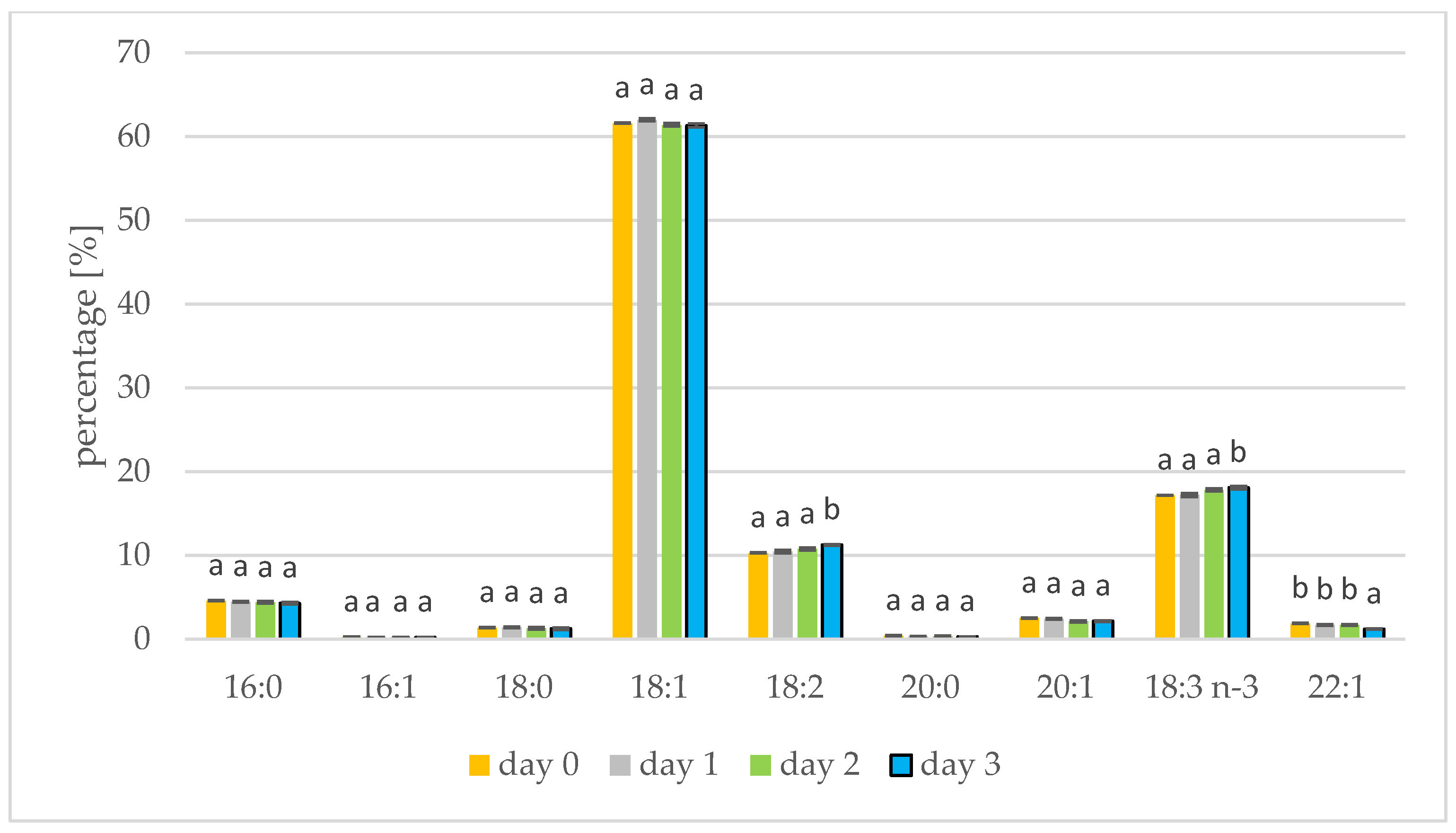Abstract
Vegetable oils are selected by consumers based on the presence of multiple bioactive substances, including polyunsaturated fatty acids, sterols, and tocopherols. Another important factor in oils is their quality. This research involved analyzing the oils quality and quantity of bioactive substances obtained from rape seeds and white mustard seeds that underwent germination. The quality of the oils was compared by determining the acid and peroxide values. Germination lowered the peroxide value by 86.3% and 71.4% for rapeseed oil and mustard oil, respectively. This was due to the germination step of the seed use oxygen, which was the substrate for lipid peroxidation. The activity of peroxidase increased by 95% for rapeseed oil and 94% for mustard oil during germination. An increase in the amount of polyunsaturated fatty acids in mustard oil also was noted during germination.
1. Introduction
Oil quality is one of the determinants of whether an oil can be used for food purposes. The quality of an oil depends on the degree of maturity of the seeds, the amount of impurities contained, the quantity of damaged seeds, the moisture of the seeds during and after storage, and the storage conditions [1]. The basic parameters that describe oil quality are the acid value and the peroxide value. The peroxide value describes the oil’s content of peroxides, which form through the process of oxidation [2]. The acid value describes the quantity of free fatty acids formed through the hydrolysis of ester bonds in fats [3]. The maximum permissible values of these parameters are specified in the World Health Organization’s Codex Alimentarius [4]. For refined oils, the maximum peroxide and acid values are 10 meq O2/kg and 0.6 mg KOH/kg, respectively. For cold-pressed and virgin oils, the maximum peroxide and acid values are 15 meq O2/kg and 4 mg KOH/kg, respectively.
Apart from the oil quality, the quantity of biologically active compounds in an oil is also important to the consumer. The biologically active compounds found in oils include polyunsaturated fatty acids, tocopherols, sterols, and carotenoids [5]. The quantity of biologically active compounds is also affected by a number of factors—particularly by the method of pressing and the method of preparing the seeds for pressing; these affect the oil composition. Both roasting and germinating seeds can affect the components of the oil [6]. During roasting, many physical and chemical changes take place in the product, which include flavor, color and texture changes. Changing these characteristics improves the palatability of the raw material [7]. Additionally, roasting conditions the seeds, which improves extraction and increases oil yield [8]. At the same time, Maillard reaction products may be formed during roasting, which may reduce the nutritional value of the raw material [9]. Germination is the process by which seeds that were previously dormant become imbibe and begin development by piercing the rootlets through the seed coat. Due to the changes taking place during germination, the level of fat is reduced, so the yield of the obtained fat is reduced [10]. Research on germination and metabolic mechanisms inside seeds is still ongoing [11,12]. However, the number of studies related to the quality of oil obtained from germinating oilseeds is insufficient. In the literature, we found some information about changes in bioactive compounds in some different seeds during germination, for example: quinoa [13], amaranth [14], sacha inchi [15], sunflower seeds [16], soybean [17] or flaxseed [18]. Depending on the type of the seeds we can see different changes during germination. However, most often, seed germination leads to the accumulation of bioactive ingredients and the improvement of oil quality. In some cases, germination improved the fatty acid profile [13,14,15], tocopherol content [15,16,17] or increase concentration of phytosterols [16,17].
Peroxidases are family of isoenzymes and structurally can be described as heme-containing monomeric glycoproteins. Peroxidases oxidize some compounds in the presence of H2O2 [19,20]. During germination, peroxidases play a significant role in axis growth by producing ˙OH from H2O2 [21]. Reactive oxygen species such as ˙OH, O2−, and H2O2 are usually considered toxic [22], but for seeds, H2O2 is a germination promoting factor. The scavenging activity of H2O2 is sufficiently high to result in the production of O2 for mitochondrial respiration [23].
Rapeseed oil is the third oil in the world in terms of consumption (first place—palm oil, second place—soybean oil) [24]. In Poland, rapeseed oil is a very popular oil due to its versatility, availability and affordable price. In addition, it is characterized by a high content of unsaturated fatty acids. Mustard oil is a niche oil, but it is also an oil characterized by a high content of unsaturated fatty acids. The new double-improved variety (less glucosinolates and less erucic acid) is a variety less sensitive to temperature changes during cultivations and can also be used for consumption purposes.
In this study, we investigated the effects of germination of rape and mustard seed on the oil’s quality and concentration of bioactive substances. The hypothesis is that the quality of the oils obtained from the seeds after germination will be improved. So far, there have been no studies of oil from germinating seeds combining both oil quality, changes in bioactive compounds and changes in the amount of peroxidase in rapeseed and mustard.
2. Materials and Methods
2.1. Chemicals
We purchased 96% Ethanol, Na2CO3, NaOH, CuSO4·5H2O, KNaC4H4O6·4H2O, NaH2PO4, Na2HPO4 from POCH (Poland). Bovine albumin (BSA), Folin reagent, hexane for GC, 30% sodium methoxide in methanol, pyrocatechol, hydrogen peroxide, 5α-cholestane, tert-butyl methyl ether, BSTFA +1% TCMS and 2-propanol were purchased from Sigma Aldrich (St. Louis, MO, USA), and 1M KOH in methanol was obtained from Chempur (Piekary Śląskie, Poland).
2.2. Materials
Rape seeds (Brassica napus L.) “Karo” variety—humidity 5.65% and white mustard seeds (Sinapis alba, “Warta” variety, with zero erucic acid and zero glucosinolates)—humidity 6.00% obtained from The Plant Breeding and Acclimatization Institute in Poznań were used in this study. Both varieties are in the Research Center for Cultivar Testing in Poland. Some of both types of seeds remained unprocessed as control seeds. The rest of the seeds were washed with 70% ethanol to remove fungi and mold. The seeds were covered in water to swell them. Once this had been achieved, the seeds were spread in a single layer to sprout and placed in heaters without access to light. The humidity in both incubators was 90%. The germination conditions for both types of seed differed only in terms of temperature: 22.5 °C for rape seeds and 10 °C for mustard seeds. Samples of the germinating seeds were withdrawn daily for three days and frozen until needed for analysis. After three days of germination, all seeds had sprouts. The experiment was performed in triplicate.
2.3. Determination of Protein Content
Seeds (100 mg) were placed in a cold mortar. The seeds were treated with 0.1 M phosphate buffer pH 7.8 and crushed. The suspension was filtered through a filter. The Lowry method [25] was used to determine the protein content of the seed. First, a standard curve was prepared covering the range of 20–100 µg proteins. Individual solutions were prepared from a solution of BSA at a concentration of 200 µg/mL. The following reagents were prepared: A: 2% Na2CO3 in 0.1 M NaOH; B: 0.5% CuSO4·5H2O in 1% KNaC4H4O6·4H2O; C: a mixture of reagent A and reagent B; D: diluted Folin reagent (1:1 v:v with distilled water). Reagent C was added to 50 µL of the protein extracts, mixed, and left for 30 min at room temperature. After that, 200 µL of D reagent was added, mixed, and again left for 30 min at room temperature. Absorbance was measured at 750 nm against a blank (to which water was added instead of the proteins). The determination coefficient was 0.926. In preparing samples from the mustard and rape seeds, the determination procedure was the same as for the preparation of the standard curve, except that the protein extracts that had earlier been obtained from the seeds were used instead of BSA solutions.
2.4. Peroxidase Enzyme Activity
Peroxidase enzymatic activity was determined following the method of Bhattacharya et al. [26]. First, 2.6 mL of 0.1 M phosphate buffer at pH 7.8, 400 µL of 5mM pyrocatechol, and 400 µL of 0.8 mM hydrogen peroxide at 4 °C were added to the tubes. Then, 100 µL of the previously obtained protein extract was added to them. The samples were mixed and the absorbance was measured at a wavelength of 470 nm every minute for three minutes against a blank sample.
2.5. Extraction of Oil from Seeds
Ground seeds (50 g) were placed in a Soxhlet extractor and 200 mL of hexane was added. The seed oil was allowed to extract for six hours from the moment the solvent began to boil. Then hexane was evaporated using a vacuum evaporator at a temperature of 40 °C [27]. Before and after the extraction was started, the weight of the seeds was weighed in order to calculate the fat content of the seeds. The oils thus produced were then analyzed.
2.6. Acid Value and Peroxide Value
The acid value was determined using International standard method PN-EN ISO 660:2010 [28]. The peroxide value was determined using International standard method PN-EN ISO 3960:2017-03 [29].
2.7. Fatty acid Analysis
The methodology described by Raczyk et al. [30] was used to analyze the fatty acids. Two drops of oils were used to perform the esterification reaction. Then 1 mL of hexane and 1 mL of 0.4 M sodium methoxide were added, mixed, and left for 15 min at room temperature. Five (5) mL of distilled water was added to stop the reaction. After separation of the phases, the upper phase was transferred to chromatographic vials. For analysis we used a gas chromatograph Thermo 1300 with a flame ionization detector (Thermo Scientific, Waltham, MA, USA) and a SP-2560 capillary column (100 m × 0.25 mm × 0.2 µm) (Supelco, Bellefonte, PA, USA). The inlet and detector temperatures were both 240 °C. Hydrogen was used as carrier gas with a flow rate of 1.5 mL/min and the analysis was performed in split mode. The initial oven temperature was 160 °C; this was maintained for 1 min, and then increased to 220 °C at 6 °C/min, where it was held for 17 min. The injector and detector were set to 240 °C. One (1) µL samples were used for the analysis. Finally, retention times were compared with those obtained from a run using 37 Component Fame Mix (Supelco, Bellefonte, PA, USA).
2.8. Sterol Analysis
The method described by Sahu et al. [31] was used to analyze the sterol content. About 0.05 g of oil was weighed and 0.1 mg of 5α-cholestane (internal standard) and 1M KOH methanol were added for saponification. The sample was mixed, placed in a thermoblock for 1 h, and left for 18 h at room temperature. Water was added and extraction was carried out twice with hexane:tert-butyl methyl ether (1:1, v/v). The solvents were evaporated from the extract under a stream of nitrogen. Finally, silylation was performed by adding 100 µL of pyridine and 400 µL of BSTFA + 1% TMCS. The samples were then analyzed on using a HP6890 gas chromatograph with a flame ionization detector (Agilent, Santa Clara, CA, USA). A DB-35ms capillary column (25 m × 0.20 mm × 0.33 μm, Agilent, Santa Clara, CA, USA) was employed. The oven temperature was increased from 100 °C (held for 5 min) to 250 °C at 25 °C/min (held for 1 min), and then at a speed of 3 °C/min to 290 °C, which was maintained for 20 min. Hydrogen was the carrier gas and the flow rate was of 1.5 mL/min; the temperature of the injection port and flame ionization detector were set to 290 °C and 300 °C, respectively.
2.9. Tocopherol Analysis
The method described by Górnaś et al. [27] was used to analyze tocopherols. First, 2-propanol was added to the oil sample and filtered through a nylon syringe filter (0.22 µm) into vials. The analysis was performed using a HPLC system (Shimadzu Corporation, Kyoto, Japan) consisting of a pump (LC-10ADvp), a degasser (DGU-14A), a low-pressure gradient unit (FCV-10ALvp), a system controller (SCL-10Avp), an auto injector (SIL-10AF), a column oven (CTO-10ASvp), and a fluorescence detector (RF-10AXL) in reversed phase mode using a Luna PFP column (150 × 4.6 mm, 3 mm) with a guard column (4 × 3 mm) (Phenomenex, Torrance, CA, USA). The column oven temperature was 40 °C. The mobile phase was methanol:water (93:7; v/v) and the flow rate was 1.0 mL min−1. The excitation wavelength was 295 nm and the emission wavelength was 330 nm.
2.10. Statistical Analysis
The samples were analyzed in triplicate. All the data was presented as a mean with standard deviations. The statistical program Statistica 13.3 (TIBCO Software Inc., Palo Alto, CA, USA) was used to prepare one-way analysis of variance (ANOVA). The Tukey test was used, with a significance level of α = 0.05. R software (version 4.1 with packages FactoMineR v.2.4 and factoextra v.1.0.7) was the software used for principal components analysis (PCA).
3. Results and Discussion
3.1. Changes in Fat Content during Seed Germination
During the germination of rapeseeds, there was a sharp drop in the percentage of fat in the seeds. Initially, the fat in the seeds accounted for 41.5%, and on the third day of germination, 22.1% of the fat (Table 1). In soybean seeds, also during germination, the percentage of fat decreased from 20% to 15% [17]. During the sprouting of the legumes, the percentage of fat in the seeds is also lowered [32]. During germination, fat is used to produce energy to grow. Lipids are used as respiratory substrates and in the pathways of sugar and amino acid synthesis [33]. In the case of mustard seeds, only an increase in the percentage of fat in seeds was observed during the 3 days of germination. The percentage increased from 26.0% to 27.0%, this is due to a slower development of germ and an earlier stage of germination [34].

Table 1.
Lipid content (%) and standard deviation of rapeseeds (R) and mustard seeds (M) during 3 days of germination. Values with different letters are significantly different (p < 0.05).
3.2. Changes in Fatty Acids during Seed Germination
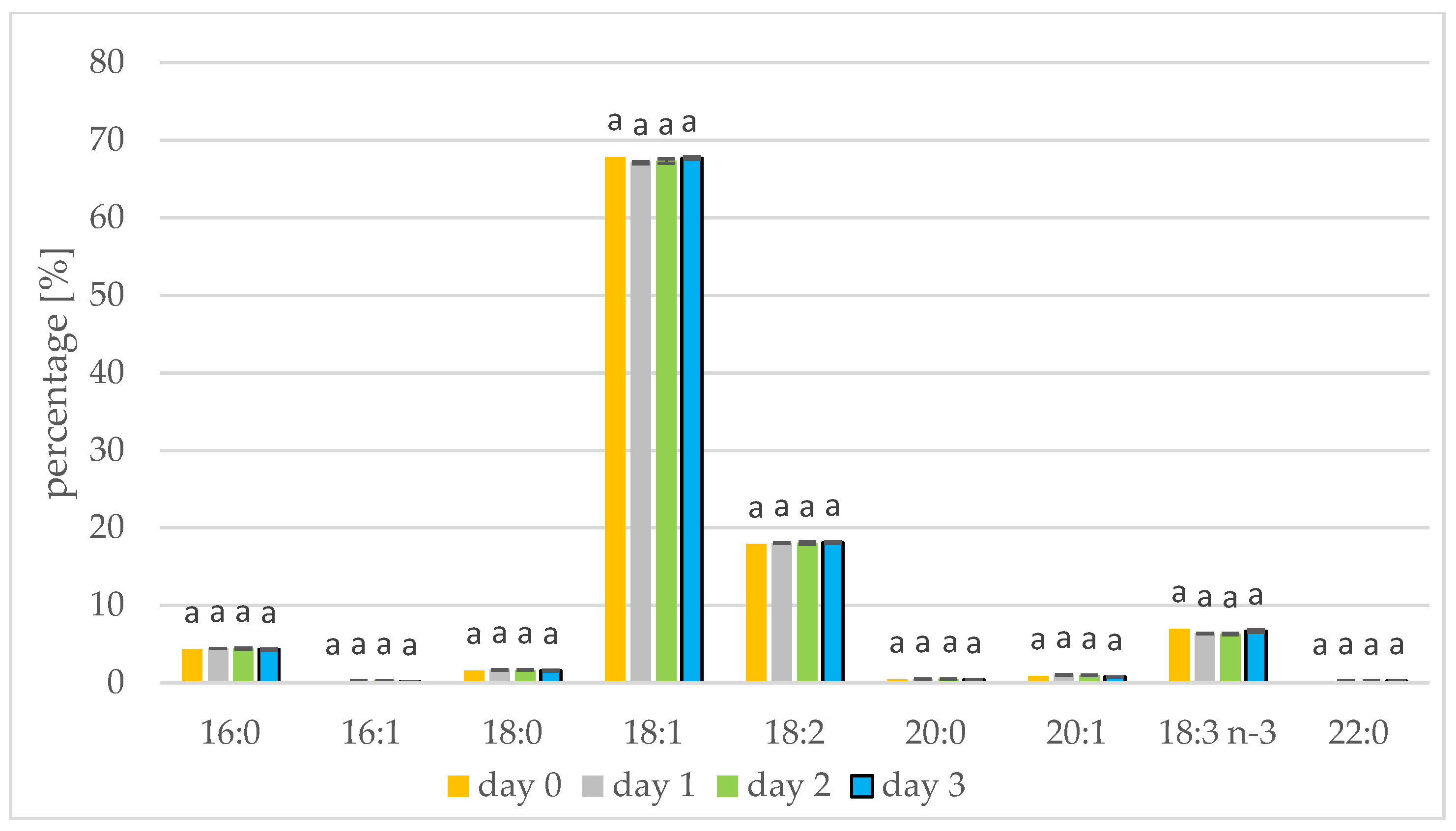
The rapeseed and white mustard oils showed their typical species-specific abundances of individual fatty acids as present in Figure 1 and Figure 2, respectively. In rapeseed and mustard, oleic, linolenic, and linoleic acids were most common. Longer germination time resulted in increases in linoleic and linolenic acids in mustard oil. The linoleic acid concentration increased from 10.29% to 11.24% in mustard seed and the linolenic acid percentage increased from 17.17% to 18.07%. Linolenic acid also increased during sesame germination [35]. In mustard oil, the remaining acids represented less than 5% and did not alter with germination. A similar relationship has been shown when Peruvian quinoa (Chenopodium quinoa Willd.) was germinated. Three varieties of Peruvian quinoa showed an increase in the percentage of linoleic and linolenic acids after two days of seed germination [13]. The concentration of linoleic acid in amaranth seeds also increased from 1.84% to 1.99% when germination was performed [14]. The percentage of fatty acids seen during germination has also been tested in seeds of sacha inchi (Plukenetia volubilis L.), with an increase in linoleic and linolenic acids seen until day 6 of germination. A decrease in linoleic and linolenic acid abundances was also seen after the sixth day of germination, on account of the longer duration of this study. From the tenth day of germination, levels of palmitic and oleic acid began to increase [15]. In the initial period of germination, fatty acids are transformed into sugars, but first saturated fatty acids and monoenoic fatty acids are transformed, which increases the percentage of polyene fatty acids. Consuming polyunsaturated fatty acids protects, for example, against the inflammatory diseases, cancer and cardiovascular diseases [36].
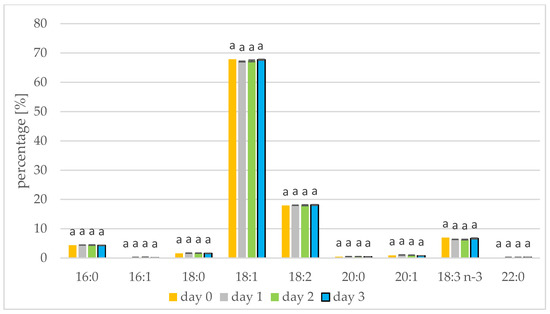
Figure 1.
Fatty acid composition [%] of rapeseed oil on days 1, 2, and 3 of germination. 16:0—palmitic acid; 16:1—palmitoleic acid; 18:0—stearic acid; 18:1—oleic acid; 18:2—linoleic acid; 20:0—arachidic acid; 20:1—eicosenoic acid; 18:3 n-3—linolenic acid; 22:0—behenic acid (values are means ± SD from three experiments. Values with different letters are significantly different (p < 0.05)).
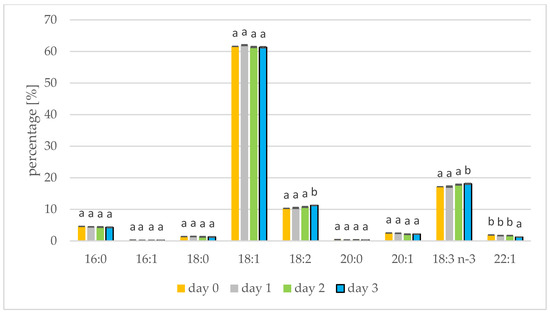
Figure 2.
Fatty acid composition [%] of mustard oil on days 1, 2 and 3 of germination. 16:0—palmitic acid; 16:1—palmitoleic acid; 18:0—stearic acid; 18:1—oleic acid; 18:2—linoleic acid; 20:0—arachidic acid; 20:1—eicosenoic acid; 18:3 n-3—linolenic acid; 22:1—erucic acid (values are means ± SD from three experiments. Values with different letters are significantly different (p < 0.05)).
3.3. Changes in Sterols during Germination
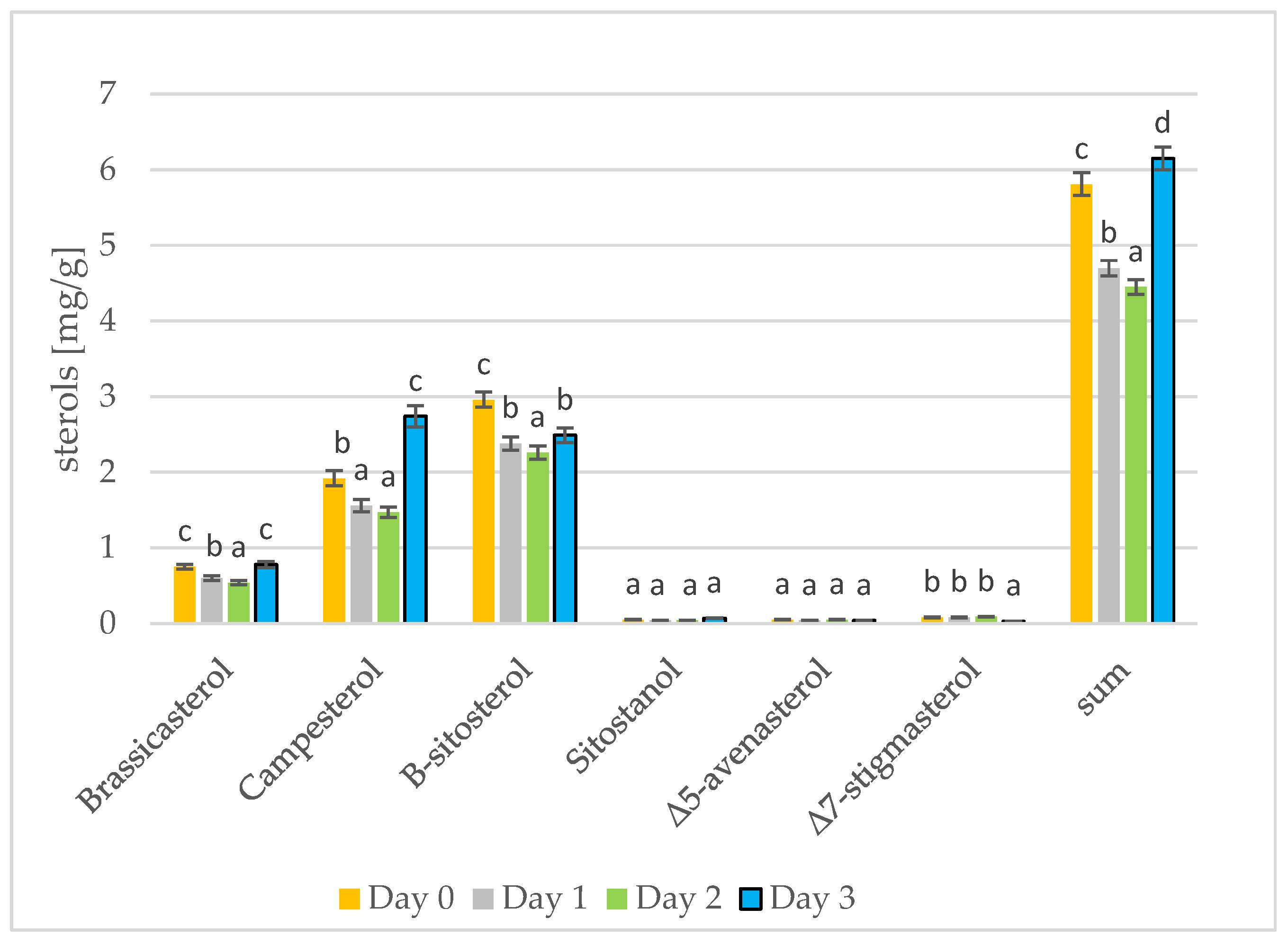
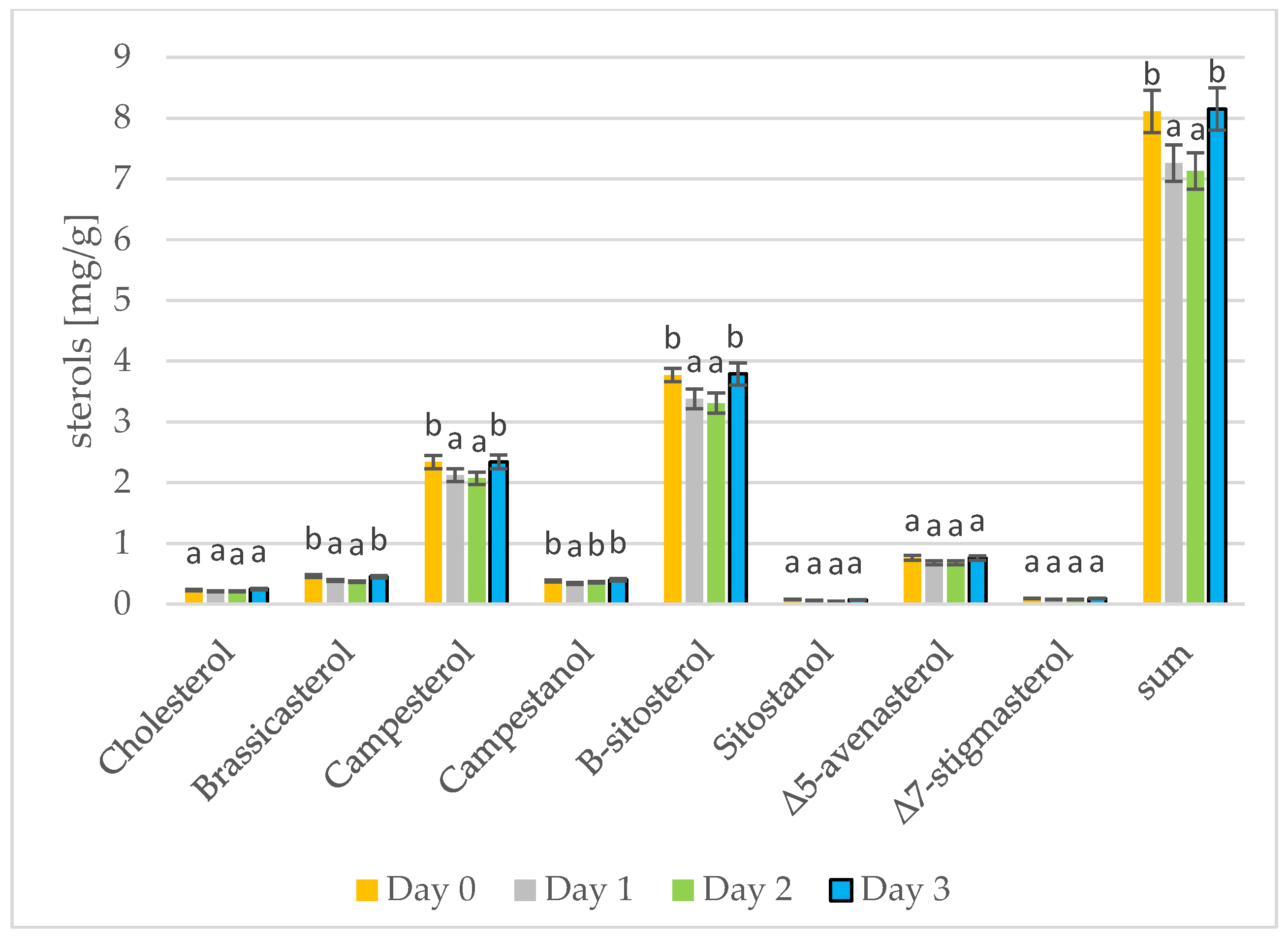
Phytosterols have been proven to lower blood cholesterol levels by blocking places for cholesterol [37]. The results of phytosterol analysis are presented in Figure 3 and Figure 4. The sterol levels found in the rape and mustard seeds prior to sprouting are what would be expected for the type of seed: the rape seeds contained mostly β-sitosterol, campesterol, and brassicasterol, while the mustard seeds contained mostly β-sitosterol, campesterol, and Δ5-avenasterol. Summarizing the results from three days of seed germination, differences can only be seen for the most abundant sterols. On the first and second day of seed germination, a decrease in the amount of individual sterols can be seen. A change occurs on the third day of germination in the rapeseeds, but the sterol levels in the mustard seeds firstly decreased and then increased and remained the same as on day 0 (prior to germination). In addition, in the case of tobacco seeds, depending on the tested sterols, there were different changes in the amount of sterols over time [38]. The amount of β-sitosterol in the rapeseed oil was lower on day 3 of germination than on day 0; the amount of campesterol increased from day 0 to day 3; the amount of brassicasterol remained the same as on day 0. During the roasting of rapeseeds, the amount of sterols in the oil also increases. Changes in the amount of total sterols are greater. During 100 min of roasting, the amount of sterols increased by 6% [39]. Stigmasterol and β-sitosterol are important contributors to cell differentiation and proliferation [40]. Initially, the sterols present in the seeds are used for cell differentiation and proliferation and their amount in the fat fraction of the seed decreases. On the third day of germination, the synthesis of additional amounts of sterols began and the sterol level in the germinating seeds thus increases [41]. The germination of sunflower and rice seeds has been examined, and there is a gradual increase in sterol levels during the 5 days or 4 days of germination, respectively [16,42].
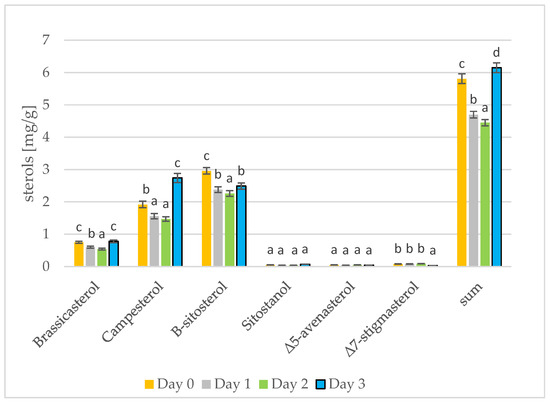
Figure 3.
The level of individual phytosterols [mg/g oil] in rapeseed oils at different stages of germination (days 0, 1, 2, 3) (values are means ± SD from three experiments. Values with different letters are significantly different (p < 0.05)).
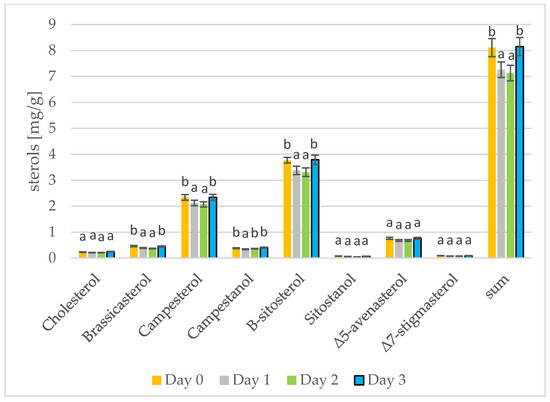
Figure 4.
Levels of individual phytosterols [mg/g oil] in mustard oils at different stages of germination (days 0, 1, 2, 3) (values are means ± SD from three experiments. Values with different letters are significantly different (p < 0.05)).
3.4. Changes in Tocochromanols during Germination
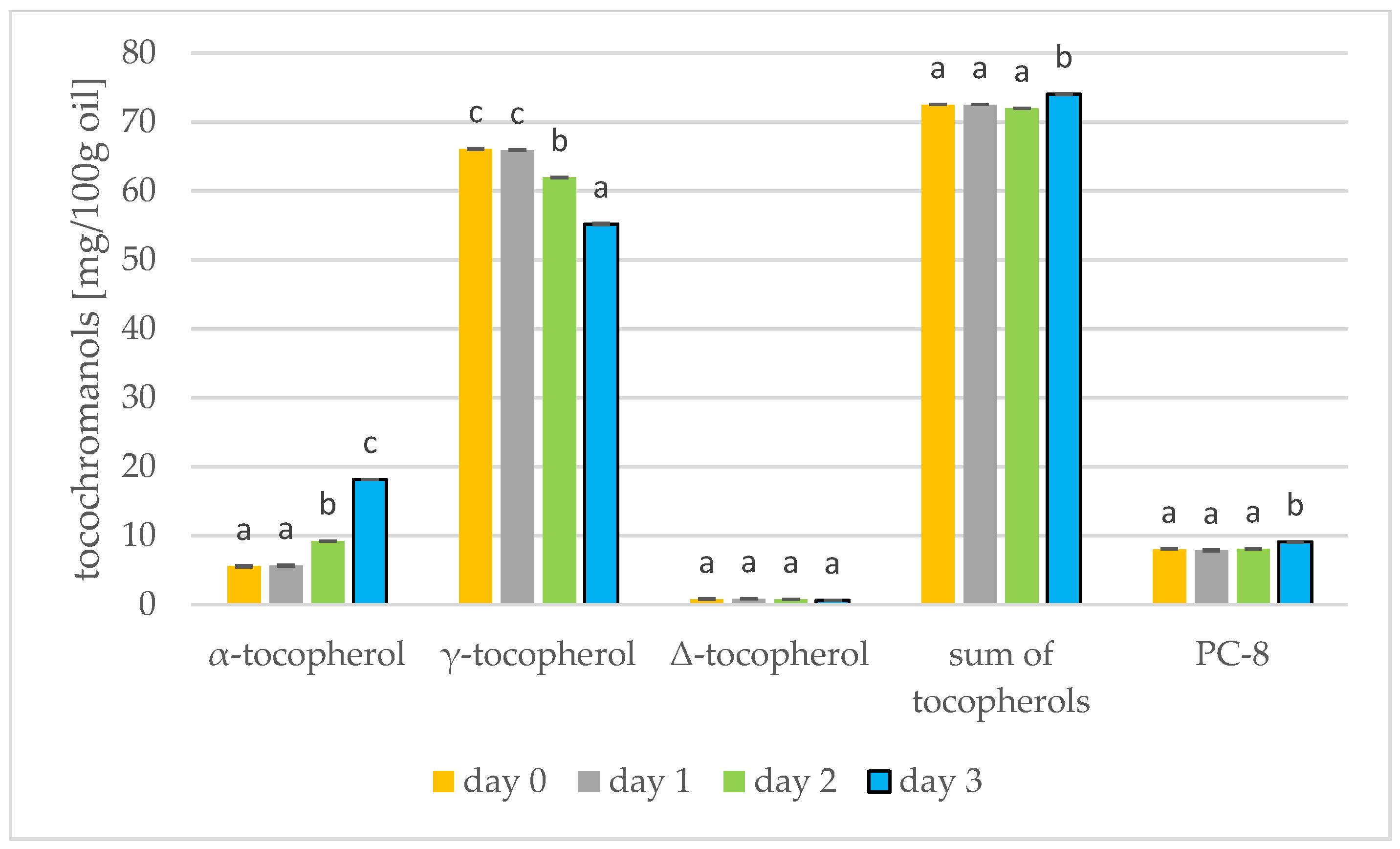
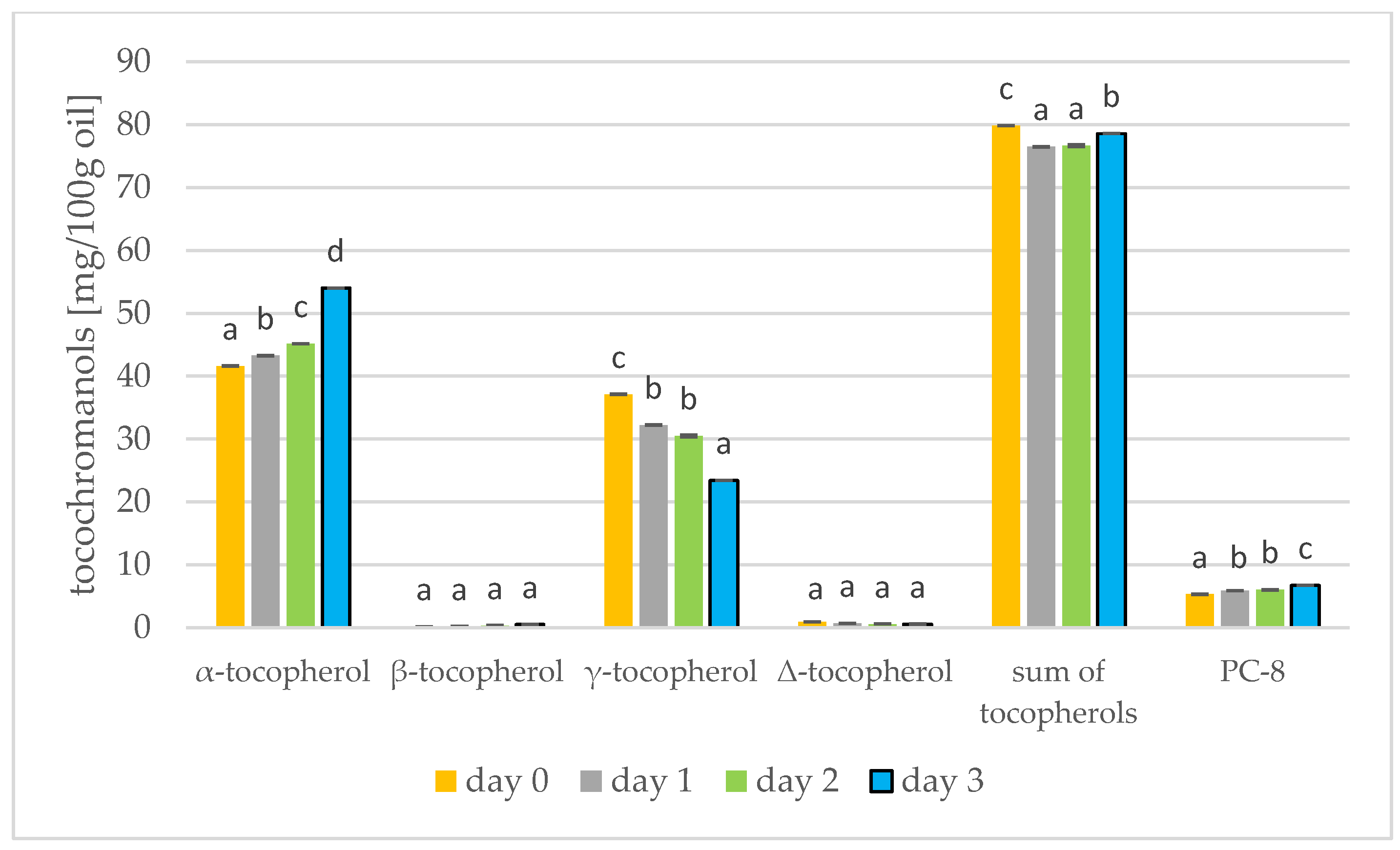
Results of the tocochromanols analysis were shown in Figure 5 and Figure 6. In mustard, it is γ-tocopherol (day 0: 66.10 mg/100 g) that dominates, but this decreases during germination (day 3: 55.24 mg/100 g). On the other hand, α-tocopherol increases during germination (day 0: 5.61 mg/100 g; day 3: 18.17 mg/100 g). The tocopherols thus increase during germination of mustard seed. In the study by García-Navarro et al. [43] on mustard seeds, a similar relationship was found: α-tocopherol increased and γ-tocopherol decreased in sprouts left in the dark. α-Tocopherol is predominant in photosynthetic tissues, e.g., in leaves, and γ-tocopherol is found primarily in seeds, fruits and tubers [43]. Therefore, at the start of germination, the amount of γ-tocopherol begins to decrease and the amount of α-tocopherol begins to increase. For soybeans, however, the tocopherol level had increased by the third day of germination, but began to decrease from day 5 [17]. In rapeseed, α-tocopherol predominates (41.61 mg/100 g), increasing during germination (day 3: 54.02 mg/100 g) while γ-tocopherol decreases. The total tocopherols did not change over the three days of germination, but in the longer study of Zhang et al. [44] the tocopherols increased by a factor of over four. During the roasting of rapeseeds, the amount of tocopherols in the oil also increases. Changes in the amount of total tocopherols are greater. During 100 min of roasting, the amount of tocopherols can increase by 11% [39]. Consuming tocopherols in the diet protects against cardiovascular disease, neurodegenerative disease and cancer [45].
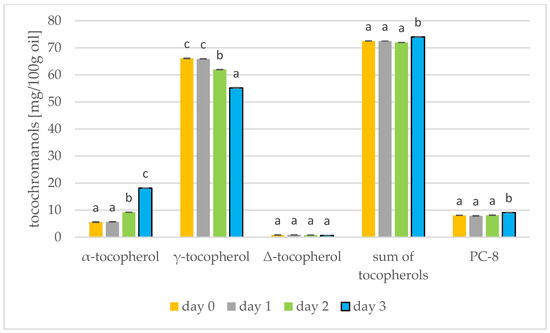
Figure 5.
Level of tocochromanols [mg/100 g oil] during mustard seed germination (days 0, 1, 2, 3) PC-8—plastochromanol-8 (values are means ± SD from three experiments. Values with different letters are significantly different (p < 0.05)).
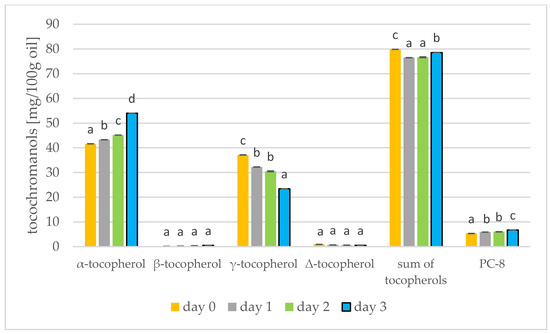
Figure 6.
Levels of tocochromanols [mg/100 g oil] during rapeseed germination (days 0, 1, 2, 3) PC-8—plastochromanol-8 (values are means ± SD from three experiments. Values with different letters are significantly different (p < 0.05)).
3.5. Oil Quality
The peroxide value reflects the quantity of peroxides present in the oil. For rapeseed oil from ordinary seeds, it equaled 11.53 meq O2/kg, while for mustard oil, it was value of 7.47 meq O2/kg. For both mustard and rapeseed, the peroxide value decreased in the oils obtained from seeds after days 1, 2, and 3 of germination. After day 3, it was 1.58 meq O2/kg for rapeseed oil, and 2.14 meq O2/kg for mustard seed. All peroxide values do not exceed the maximum values set by the Codex Alimentarius [4]. However, the initial peroxide value (day 0) is closer to the upper limit of the maximum value. Reducing the peroxide value during seed germination improves the quality of the oil, it contains less compounds of primary fat oxidation. Peroxide and acid values are presented in Table 2. Peroxides formed during the oxidation process easily break up into secondary compounds of the oxidation process, e.g., aldehydes or esters. For this reason, the amount of peroxides decreased during seed germination. In addition, the presence of the peroxidase enzyme (Section 3.6) reduced fat oxidation. The reverse properties can be seen in oils obtained from roasted rapeseeds—the longer the seeds are roasted, the more the peroxide value increases [38]. The acid value describes the quantity of free fatty acids in an oil; it was 5.61 mg KOH/g for the oil from ordinary mustard seeds and 0.67 mg KOH/g for the rapeseed oil. By the first day of germination, the acid value had decreased significantly in the mustard oil to 3.80 mg KOH/g, but then increased in the days following germination, reaching 5.61 mg KOH/g on the third day. In the case of rapeseed oil, the acid value increased on each subsequent day of germination, until it reached 10.31 mg KOH/g on the third day. The increase in the amount of free fatty acids is associated with the start of hydrolysis, which leads to the breaking of ester bonds between glycerol and fatty acid. The effect of this is an increasing amount of free fatty acids, which are determined by the acid number [46]. The acid values of rapeseed oil exceed the permissible values specified by the Codex Alimentarius only on the third day of germination. In the case of mustard, all values exceed the permissible value set by the Codex Alimentarius [4], except for the oil obtained from the seeds on the first day of germination. Similar results have been seen for flax seeds, but there both the acid value and the peroxide value increasing over each of four days of germination [18]. There was also a significant increase in the acid number during the germination of Moringa peregrina seeds [46]. The quality of the oil depends primarily on the oil extraction technique, but also on the quality of the seeds used to obtain the oil. In order to be able to use inferior quality seeds, the oil of which will be characterized by high peroxide and acid values, it is possible to apply earlier treatment of these seeds, i.e., seed germination, which will reduce the peroxide and acid values. The possibility of lowering the peroxide and acid number by germination can be also used in subsequent studies on other seeds with high peroxide and acid values.

Table 2.
Acid [mg KOH/g] and peroxide values [meq O2/kg] of rapeseed and mustard oils.
3.6. Comparison of Enzymatic Activity in Seeds and Sprouts of Rape and Mustard
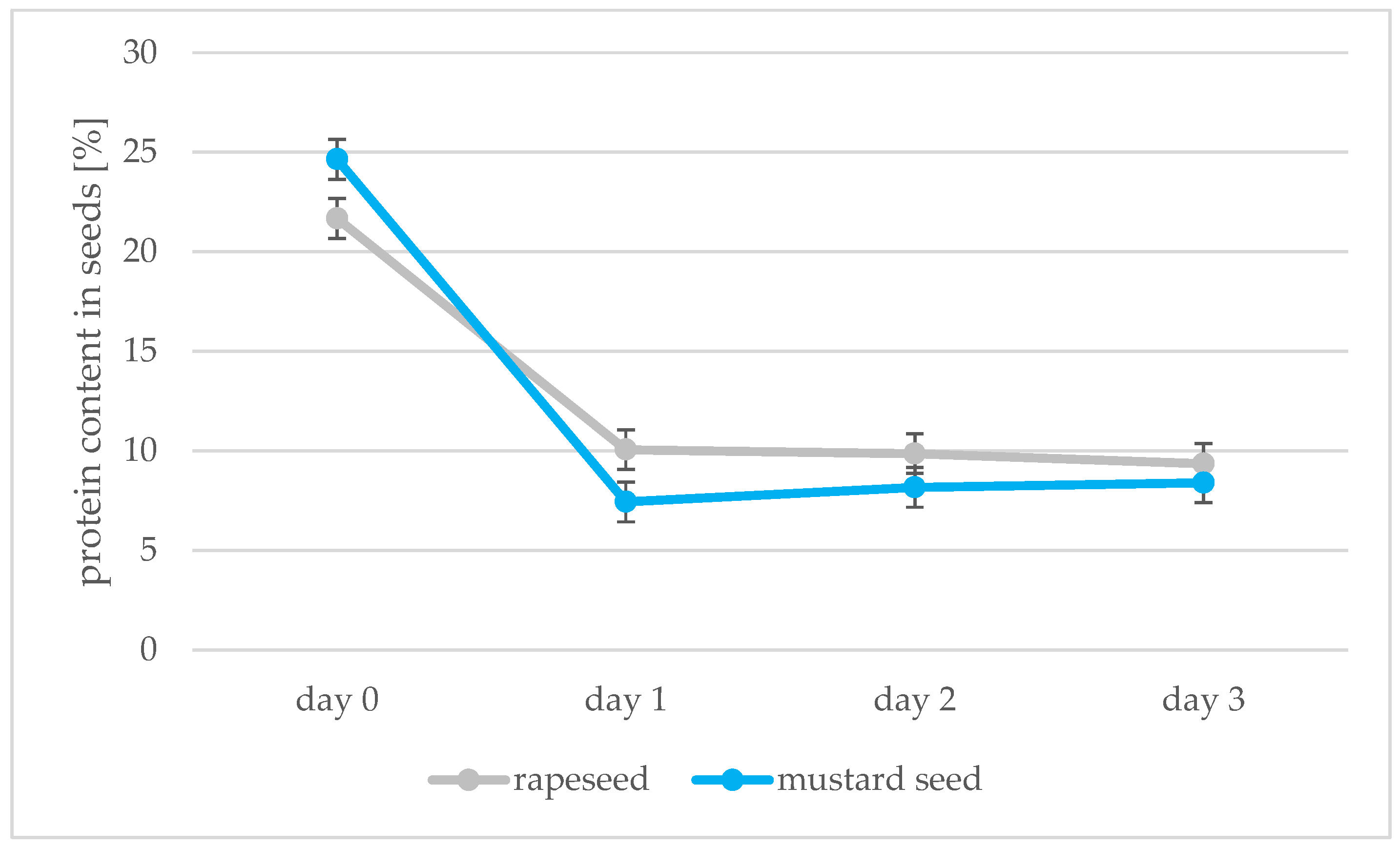
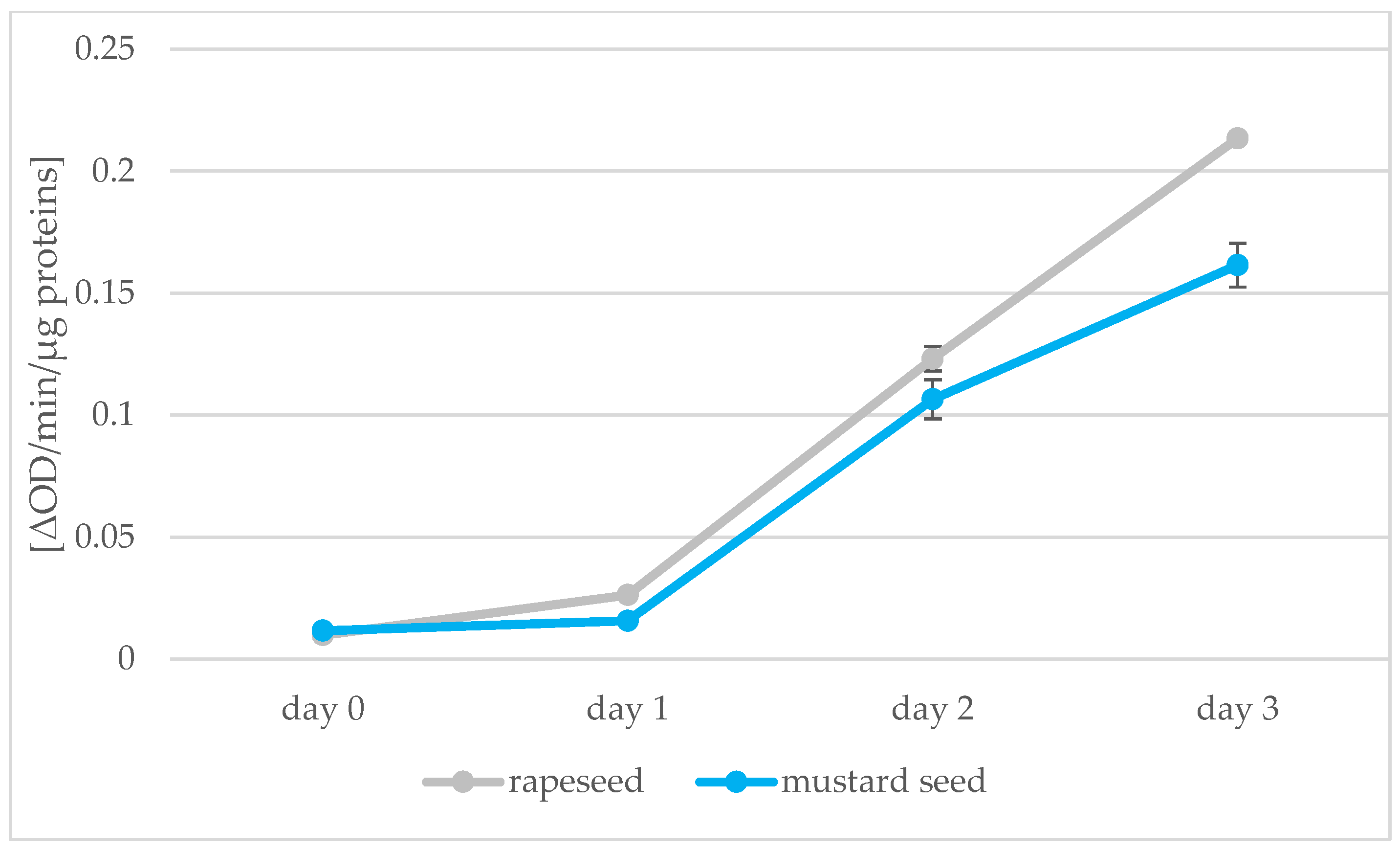
Absorbance measurements of samples prepared by the Lowry method allowed the amount of protein in the seeds to be calculated; the peroxidase activity was also determined using the spectrophotometric method. The results of these determinations are shown in Figure 7 and Figure 8. Protein decreases during germination in both types of seed from 21.68 to 9.36 protein content in seeds [%] for rapeseed oil and from 24.65 to 8.40 protein content in seeds [%] for mustard oil. The greatest decrease in protein was observed between days 0 and 1 of germination. Changes in protein after this were not significant. Two opposing processes take place during germination: as soon as the germinal root breaks through the seed coat, storage proteins begin to be broken down by proteolytic enzymes. Proteins then begin to be synthesized in the embryo [47]. In our experiment, we saw a decrease in the amount of protein associated with root puncture, but the process of synthesizing new proteins in the sprouts had not yet begun. During 7 days of peanut kernel germination, the percentage of proteins also decreased from 37.6 to 27.1% [48]. The enzyme peroxidase was found to be present. During the following days of sprouting of rape and mustard seeds, there was an increase in the activity of the enzyme peroxidase, which is synonymous with an increase in the vigor of germinating seeds. Peroxidase activity then decreases in mature seeds [49]. An increase in the level of peroxidase was also found in the research of Palmiano and Juliano [50]. In rice seeds exposed to light during germination, a significant increase in peroxidase was found on the fourth day of germination. The increase in peroxidase was faster for seeds that grew in the dark: on day 3, there was already a significant increase in the amount of peroxidase. The increase in peroxidase during germination was also confirmed by the studies of Omidiji et al. [51] and Ramaiah et al. [52] in which Sorghum bicolor and Pinus banksiana seeds were tested. The increase in the activity of the peroxidase enzyme and the use of O2 for germination affected the quality of the oil obtained from the germinating seeds. In subsequent stages of germination, the peroxide value of the sprout oil decreased, which means that the fat present in the seeds was not oxidized due to the peroxidase activity.
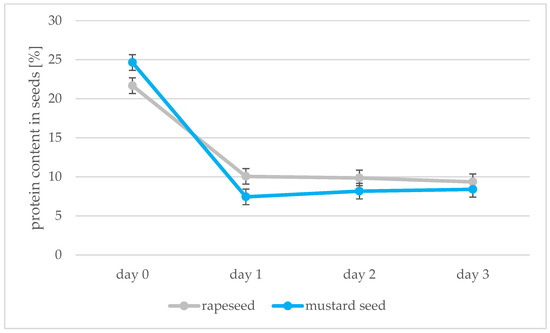
Figure 7.
Protein content [%] in rapeseeds and mustard seeds during germination (days 0, 1, 2, 3).
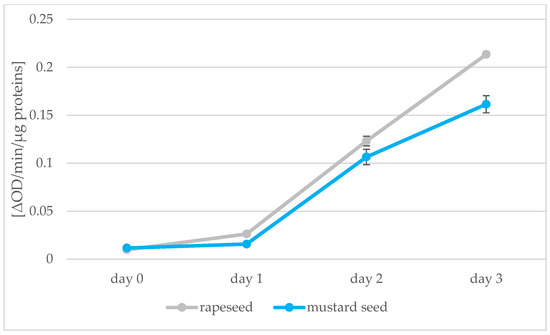
Figure 8.
Peroxidase activity [ΔOD/min/µg proteins] in rapeseeds and mustard seeds during germination (days 0, 1, 2, 3) (values are means ± SD from three experiments.).
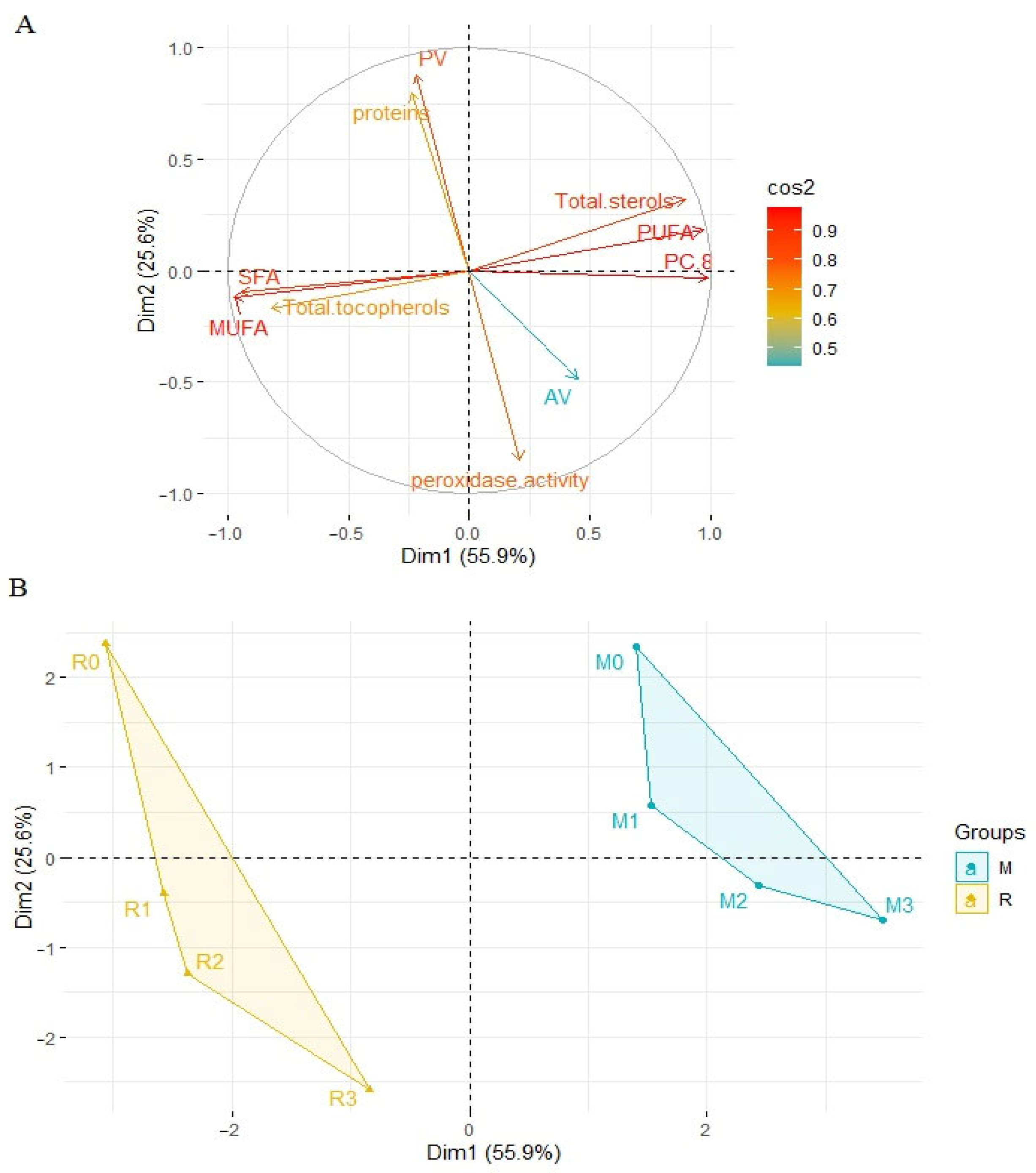
3.7. Principal Component Analysis (PCA)
Principal component analysis (PCA) was applied to observe possible clusters in oil samples obtained from rape and white mustard seeds. The first two principal factors accounted for 81.5% (Dim1 = 55.9% and Dim2 = 25.6%) of the total variation. The PCA analysis showed differences between the samples of oil obtained from rape seed and mustard seed but also within the individual oils (Figure 9). Factor 1 was mainly correlated with the content of plastochromanol-8 (PC-8) (r = 0.990), PUFA (r = 0.969) and total sterols (r = 0.893). It was also negatively correlated with the MUFA (r = −0.975), SFA (r = −0.946) and total tocopherols (r = −0.823). Factor 2 was mainly correlated with peroxide value (r = 0.880) and negatively correlated with peroxidase activity (r = −0.854). The data shown in the score plot divided samples of oil into two groups. On the left side of the Y axis, there are oil samples obtained from rape seeds. On the right side of the axis, there are oil samples obtained from mustard seeds. Additionally, the observed distances between individual samples presented in the score plot are bigger in the case of rapeseed oil. This is especially true for the oil obtained from seeds after the 1st and 3rd day of germination. In the score plot, the samples of oil from non-sprouted seeds (R0, M0) are above the x-axis, but in two different quarters of the diagram. This is mainly due to differences in the total content of phytosterols, tocopherols, and PC-8 as well as the peroxide and acid numbers. Rapeseed oil was characterized by a lower content of sterols and PC-8 and a higher content of tocochromanols compared to mustard oil. In rapeseed oil, the highest peroxide value and the lowest acid value were observed. The germination process changed the composition of the obtained oil and the seeds used. The shape of the surface area of all samples is similar in both cases (rape and mustard seeds). However, the distance between the non-germinated sample and after three days of germination is shorter for the mustard seed and its oil (Figure 9). After three days of germination, the mustard seeds were characterized by lower peroxidase activity (0.162 and 0.213 ΔOD/min/μg protein for mustard and rapeseed oil, respectively) and protein content (8.40% and 9.36% for mustard and rapeseed oil, respectively). The mustard seed oil was characterized by higher sterols and PC-8 content, higher level of peroxide value, and lower acid value, compared to rapeseed oil.
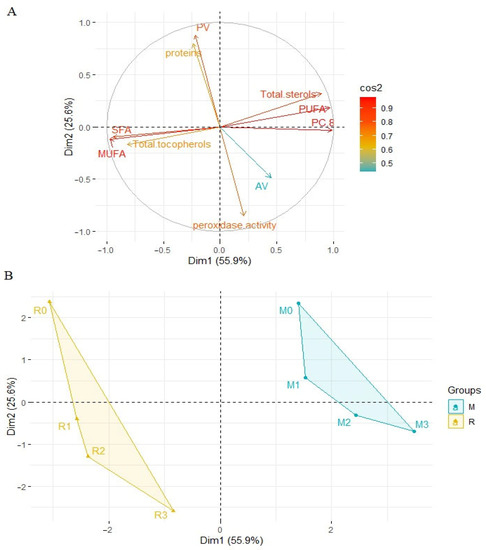
Figure 9.
Principal component analysis (PCA) of the loading plot (A) and the score plot (B) of data from SFA, MUFA, PUFA, total sterols and tocopherols content, plastochromanol-8 (PC-8), peroxide value, acid value in rapeseed and mustard seed oils, and proteins content and peroxidase activity in rape and mustard seeds. M—mustard samples, R—rape samples, 1, 2, 3—days of germination of seeds.
4. Conclusions
In summary, our research shows that sprouting of rapeseeds and mustard seeds is an effective method of reducing peroxide levels in oil, but not reducing the oil’s bioactive components (such as polyunsaturated fatty acids, tocopherols, and sterols). The oils obtained from the second day of seed germination are characterized by the lowest acid value and peroxide value and can be used as a good quality oil for consumption. Sprouting can be used to lower the peroxide value of oils without changing the amount of bioactive ingredients in the oil. In mustard oil, we can see slight increase in polyunsaturated fatty acids and tocopherols and percentage of oil in the seeds, which has a positive effect on the value of mustard sprouts as a source of polyunsaturated fatty acids. However, in the case of rapeseed oil, a decrease in the percentage of oil was observed, but the amount of sterols increased. The obtained results regarding the reduction of the peroxide value of oil from germinating seeds can be tested in the case of other oils that are characterized by a high value of primary oxidation compounds.
Author Contributions
Conceptualization, M.R.; methodology, A.G., D.K. and A.S.; software, A.G., D.K. and A.S.; validation, A.S. and D.K.; formal analysis, D.B.; investigation, D.B. and A.G.; resources, M.R. and S.S.; data curation, A.G. and D.B.; writing—original draft preparation, A.G. and D.K.; writing—review and editing, M.R.; visualization, D.K.; supervision, M.R.; project administration, A.G.; funding acquisition, M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a statutory research fund (grant number 506.752.03.00) from the Faculty of Food Science and Nutrition at Poznań University of Life Sciences.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wroniak, M.; Chlebowska-Śmigiel, A. Impact of purity of rapeseed and oil purification method on selected properties of cold-pressed oils. Zywnosc Nauka Technol. Jakosc 2013, 4, 133–149. [Google Scholar] [CrossRef]
- Zhang, N.; Li, Y.; Wen, S.; Sun, Y.; Chen, J.; Gao, Y.; Sagymbek, A.; Yu, X. Analytical methods for determining the peroxide value of edible oils: A mini-review. Food Chem. 2021, 358, 129834. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Huyan, Z.; Geng, Q.; Yu, X. Rapid Determination of Acid Value of Edible Oils via FTIR Spectroscopy Using Infrared Quartz Cuvette. J. Oleo Sci. 2019, 68, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of The United Nations. The Codex Alimentarius Fats, Oils and Related Products; World Health Organization: Rome, Italy, 2001. [Google Scholar]
- Czwartkowski, K.; Wierzbic, A.; Golimowski, W. Quality, key production factors, and consumption volume of niche edible oils marketed in the European Union. Sustainability 2022, 14, 1846. [Google Scholar] [CrossRef]
- Rękas, A.; Wroniak, M.; Siger, A.; Ścibisz, I. Chemical composition and resistance to oxidation of high-oleic rapeseed oil pressed from microwave pre-treated intact and de-hulled seeds. Grasas Aceites 2017, 68, e225. [Google Scholar] [CrossRef]
- Corrales, C.V.; Achir, N.; Forestier, N.; Lebrun, M.; Maraval, I.; Dornier, M.; Perez, A.M.; Vaillant, F.; Fliedel, G. Innovative process combining roasting and tempering to mechanically dehull jicaro seeds (Crescentia alata K.H.B). J. Food Eng. 2017, 212, 283–290. [Google Scholar] [CrossRef]
- Nederal, S.; Skevin, D.; Kraljić, K.; Obranović, M.; Papesa, S.; Bataljaku, A. Chemical Composition and Oxidative Stability of Roasted and Cold Pressed Pumpkin Seed Oils. J. Am. Oil Chem. Soc. 2012, 89, 1763–1770. [Google Scholar] [CrossRef]
- Luo, F.; Fei, X. Maillard reaction derived from oil-tea camellia seed through roasting. J. Sci. Food Agric. 2019, 99, 5000–5007. [Google Scholar] [CrossRef]
- Vo, B.V.; Siddik, M.A.B.; Fotedar, R.; Chaklader, M.R.; Foysal, M.J.; Pham, H.D. Digestibility and water quality investigations on the processed peanut (Arachis hypogaea) meal fed barramundi (Lates calcarifer) at various inclusion levels. Aquac. Rep. 2020, 18, 100474. [Google Scholar] [CrossRef]
- Cui, J.; Lamade, E.; Tcherkez, G. Seed Germination in Oil Palm (Elaeis guineensis Jacq.): A Review of Metabolic Pathways and Control Mechanisms. Int. J. Mol. Sci. 2020, 21, 4227. [Google Scholar] [CrossRef]
- Gu, J.; Hou, D.; Li, Y.; Chao, H.; Zhang, K.; Wang, H.; Xiang, J.; Raboanatahiry, N.; Wang, B.; Li, M. Integration of proteomic and genomic approaches to dissect seed germination vigor in Brassica napus seeds differing in oil content. BMC Plant Biol. 2019, 19, 21. [Google Scholar] [CrossRef]
- Pachari Vera, E.; Alca, J.J.; Rondón Saravia, G.; Callejas Campioni, N.; Jachmanián Alpuy, I. Comparison of the lipid profile and tocopherol content of four Peruvian quinoa (Chenopodium quinoa Willd.) cultivars (‘Amarilla de Maranganí’, ‘Blanca de Juli’, INIA 415 ‘Roja Pasankalla’, INIA 420 ‘Negra Collana’) during germination. J. Cereal Sci. 2019, 88, 132–137. [Google Scholar] [CrossRef]
- Chauhan, A.; Kumari, N.; Saxena, D.C.; Singh, S. Effect of germination on fatty acid profile, amino acid profile and minerals of amaranth (Amaranthus spp.) grain. J. Food Meas. Charact. 2022, 16, 1777–1786. [Google Scholar] [CrossRef]
- Chandrasekaran, U.; Liu, A. Stage-specific metabolization of triacylglycerols during seed germination of Sacha Inchi (Plukenetia volubilis L.). J. Sci. Food Agric. 2015, 95, 1764–1766. [Google Scholar] [CrossRef]
- Guo, S.; Klinkesorn, U.; Lorjaroenphon, Y.; Ge, Y.; Na Jom, K. Effects of germinating temperature and time on metabolite profiles of sunflower (Helianthus annuus L.) seed. Food Sci. Nutr. 2021, 9, 2810–2822. [Google Scholar] [CrossRef]
- Shi, H.; Nam, P.K.; Ma, Y. Comprehensive Profiling of Isoflavones, Phytosterols, Tocopherols, Minerals, Crude Protein, Lipid, and Sugar during Soybean (Glycine max) Germination. J. Agric. Food Chem. 2010, 58, 4970–4976. [Google Scholar] [CrossRef]
- Herchi, W.; Bahashwan, S.; Sebei, K.; Saleh, H.B.; Kallel, H.; Boukhchina, S. Effects of germination on chemical composition and antioxidant activity of flaxseed (Linum usitatissimum L.) oil. Grasas Aceites 2015, 66, 1–8. [Google Scholar] [CrossRef]
- Kohli, S.K.; Khanna, K.; Bhardwaj, R.; Abd_Allah, E.F.; Ahmad, P.; Corpas, F.J. Assessment of Subcellular ROS and NO Metabolism in Higher Plants: Multifunctional Signaling Molecules. Antioxidants 2019, 8, 641. [Google Scholar] [CrossRef]
- Mansoor, S.; Wani, O.A.; Lone, J.K.; Manhas, S.; Kour, N.; Alam, P.; Ahmad, A.; Ahmad, P. Reactive Oxygen Species in Plants: From Source to Sink. Antioxidants 2022, 11, 225. [Google Scholar] [CrossRef]
- Singh, K.L.; Chaudhuri, A.; Kar, R.K. Role of peroxidase activity and Ca2+ in axis growth during seed germination. Planta 2015, 242, 997–1007. [Google Scholar] [CrossRef]
- Ahmad, P.; Tripathi, D.K.; Deshmukh, R.; Singh, V.P.; Corpas, F.J. Revisiting the role of ROS and RNS in plants under changing environment. Environ. Exp. Bot. 2019, 161, 1–3. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Yamamoto, K.; Tawaratsumida, T.; Yuasa, T.; Iwaya-Inoue, M. Hydrogen peroxide scavenging regulates germination ability during wheat (Triticum aestivum L.) seed maturation. Plant Signal. Behav. 2008, 3, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Consumption of Vegetable Oils Worldwide from 2013/14 to 2021/2022, by Oil Type. Available online: https://www.statista.com/statistics/263937/vegetable-oils-global-consumption/ (accessed on 17 January 2023).
- Lowry, O.H.; Rosenbrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Guha, P.; Mandal, A.K. Deteriorative changes in enzyme activity of non-invigorated and invigorated soybean seeds (Glycine max [l.] merrill, cv. soyamax). Legum. Res. 2019, 42, 633–639. [Google Scholar] [CrossRef]
- Górnaś, P.; Siger, A.; Rudzińska, M.; Grygier, A.; Marszałkiewicz, S.; Ying, Q.; Sobieszczańska, N.; Segliņa, D. Impact of the Extraction Technique and Genotype on the Oil Yield and Composition of Lipophilic Compounds in the Oil Recovered from Japanese Quince (Chaenomeles japonica) Seeds. Eur. J. Lipid Sci Technol. 2019, 121, 1–10. [Google Scholar] [CrossRef]
- ISO 660:2010; Animal and Vegetable Fats and Oils—Determination of Acid Value and Acidity. Polish Committee of Standardization International: Warsaw, Poland, 2010. Available online: https://www.iso.org/standard/75594.html (accessed on 17 January 2023).
- ISO 3960:2017; Animal and Vegetable Fats and Oils—Determination of Peroxide Value—Iodometric (Visual) Endpoint Determination. Polish Committee for Standardization: Warsaw, Poland, 2017. Available online: https://www.iso.org/standard/71268.html (accessed on 17 January 2023).
- Raczyk, M.; Polanowska, K.; Kruszewski, B.; Grygier, A.; Michałowska, D. Effect of spirulina (Arthrospira platensis) supplementation on physical and chemical properties of semolina (Triticum durum) Based Fresh Pasta. Molecules 2022, 27, 355. [Google Scholar] [CrossRef]
- Sahu, P.K.; Chakradhari, S.; Sipeniece, E.; Mišina, I.; Qian, Y.; Grygier, A.; Rudzińska, M.; Patel, K.S.; Górnaś, P. Fatty Acids, Tocopherols, Tocotrienols, Phytosterols, Carotenoids, and Squalene in Seed Oils of Hyptis suaveolens, Leonotis nepetifolia, and Ocimum sanctum. Eur. J. Lipid Sci. Technol. 2020, 122, 1–5. [Google Scholar] [CrossRef]
- Xu, M.; Jin, Z.; Simsek, S.; Hall, C.; Rao, J.; Chen, B. Effect of germination on the chemical composition, thermal, pasting, and moisture sorption properties of flours from chickpea, lentil, and yellow pea. Food Chem. 2019, 295, 579–587. [Google Scholar] [CrossRef]
- Borek, S.; Kubala, S.; Kubala, S.; Ratajczak, L. Comparative study of storage compound breakdown in germinating seeds of three lupine species. Acta Physiol. Plant 2011, 33, 1953–1968. [Google Scholar] [CrossRef]
- Sakiroh, S.; Taryono, T.; Purwanti, S. Dynamics of storage materials in cotyledon during cocoa seed germination. Ilmu Pertan. (Agric. Sci.) 2018, 3, 12–20. [Google Scholar] [CrossRef]
- Hahm, T.; Park, S.; Lo, M.Y. Effects of germination on chemical composition and functional properties of sesame (Sesamum indicum L.) seeds. Bioresour. Technol. 2009, 100, 1643–1647. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Marangoni, F.; Poli, A. Phytosterols and cardiovascular health. Pharmacol. Res. 2010, 61, 193–199. [Google Scholar] [CrossRef]
- Bush, P.B.; Grunwald, C. Sterol Changes during Germination of Nicotiana tabacum Seeds. Plant Physiol. 1972, 50, 69–72. [Google Scholar] [CrossRef][Green Version]
- Rękas, A.; Siger, A.; Wroniak, M.; Ścibisz, I.; Derewiaka, D.; Anders, A. Influence of de-hulled rapeseed roasting on the physicochemical composition and oxidative state of oil. Grasas Aceites 2017, 68, e176. [Google Scholar] [CrossRef]
- Piironen, V.; Lindsay, D.; Miettinen, T.; Toivo, J.; Lampi, A.M. Plant sterols: Biosynthesis, biological function and their importance to human nutrition. J. Sci. Food Agric. 2020, 80, 939–966. [Google Scholar] [CrossRef]
- Valitova, J.N.; Sulkarnayeva, A.G.; Minibayeva, F.V. Plant Sterols: Diversity, Biosynthesis, and Physiological Functions. Biochem. Biokhimiia 2016, 81, 819–834. [Google Scholar] [CrossRef]
- Shu, X.L.; Frank, T.; Shu, Q.Y.; Engel, K.H. Metabolite profiling of germinating rice seeds. J. Agric. Food Chem. 2008, 56, 11612–11620. [Google Scholar] [CrossRef]
- García-Navarro, E.; Pérez-Vich, B.; Velasco, L. Changes in plastochromanol-8 and tocopherols during germination in Ethiopian mustard lines with contrasting tocopherol levels. Seed Sci. Res. 2014, 24, 101–112. [Google Scholar] [CrossRef]
- Zhang, H.; Vasanthan, T.; Wettasinghe, M. Enrichment of tocopherols and phytosterols in canola oil during seed germination. J. Agric. Food Chem. 2007, 55, 355–359. [Google Scholar] [CrossRef]
- Azzi, A. Many tocopherols, one vitamin E. Mol. Aspects Med. 2018, 61, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, H.A.; Ahmad, E.E.M.; Mariod, A.A.; Matthäus, B.; Salaheldeen, M. Effect of pretreatment on the proximate composition, physicochemical characteristics and stability of Moringa peregrina oil. Grasas Aceites 2017, 68, e227. [Google Scholar] [CrossRef]
- Lewicki, P.P. Kiełki nasion jako źródło cennych składników odżywczych. Zywn. Nauka Technol. Jakosc 2010, 6, 18–33. [Google Scholar]
- Wang, K.H.; Lai, Y.H.; Chang, J.C.; Ko, T.F.; Shyu, S.L.; Chiou, R.Y. Germination of Peanut Kernels to Enhance Resveratrol Biosynthesis and Prepare Sprouts as a Functional Vegetable. Agric. Food Chem. 2005, 53, 242–246. [Google Scholar] [CrossRef]
- Scilabba, A.; Bellani, L.M.; Dell’Aquila, A. Effects of ageing on peroxidase activity and localization in radish (Raphanus sativus L.) seeds. Eur. J. Histochem. 2002, 46, 351–358. [Google Scholar] [CrossRef]
- Palmiano, E.P.; Juliano, B.O. Changes in the activity of some hydrolases, peroxidase, and catalase in the rice seed during germination. Plant Physiol. 1973, 52, 274–277. [Google Scholar] [CrossRef]
- Omidiji, O.; Okpuzor, J.; Otubu, O. Peroxidase activity of germinating Sorghum bicolor grains: Effect of some cations. J. Sci. Food Agric. 2002, 82, 1881–1885. [Google Scholar] [CrossRef]
- Ramaiah, P.K.; Durzan, D.J.; Mia, A.J. Amino acids, soluble proteins, and isoenzyme patterns of peroxidase during the germination of jack pine. Can. J. Plant Sci. 1971, 49, 2151–2161. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).