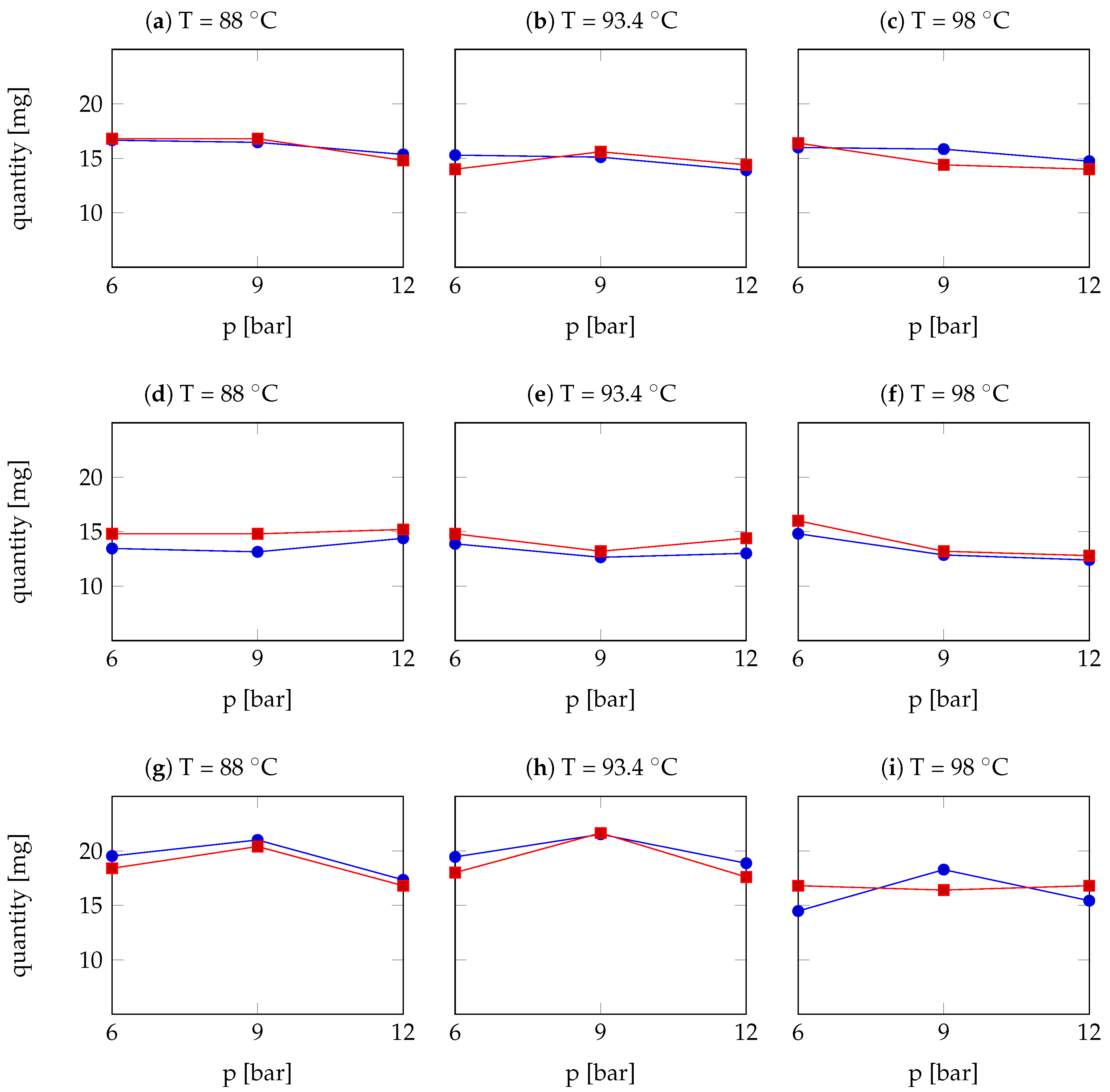
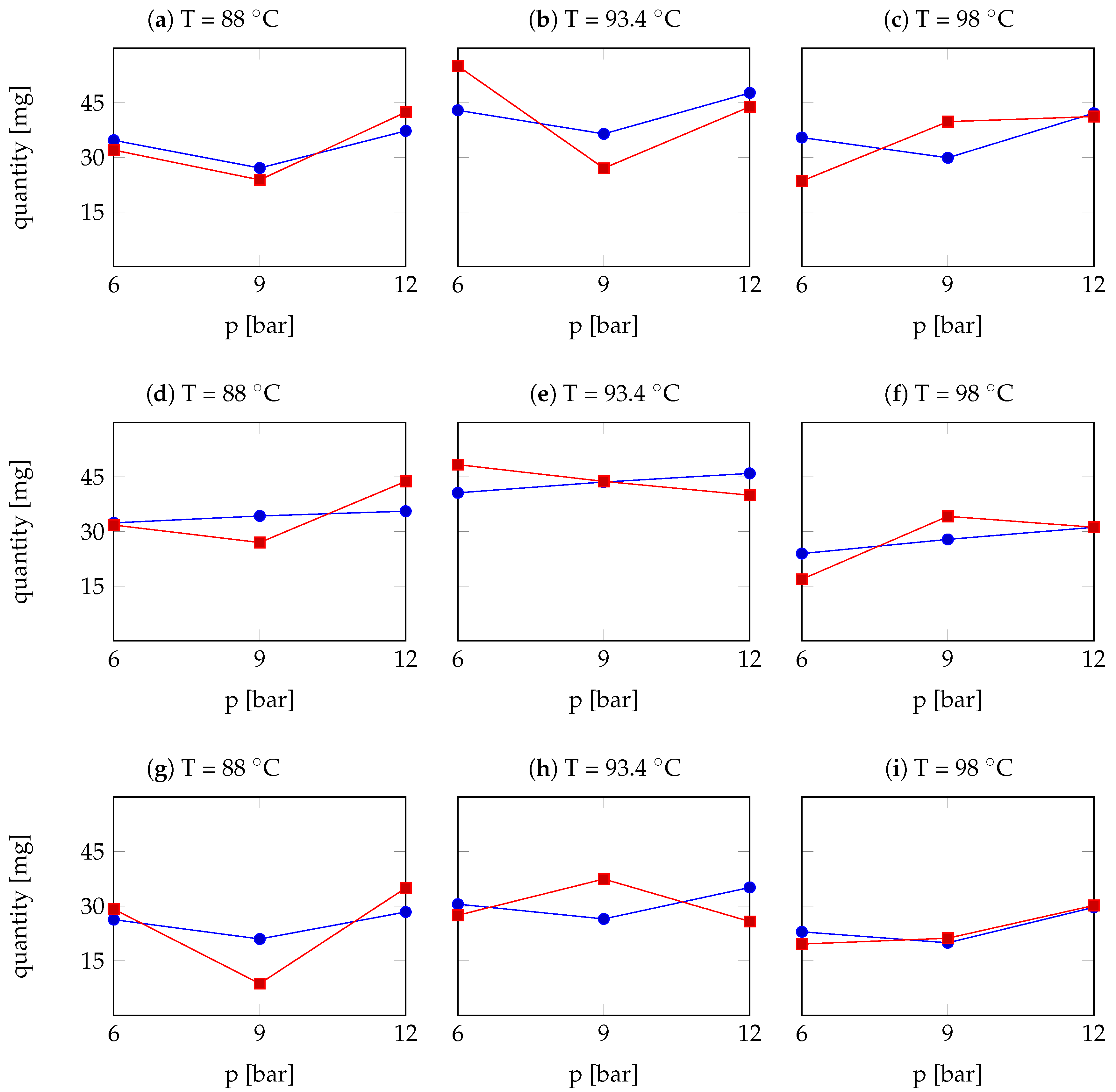
| Arabica Sample | T [°C] | p [bar] | r | Robusta Sample | T [°C] | p [bar] | r |
|---|---|---|---|---|---|---|---|
| A1 | 93.4 | 9 | O | R1 | 93.4 | 9 | O |
| A2 | 93.4 | 6 | O | R2 | 93.4 | 12 | O |
| A3 | 93.4 | 12 | O | R3 | 93.4 | 6 | O |
| A4 | 88 | 9 | O | R4 | 88 | 9 | O |
| A5 | 88 | 6 | O | R5 | 88 | 12 | O |
| A6 | 88 | 12 | O | R6 | 88 | 6 | O |
| A7 | 98 | 12 | O | R7 | 98 | 9 | O |
| A8 | 98 | 9 | O | R8 | 98 | 12 | O |
| A9 | 98 | 6 | O | R9 | 98 | 6 | O |
| A10 | 96 | 9 | O | R10 | 96 | 9 | O |
| A11 | 91 | 8 | O | R11 | 91 | 8 | O |
| A12 | 93.4 | 9 | C | R12 | 93.4 | 9 | C |
| A13 | 93.4 | 6 | C | R13 | 93.4 | 12 | C |
| A14 | 93.4 | 12 | C | R14 | 93.4 | 6 | C |
| A15 | 98 | 9 | C | R15 | 88 | 9 | C |
| A16 | 98 | 12 | C | R16 | 88 | 12 | C |
| A17 | 98 | 6 | C | R17 | 88 | 6 | C |
| A18 | 88 | 9 | C | R18 | 98 | 9 | C |
| A19 | 88 | 12 | C | R19 | 98 | 12 | C |
| A20 | 88 | 6 | C | R20 | 98 | 6 | C |
| A21 | 89 | 10 | C | R21 | 97 | 7 | C |
| A22 | 97 | 7 | C | R22 | 89 | 10 | C |
| A23 | 93.4 | 9 | F | R23 | 93.4 | 9 | F |
| A24 | 93.4 | 6 | F | R24 | 93.4 | 12 | F |
| A25 | 93.4 | 12 | F | R25 | 93.4 | 6 | F |
| A26 | 98 | 12 | F | R26 | 88 | 9 | F |
| A27 | 98 | 6 | F | R27 | 88 | 12 | F |
| A28 | 98 | 9 | F | R28 | 88 | 6 | F |
| A29 | 88 | 9 | F | R29 | 98 | 6 | F |
| A30 | 88 | 12 | F | R30 | 98 | 9 | F |
| A31 | 88 | 6 | F | R31 | 98 | 12 | F |
| A32 | 90 | 8 | F | R32 | 95 | 11 | F |
| A33 | 95 | 11 | F | R33 | 90 | 8 | F |
| Arabica Sample | TS (g/100 mL) | RSD (%) | Robusta Sample | TS (g/100 mL) | RSD (%) |
|---|---|---|---|---|---|
| A1 | 10.03 | 2.9 | R1 | 9.78 | 17.9 |
| A2 | 9.65 | 2.6 | R2 | 10.12 | 16.9 |
| A3 | 10.42 | 1.0 | R3 | 10.15 | 18.8 |
| A4 | 9.69 | 4.7 | R4 | 10.31 | 9.7 |
| A5 | 9.59 | 2.4 | R5 | 10.72 | 9.1 |
| A6 | 9.94 | 5.4 | R6 | 11.20 | 0.9 |
| A7 | 9.87 | 10.7 | R7 | 11.19 | 3.8 |
| A8 | 9.80 | 3.2 | R8 | 9.63 | 14 |
| A9 | 9.38 | 1.9 | R9 | 10.88 | 3.7 |
| A10 | 9.57 | 0.4 | R10 | 12.09 | 16.9 |
| A11 | 10.21 | 4.5 | R11 | 9.83 | 14.5 |
| A12 | 8.79 | 2.2 | R12 | 9.46 | 3.6 |
| A13 | 10.08 | 11.2 | R13 | 8.62 | 17.1 |
| A14 | 10.38 | 14.4 | R14 | 10.86 | 0.7 |
| A15 | 9.15 | 8.9 | R15 | 9.69 | 4.7 |
| A16 | 9.38 | 0.9 | R16 | 11.03 | 5.6 |
| A17 | 9.49 | 4.5 | R17 | 9.13 | 2.7 |
| A18 | 9.59 | 6.8 | R18 | 9.53 | 1.8 |
| A19 | 9.05 | 1.2 | R19 | 8.85 | 19.4 |
| A20 | 9.79 | 0.9 | R20 | 11.30 | 8.0 |
| A21 | 8.80 | 6.4 | R21 | 10.88 | 11.0 |
| A22 | 9.22 | 1.2 | R22 | 8.53 | 9.6 |
| A23 | 10.73 | 5.0 | R23 | 12.55 | 3.5 |
| A24 | 10.84 | 7.6 | R24 | 10.05 | 18.3 |
| A25 | 10.50 | 2.3 | R25 | 11.95 | 6.7 |
| A26 | 10.90 | 9.9 | R26 | 11.63 | 18.6 |
| A27 | 11.61 | 2.3 | R27 | 11.28 | 3.1 |
| A28 | 10.93 | 3.5 | R28 | 10.36 | 3.5 |
| A29 | 10.87 | 6.2 | R29 | 12.37 | 8.3 |
| A30 | 10.65 | 0.6 | R30 | 11.23 | 0.3 |
| A31 | 11.52 | 5.6 | R31 | 11.73 | 13.7 |
| A32 | 11.42 | 4.5 | R32 | 10.90 | 13.8 |
| A33 | 10.31 | 1.9 | R33 | 11.19 | 3.7 |
| Arabica Sample | Total Lipids (g/100 mL) | RSD (%) | Robusta Sample | Total Lipids (g/100 mL) | RSD (%) |
|---|---|---|---|---|---|
| A1 | 0.45 | 6.6 | R1 | 0.07 | 16.8 |
| A2 | 0.53 | 14.8 | R2 | 0.11 | 17.1 |
| A3 | 0.55 | 16.9 | R3 | 0.14 | 15.7 |
| A4 | 0.59 | 19.2 | R4 | 0.06 | 13.1 |
| A5 | 0.38 | 17.4 | R5 | 0.11 | 12.0 |
| A6 | 0.47 | 15.8 | R6 | 0.08 | 8.8 |
| A7 | 0.39 | 16.0 | R7 | 0.10 | 6.4 |
| A8 | 0.41 | 12.3 | R8 | 0.10 | 9.6 |
| A9 | 0.38 | 18.1 | R9 | 0.06 | 4.2 |
| A10 | 0.30 | 14.5 | R10 | 0.03 | 19.0 |
| A11 | 0.29 | 19.2 | R11 | 0.07 | 6.5 |
| A12 | 0.33 | 11.5 | R12 | 0.11 | 20.0 |
| A13 | 0.34 | 9.0 | R13 | 0.10 | 8.5 |
| A14 | 0.44 | 12.1 | R14 | 0.12 | 19.9 |
| A15 | 0.43 | 17.9 | R15 | 0.07 | 5.2 |
| A16 | 0.52 | 8.3 | R16 | 0.11 | 18.7 |
| A17 | 0.55 | 12.9 | R17 | 0.08 | 16.9 |
| A18 | 0.29 | 3.5 | R18 | 0.09 | 19.0 |
| A19 | 0.65 | 1.1 | R19 | 0.08 | 1.8 |
| A20 | 0.43 | 18.2 | R20 | 0.04 | 14.2 |
| A21 | 0.36 | 0.0 | R21 | 0.06 | 0.6 |
| A22 | 0.35 | 12.1 | R22 | 0.09 | 10.4 |
| A23 | 0.52 | 12.2 | R23 | 0.09 | 13.1 |
| A24 | 0.45 | 8.7 | R24 | 0.07 | 15.3 |
| A25 | 0.45 | 2.1 | R25 | 0.07 | 18.0 |
| A26 | 0.41 | 12.8 | R26 | 0.02 | 16.2 |
| A27 | 0.68 | 4.6 | R27 | 0.09 | 12.1 |
| A28 | 0.69 | 18.2 | R28 | 0.07 | 17.4 |
| A29 | 0.66 | 19.5 | R29 | 0.05 | 11.5 |
| A30 | 0.47 | 8.4 | R30 | 0.05 | 1.3 |
| A31 | 0.62 | 17.8 | R31 | 0.08 | 9.4 |
| A32 | 0.59 | 4.8 | R32 | 0.09 | 5.4 |
| A33 | 0.47 | 14.8 | R33 | 0.09 | 7.9 |
| Sample | TR | TA | AA | CA | 3-CQA | 5-CQA | CF | FA | 3,5- diCQA | Tot CQA | Tot OA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | 3.41 | 3.45 | 7.27 | 16.18 | 2.95 | 5.89 | 5.27 | 0.27 | 0.30 | 9.13 | 26.89 |
| A2 | 3.55 | 3.53 | 6.98 | 17.92 | 3.05 | 5.74 | 5.45 | 0.32 | 0.32 | 9.11 | 28.43 |
| A3 | 3.55 | 3.56 | 7.94 | 17.40 | 3.11 | 6.01 | 5.40 | 0.29 | 0.32 | 9.44 | 28.89 |
| A4 | 3.54 | 3.54 | 7.55 | 16.56 | 2.94 | 5.95 | 5.44 | 0.30 | 0.30 | 9.20 | 27.66 |
| A5 | 3.47 | 3.47 | 7.75 | 14.86 | 2.86 | 5.44 | 5.19 | 0.30 | 0.30 | 8.59 | 26.07 |
| A6 | 3.28 | 3.29 | 5.34 | 15.58 | 2.69 | 5.21 | 4.77 | 0.30 | 0.28 | 8.17 | 24.21 |
| A7 | 3.52 | 3.53 | 4.52 | 11.25 | 3.02 | 5.71 | 5.34 | 0.34 | 0.31 | 9.04 | 19.31 |
| A8 | 3.39 | 3.38 | 6.11 | 14.08 | 2.82 | 5.24 | 5.20 | 0.28 | 0.30 | 8.36 | 23.57 |
| A9 | 3.36 | 3.35 | 7.02 | 13.65 | 2.81 | 5.20 | 5.10 | 0.28 | 0.30 | 8.31 | 24.02 |
| A10 | 3.19 | 3.19 | 6.50 | 13.01 | 2.66 | 5.00 | 4.85 | 0.27 | 0.28 | 7.94 | 22.70 |
| A11 | 3.86 | 3.84 | 4.67 | 8.89 | 3.18 | 5.93 | 5.80 | 0.32 | 0.32 | 9.42 | 17.40 |
| A12 | 3.13 | 3.17 | 6.83 | 12.98 | 2.47 | 4.53 | 4.76 | 0.25 | 0.27 | 7.26 | 22.98 |
| A13 | 3.37 | 3.38 | 10.97 | 16.04 | 2.58 | 4.79 | 5.00 | 0.27 | 0.27 | 7.65 | 30.39 |
| A14 | 3.53 | 3.53 | 7.12 | 10.73 | 2.73 | 5.00 | 5.24 | 0.27 | 0.29 | 8.02 | 21.38 |
| A15 | 3.20 | 3.21 | 3.80 | 6.32 | 2.44 | 4.62 | 4.85 | 0.25 | 0.27 | 7.32 | 13.33 |
| A16 | 3.12 | 3.11 | 8.04 | 10.50 | 2.37 | 4.37 | 4.61 | 0.23 | 0.25 | 7.00 | 21.65 |
| A17 | 3.19 | 3.18 | 5.92 | 13.10 | 2.45 | 5.76 | 4.79 | 0.23 | 0.21 | 8.42 | 22.19 |
| A18 | 3.23 | 3.21 | 11.69 | 15.90 | 2.43 | 4.51 | 4.71 | 0.25 | 0.25 | 7.19 | 30.80 |
| A19 | 2.99 | 2.98 | 7.55 | 13.53 | 2.26 | 4.14 | 4.41 | 0.23 | 0.23 | 6.64 | 24.07 |
| A20 | 3.39 | 3.37 | 4.57 | 11.46 | 2.55 | 4.77 | 5.00 | 0.24 | 0.27 | 7.59 | 19.40 |
| A21 | 3.32 | 3.31 | 13.09 | 16.42 | 2.53 | 4.83 | 4.82 | 0.27 | 0.26 | 7.62 | 32.81 |
| A22 | 3.00 | 3.01 | 6.64 | 10.19 | 2.37 | 4.43 | 4.45 | 0.24 | 0.25 | 7.05 | 19.84 |
| A23 | 3.48 | 3.50 | 5.42 | 11.40 | 2.97 | 5.51 | 5.43 | 0.30 | 0.32 | 8.80 | 20.32 |
| A24 | 3.54 | 3.54 | 4.47 | 7.94 | 2.96 | 5.64 | 5.53 | 0.32 | 0.32 | 8.93 | 15.95 |
| A25 | 3.49 | 3.48 | 8.04 | 16.56 | 2.93 | 5.50 | 5.44 | 0.31 | 0.32 | 8.75 | 28.08 |
| A26 | 3.49 | 3.49 | 5.53 | 18.06 | 2.99 | 5.64 | 5.50 | 0.33 | 0.33 | 8.95 | 27.09 |
| A27 | 3.71 | 3.72 | 7.07 | 20.49 | 3.20 | 5.99 | 5.98 | 0.33 | 0.32 | 9.51 | 31.28 |
| A28 | 3.48 | 3.49 | 13.28 | 17.08 | 2.98 | 5.54 | 5.41 | 0.32 | 0.31 | 8.84 | 33.86 |
| A29 | 3.38 | 3.35 | 9.53 | 16.36 | 2.87 | 5.30 | 5.65 | 0.30 | 0.30 | 8.47 | 29.24 |
| A30 | 3.46 | 3.45 | 5.53 | 16.51 | 2.96 | 5.53 | 5.49 | 0.29 | 0.30 | 8.80 | 25.49 |
| A31 | 3.70 | 3.69 | 6.25 | 18.78 | 3.15 | 5.91 | 5.83 | 0.34 | 0.33 | 9.38 | 28.73 |
| A32 | 3.71 | 3.69 | 5.68 | 18.44 | 3.17 | 5.88 | 5.73 | 0.30 | 0.32 | 9.37 | 27.80 |
| A33 | 2.75 | 2.77 | 4.67 | 14.02 | 2.36 | 4.52 | 4.40 | 0.24 | 0.26 | 7.14 | 21.46 |
| Average | 3.39 | 3.39 | 7.07 | 14.31 | 2.78 | 5.27 | 5.18 | 0.28 | 0.29 | 8.35 | 24.77 |
| Sample | TR | TA | AA | CA | 3-CQA | 5-CQA | CF | FA | 3,5- diCQA | Tot CQA | Tot OA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| R1 | 2.83 | 2.75 | 15.06 | 18.93 | 2.28 | 3.41 | 7.88 | 0.39 | 0.45 | 6.14 | 36.74 |
| R2 | 2.79 | 2.58 | 11.55 | 17.02 | 2.19 | 3.21 | 7.46 | 0.36 | 0.48 | 5.88 | 31.15 |
| R3 | 2.66 | 2.59 | 7.17 | 19.07 | 2.23 | 3.26 | 7.46 | 0.35 | 0.47 | 5.96 | 28.83 |
| R4 | 2.90 | 2.72 | 9.86 | 20.26 | 2.44 | 4.40 | 8.35 | 0.42 | 0.57 | 7.42 | 32.84 |
| R5 | 2.62 | 2.51 | 7.17 | 19.22 | 2.08 | 3.01 | 6.98 | 0.37 | 0.45 | 5.54 | 28.90 |
| R6 | 3.17 | 3.06 | 14.43 | 17.95 | 2.56 | 4.85 | 8.54 | 0.42 | 0.60 | 8.01 | 35.44 |
| R7 | 2.64 | 2.52 | 7.46 | 18.21 | 2.09 | 3.00 | 6.97 | 0.36 | 0.46 | 5.55 | 28.18 |
| R8 | 2.73 | 2.65 | 10.01 | 21.04 | 2.20 | 4.27 | 7.58 | 0.35 | 0.50 | 6.97 | 33.69 |
| R9 | 2.76 | 2.66 | 10.10 | 21.15 | 2.24 | 3.22 | 7.48 | 0.41 | 0.48 | 5.95 | 33.91 |
| R10 | 2.45 | 2.41 | 8.90 | 19.19 | 2.02 | 3.47 | 6.92 | 0.39 | 0.48 | 5.97 | 30.50 |
| R11 | 2.93 | 2.86 | 16.94 | 23.81 | 2.39 | 3.46 | 8.12 | 0.46 | 0.51 | 6.35 | 43.60 |
| R12 | 2.50 | 2.43 | 13.38 | 18.44 | 1.93 | 2.79 | 6.82 | 0.33 | 0.40 | 5.12 | 34.25 |
| R13 | 2.48 | 2.40 | 12.85 | 18.50 | 2.23 | 2.65 | 6.44 | 0.36 | 0.36 | 5.24 | 33.74 |
| R14 | 2.67 | 2.64 | 12.32 | 15.64 | 2.23 | 3.15 | 7.37 | 0.37 | 0.44 | 5.82 | 30.59 |
| R15 | 2.68 | 2.69 | 11.84 | 21.81 | 2.21 | 3.13 | 7.22 | 0.37 | 0.42 | 5.75 | 36.34 |
| R16 | 2.93 | 2.75 | 15.73 | 19.16 | 2.23 | 3.31 | 7.20 | 0.38 | 0.40 | 5.95 | 37.64 |
| R17 | 2.50 | 2.41 | 9.57 | 15.78 | 2.19 | 2.87 | 6.53 | 0.37 | 0.39 | 5.46 | 27.77 |
| R18 | 2.56 | 2.41 | 9.67 | 15.73 | 2.05 | 2.95 | 6.57 | 0.33 | 0.37 | 5.36 | 27.81 |
| R19 | 2.30 | 2.26 | 11.35 | 14.17 | 1.93 | 2.67 | 6.22 | 0.32 | 0.35 | 4.94 | 27.79 |
| R20 | 2.70 | 2.55 | 11.14 | 17.64 | 2.25 | 3.04 | 7.33 | 0.40 | 0.44 | 5.73 | 31.33 |
| R21 | 2.67 | 2.60 | 12.75 | 16.10 | 2.26 | 3.16 | 7.31 | 0.36 | 0.43 | 5.84 | 31.45 |
| R22 | 2.57 | 2.56 | 13.62 | 16.30 | 2.40 | 2.83 | 6.99 | 0.35 | 0.38 | 5.61 | 32.48 |
| R23 | 3.17 | 2.99 | 6.54 | 18.41 | 2.64 | 3.92 | 8.36 | 0.54 | 0.51 | 7.07 | 27.95 |
| R24 | 2.82 | 2.79 | 12.51 | 19.56 | 2.48 | 3.57 | 8.28 | 0.44 | 0.53 | 6.58 | 34.87 |
| R25 | 2.99 | 2.40 | 8.37 | 16.30 | 2.21 | 3.17 | 7.39 | 0.45 | 0.40 | 5.78 | 27.08 |
| R26 | 3.19 | 3.19 | 12.70 | 21.47 | 3.09 | 3.85 | 8.80 | 0.51 | 0.52 | 7.46 | 37.36 |
| R27 | 2.85 | 2.71 | 14.29 | 18.76 | 2.35 | 3.48 | 7.81 | 0.42 | 0.49 | 6.32 | 35.76 |
| R28 | 3.19 | 3.14 | 16.36 | 20.66 | 2.83 | 3.96 | 9.15 | 0.46 | 0.53 | 7.32 | 40.16 |
| R29 | 2.59 | 2.45 | 8.71 | 16.76 | 2.26 | 2.99 | 7.39 | 0.42 | 0.51 | 5.77 | 27.92 |
| R30 | 2.70 | 2.52 | 10.97 | 17.52 | 2.26 | 3.34 | 7.51 | 0.41 | 0.48 | 6.08 | 31.01 |
| R31 | 2.57 | 2.46 | 11.74 | 16.71 | 2.33 | 3.19 | 7.10 | 0.42 | 0.44 | 5.96 | 30.90 |
| R32 | 2.54 | 2.49 | 8.80 | 18.06 | 2.30 | 3.26 | 7.68 | 0.40 | 0.45 | 6.01 | 29.35 |
| R33 | 2.78 | 2.64 | 9.00 | 18.09 | 2.22 | 3.33 | 7.58 | 0.41 | 0.49 | 6.05 | 29.73 |
| Average | 2.74 | 2.63 | 11.30 | 18.41 | 2.29 | 3.34 | 7.48 | 0.40 | 0.46 | 6.09 | 32.33 |
| p (bar) | |||
|---|---|---|---|
| 6 | 9 | 12 | |
| 61.18 | 91.78 | 122.37 | |
| 6.5 × | 6.5 × | 6.5 × | |
| 0 | 0 | 0 | |
| CF | CQA | TR | CA | AA | TA | FA | LP | |
|---|---|---|---|---|---|---|---|---|
| 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 12,540 | 22,130 | 7970 | 39,970 | 11,870 | 7700 | 790 | 138,700 | |
| 18,580 | 16,080 | 5960 | 40,920 | 27,910 | 5970 | 1060 | 96,200 |
| (91, 8, O) | (96, 9, O) | (89, 10, G) | (97, 7, G) | (90, 8, F) | (95, 11, F) | |
|---|---|---|---|---|---|---|
| CF | 215.2 (7.2) | 216.0 (11.4) | 186.9 (3.0) | 192.0 (7.9) | 221.2 (3.5) | 213.9 (21.5) |
| CQA | 369.6 (1.9) | 351.4 (10.6) | 324.8 (6.6) | 304.5 (8.0) | 347.6 (7.3) | 349.5 (22.4) |
| TR | 135.4 (12.2) | 136.1 (6.5) | 125.8 (5.4) | 126.9 (5.7) | 140.3 (5.3) | 140.2 (27.2) |
| CA | 697.2 (96.1) | 634.2 (21.8) | 542.2 (17.4) | 493.4 (21.1) | 523.2 (29.0) | 541.4 (3.5) |
| AA | 297.8 (59.6) | 275.2 (5.9) | 299.0 (42.9) | 298.8 (12.5) | 336.3 (48.1) | 321.4 (72.2) |
| TA | 138.4 (10.0) | 138.8 (8.6) | 123.8 (6.5) | 123.4 (2.5) | 138.1 (6.4) | 137.6 (24.3) |
| FA | 10.5 (17.2) | 10.8 (0.3) | 9.8 (9.7) | 9.7 (0.2) | 12.0 (1.4) | 12.3 (30.0) |
| LP | 207.0 (76.0) | 188.9 (55.8) | 151.9 (6.7) | 172.2 (24.6) | 232.4 (1.2) | 188.5 (0.9) |
| (91, 8, O) | (96, 9, O) | (89, 10, G) | (97, 7, G) | (90, 8, F) | (95, 11, F) | |
|---|---|---|---|---|---|---|
| CF | 322.5 (0.7) | 303.3 (9.6) | 261.2 (6.6) | 264.0 (9.7) | 362.0 (19.4) | 336.1 (9.4) |
| CQA | 270.6 (6.4) | 228.5 (4.3) | 208.4 (7.1) | 207.0 (11.5) | 318.1 (31.7) | 290.4 (20.8) |
| TR | 115.1 (1.8) | 109.6 (11.8) | 99.8 (2.9) | 96.5 (9.7) | 136.7 (22.9) | 122.7 (20.8) |
| CA | 729.5 (23.4) | 763.7 (0.5) | 772.4 (18.4) | 648.3 (0.7) | 844.9 (16.8) | 781.6 (8.2) |
| AA | 520.9 (23.1) | 465.3 (30.7) | 505.1 (7.3) | 453.7 (11.0) | 503.4 (39.8) | 370.3 (5.2) |
| TA | 108.9 (4.8) | 104.0 (7.9) | 99.7 (2.6) | 92.6 (10.9) | 131.7 (24.7) | 115.3 (15.8) |
| FA | 15.6 (15.2) | 15.3 (1.8) | 13.2 (5.8) | 13.8 (3.9) | 21.6 (31.9) | 19.8 (23.6) |
| LP | 34.9 (29.1) | 34.4 (157) | 38.9 (14.6) | 30.8 (24.5) | 24.9 (30.0) | 30.0 (18.0) |
| CF | CQA | TR | CA | AA | TA | FA | LP | |
|---|---|---|---|---|---|---|---|---|
| A | 9.1 | 9.4 | 10.4 | 31.0 | 40.2 | 9.7 | 9.8 | 27.5 |
| R | 9.2 | 13.6 | 11.7 | 11.3 | 19.5 | 11.1 | 13.7 | 45.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angeloni, S.; Giacomini, J.; Maponi, P.; Perticarini, A.; Vittori, S.; Cognigni, L.; Fioretti, L. Computer Percolation Models for Espresso Coffee: State of the Art, Results and Future Perspectives. Appl. Sci. 2023, 13, 2688. https://doi.org/10.3390/app13042688
Angeloni S, Giacomini J, Maponi P, Perticarini A, Vittori S, Cognigni L, Fioretti L. Computer Percolation Models for Espresso Coffee: State of the Art, Results and Future Perspectives. Applied Sciences. 2023; 13(4):2688. https://doi.org/10.3390/app13042688
Chicago/Turabian StyleAngeloni, Simone, Josephin Giacomini, Pierluigi Maponi, Alessia Perticarini, Sauro Vittori, Luca Cognigni, and Lauro Fioretti. 2023. "Computer Percolation Models for Espresso Coffee: State of the Art, Results and Future Perspectives" Applied Sciences 13, no. 4: 2688. https://doi.org/10.3390/app13042688
APA StyleAngeloni, S., Giacomini, J., Maponi, P., Perticarini, A., Vittori, S., Cognigni, L., & Fioretti, L. (2023). Computer Percolation Models for Espresso Coffee: State of the Art, Results and Future Perspectives. Applied Sciences, 13(4), 2688. https://doi.org/10.3390/app13042688








