Study on the Mechanical Properties and Durability of Hydraulic Lime Mortars Based on Limestone and Potassium Feldspar
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Sample Preparations
2.2.1. Calcination
2.2.2. Specimens’ Preparations
2.3. Test Methods
2.3.1. Microstructure Analysis
2.3.2. Mechanical Properties Test
2.3.3. Durability Test
3. Results and Discussions
3.1. Changes in Hydraulic Lime Composition with Mixing Percentage
3.2. Physical and Mechanical Performance
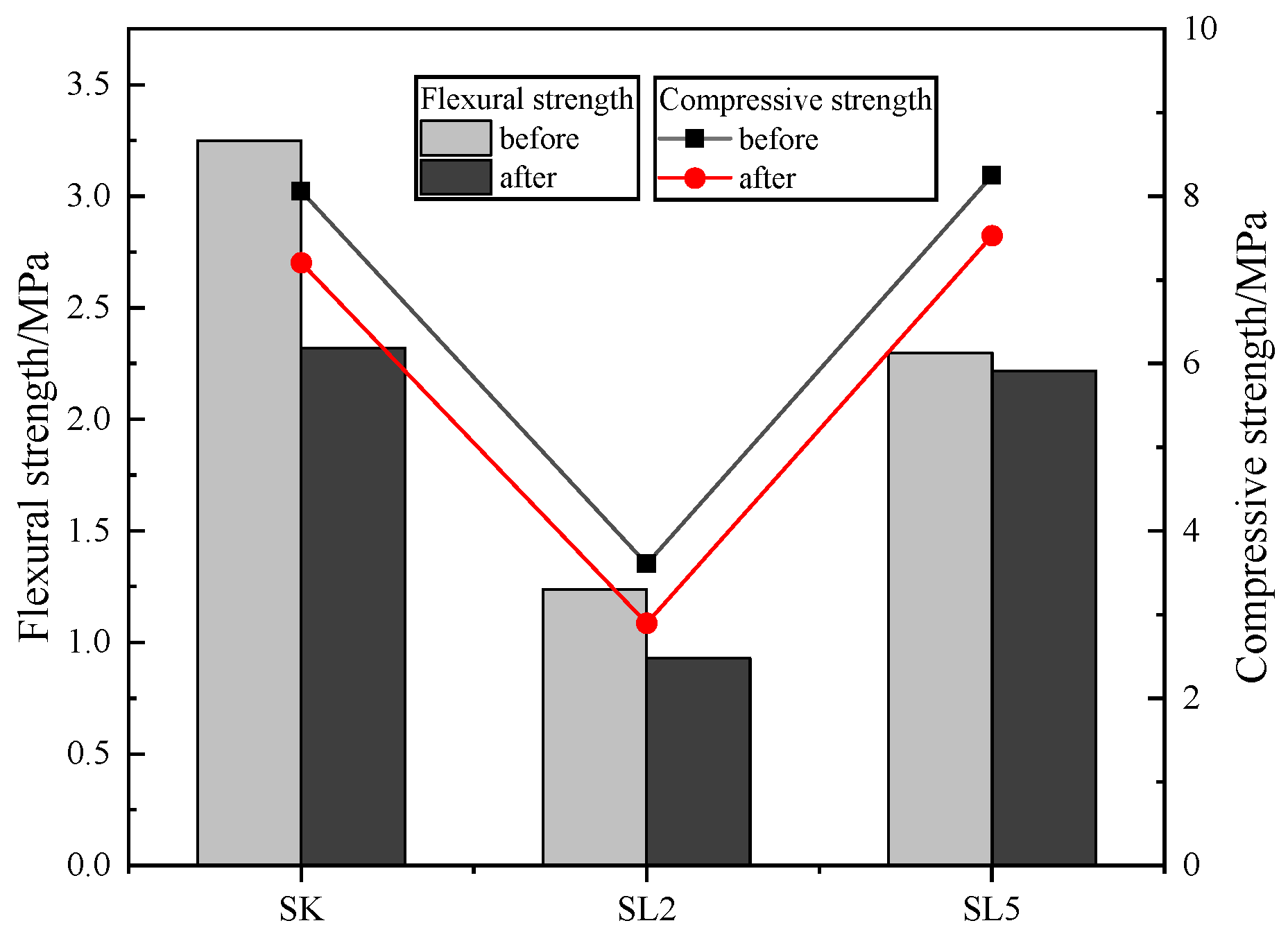
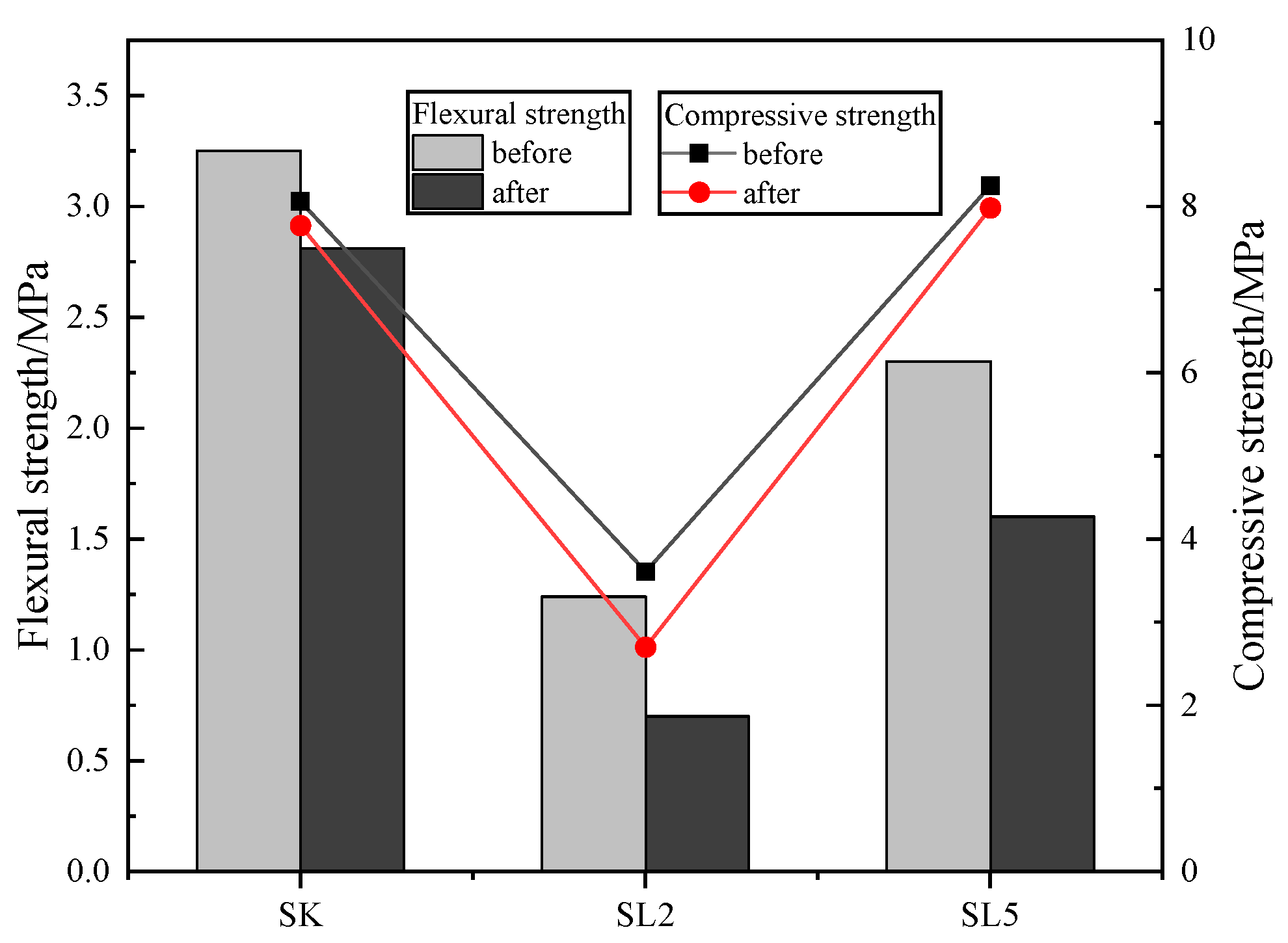
3.2.1. Flexural and Compressive Strength of Mortars
3.2.2. Shrinkage Rate
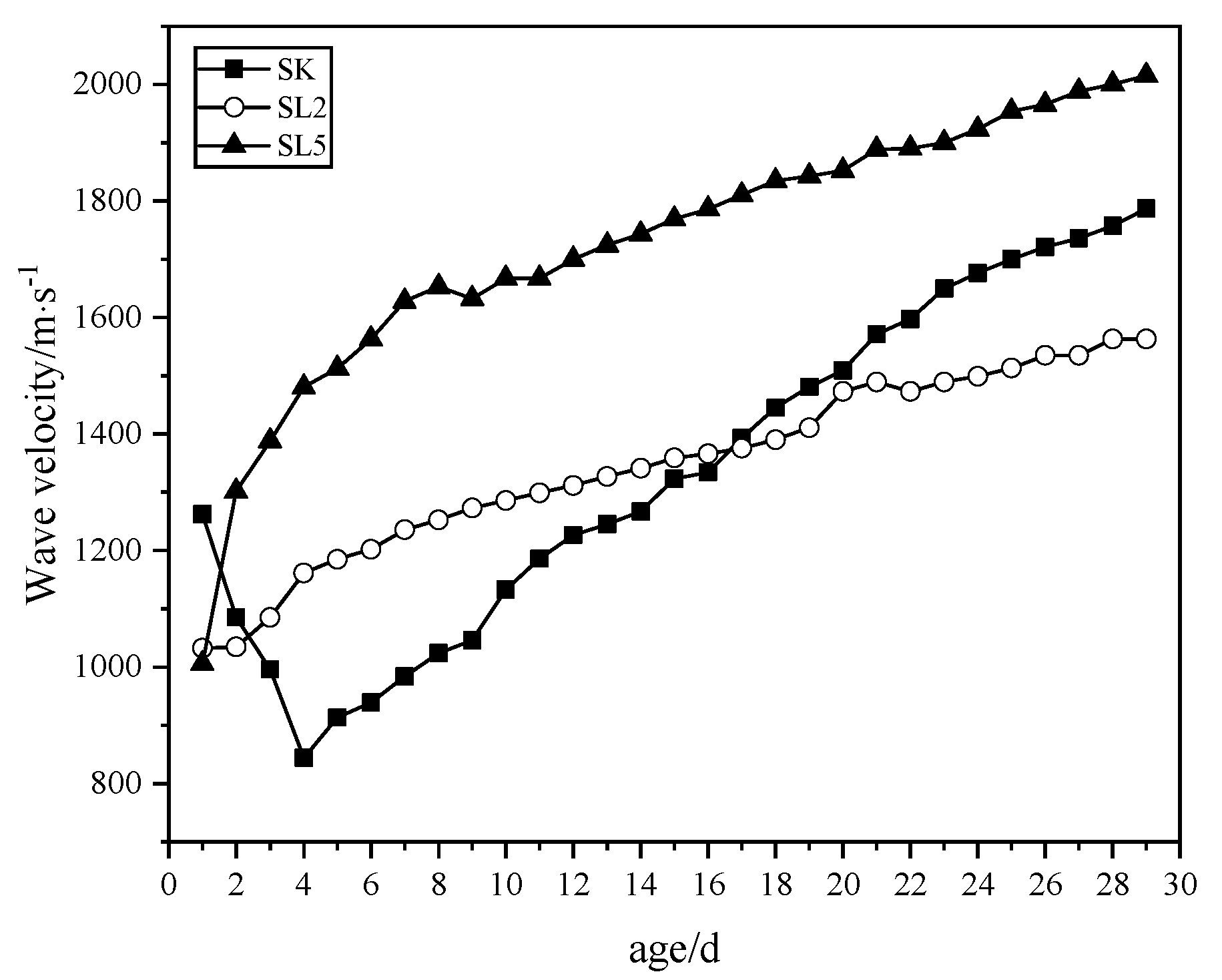
3.2.3. Relationship between the Elastic Wave Velocity and Age
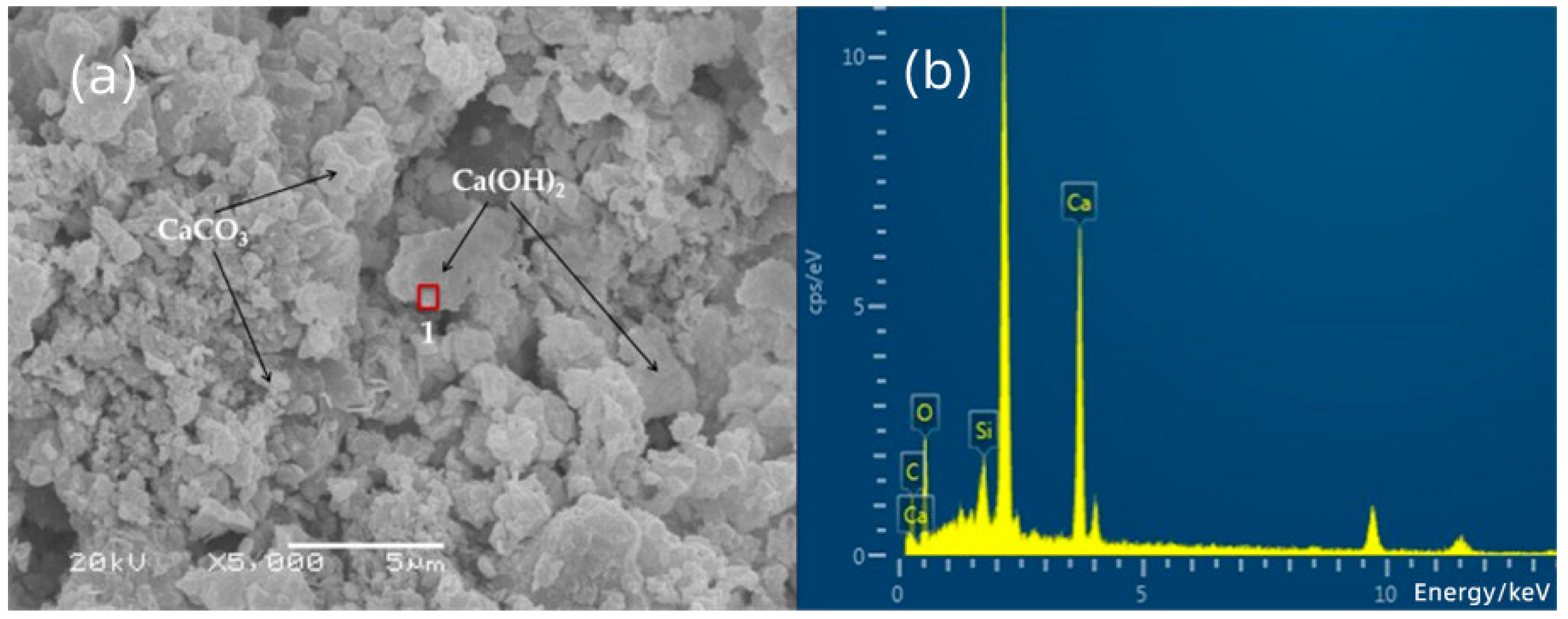
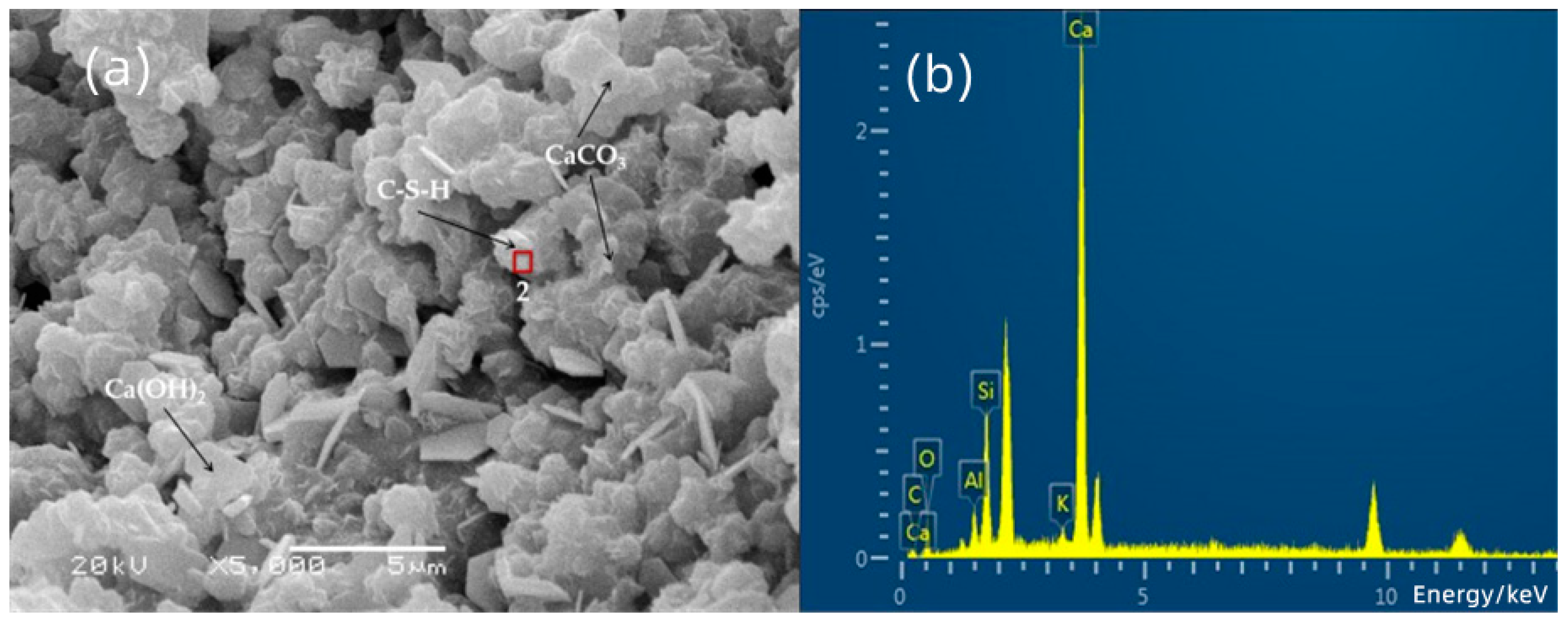
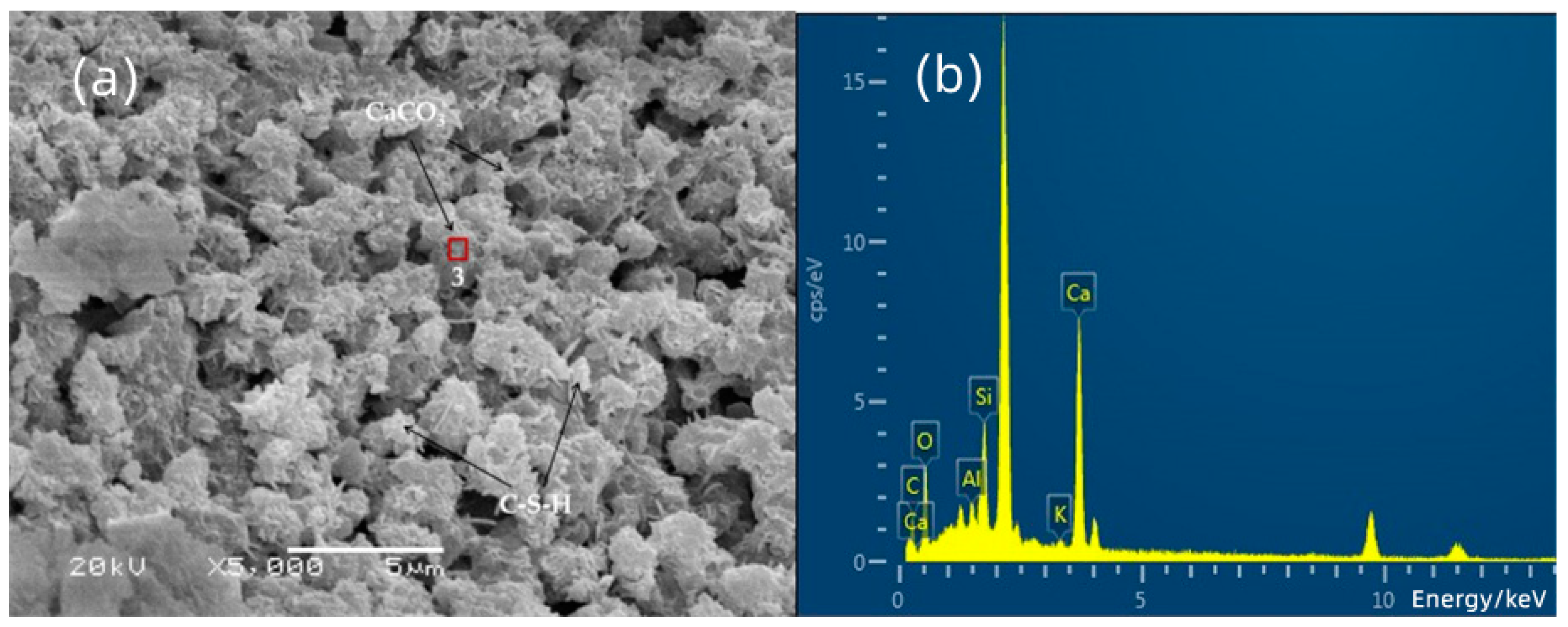
3.3. SEM-EDS Analysis
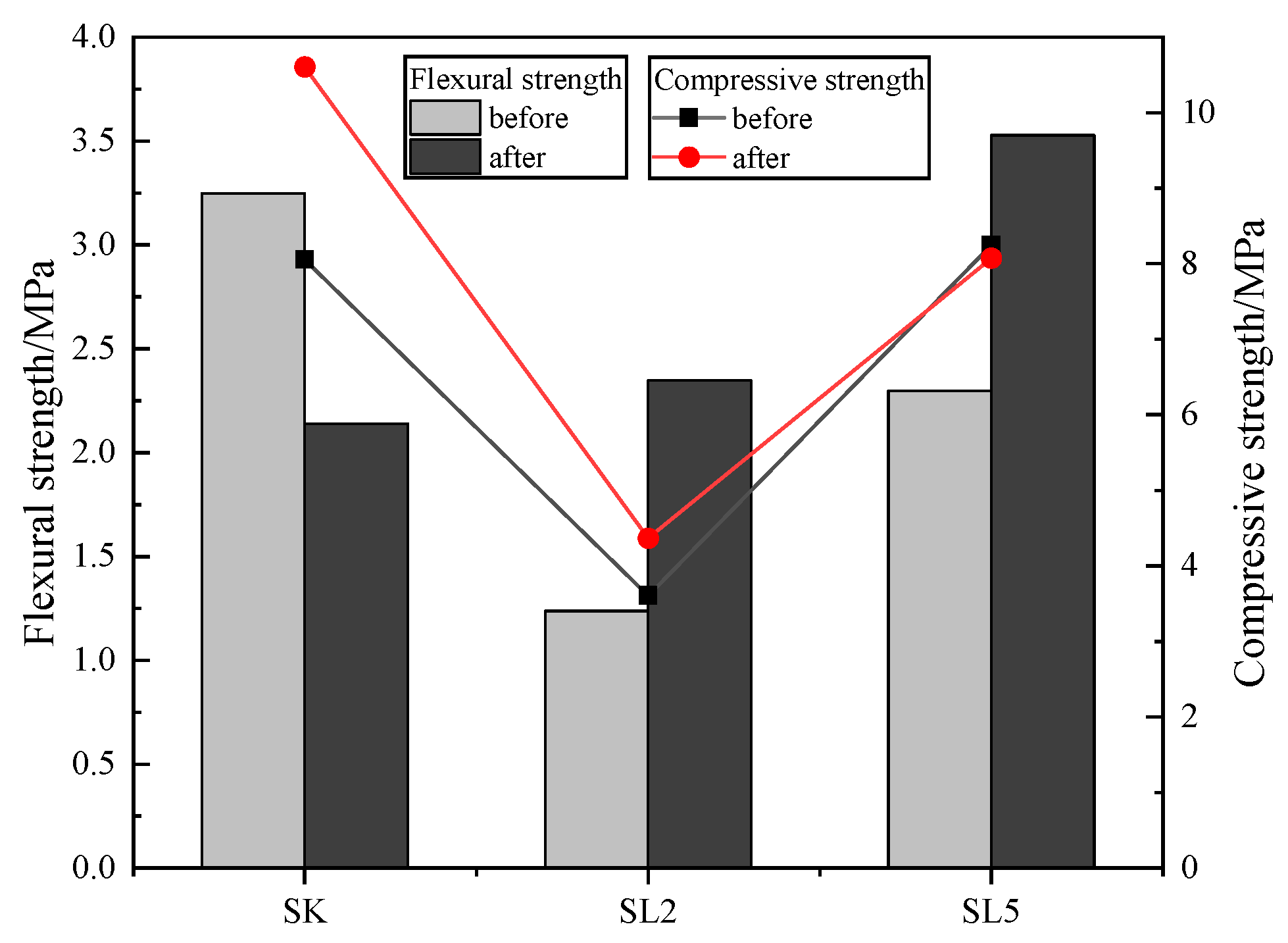
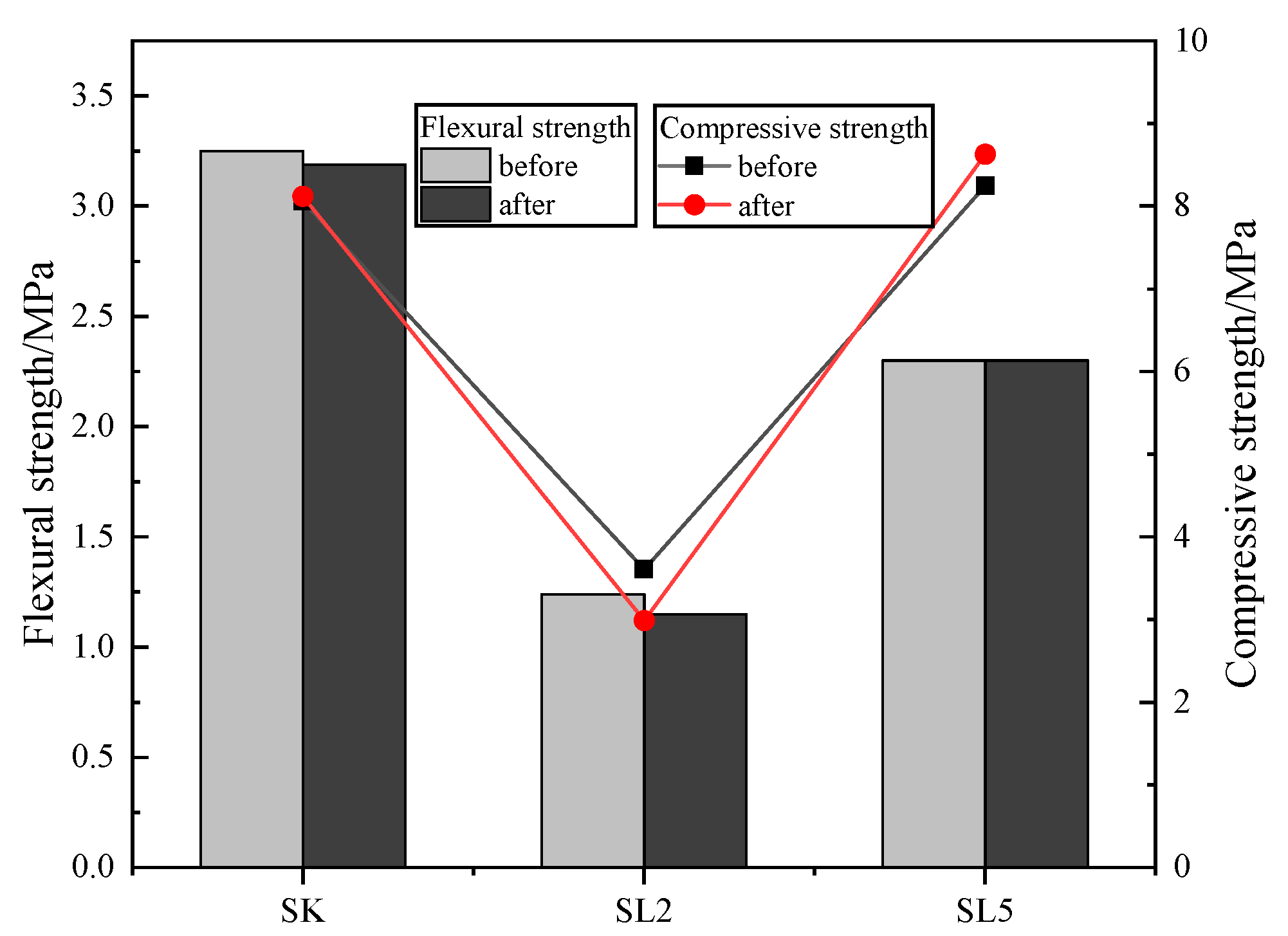
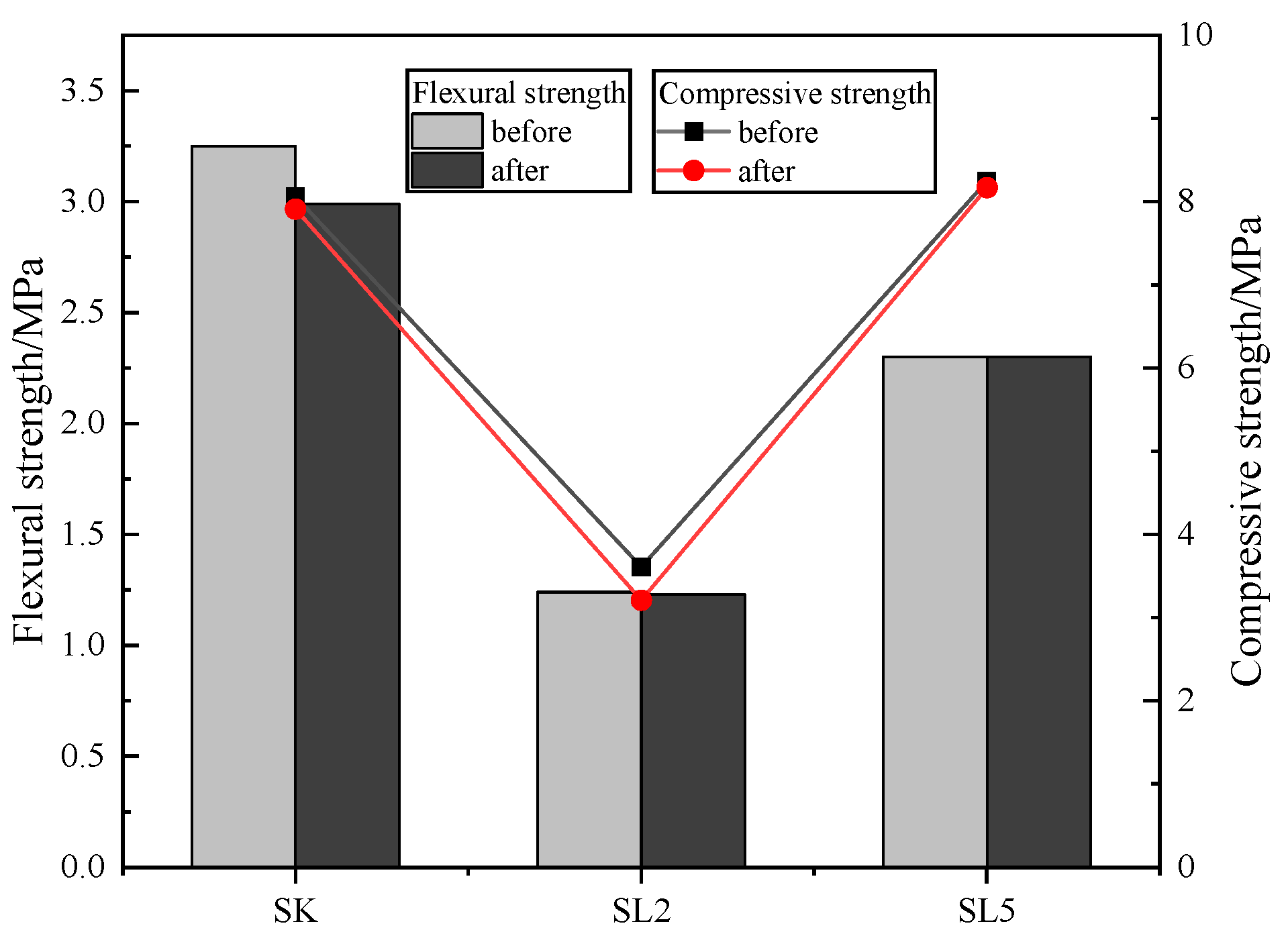
3.4. Durability of the Specimens
3.4.1. Water Stability Test
3.4.2. Soundness Test
3.4.3. Alkali Resistivity Test
3.4.4. Frost–Thaw Cycle Test
3.4.5. Temperature and Humidity Cycle Test
3.4.6. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silva, B.A.; Pinto, A.P.F.; Gomes, A. Natural hydraulic lime versus cement for blended lime mortars for restoration works. Constr. Build. Mater. 2015, 94, 346–360. [Google Scholar] [CrossRef]
- Frew, C. Practical building conservation: Mortars, renders & plasters. J. Archit. Conser. 2014, 19, 282–283. [Google Scholar]
- Gulotta, D.; Goidanich, S.; Tedeschi, C.; Nijland, T.G.; Toniolo, L. Commercial NHL-containing mortars for the preservation of historical architecture. Part 1: Compositional and mechanical characterization. Constr. Build. Mater. 2013, 38, 31–42. [Google Scholar] [CrossRef]
- Xu, S.; Wang, J.; Sun, Y. Effect of water binder ratio on the early hydration of natural hydraulic lime. Mater Struct. 2015, 48, 3431–3441. [Google Scholar] [CrossRef]
- Dai, S.B.; Zhong, Y.; Hu, Z.Y. Building Lime and Its Application for the Restoration of Built Heritage; Tongji University: Shanghai, China, 2016; pp. 63–74. [Google Scholar]
- Silva, B.A.; Pinto, A.P.F.; Gomes, A. Influence of natural hydraulic lime content on the properties of aerial lime-based mortars. Constr. Build. Mater. 2014, 72, 208–218. [Google Scholar] [CrossRef]
- Grilo, J.; Silva, S.A.; Faria, P.; Gameiro, A.; Veiga, R.; Velosa, A. Mechanical and mineralogical properties of natural hydraulic lime-metakaolin mortars in different curing conditions. Constr. Build. Mater. 2014, 51, 287–294. [Google Scholar] [CrossRef]
- Vavricuk, A.; Bokan-Bosiljkov, V.; Kramar, S. The influence of metakaolin on the properties of natural hydraulic lime-based grouts for historic masonry repair. Constr. Build. Mater. 2018, 172, 706–716. [Google Scholar] [CrossRef]
- Iucolano, F.; Liguori, B.; Colella, C. Fiber-reinforced lime-based mortars: A possible resource for ancient masonry restoration. Constr. Build. Mater. 2013, 38, 785–789. [Google Scholar] [CrossRef]
- Liguori, B.; Caputo, D.; Iucolano, F. Fiber-reinforced lime-based mortars: Effect of zeolite addition. Constr. Build. Mater. 2015, 77, 455–460. [Google Scholar] [CrossRef]
- Barbero-Barrera, M.M.; Medina, N.F. The effect of polypropylene fibers on graphite-natural hydraulic lime pastes. Constr. Build. Mater. 2018, 184, 591–601. [Google Scholar] [CrossRef]
- Faria, P.; Duarte, P.; Barbosa, D.; Ferreira, I. New composite of natural hydraulic lime mortar with graphene oxide. Constr. Build. Mater. 2017, 156, 1150–1157. [Google Scholar] [CrossRef]
- Maravelaki-Kalaitzaki, P.; Bakolas, A.; Karatasios, I. Hydraulic lime mortars for the restoration of historic masonry in Crete. Cem. Concr. Res. 2004, 35, 1577–1586. [Google Scholar] [CrossRef]
- Zhou, X.; Hu, Y.; Wang, J.H.; Dai, S.B. Study on hydraulic lime mortar used for consolidation of Hua shan rock paintings. Sci. Conser Arc. 2011, 23, 1–7. [Google Scholar]
- Dai, S.B.; Wang, J.H. Selection and research and development of rescue and reinforcement materials of Zuojiang Huashan rock paintings. Chinese Cult. Herit. 2016, 4, 55–59. [Google Scholar]
- Zhang, Y.S.; Wang, X.H.; Xiao, J.Q.; Yang, L. Preparation and Durability of Hydraulic Lime Used as Repairing Materials for Architectural Heritage. J. Build Mater. 2018, 21, 143–149. [Google Scholar]
- Moropoulu, A.; Bakolas, A.; Bisbikou, K. Investigation of historic mortars. J. Cult Herit. 2000, 1, 45–58. [Google Scholar] [CrossRef]
- Wang, L.L.; Liu, Z.; Wang, D.M.; Jiang, Q.Y.; Zhang, C. Process optimization and properties of natural hydraulic lime from marlite. Bullet. Chin. Cera. Soc. 2019, 38, 853–857. [Google Scholar]
- Shen, X.F.; Xue, Q.H.; Xu, L.; Shi, Z.W.; Zhang, H. Research of the feasibility to prepare the natural hydraulic limes from the lead and zinc mine tailing. Bullet. Chin. Cera. Soc. 2013, 32, 1793–1798. [Google Scholar]
- CEN. EN 459–1:2010; Building Lime. Part 1: Definitions, Specifications and Conformity Criteria. iTeh Standards: Brussels, Switzerland, 2010.
- Li, L.; Zhao, L.Y. Study on Ancient Chinese Calcareous Materials; Cultural Relics Press: Beijing, China, 2015; pp. 58–64. [Google Scholar]
- Válek, J.; Halem, E.V.; Viani, A.; Pérez-Estébanez, M.; Ševcík, R.; Šašek, P. Determination of optimal burning temperature ranges for production of natural hydraulic limes. Constr. Build. Mater. 2014, 66, 771–780. [Google Scholar] [CrossRef]
- Zhang, D.J.; Zhao, J.H.; Wang, D.M.; Xu, C.Y.; Zhai, M.Y.; Ma, X.D. Comparative study on the properties of three hydraulic lime mortar systems: Natural hydraulic lime mortar, cement-aerial lime-based mortar and slag-aerial lime-based mortar. Constr. Build. Mater. 2018, 186, 42–52. [Google Scholar] [CrossRef]
- Frankeová, D.; Koudelková, V. Influence of ageing conditions on the mineralogical micro-character of natural hydraulic lime mortars. Constr. Build. Mater. 2020, 264, 120205. [Google Scholar] [CrossRef]
- CEN. EN 1015-11:1999/A1:2006; Methods of Test for Mortars for Masonry. Part 11: Determination of Flexural and Compressive Strength of Hardened Mortar. iTeh Standards: Luxembourg, 2006.
- ASTMC 1148-92a:2008; Standard Test Method for Measuring the Drying Shrinkage of Masonry Mortar. ANSI Webstore: New York, NY, USA, 2008.
- Wang, H.X.; Diao, G.Z.; Liu, G.H. Effect of doping on structure and hydration reactivity of dicalcium aluminosilicate. China Build. Mater. Sci. Technol. 2019, 28, 47–49. [Google Scholar]
- Zhang, J.K.; Chen, W.W.; Li, Z.X.; Wang, X.D.; Guo, Q.L.; Wang, N. Study on workability and durability of calcined ginger nuts-based grouts used in anchoring conservation of earthen sites. J. Cult Herit. 2015, 16, 831–837. [Google Scholar] [CrossRef]
- Li, Z.X.; Zhao, L.Y.; Li, L. On new fracture grouting material for conglomerate grottoes rock. Dunhuang Res. 2011, 130, 59–64. [Google Scholar]
- Wang, J.F.; Yan, G.S.; Yang, S.L. Distribution of soluble salts in the cliff strata of the Mogao Grottoes. Hydrogeol Eng. Geol. 2010, 37, 116–120. [Google Scholar]
- Li, L.; Zhao, L.Y.; Wang, J.H.; Li, Z.X. Research on physical and mechanical characteristics of two traditional silicate materials in Chinese ancient building. Chin. J. Rock. Mech. Eng. 2011, 30, 2020–2027. [Google Scholar]

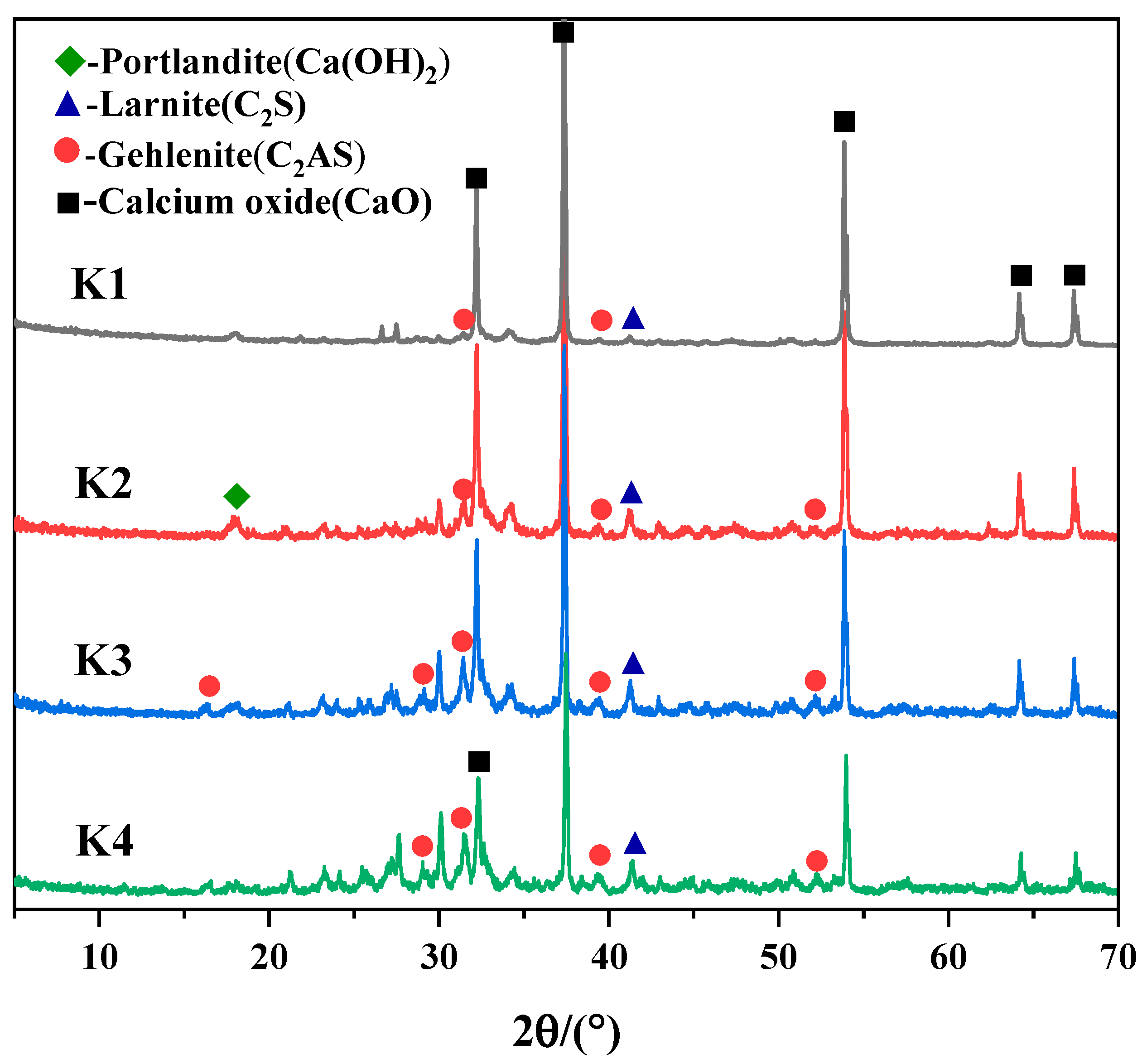
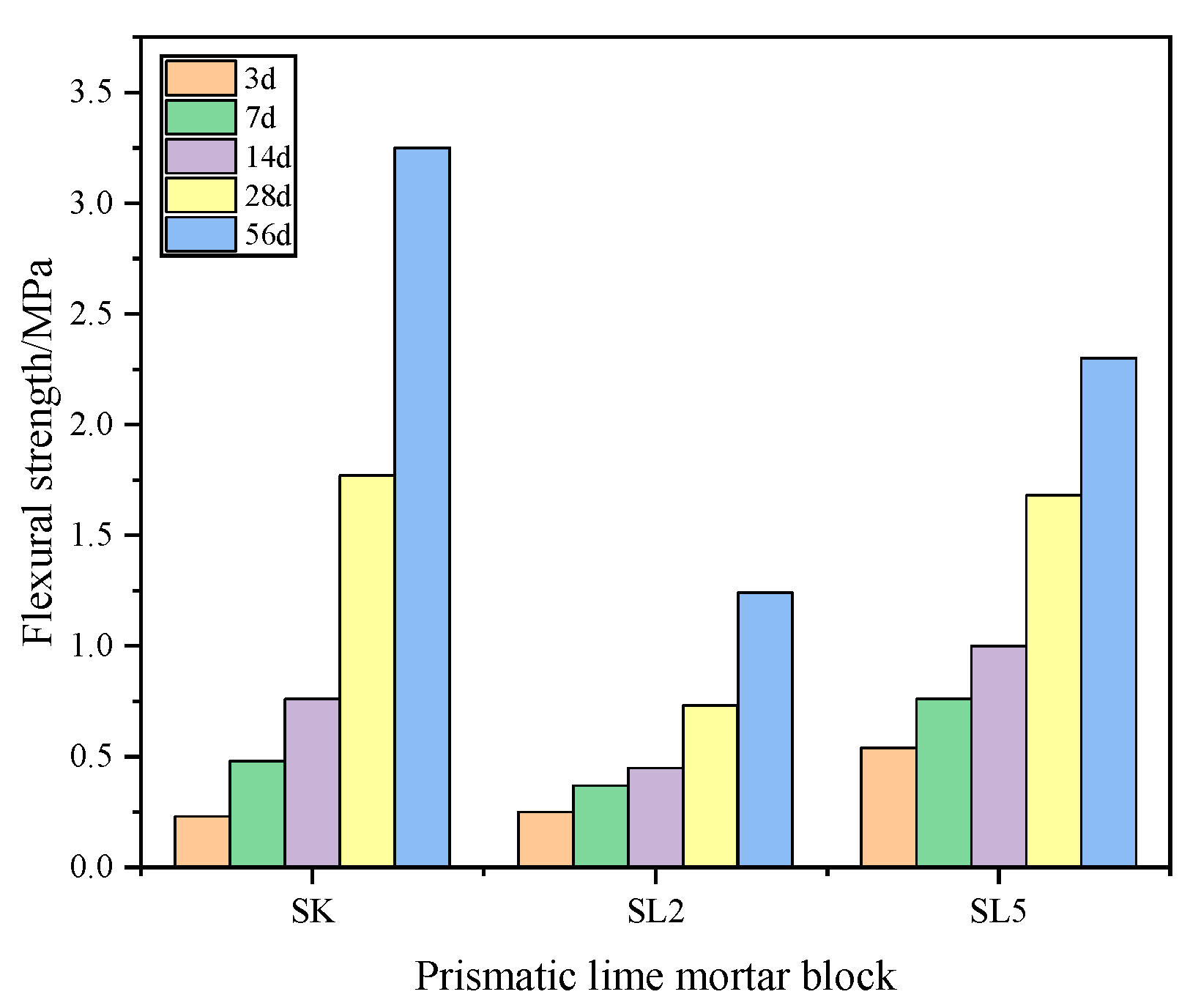
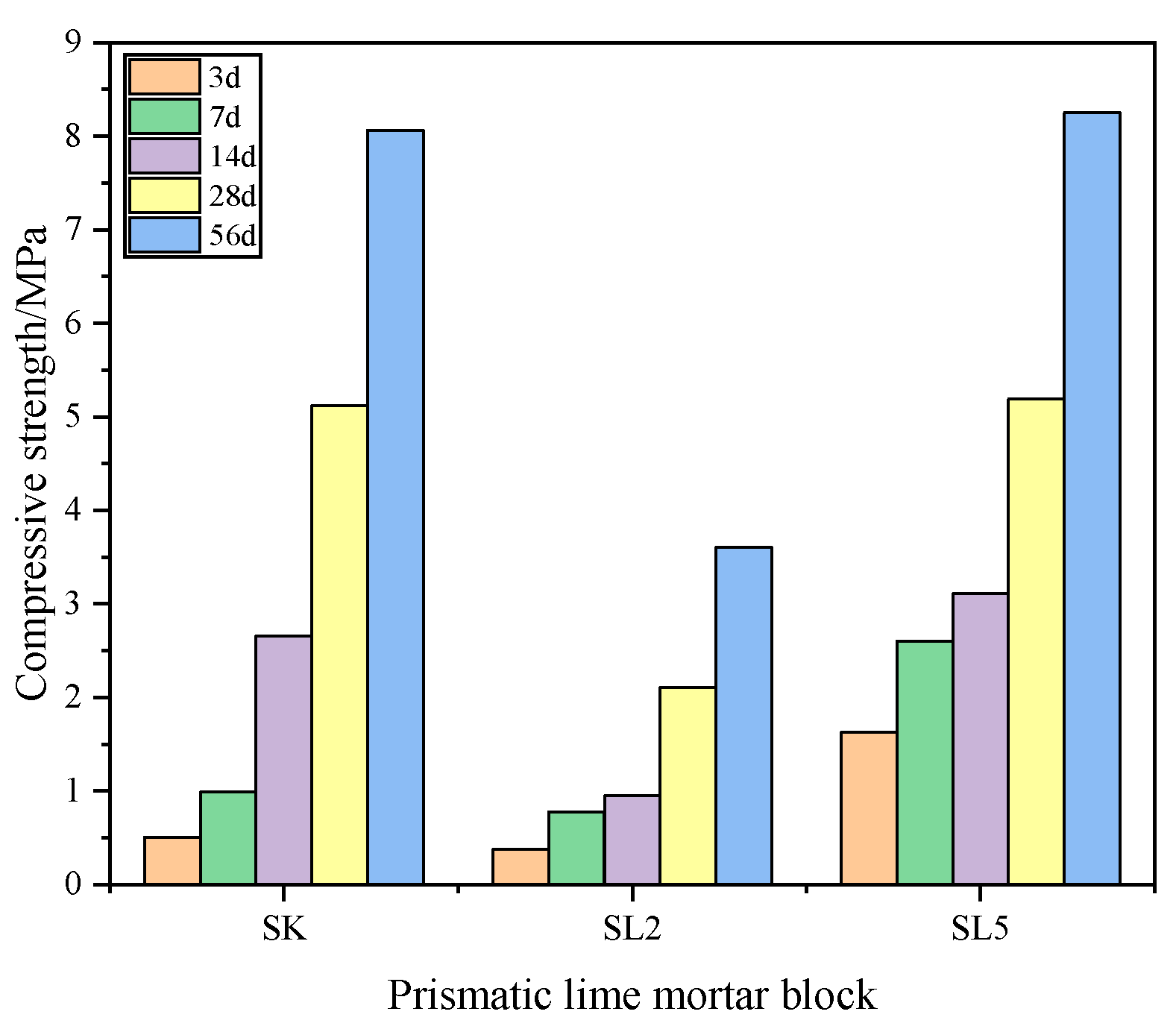
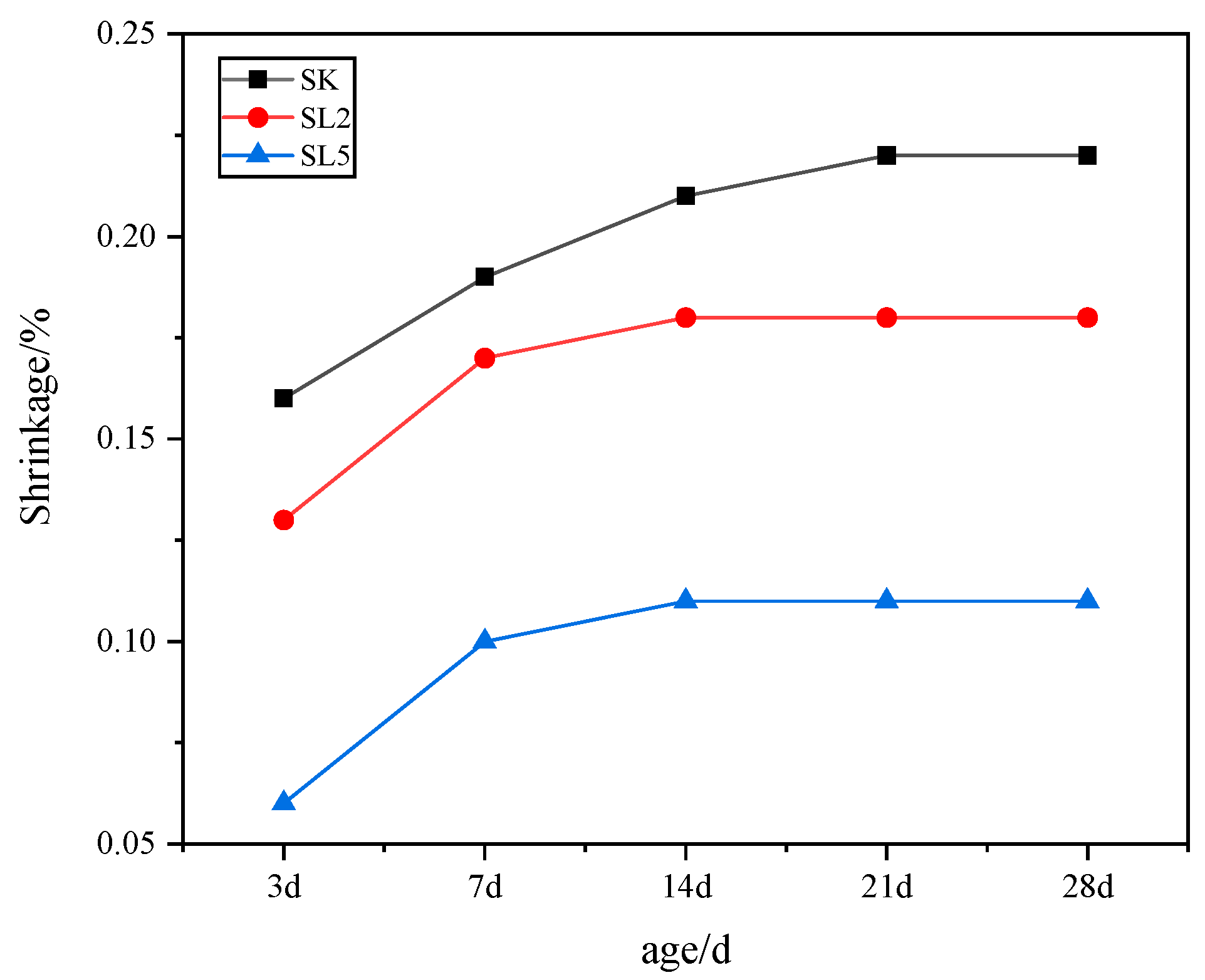
| Composition | SiO2 | Al2O3 | K2O | Na2O | CaO | Fe2O3 | MgO | Total | LOI |
|---|---|---|---|---|---|---|---|---|---|
| Potassium feldspar | 63.36 | 15.74 | 15.32 | 2.36 | 0.53 | 0.91 | 0.47 | 98.69 | 1.31 |
| Limestone | 1.34 | 0.29 | 0.00 | 0.00 | 54.13 | 0.17 | 1.53 | 57.46 | 42.54 |
| Components and Content | Mass Percentage (%) | |||||
|---|---|---|---|---|---|---|
| Air-Hardening Component | Hydraulic Component | |||||
| No. | CaO | Ca(OH)2 | Total | C2S | C2AS | Total |
| K1 | 66.5 | 3.5 | 70.0 | 17.6 | 3.3 | 20.9 |
| K2 | 40.9 | 10.2 | 51.1 | 28.4 | 10.1 | 38.5 |
| K3 | 33.1 | 5.1 | 38.2 | 31.0 | 12.9 | 43.9 |
| K4 | 29.6 | 5.1 | 34.7 | 27.4 | 19.3 | 46.7 |
| NHL2 | 23.9 | 56.8 | 80.7 | 18.3 | 0.0 | 18.3 |
| NHL5 | 45.0 | 20.2 | 65.2 | 33.8 | 0.0 | 33.8 |
| Area | Element, Atomic (%) | |||||
|---|---|---|---|---|---|---|
| C | O | Al | Si | K | Ca | |
| 1 | 2.89 | 60.54 | 0.00 | 2.51 | 0.00 | 34.06 |
| 2 | 2.43 | 26.59 | 2.72 | 9.06 | 1.22 | 57.99 |
| 3 | 16.55 | 43.99 | 2.66 | 7.56 | 0.73 | 28.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Sun, M.; Guo, Q.; Zhao, L.; Li, Z. Study on the Mechanical Properties and Durability of Hydraulic Lime Mortars Based on Limestone and Potassium Feldspar. Appl. Sci. 2023, 13, 2412. https://doi.org/10.3390/app13042412
Zhang S, Sun M, Guo Q, Zhao L, Li Z. Study on the Mechanical Properties and Durability of Hydraulic Lime Mortars Based on Limestone and Potassium Feldspar. Applied Sciences. 2023; 13(4):2412. https://doi.org/10.3390/app13042412
Chicago/Turabian StyleZhang, Shaoyun, Manli Sun, Qinglin Guo, Linyi Zhao, and Zhipeng Li. 2023. "Study on the Mechanical Properties and Durability of Hydraulic Lime Mortars Based on Limestone and Potassium Feldspar" Applied Sciences 13, no. 4: 2412. https://doi.org/10.3390/app13042412
APA StyleZhang, S., Sun, M., Guo, Q., Zhao, L., & Li, Z. (2023). Study on the Mechanical Properties and Durability of Hydraulic Lime Mortars Based on Limestone and Potassium Feldspar. Applied Sciences, 13(4), 2412. https://doi.org/10.3390/app13042412






