Abstract
In recent years, foliar application of elicitors to the vineyard has been increasingly used, in particular, elicitation with methyl jasmonate (MeJ). However, due to the high cost of this compound, it is necessary to find a form of application in which the amount to be used is considerably reduced. Therefore, the aim of this work was study for the first time the influence of foliar application of nanoparticles doped with MeJ (ACP-MeJ) and foliar application of methyl jasmonate (MeJ), using a dose of 1 mM versus 10 mM, respectively, on volatile composition of Tempranillo grapes during two consecutive vintages. Grape volatile composition was determined by SPME-GC-MS. The obtained results reveal that MeJ application increased the concentration of terpenoids, and total C6 compounds in 2019 and 2020, and C13 norisoprenoids in 2019. In addition, ACP-MeJ enhanced the amount of terpenoids, and benzenoids in 2020. These are encouraging results considering that the ACP-MeJ dose was 10 times lower than that of MeJ. Therefore, the foliar application of MeJ supported on nanoparticles could be a tool in order to improve grape volatile composition, favoring a more viable and sustainable viticulture.
1. Introduction
Aroma is one of the most important parameters to determine must and wine quality, also influences the grape flavor and contributes to the sensory character of the wine [1,2,3,4]. The grape volatile compounds belong to several groups: terpenoids, C13 norisoprenoides, benzenoid compounds, esters, C6 compounds, alcohols, thiols and methoxypyrazines [5,6,7]. The amount of these compounds depend on several factors such as grape variety, season, terroir, grape maturity, viticultural practices, etc. [1,8,9,10]. Foliar fertilization is a technique that is increasingly used as allows a quick and efficient assimilation of the products applied to the plant, reducing soil contamination and costs [10,11,12].
Foliar application of elicitors, molecules capable of activating the defensive systems of plants, can increase the synthesis of secondary metabolites [12,13,14]. Methyl jasmonate is a volatile organic compound derived from jasmonic acid [15,16]. This elicitor has been mainly implicated as a mediator of plant responses triggered by wounding and insect feeding and is involved in the pathogen resistance [15,17,18]. Foliar application of methyl jasmonate has been shown to increase the synthesis of secondary metabolites such as amino acids [19], phenolic compounds [14,18,20], and volatile compounds [5,18,19,20,21]. Despite this, methyl jasmonate is a compound with a very high cost and with low chemical stability.
In the last decade, nanotechnology has opened new horizons in several disciplines, including and agriculture [22]. Nanotechnology is providing very interesting results in agriculture by improving the efficiency of agrochemicals [23,24]. Specifically in viticulture, there are few studies [25] in which nanocarriers have been applied to vineyards to improve the efficiency of fertilizers (i.e., urea) or elicitors (i.e., MeJ) [26,27,28,29,30]. In this line, biomimetic calcium phosphate nanoparticles (ACP-NPs) have been proposed as promising MeJ nanocarrier providing slow release kinetic and protection against thermal degradation [31]. ACP-NPs are non-toxic and biocompatible nanomaterials widely used in biomedicine for drug delivery, dental remineralization or bone tissue engineering [32]. But, the effect of the foliar application of this nanoelicitor on grape aromatic composition has not been studied so far, only in the wine volatile composition, i.e., fermentative aromas [33].
Hence, this work aims at evaluating the influence of foliar application of nanoparticles doped with MeJ (ACP-MeJ) and foliar application of methyl jasmonate (MeJ) in conventional form on volatile composition of Vitis vinifera L. cv. Tempranillo grapes during two vintages.
2. Materials and Methods
2.1. Vineyard Site, Grapevine Treatments and Samples
This study was conducted, during the 2019 and 2020 vintages, on Vitis vinifera L. cv. Tempranillovines belonging to an experimental vineyard located at Finca La Grajera, Logroño, La Rioja (Spain). These vines were planted in 1997 using R-110 rootstock and treated according to local viticultural practices. They were trained in a VSP (vertical shoot positioned) trellis system, with a spacing between vines of 2.80 m between rows, and 1.25 m within the same row. For further information, climatic data were obtained from the Agroclimatic Information Service (SIAR), which were collected by an automatic weather station located near the area. During 2019, from the beginning of April to 1 September, the accumulated rainfall was 247.8 L/m2, and the average temperatures were: 27 °C the maximum, 13.8 °C the mean, and 3.7 °C the minimum. For the year 2020, in the same period, the accumulated rainfall was 217.8 L/m2, and the average temperatures were: 26.3 °C the maximum, 13.8 °C the mean, and 3.7 °C the minimum. Foliar applications of free methyl jasmonate (MeJ) and amorphous calcium phosphate nanoparticles functionalized with MeJ (ACP-MeJ) were studied. To carry out the field experiments, free MeJ aqueous solution (10 mM) and ACP-MeJ aqueous suspension (1 mM MeJ) were prepared following previous [19,27,34]. Tween 80 were used in both cases as wetting agent (1 mL/L). ACP-MeJ nanoparticles were synthesized and fully characterized as described in detail elsewhere [31]. All treatments were applied first at veraison and second one week later. The concentration of treatment applied to the leaves of each plant was 200 mL/plant in each of the two applications. For the control only the plants were sprayed with the aqueous solution of Tween 80. Each of the treatments was carried out in triplicate, and each replicate consisted of 10 vines. All treatments were arranged in a complete randomized block design.
The berries were harvested at their optimum point of technological maturity (weight of 100 berries constant, and 13% (v/v) of probable alcohol). Once harvested, they were destemmed and crushed until the must was obtained. The general parameters of all the musts were then measured, and aliquots of each must sample were frozen (−20 °C) for subsequent analysis of the aromatic composition.
2.2. General Parameters Determination
Enological parameters (°Brix, probable alcohol, pH, total acidity…) were determined by official methods established by the OIV [35]. The remaining general parameters such as glucose + fructose fractions, glucose (and fructose indirectly, as subtraction of glucose + fructose − glucose), malic acid, total phenols and nitrogen, were determined by enzymatic methods, with the Miura One equipment (TDI, Barcelona, Spain). The results obtained for these parameters are shown as the mean ± standard deviation (n = 3).
2.3. Analysis of Grape Volatile Compounds by HS-SPME-GC-MS
Determination of volatile compounds in the musts was carried out by head space solid-phase microextraction (HS-SPME) and their subsequent analysis by gas chromatography (GC) coupled to mass spectrometry (MS), according to the method described by Garde-Cerdán et al., 2018 [34]. The SPME fiber used was divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS, 50/30 µm) (Supelco, Bellenfonte, PA, USA). In 20 mL vials (Supelco), 9 mL of sample, 2.5 g NaCl and 10 µL of internal standard (2-octanol) were added. After adding a stir bar, the vial was closed and placed in the GC-MS (Agilent, Palo Alto, CA, USA). Sample conditioning was done at 60 °C, for 15 min and with stirring. After this step, the fiber was automatically inserted into the headspace in order to the extraction of the volatile compounds could take place, for 105 min, with agitation.
After the extraction process was completed, the fiber was immediately introduced into the GC injection port at 250 °C and held for 15 min for desorption of the compounds of interest. The capillary column used for analyte separation is SPB™-20 (30 m × 0.25 mm I.D. × 0.25 μm film thickness) (Supelco). Helium was used as the carrier gas at a flow rate of 1.2 mL/min. The chromatographic conditions used were: initial temperature, 40 °C for 5 min, a temperature gradient of 2 °C/min, up to a final temperature of 220 °C, to be maintained for 20 min (total time = 115 min). The ionization of the volatile compounds was performed at 70 eV. The detector worked at full scan (35—300 m/z). Identification was carried out using the NIST library and comparing with mass spectra and retention time of chromatographic standards, when available, as well as with data found in the literature. Semi-quantification was performed by relating the areas of each compound to the area and known concentration of the internal standard.
Since the treatments were performed in triplicate, the results of grape volatile compounds are expressed as the mean concentration and standard deviation of the three replicates (n = 3).
2.4. Statistical Analyses
Statistical analysis of the data was performed with the SPSS statistical package version 21.0 for Windows (SPSS, Chicago, IL, USA). Analysis of variance (ANOVA) (p < 0.05) was performed for general parameters and volatile compound data. To evaluate possible differences between treatments, the Duncan test was performed at the 95% probability level. A multivariate factor analysis was also performed (with treatment and season as factors) considering oenological parameters and grape aromatic compounds. Finally, a discriminant analysis was performed to classify the samples according to their volatile composition.
3. Results and Discussion
3.1. Effect of the Foliar MeJ and ACP-MeJ Treatments on the Must General Parameters
Table 1 shows the enological parameters in the grapes from control and vines treated with methyl jasmonate (MeJ) and with nanoparticles doped with MeJ (ACP-MeJ), in 2019 and 2020 seasons. In 2019, MeJ treatment significantly decreased °Brix, probable grade, glucose + fructose (Glu + Fru), glucose (Glu), and fructose (Fru) content with respect to control grapes, while total acidity, total phenols, amino nitrogen, and yeast assimilable nitrogen (YAN) increased when vines were foliarly treated with MeJ. However, ACP-MeJ treatment showed no significant differences with respect to the control in any of the parameters studied except for total phenols, which concentration increased (Table 1). In 2020 season, MeJ and ACP-MeJ foliar application did not affect must enological parameters. This result are similar to those reported by Garde-Cerdán et al., 2018 [34] which found only small or no differences in these parameters after MeJ application. Although overall precipitation and average temperatures were similar in 2019 and 2020, August rainfall was 11.5 L/m2 in 2019 and 32.9 L/m2 in 2020. Since this month is where the berry ripening process is completed, this may be the reason why the weight of 100 berries is higher in 2020 than in 2019 (Table 1).

Table 1.
General parameters in grapes from control, methyl jasmonate (MeJ) and nanoparticles doped with MeJ (ACP-MeJ) foliar treatments, in 2019 and 2020 seasons.
3.2. Influence of the Foliar MeJ and ACP-MeJ Treatments on Must Volatile Compounds
Figure 1, Figure 2 and Figure 3 and Table 2 show the results of must volatile primary aroma content in control and in samples from treated grapevines with methyl jasmonate (MeJ) and with nanoparticles doped with MeJ (ACP-MeJ), in 2019 and 2020 seasons. A total of 37 compounds were identified and semi-quantified, including terpenoids, C13 norisoprenoids, benzenoid compounds, alcohols, carbonyl compounds, C6 compounds, and other compounds.
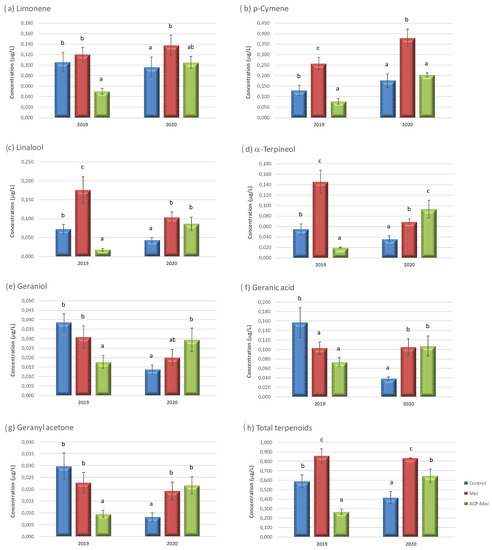
Figure 1.
Terpenoids concentration (µg/L) in grapes from control, methyl jasmonate (MeJ) and nanoparticles doped with MeJ (ACP-MeJ) foliar treatments, in 2019 and 2020 seasons. All parameters listed with their standard deviation (n = 3). For each season and compound, different letters indicate significant differences between samples (p ≤ 0.05).
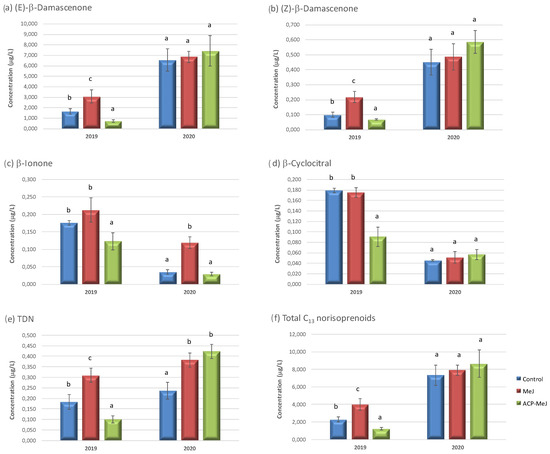
Figure 2.
C13 norisoprenoids concentration (µg/L) in grapes from control, methyl jasmonate (MeJ) and nanoparticles doped with MeJ (ACP-MeJ) foliar treatments, in 2019 and 2020 seasons. All parameters listed with their standard deviation (n = 3). For each season and compound, different letters indicate significant differences between samples (p ≤ 0.05). TDN: 1,1,6-trimethyl-1,2-dihydronaphthalene.
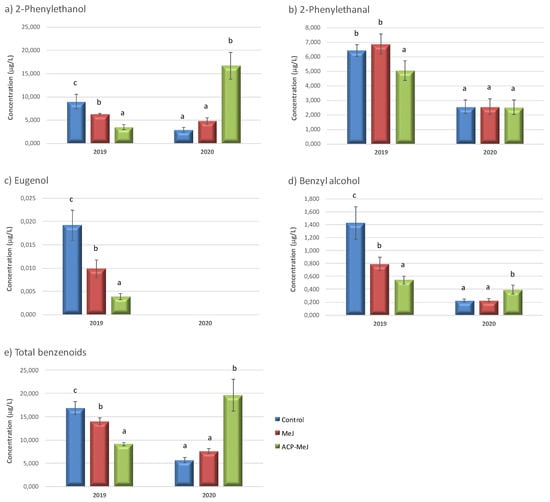
Figure 3.
Benzenoid compounds concentration (µg/L) in grapes from control, methyl jasmonate (MeJ) and nanoparticles doped with MeJ (ACP-MeJ) foliar treatments, in 2019 and 2020 seasons. All parameters listed with their standard deviation (n = 3). For each season and compound, different letters indicate significant differences between samples (p ≤ 0.05).

Table 2.
Alcohols, carbonyl compounds, C6 compounds and other compounds concentration (µg/L) in grapes from control, methyl jasmonate (MeJ) and nanoparticles doped with MeJ (ACP-MeJ) foliar treatments, in 2019 and 2020 seasons.
Figure 1 shows the concentration of terpenoids found in the control samples and in the grapes from the treatments with MeJ and ACP-MeJ, in 2019 and 2020 seasons. In 2019, limonene (Figure 1a), geraniol (Figure 1e), and geranyl acetone (Figure 1g) decreased their concentration in ACP-MeJ grapes relative to control and MeJ grapes. This effect had already been observed in Vitis vinifera L. cv. Tempranillo variety after the application of MeJ [34]. The MeJ-based treatment showed no significant differences with the control for these compounds.
For the same year, p-cymene (Figure 1b), linalool (Figure 1c), and α-terpineol (Figure 1d), which are very important terpenoids for grape and wine aroma [36], increased their content in MeJ grapes, and decreased it in ACP-MeJ samples with respect to control one. In the case of geranic acid (Figure 1f), both treatments significantly decreased the amount of this compound. Finally, in the same year, total terpenoids concentration (Figure 1h) increased in grapes from the foliar application of MeJ, and decreased in grapes treated with ACP-MeJ with respect to control grapes.
In 2020, limonene and p-cymene (Figure 1a,b) increased their concentration in MeJ grapes relative to control grapes. This effect had already been observed in Vitis vinifera ‘Garnacha’ variety after the application of MeJ [12]. The MeJ-doped nanoparticles treatment showed no significant differences in those compounds with the control samples. In the Garde-Cerdán et al., 2018 [34] study, it is shown that the synthesis of p-cymene increases upon application of MeJ. Moreover, for the same year, linalool (Figure 1c), geranic acid (Figure 1f) and geranyl acetone (Figure 1g), significantly increased their concentration in MeJ-treated and ACP-MeJ-treated samples with respect to the control. In the case of α-terpineol (Figure 1d), both foliar treatments increased the content of this compound in the grapes, with ACP-MeJ increasing to a greater extent. Regarding geraniol (Figure 1e), only the ACP-MeJ treatment significantly increased the amount of this compound with respect to the control grapes, despite being a treatment with a concentration 10 times lower. In this season, the total concentration of terpenoids (Figure 1h) increased significantly with both treatments with respect to the control grapes, being more effective the application with MeJ. The increase in the amount of terpenoids after foliar application with MeJ has been previously demonstrated by other groups [5,12,37]. However, some studies have also found that the content of total terpenoids decreases when MeJ is applied [34,38]. In general, the treatments increased the amount of several terpenoids found in the grapes (Figure 1). This may be due to the foliar treatments were applied during veraison, moment when free terpenoids start to be produced [12,39]. These compounds are high volatile compounds, and have very low perception threshold, and therefore represent one of the most important group of aromatic compounds [12,40], and among these, linalool, α-terpineol, and geraniol, which are some of the most odoriferous monoterpenes [5].
Figure 2 shows the concentration of C13 norisoprenoids found in the control and in the grapes from the applications to vines of MeJ and ACP-MeJ, in 2019 and 2020 seasons. In 2019, (E)-β-damascenone (Figure 2a), (Z)-β-damascenone (Figure 2b), and 1,1,6-trimetil-1,2-dihidronaftaleno (TDN) (Figure 2e), increased their concentration in MeJ grapes and decreased their concentration in ACP-MeJ samples with respect to control. The (E)-β-damascenone (Figure 2a) was the most abundant C13 norisoprenoid in the samples, predominantly over the rest of the compounds of this group. This fact is expected because this compound is one of the most abundant norisoprenoid in the grapes [12,41]. TDN is one of the most polarising, and maybe the less studied C13 norisoprenoid [39], its typical aroma descriptor is pretolor kerosene. In the case of β-ionone (Figure 2c), which provides violet notes [42], and β-cyclocitral (Figure 2d), both significantly decreased their amount in ACP-MeJ grapes relative to control and MeJ samples. Regarding the total C13 norisoprenoids (Figure 2f) in 2019, MeJ treatment increased its content with respect to control grapes. This could be probably due to the fact that the MeJ increases the activity of the enzymes involved in the synthesis of these compounds [43], which derive from biodegradation of carotenoids [40,44], whereas ACP-MeJ treatment decreased it. Therefore, despite applying the same product, the dose was 10 times lower, and maybe it was too low to affect enzyme activity.
In 2020, (E)-β-damascenone (Figure 2a), (Z)-β-damascenone (Figure 2b), and β-cyclocitral (Figure 2d), did not suffer variations in their content in MeJ and ACP-MeJ grapes with respect to control samples. In this season, β-ionone (Figure 2c) significantly increased its amount in MeJ grapes with respect to ACP-MeJ and control grapes. The increase of β-ionone with MeJ may be justified because β-ionone is a derivative of β-carotene [39], and MeJ accelerates its degradation [45]. TDN (Figure 2e) increased its concentration in grapes from both treatments (MeJ and ACP-MeJ) with respect to control grapes. As for total C13 norisoprenoids (Figure 2f), in 2020 neither treatment had a significant effect on its amount with respect to control samples. Interestingly, C13 norisoprenoids, derived from the breakdown of carotenoids via chemical, photochemical and oxidase-coupled degradation or enzymatic cleavage [5], generally unchanged with the MeJ treatments in 2020. These results contrast with those obtained by Gutiérrez-Gamboa et al., 2019 [38], where the application with MeJ decreased the amount of C13 norisoprenoids.
Terpeneoids and C13 norisoprenoids are very important in the floral aroma of grapes. Respect to the C13 norisoprenoids, β-damascenone and β-ionone are the most important, since they strongly contribute to the desirable flavor and odor in wines, due to their low perception thresholds [40,46].
Figure 3 shows the concentration of benzenoids found in the control and in the grapes from the foliar treatments with MeJ and ACP-MeJ, in 2019 and 2020 seasons. In 2019, 2-phenylethanol (Figure 3a), eugenol (Figure 3c) and benzyl alcohol (Figure 3d) decreased their concentration in grapes from both treatments (MeJ and ACP-MeJ) with respect to control grapes, with significantly lower content in ACP-MeJ samples. In the study of Marín-San Román et al., 2020 [12], 2-phenylethanol also decreased when MeJ is applied to vines. 2-Phenylethanal (Figure 3b) significantly decreased its amount in grapes from ACP-MeJ treatment with respect to control and MeJ samples. Finally, in 2019, the content of total benzenoids (Figure 3e) decreased with both treatments respect to control samples, with significantly lower content in ACP-MeJ grapes. This trend was also observed in the work of Gutiérrez-Gamboa et al., 2019 [38].
Regarding to the 2020 season, the concentration of 2-phenylethanol (Figure 3a) and benzyl alcohol (Figure 3d) increased in ACP-MeJ grapes with respect to those from the other two samples. 2-Phenylethanol and benzyl alcohol, which in grapes derive from aromatic amino acids, were the principal benzenoid compounds [5]. The content of 2-phenylethanal in grapes (Figure 3b) showed no significant differences in MeJ treatments with respect to the control. Eugenol was not detected in grapes in this second season (Figure 3c). The concentration of total benzenoids (Figure 3e) significantly increased with the ACP-MeJ treatment with respect to the control and MeJ ones.
Terpenoids, C13 norisoprenoids, and some benzenoid compounds are the most important grape aroma compounds present in the pulp and skin of the berries in both free and glycoside forms. These compounds are transferred to the wine during the winemaking and depend on the process used [47].
Table 2 shows the concentration of alcohols, carbonyl compounds, C6 compounds, and other compounds in grapes from the control, MeJ, and ACP-MeJ foliar treatments, in 2019 and 2020 seasons.
As regards to the alcohols in the 2019 season, the concentration of n-heptanol decreased with both MeJ and ACP-MeJ treatments with respect to the control grapes, being significantly lower the concentration in ACP-MeJ grapes. Both, n-octanol and n-nonanol decreased their concentrations in ACP-MeJ grapes with respect to control and ACP-MeJ samples; while the content of 1-octen-3-ol and 2-ethyl-1-hexanol decreased in grapes from both treatments (MeJ and ACP-MeJ) to the same extent. In 2019, the concentration of total alcohols decreased with both MeJ treatments respect to the control one (Table 2). In 2020, n-heptanol concentration did not change with either treatment (MeJ and ACP-MeJ). The content of n-octanol decreased in samples from both treatments (MeJ and ACP-MeJ) with respect to the control grapes. The n-nonanol content decreased only in the ACP-MeJ samples with respect to the control and MeJ grapes. In contrast, 1-octen-3-ol and 2-ethyl-1-hexanol only decreased in MeJ grapes relative to control and ACP-MeJ samples. The total alcohols content, in 2020, decreased in MeJ grapes, while ACP-MeJ did not show significant differences with the control (Table 2).
Among the carbonyl compounds, in 2019, heptanal and nonanal decreased their concentrations in the grapes from both treatments with respect to the control. The concentration of (E)-2-octenal and (E)-2-nonenal did not show significant differences between treatments. The content of decanol, (E,E)-2,4-hexadienal, (E,E)-2,4-nonadienal, γ-decalactone, and 6-methyl-3,5-heptadien-2-one, decreased only with the ACP-MeJ treatment with respect to control and MeJ grapes. This same trend was followed by the content of total carbonyl compounds in grapes (Table 2). In 2020, heptanal, nonanal, (E)-2-nonenal, and decanal decreased their concentration when grapevines were treated with MeJ and ACP-MeJ with respect to control grapes. Moreover, the concentration of (E)-2-octenal, (E,E)-2,4-hexadienal, and (E,E)-2,4-nonadienal decreased only when grapevines were treated with the MeJ with respect to the control and ACP-MeJ samples. On the contrary, γ-decalactone decreased its content in ACP-MeJ treated grapes with respect to control and MeJ ones. The concentration of 6-methyl-3,5-heptadien-2-one in grapes was not affected by the foliar applications. Total carbonyl compounds, in 2020, decreased in MeJ grapes compared to control and ACP-MeJ samples (Table 2).
Regarding the C6 compounds, which, in high concentrations, can provide negative notes, in 2019, the concentration of n-hexanol and n-hexanal decreased in ACP-MeJ grapes compared to control and MeJ samples. The content of (Z)-3-hexen-1-ol + (E)-2-hexen-1-ol decreased in both MeJ and ACP-MeJ grapes relative to the control ones. The concentration of (E)-2-hexenal significantly increased in MeJ grapes respect to the control, whereas its content decreased in ACP-MeJ samples (Table 2). Total C6 compounds followed the same trend as the latter compound, as well as was observed by Gutiérrez-Gamboa et al., 2019 [38]. In 2020, n-hexanol, (Z)-3-hexen-1-ol + (E)-2-hexen-1-ol, and (E)-2-hexenal increased their concentration in MeJ grapes respect to the other samples; since these compounds were the majority, total C6 compounds followed the same trend. Garde-Cerdán et al., 2018 [34] demonstrated that the application of MeJ increased the content of C6 compounds in the Vitis vinifera L. cv. Tempranillo variety. However, n-hexanal content increased in MeJ grapes, but decreased in ACP-MeJ samples compared to control. In this way, the increase in C6 aldehydes observed in the studied grapes, as a consequence of MeJ treatment, could be due to modification of the pathways involved in the formation of fatty acids [21]. These compounds are responsible for green aromas [40,48].
For the rest of the aroma compounds determined in the Vitis vinifera L. cv. Tempranillo grapes, in 2019, only methyl jasmonate was quantified, which increased its concentration in grapes from ACP-MeJ foliar treatment with respect to control and MeJ samples (Table 2). In 2020, hexyl acetate, which increased its concentration with both treatments (MeJ and ACP-MeJ), and methyl jasmonate, which decreased its concentration with both treatments (MeJ and ACP-MeJ), were quantified in grapes.
The differences in the amount of volatile compounds found between vintages may be due to climatic differences between them. For example, the difference in average precipitation in August (11.5 L/m2 in 2019 and 32.9 L/m2 in 2020). Moreover, as can be seen in Table 1, in the case of control berries and ACP-Mej treated berries, the probable alcohol content is higher in 2019 than in 2020, which may affect the content of volatile compounds in the grapes.
3.3. Factorial (Treatment, Season and Their Interaction) and Discriminant Analysis of the Aroma Compounds in Vitis vinifera L. cv. Tempranillo Grapes from 2019 and 2020 Seasons
Table 3 and Table 4 show the factorial analysis of the general parameters (Table 3) and volatile compounds (Table 4) of the grapes with the two factors studied: treatment (control, MeJ, ACP-MeJ) and season (2019 and 2020).

Table 3.
Multifactor analysis of variance of general parameters of the musts with the two factors studied: treatment (control, MeJ, ACP-MeJ) and season (2019 and 2020).

Table 4.
Multifactor analysis of variance of grape aroma compounds (expressed as µg/L) with the two factors studied: treatment (control, MeJ, ACP-MeJ) and season (2019 and 2020).
In Table 3, it can be seen that MeJ foliar treatments did not affect must enological parameters, except in the amount of total phenols, where the application of MeJ and ACP-MeJ increased its concentration with respect to those from the control grapes. However, some annual differences were observed. In fact, the weight of 100 berries, and the ammonium nitrogen were significantly lower in the grapes harvested in the 2019 than in 2020, while values of total acidity, fructose (Fru), malic acid, and total phenols were significantly lower in 2020 than in 2019. For any enological parameter, there was no significant interaction between the two factors (treatment and season) but for the ammonium nitrogen, amino nitrogen, and YAN.
Regarding to the treatment factor, Table 4 shows that, for terpenoids, MeJ foliar application increased the grape concentration of limonene, p-cymene, linalool, α-terpineol, and total terpenoids with respect to control and ACP-MeJ grapes. For the remaining terpenoids, MeJ had no significant effect respect to control samples. On the other hand, ACP-MeJ applications showed no effect on the studied terpenoids. For C13 norisoprenoids, MeJ foliar application increased the concentration of β-ionone, and TDN with respect to the other two samples (control and ACP-MeJ). β-Cyclocitral content was similar in control and MeJ samples but higher than in ACP-MeJ one. The application of ACP-MeJ only increased the concentration of TDN with respect to the control grapes, being MeJ the most effective treatment (Table 4). MeJ treatment did not increase the concentration of benzenoid compounds. However, this treatment decreased the concentration of eugenol and benzyl alcohol with respect to the control samples. On the other hand, ACP-MeJ treatment increased the 2-phenylethanol concentration, and, since this is the most abundant benzenoid, ACP-MeJ foliar application also increased the concentration of total benzenoids respect to the control and MeJ treatments. On the other hand, foliar application of ACP-MeJ decreased the concentration of 2-phenylethanal, eugenol, and benzyl alcohol with respect to control and MeJ grapes (Table 4). For alcohols, MeJ treatment decreased the amount of n-octanol, 1-octen-3-ol, 2-ethyl-1-hexanol, and total alcohols, while the other compounds remained unaffected. In the case of the ACP-MeJ treatment, its application to vines decreased the grape concentration of all alcohols respect to the control one. For carbonyl compounds, MeJ foliar application did not increase the concentration of any of them respect to the control. However, MeJ application decreased the concentration of heptanal, (E)-2-octenal, nonanal, decanal, and total carbonyl compounds with respect to the control. ACP-MeJ foliar application decreased the concentration of heptanal, nonanal, (E)-2-nonenal, decanal, 6-methyl-3,5-heptadien-2-one, and total carbonyl compounds with respect to the control and increased the content of (E)-2-octenal respect to MeJ application (Table 4). Regarding C6 compounds, MeJ treatment increased the concentration of n-hexanol, n-hexanal, (E)-2-hexenal, and total C6 compounds with respect to the control and ACP-MeJ grapes. Foliar application of ACP-MeJ did not increase the concentration of C6 compounds, but decreased the amount of the n-hexanal, (Z)-3-hexen-1-ol + (E)-2-hexen-1-ol, (E)-2-hexenal, and total C6 compounds with respect to control grapes. For other aroma compounds, like hexyl acetate and methyl jasmonate, both MeJ treatments increased the concentration of hexyl acetate and decreased the concentration of methyl jasmonate with respect to the control samples. As regards the season factor, some compounds were found in greater quantities in 2019 and others in 2020 (Table 4). In 2019, the terpenoids: geraniol, geranic acid, and geranyl acetone; the C13 norisoprenoids: β-ionone, and β-cyclocitral; the benzenoid compounds: 2-phenylethanal, eugenol, benzyl alcohol and the total benzenoid compounds; the alcohols: 1-octen-3-ol, and 2-ethyl-1-hexanol, and the total alcohols; the carbonyl compounds: heptanal, (E)-2-octenal, (E)-2-nonenal, (E,E)-2,4-hexadienal, (E,E)-2,4-nonadienal, and 6-methyl-3,5-heptadien-2-one, and the total carbonyl compounds; and the C6 compound: n-hexanal, were found in greater quantities than in 2020. On the contrary, in the second season, the terpenoids: limonene, and p-cymene and the total terpenoids; the C13 norisoprenoids: (E) and (Z)-β-damascenone, and TDN, and the total C13 norisoprenoids; the benzenoid compound: 2-phenylethanol; the alcohols: n-octanol, and n-nonanol; the carbonyl compounds: nonanal, and γ-decalactone; the C6 compounds: n-hexanol, (Z)-3-hexel-1-ol + (E)-2-hexen-1-ol, and (E)-2-hexenal, and the total C6 compounds; and the hexyl acetate and methyl jasmonate were found in higher amounts than those from 2019. In this case, the treatment-season interaction was significant for all compounds except β-ionone, n-octanol, (E)-2-octenal, and (E)-2-hexenal (Table 4).
In order to classify the different samples, discriminant analysis was performed on data expressing the concentration of volatile compounds in control, MeJ, and ACP-MeJ samples. The results are shown in Figure 4. In 2019 season (Figure 4a), Function 1 explained a very high percentage of variance 90.5% and Function 2 explained only 9.5%, so the total of variance explained was 100%. The variables that contributed the most to the discriminant model were α-terpineol, 2-ethyl-1-hexanol, geraniol, and 1-hexanol (Function 1) and 2-ethyl-1-hexanol, geraniol, and TDN (Function 2). The discriminant model showed a good separation among the different samples. In the case of the data from 2020 (Figure 4b), Function 1 explained 89.8% and Function 2 explained 10.2%, (total variance explained = 100%). The variables that contributed the most to the discriminant model were: (Z)-3-hexen-1-ol, β-ionone, 2-phenylethanol, and 6-methyl-3,5-heptadiene-2-one for Function 1 and 2-phenylethanol, 2-ethyl-1-hexanol, and (E)-2-octenal for Function 2. Again, the discriminant showed a very good separation among the different samples. Figure 4c shows the discriminant analysis for both seasons, with treatment as factor. It can be observed that there is a good separation between the treatments. Function 1 explained 56.4% of the variance and Function 2 explained 43.6% (total variance = 100%). The variables with the highest contribution were p-cymene, and (E)-2-nonenal for Function 1, and heptanal, (E)-2-nonenal, and decanal for Function 2. Considering the sample as factor (Figure 4d), Function 1 explained almost all the variance 98.7%, and Function 2 only 0.7%, so the total of variance explained was 99.4%. Again, the discriminant shows a good separation between all samples. The variables that contributed the most to the discriminant model were (E)-β-damascenone, p-cymene, 1-octanol, and linalool for Function 1, and p-cymene, 2-phenylethanol, 1-octanol, and 1-hexanol for Function 2.
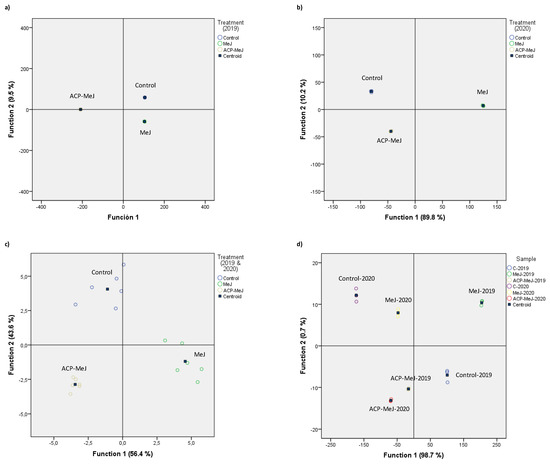
Figure 4.
Discriminant analysis of volatile compounds content (µg/L) in grapes from control, methyl jasmonate (MeJ) and nanoparticles doped with this elicitor (ACP-MeJ) treatments, in (a) 2019, (b) 2020, and (c) 2019 & 2020 seasons, carried out with the treatment as factor; and (d) carried out with the sample as factor.
4. Conclusions
The use of elicitors through foliar applications to Vitis vinifera L. cv. Tempranillo grapevines affected grape volatile composition. Methyl jasmonate (MeJ) treatment increased the concentration in the grapes of total terpenoids, and total C6 compounds in 2019 and 2020, and the total C13 norisoprenoids in 2019; while decreased the concentration of total benzenoid compounds in 2019, total carbonyl compounds in 2020, and total alcohols in both seasons. In addition, ACP-MeJ increased the amount in the grapes of total terpenoids, and total benzenoid compounds in 2020; whereas decreased the content of total terpenoids, total C13 norisoprenoids, total benzenoid compounds, total alcohols, total carbonyl compounds, and C6 compounds in 2019. These results are not completely conclusive since this is the first time that foliar application of ACP-MeJ has been performed in Vitis vinifera L. cv. Tempranillo grapevines to evaluate the effect on grape aroma. Nevertheless, the results suggest that MeJ is still a better option than ACP-MeJ in order to enhance the grape volatile composition, but considering that the applied dose in the ACP-MeJ treatment was 10 times lower than that applied in the MeJ conventional treatment, it can be said that nanotechnology has given very positive results in order to improve the grape aromatic quality.
Author Contributions
Conceptualization, T.G.-C., J.M.D.-L. and E.P.P.-Á.; methodology, T.G.-C., J.M.D.-L., E.P.P.-Á. and G.B.R.-R.; formal analysis, S.M.-S.R., I.S.d.U. and T.G.-C.; investigation, B.P.-T., G.B.R.-R., S.M.-S.R. and I.S.d.U.; data curation, S.M.-S.R.; writing—original draft preparation, S.M.-S.R. and T.G.-C.; writing—review and editing, all authors; supervision, T.G.-C., J.M.D.-L. and E.P.P.-Á.; funding acquisition, T.G.-C. and J.M.D.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been carried out thanks to funding from the Ministerio de Ciencia, Innovación y Universidades (FEDER/MCIU/AEI, Spain) through the Projects RTI2018-096549-B-I00 and RTI-2018-095794-A-C22. S.M.-S.R. thanks Gobierno de La Rioja for her predoctoral contract. J.M.D.-L. and E.P.P.-Á. acknowledge the Ministerio de Ciencia, Innovación y Universidades for their Ramón y Cajal and Juan de la Cierva-Incorporación contracts, respectively. G.B.R.-R. would like to thank to Junta de Andalucía for her postdoctoral contract (PAIDI 2020, DOC_01383).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Perestrelo, R.; Barros, A.S.; Rocha, S.M.; Câmara, J.S. Optimisation of solid-phase microextraction combined with gas chromatography–mass spectrometry based methodology to establish the global volatile signature in pulp and skin of Vitis vinifera L. grape varieties. Talanta 2011, 85, 1483–1493. [Google Scholar] [CrossRef] [PubMed]
- Aleixandre-Tudo, J.L.; Weightman, C.; Nieuwoudt, H.; Du Toit, W. Effect of skin contact before and during alcoholic fermentation on the chemical and sensory profile of south african chenin blanc white wines. S. Afr. J. Enol. Vitic. 2015, 36, 366–377. [Google Scholar] [CrossRef]
- Román, S.M.-S.; Rubio-Bretón, P.; Pérez-Álvarez, E.P.; Garde-Cerdán, T. Advancement in analytical techniques for the extraction of grape and wine volatile compounds. Food Res. Int. 2020, 137, 109712. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, S.; Wang, Y.; Zeng, W.; Jin, B. Floral scents and fruit aromas: Functions, compositions, biosynthesis, and regulation. Front. Plant Sci. 2022, 13, 860157. [Google Scholar] [CrossRef]
- D’Onofrio, C.; Matarese, F.; Cuzzola, A. Effect of methyl jasmonate on the aroma of Sangiovese grapes and wines. Food Chem. 2018, 242, 352–361. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Gutiérrez-Gamboa, G.; López, R.; Rubio-Bretón, P.; Pérez-Álvarez, E.P. Influence of foliar application of phenylalanine and urea at two doses to vineyards on grape volatile composition and amino acids content. Vitis 2018, 141, 137–141. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Rubio-Bretón, P.; Román, S.M.-S.; de Urturi, I.S.; Pérez-Álvarez, E.P. Pre-fermentative maceration with SO2 enhanced the must aromatic composition. Food Chem. 2020, 345, 128870. [Google Scholar] [CrossRef]
- Gambetta, J.M.; Bastian, S.E.P.; Cozzolino, D.; Jeffery, D.W. Factors influencing the aroma composition of chardonnay wines. J. Agric. Food Chem. 2014, 62, 6512–6534. [Google Scholar] [CrossRef]
- Darriet, P.; Thibon, C.; Dubourdieu, D. Aroma and Aroma Precursors in Grape Berry. In The Biochemistry of the Grape Berry; Bentham Science: Sharjah, United Arab Emirates, 2012; ISBN 9781608055401. [Google Scholar]
- Garde-Cerdán, T.; Santamaría, P.; Rubio-Bretón, P.; González-Arenzana, L.; López-Alfaro, I.; López, R. Foliar application of proline, phenylalanine, and urea to Tempranillo vines: Effect on grape volatile composition and comparison with the use of commercial nitrogen fertilizers. LWT Food Sci. Technol. 2015, 60, 684–689. [Google Scholar] [CrossRef]
- Portu, J.; González-Arenzana, L.; Hermosín-Gutiérrez, I.; Santamaría, P.; Garde-Cerdán, T. Phenylalanine and urea foliar applications to grapevine: Effect on wine phenolic content. Food Chem. 2015, 180, 55–63. [Google Scholar] [CrossRef]
- Román, S.M.-S.; Garde-Cerdán, T.; Baroja, E.; Rubio-Bretón, P.; Pérez-Álvarez, E.P. Foliar application of phenylalanine plus methyl jasmonate as a tool to improve Grenache grape aromatic composition. Sci. Hortic. 2020, 272, 109515. [Google Scholar] [CrossRef]
- Paladines-Quezada, D.; Moreno-Olivares, J.; Fernández-Fernández, J.; Bautista-Ortín, A.; Gil-Muñoz, R. Influence of methyl jasmonate and benzothiadiazole on the composition of grape skin cell walls and wines. Food Chem. 2019, 277, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Portu, J.; Santamaría, P.; López-Alfaro, I.; López, R.; Garde-Cerdán, T. Methyl jasmonate foliar application to tempranillo vineyard improved grape and wine phenolic content. J. Agric. Food Chem. 2015, 63, 2328–2337. [Google Scholar] [CrossRef] [PubMed]
- Gil-Muñoz, R.; Fernández-Fernández, J.I.; Crespo-Villegas, O.; Garde-Cerdán, T. Elicitors used as a tool to increase stilbenes in grapes and wines. Food Res. Int. 2017, 98, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Portu, J.; López, R.; Santamaría, P.; Garde-Cerdán, T. Elicitation with methyl jasmonate supported by precursor feeding with phenylalanine: Effect on Garnacha grape phenolic content. Food Chem. 2017, 237, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Gamboa, G.; Román, S.M.-S.; Jofré, V.; Rubio-Bretón, P.; Pérez-Álvarez, E.; Garde-Cerdán, T. Effects on chlorophyll and carotenoid contents in different grape varieties (Vitis vinifera L.) after nitrogen and elicitor foliar applications to the vineyard. Food Chem. 2018, 269, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Esplá, A.; Valero, D.; Martínez-Romero, D.; Castillo, S.; Giménez, M.J.; García-Pastor, M.E.; Serrano, M.; Zapata, P.J. Preharvest application of methyl jasmonate as an elicitor improves the yield and phenolic content of artichoke. J. Agric. Food Chem. 2017, 65, 9247–9254. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Portu, J.; López, R.; Santamaría, P. Effect of methyl jasmonate application to grapevine leaves on grape amino acid content. Food Chem. 2016, 203, 536–539. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Bautista-Ortín, A.B.; Ruiz-García, Y.; I Fernández-Fernández, J.; Gil-Muñoz, R. Effect of elicitors on the evolution of grape phenolic compounds during the ripening period. J. Sci. Food Agric. 2016, 97, 977–983. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Mestre-Ortuño, L.; Ruiz-García, Y.; Fernández-Fernández, J.I.; López-Roca, J.M. Effect of benzothiadiazole and methyl jasmonate on the volatile compound composition of Vitis vinifera L. Monastrell grapes and wines. Am. J. Enol. Vitic. 2012, 63, 394–401. [Google Scholar] [CrossRef]
- Rahim, H.U.; Qaswar, M.; Uddin, M.; Giannini, C.; Herrera, M.L.; Rea, G. Nano-enable materials promoting sustainability and resilience in modern agriculture. Nanomaterials 2021, 11, 2068. [Google Scholar] [CrossRef] [PubMed]
- Fellet, G.; Pilotto, L.; Marchiol, L.; Braidot, E. Tools for nano-enabled agriculture: Fertilizers based on calcium phosphate, silicon, and chitosan nanostructures. Agronomy 2021, 11, 1239. [Google Scholar] [CrossRef]
- Fincheira, P.; Tortella, G.; Seabra, A.B.; Quiroz, A.; Diez, M.C.; Rubilar, O. Nanotechnology advances for sustainable agriculture: Current knowledge and prospects in plant growth modulation and nutrition. Planta 2021, 254, 1–25. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Costa, B.S.; Rubio-Bretón, P.; Pérez-Álvarez, E.P. Nanotechnology: Recent advances in viticulture and enology. J. Sci. Food Agric. 2021, 101, 6156–6166. [Google Scholar] [CrossRef]
- Pérez-Álvarez, E.P.; Ramírez-Rodríguez, G.B.; Carmona, F.J.; Martínez-Vidaurre, J.M.; Masciocchi, N.; Guagliardi, A.; Garde-Cerdán, T.; Delgado-López, J.M. Towards a more sustainable viticulture: Foliar application of N-doped calcium phosphate nanoparticles on Tempranillo grapes. J. Sci. Food Agric. 2020, 101, 1307–1313. [Google Scholar] [CrossRef]
- Pérez-Álvarez, E.; Rubio-Bretón, P.; Intrigliolo, D.; Parra-Torrejón, B.; Ramírez-Rodríguez, G.; Delgado-López, J.; Garde-Cerdán, T. Year, watering regime and foliar methyl jasmonate doped nanoparticles treatments: Effects on must nitrogen compounds in Monastrell grapes. Sci. Hortic. 2022, 297, 110944. [Google Scholar] [CrossRef]
- Gaiotti, F.; Lucchetta, M.; Rodegher, G.; Lorenzoni, D.; Longo, E.; Boselli, E.; Cesco, S.; Belfiore, N.; Lovat, L.; Delgado-López, J.; et al. Urea-doped calcium phosphate nanoparticles as sustainable nitrogen nanofertilizers for viticulture: Implications on yield and quality of pinot gris grapevines. Agronomy 2021, 11, 1026. [Google Scholar] [CrossRef]
- Gil-Muñoz, R.; Giménez-Bañón, M.J.; Moreno-Olivares, J.D.; Paladines-Quezada, D.F.; Bleda-Sánchez, J.A.; Fernández-Fernández, J.I.; Parra-Torrejón, B.; Ramírez-Rodríguez, G.B.; Delgado-López, J.M. Effect of methyl jasmonate doped nanoparticles on nitrogen composition of Monastrell grapes and wines. Biomolecules 2021, 11, 1631. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Sáenz de Urturi, I.; Rubio-Bretón, P.; Marín-San Román, S.; Baroja, E.; Ramírez-Rodríguez, G.; Delgado-López, J.; Pérez-Álvarez, E. Foliar application of methyl jasmonate and methyl jasmonate supported on nanoparticles: Incidence on grape phenolic composition over two seasons. Food Chem. 2023, 402, 134244. [Google Scholar] [CrossRef]
- Parra-Torrejón, B.; Ramírez-Rodríguez, G.B.; Giménez-Bañón, M.J.; Moreno-Olivares, J.D.; Paladines-Quezada, D.F.; Gil-Muñoz, R.; Delgado-López, J.M. Nanoelicitors with prolonged retention and sustained release to produce beneficial compounds in wines. Environ. Sci. Nano 2021, 8, 3524–3535. [Google Scholar] [CrossRef]
- Epple, M. Review of potential health risks associated with nanoscopic calcium phosphate. Acta Biomater. 2018, 77, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Bañón, M.J.; Moreno-Olivares, J.D.; Paladines-Quezada, D.F.; Bleda-Sánchez, J.A.; Fernández-Fernández, J.I.; Parra-Torrejón, B.; Delgado-López, J.M.; Gil-Muñoz, R. Effects of methyl jasmonate and nano-methyl jasmonate treatments on monastrell wine volatile composition. Molecules 2022, 27, 2878. [Google Scholar] [CrossRef] [PubMed]
- Garde-Cerdán, T.; Gamboa, G.G.; Baroja, E.; Rubio-Bretón, P.; Pérez-Álvarez, E.P. Influence of methyl jasmonate foliar application to vineyard on grape volatile composition over three consecutive vintages. Food Res. Int. 2018, 112, 274–283. [Google Scholar] [CrossRef] [PubMed]
- OIV. Compendium of Internationals Methods of Wine and Must Analysis; International Organisation of Vine and Wine: Paris, France, 2009. [Google Scholar]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology, Volume 2: The Chemistry of Wine Stabilization and Treatments, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006; ISBN 9780470010372. [Google Scholar]
- Li, W.; Li, W.; Yang, S.; Ma, Z.; Zhou, Q.; Mao, J.; Han, S.; Chen, B. Transcriptome and metabolite conjoint analysis reveals that exogenous methyl jasmonate regulates monoterpene synthesis in grape berry skin. J. Agric. Food Chem. 2020, 68, 5270–5281. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Gamboa, G.; Pérez-Álvarez, E.; Rubio-Bretón, P.; Garde-Cerdán, T. Changes on grape volatile composition through elicitation with methyl jasmonate, chitosan, and a yeast extract in Tempranillo (Vitis vinifera L.) grapevines. Sci. Hortic. 2018, 244, 257–262. [Google Scholar] [CrossRef]
- Black, C.; Parker, M.; Siebert, T.; Capone, D.; Francis, I. Terpenoids and their role in wine flavour: Recent advances. Aust. J. Grape Wine Res. 2015, 21, 582–600. [Google Scholar] [CrossRef]
- Alem, H.; Rigou, P.; Schneider, R.; Ojeda, H.; Torregrosa, L. Impact of agronomic practices on grape aroma composition: A review. J. Sci. Food Agric. 2018, 99, 975–985. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; González-Arenzana, L.; López, N.; López, R.; Santamaría, P.; López-Alfaro, I. Effect of different pulsed electric field treatments on the volatile composition of Graciano, Tempranillo and Grenache grape varieties. Innov. Food Sci. Emerg. Technol. 2013, 20, 91–99. [Google Scholar] [CrossRef]
- Styger, G.; Prior, B.; Bauer, F.F. Wine flavor and aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145–1159. [Google Scholar] [CrossRef]
- Dubery, I.A.; Teodorczuk, L.G.; Louw, A.E. Early responses in methyl jasmonate-preconditioned cells toward pathogen-derived elicitors. Mol. Cell Biol. Res. Commun. 2000, 3, 105–110. [Google Scholar] [CrossRef]
- Mendes-Pinto, M.M.; Ferreira, A.C.S.; Caris-Veyrat, C.; de Pinho, P.G. Carotenoid, chlorophyll, and chlorophyll-derived compounds in grapes and port wines. J. Agric. Food Chem. 2005, 53, 10034–10041. [Google Scholar] [CrossRef] [PubMed]
- Peña-Cortés, H.; Barrios, P.; Dorta, F.; Polanco, V.; Sánchez, C.; Sánchez, E.; Ramírez, I. Involvement of jasmonic acid and derivatives in plant responses to pathogens and insects and in fruit ripening. J. Plant Growth Regul. 2004, 23, 246–260. [Google Scholar] [CrossRef]
- Sefton, M.A.; Skouroumounis, G.K.; Elsey, G.M.; Taylor, D.K. Occurrence, Sensory impact, formation, and fate of damascenone in grapes, wines, and other foods and beverages. J. Agric. Food Chem. 2011, 59, 9717–9746. [Google Scholar] [CrossRef] [PubMed]
- Flamini, R.; Traldi, P. Grape aroma compounds: Terpenes, C13-norisoprenoids, benzene compounds, and 3-Alkyl-2-methoxypyrazines. In Mass Spectrometry in Grape and Wine Chemistry; Jonh Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 97–116. [Google Scholar]
- Robinson, A.L.; Boss, P.K.; Solomon, P.S.; Trengove, R.D.; Heymann, H.; Ebeler, S.E. Origins of grape and wine aroma. Part 1. Chemical components and viticultural impacts. Am. J. Enol. Vitic. 2013, 65, 1–24. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).