Abstract
The surface gloss, radiopacity and enamel/dentin shear bond strengths (SBS)s of five commercially available contemporary universal composite resins (CR)s were examined. The tested universal CRs were as follows: G-aenial A’CHORD (GA), Charisma Diamond (CD), Neo SpectraTMST(NS), Filtek(F) and Estelite Asteria(EA). Twelve cylindrical specimens per group were fabricated and gloss measurements were performed using a gloss meter after polishing and darkening. Five specimens of each group were radiographed using a CMOS sensor alongside an aluminum stepwedge, human enamel and dentin. The mean gray values were measured with a computer program. A total of 120 bonded human enamel and dentin specimens were subjected to SBS test. To analyze surface gloss, radiopacity and SBS we used Kruskall–Wallis, Wilcoxon signed rank, Welch tests and one-way ANOVA. Games–Howell, Tukey’s HSD tests, and Dunn’s multiple comparisons were used for multiple comparisons (p < 0.05). In terms of gloss units of the materials after polishing and darkening, the highest surface gloss was achieved with GA, followed by NS, F, CD and EA (p < 0.001). All the tested CRs showed significantly higher radiopacity values in comparison with dentin (p < 0.05). CD and NS presented higher radiopacity values, while other CRs were, in decreasing order; F, GA and EA (p < 0.001). The SBS ranking varied for enamel and dentin, and the adhesive failure was detected at a higher rate. Commercially available contemporary universal CRs display different surface gloss, radiopacity and SBS properties.
1. Introduction
Composite resins (CRs) were first used as an aesthetic restorative material in the anterior region in the late 1960’s [1]. Over time, changes in chemical structures and developments in production technologies have made these materials usable also in the posterior region. It is estimated that approximately three hundred million direct composite restorations are performed annually in the world today, which means it is one of the most common interventional applications in the human body [2]. Moreover, these restorations are constantly exposed to mechanical, biological and chemical conditions in the oral environment.
For almost 20 years, the majority of the composites on the market have been universal restoratives, serving the dentist with a single material that can be used in both anterior and posterior areas [3]. These are often described as ultra-thin medium-filler CRs and integrate a slightly reduced filler load with polishability [3]. These CRs have a single shade and they are purported to match with different tooth colors [4,5]. Choosing a proper CR for a restoration is not easy for clinicians, as there are so many material options available today. It is important for the long-term prognosis of restorations to have a high bonding potential to dental tissues (ref), to be well polished, and to maintain their brightness in the long term [6]. The radiopacity of CR is also important for radiographic diagnosis, as materials with sufficient radiopacity provide an opportunity to diagnose recurrent dental caries [7].
Aesthetic, mechanical properties and surface characteristics of new generation universal CRs are still under investigation [8,9]. Among the esthetic factors, the surface gloss is of great importance. This property is often related to deterioration or wear of materials and leads to unsuitable optical properties of restorations and the need for re-polishing, repair or restoration replacement. A recent study evaluated the surface gloss of some universal CRs and reported changes in the surface gloss following thermal cycles [10]. Gurgan et al. [11] also investigated the surface roughness, microhardness, color change, and translucency of universal nanohybrid CRs and stated that mechanical and optical properties of universal CRs with different compositions showed variations.
Since the masticatory process is more of a shearing phenomenon, shear bond strength indicates the adhesive strength of the restorative material at the tooth–restoration interface. The increasing demand for simplified universal adhesives has led to many in vitro/in vivo studies [12,13,14]. Reviews about universal adhesives have suggested that bond strength may vary according to tissue and material [15,16], though manufacturers often claim, as part of their marketing strategies, that using their composites with their own adhesives will result in a better performance.
Currently, a small number of new universal CRs are available on the market promising clinicians to combine advanced features in a syringe with the claim of long-lasting gloss and to optimize the radiographic follow-up with their radiopacities over time. On the other hand, there have been very few studies that evaluate multiple different properties of contemporary universal resin composites and that shed light on clinicians from different perspectives. Therefore, the aim of this in vitro study was to evaluate and compare the aesthetic, radiographic and bonding properties of five commercially available contemporary universal CRs. The null hypothesis stated that there would be no significant differences among the tested CRs in terms of (1) surface gloss, (2) radiopacity, and (3) SBS to enamel and dentin.
2. Materials & Methods
2.1. Study Materials
Five universal CRs in A1 shades were tested in this in vitro study. The commercially available contemporary universal CRs were as follows; 1. G-aenial A’CHORD (GA, GC, Tokyo, Japan), 2. Charisma Diamond (CD, Kulzer, Gmb Hanou, Germany), 3. Neo Spectra ST (NS, Dentsply, Konstanz, Germany), 4. Filtek (F, 3M, ESPE, St. Paul, MN, USA), 5. Estelite Asteria (EA, Tokuyama Dental Corp., Tokyo, Japan) (Table 1). G*Power software (Ver 3.1, Heinrich—Heine Dusseldorf University, Dusseldorf, Germany) with a 95% confidence interval, 80% power, and 0.50 effect size values was used for one-way ANOVA-type power analysis. The sample size calculation indicated the need for approximately 11 teeth for each group in order to determine a difference of 25% among the study groups. The primary outcome was to evaluate if analyzed different materials affected SBS to dental hard tissues.

Table 1.
The composite resins, as well as their compositions and respective adhesive systems, that were used in this study.
2.2. Specimen Preparation for Surface Gloss and Radiopacity Evaluations
Twelve cylindrical specimens (8 mm diameter and 2 mm height) of each CR were prepared using a silicone mold. A single increment of the CR was inserted into the mold, covered with a glass slide and a Mylar Strip to extrude excess material and was cured with a LED light curing unit (DB-686-1b, Coxo, Guangdong Province, China, 1250 mW/cm2) for 20 s. A curing light meter (Hilux Dental Curing Light Meter, Benliglu Dental Inc., Ankara, Turkey) was used to check the irradiance of the curing unit. After light curing, the specimens were polished with aluminum-oxide abrasive discs (OptiDisc, Kerr, Bioggio, Switzerland) at a low speed, using a sequence of medium (40 μm, fine (20 μm), and extra-fine (10 μm) granulation. The polishing processes were achieved dry with a low-speed handpiece at 10,000 rpm for 20 s in a slight, uniform, intermittent pressure with circular movements by a single operator [17]. Subsequently, the specimens were washed with water for 10 s and air dried for 5 s. The abrasive discs were renewed after every three specimens. After the polishing procedure, the specimens were ultrasonically cleaned (Amsco, Reliance Sonic 250, Steris Corp., Mentor, OH, USA) in deionized water for 10 min to remove polishing residue and stored in distilled water at 37 °C for 24 h.
2.3. Surface Gloss Measurements
Surface gloss was measured with a gloss meter (Elcometer 407 Statistical glossmeter, Manchester, UK). During the measurements, a black opaque plastic mold was placed over the specimens to both eliminate the effect of ambient light and maintain the exact position of the specimen. Three measurements per specimen were performed at 60° light incidence and reflection angles [18]. The surface gloss values were recorded by taking the average of the obtained values.
Following baseline gloss measurements, the specimens were subjected to the darkening protocol. For darkening purpose, the coffee solution was used. The coffee solution was prepared by pouring 15 g of coffee powder (Nescafe® Classic, Nestle SA, Vevey, Switzerland) into 500 mL of boiling distilled water, stirring for 10 min and then filtering through a filter paper. After the solution was cooled, the specimens were stored in this solution for 7 days at 37 °C in stainless steel containers. To avoid specimen–specimen contact, each specimen was immersed separately with holders. The coffee solution was renewed daily. At the end of 7 days, the specimens were taken out of the coffee solution, washed with distilled water and air dried. Then gloss measurements were repeated.
2.4. Radiopacity Measurements
Five cylindrical specimens (8 mm diameter and 1 mm height) were prepared from each CR, paying attention to its homogeneity and non-porosity. One freshly extracted human molar was used to compare enamel and dentin radiopacity. The use of extracted human teeth was approved by the non-interventional clinical research ethics board of Hacettepe University (Approval Number 2020/20-86). The molar tooth was sectioned longitudinally using a slow speed diamond saw (Isomet1000, Buehler, Lake Bluff, IL, USA) to obtain a 1-mm thick slice and the tooth slice was stored in distilled water.
A 99% pure aluminum stepwedge with 9 incremental steps measuring 1 mm was used. Five specimens of each CR, together with the aluminum stepwedge and tooth slice, were positioned over the CMOS sensor (Digora Toto, Soredex, Milwaukee, WI, USA), and the storage phosphor plate system (VistaScan, Du¨rr Dental, Bietigheim-Bissingen, Germany) on the radiographs. All specimens were placed in a dental X-ray unit (for 32 s, 65 kVp/7 mA, Myray, Cefla Dental Group, Imola, Italy) at a distance of 30 cm.
The mean gray values of the CRs and enamel and dentin substrates (tooth slice) were measured in digital radiographs using Adobe Photoshop CS3 Extended computer program, version 10.0 (Adobe Systems, San Jose, CA, USA). To reduce the measurement deviation, five different regions without air bubbles or other anomalies, each with an area of 10 × 10 pixels, were measured. The measurements were carried out by an evaluator who was blinded to the brands of CRs.
2.5. SBS Measurements
One hundred and twenty human incisors stored in 0.2% Chloramine-T solution (Merck KGaA, Darmstadt, Germany) at 4 °C were used for SBS testing. Calculus and stains on selected teeth were removed by a hand scaler, cleaned with pumice using rubber cups and then the roots were separated from crowns using a low-speed saw (IsoMet 1000, Buehler, Lake Bluff, IL, USA) and a diamond-impregnated disc. The buccal surfaces of the teeth were grounded for 15 s with 240-grit silicon-carbide (SiC) paper (Interflex, Ankara, Turkiye) using a polishing machine under water irrigation until flat enamel surfaces were obtained. The same procedure was applied until flat dentin surfaces of 3-mm diameter were obtained in the middle thirds of the buccal surfaces of the teeth. In total, 60 enamel and 60 dentin specimens were prepared and checked under a light microscopy (Olympus SZX7, Hamburg, Germany) at a magnification of 40×. Each specimen was then embedded in cylindrical molds with a quick-set acrylic resin to expose the surfaces. The enamel and dentin surfaces were then polished with 600 grit SiC abrasive papers under running water to form a homogeneous smear layer.
The specimens were randomly allocated into 5 groups according to universal CR that was tested (n = 12). All universal CRs were used with their respective adhesive systems, as follows: Group GA (G-aenial A’CHORD + G Premio Bond); Group CD (Charisma Diamond + Gluma Bond Universal); Group NS (Neo SpectraTM ST + PrimeBbond Universal); Group F (Filtek + Single Bond Universal); Group EA (Estelite Asteria + Tokuyama Universal Bond). The adhesive procedures were performed following the manufacturers’ instructions (Table 1). For all groups, the enamel and dentin specimens were held by a bonding jig (Ultradent Inc, Salt Lake, UT, USA) with a cylindrical mold (Φ = 2.38 mm) and the mold was filled with the CR. The specimens were kept in distilled water at 37 °C for 24 h before the SBS tests.
SBS was measured using a universal testing machine (Instron, Lloyd LRXPlus, Lloyd Instruments Ltd., Fareham, UK) at a crosshead speed of 1 mm/min. The specimens were placed in a freely movable test base clamp to facilitate positioning during testing. The load that caused the bond between the CR–tooth surface break was recorded, and the mean SBS was calculated by dividing the load by the surface area of the bonded specimen and recorded in MPa. Following testing, the bonding areas between tooth surfaces and CRs were observed under a light microscopy (Olympus SZX7, Hamburg, Germany) at a magnification of 40× to determine the failure mode. Classification was made according to the types of failure observed in enamel or dentin/composite bonding; adhesive, cohesive and mixed [19]. One representative specimen for each failure types were also examined by scanning electron microscope (Nova NanoSEM 430, FEI, Hillsboro, OR, USA).
2.6. Statistical Analysis
Since the surface gloss measurement data were not normally distributed (Shapiro–Wilk test, p < 0.05), the CR groups’ data both before and after darkening were analyzed using Kruskall–Wallis ANOVA by ranks, followed by the Dunn’s Multiple Comparisons Test, while the effect of darkening for each group was tested using Wilcoxon sign rank test. The radiopacity data were normally distributed (Shapiro-Wilk test, p > 0.05) and the variances were homogenous (Levene’s test, p > 0.05), the data were tested using ANOVA with Tukey′s HSD test as a post hoc test. The SBS data were normally distributed, but the results of the test of homogeneity of variances showed that variances were not homogeneous in the dentine group and homogenous in the enamel group, therefore the data for the dentine group were analysed using the Welch test, while the data for the enamel group were tested using ANOVA followed by the Tukey’s HSD test. An independent samples t-test was used to compare the SBS of enamel and dentin. The Fisher–Freeman–Halton exact test was used to analyze the relationship between the CRs and failure mode for enamel and dentin, separately. All statistical tests were performed using SPSS 23.0 package (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Surface Gloss Measurements (GU)
Significant differences were detected among the CRs in terms of gloss units (GU) of the materials at baseline (after polishing) and after darkening (p < 0.001). After immersion in coffee solution, GUs were reduced in all groups. The highest GU was seen in GA > NS ≥ F > CD > EA after polishing and after darkening (Table 2).

Table 2.
Mean, SD, and median of gloss values (gloss units/GU) of the tested CRs.
3.2. Radiopacity Measurements
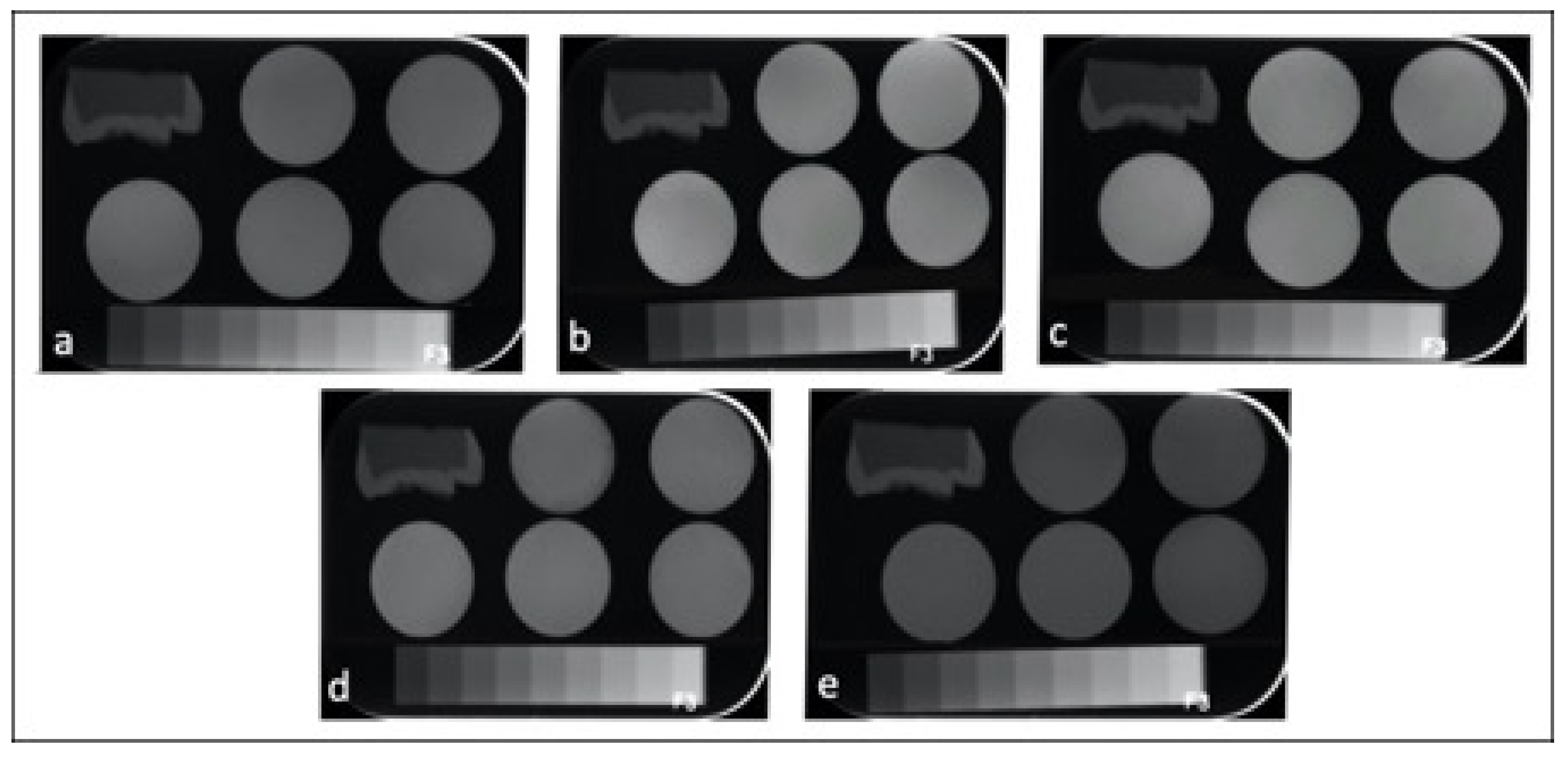
Table 3 represents the mean and standard deviations (SD)s of CRs in terms of radiopacity. All the tested CRs had radiopacity values greater than the radiopacity of enamel (63.17 ± 1.50) and dentin (39.72 ± 1.13) and 3 mm Al (58.02 ± 0.55). One-way ANOVA showed significant differences among the tested CRs in terms of radiopacity (p < 0.001). The highest radiopacity was seen in CD (126.72 ± 5.26) and NS (123.22 ± 3.25), followed by F (104.79 ± 6.37) > GA (90.80 ± 4.44) > EA (69.19 ± 4.02). Figure 1 shows representative radiographs of each CR.

Table 3.
Radiopacity and equivalent thickness (mm Al) of CRs and enamel and dentin.
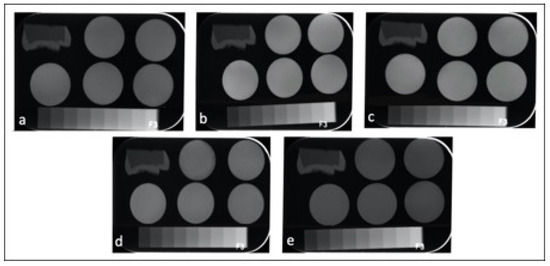
Figure 1.
Radiographic images of the enamel, dentin, aluminum stepwedge and universal CRs: (a) GA; (b) CD; (c) NS; (d) F; (e) EA.
3.3. SBS Measurements
Table 4 summarizes SBS values (the mean ± SD) of the tested CRS to enamel end dentin in MPa. There were significant differences among the CRs in terms of SBS to enamel (p < 0.001) and dentin (p = 0.002). Additionally, there was a significant difference between the SBS of CRs to enamel and dentin in all groups (<0.001). The highest SBS to enamel was seen in GA, EA and F followed by NS and CD. The highest SBS to dentin was seen as F, GA, EA and CD > NS.

Table 4.
Mean (± SD) bond strength (MPa) of the groups to enamel end dentin (n = 12).
3.4. Failure Analysis
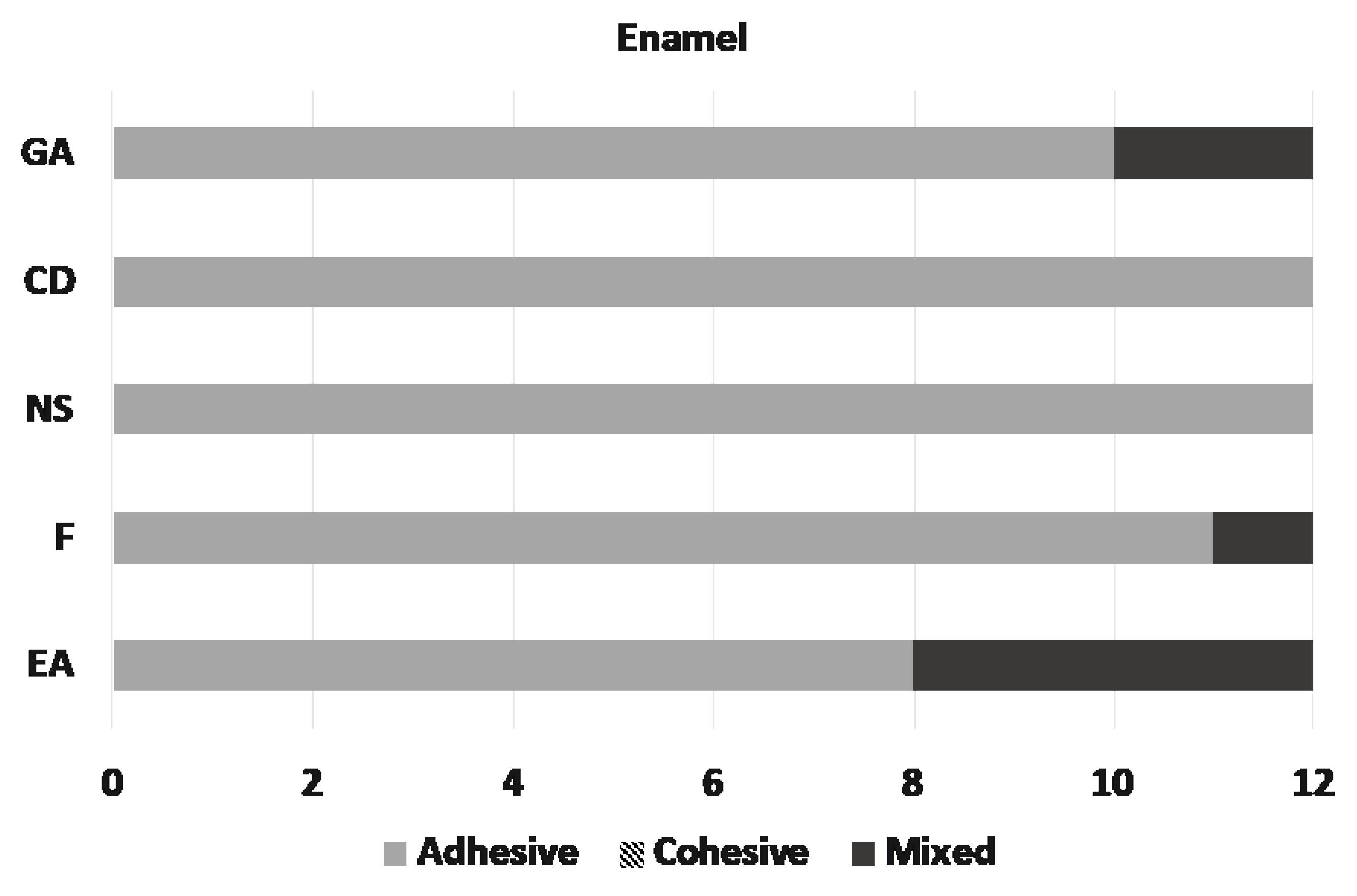
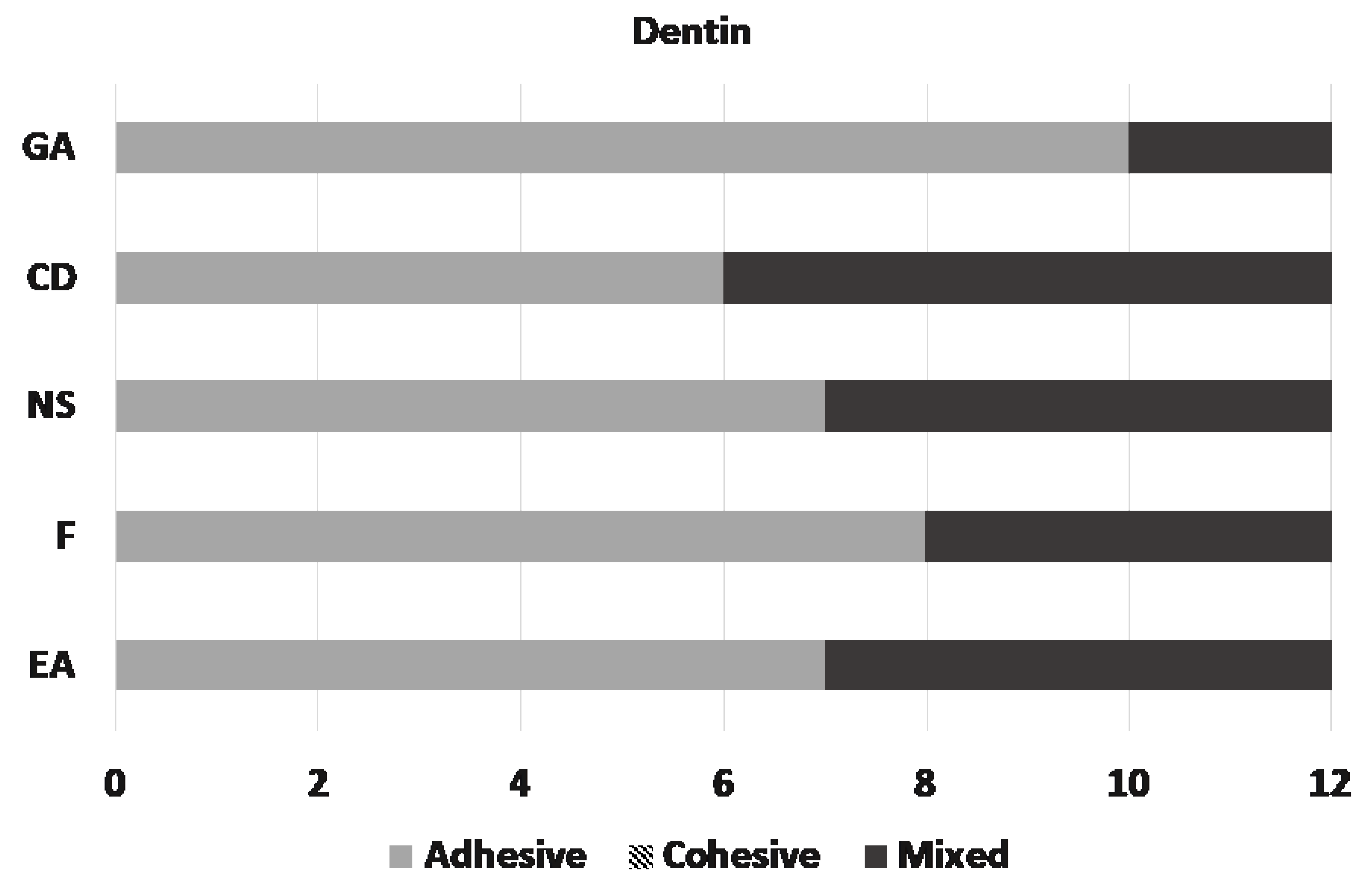
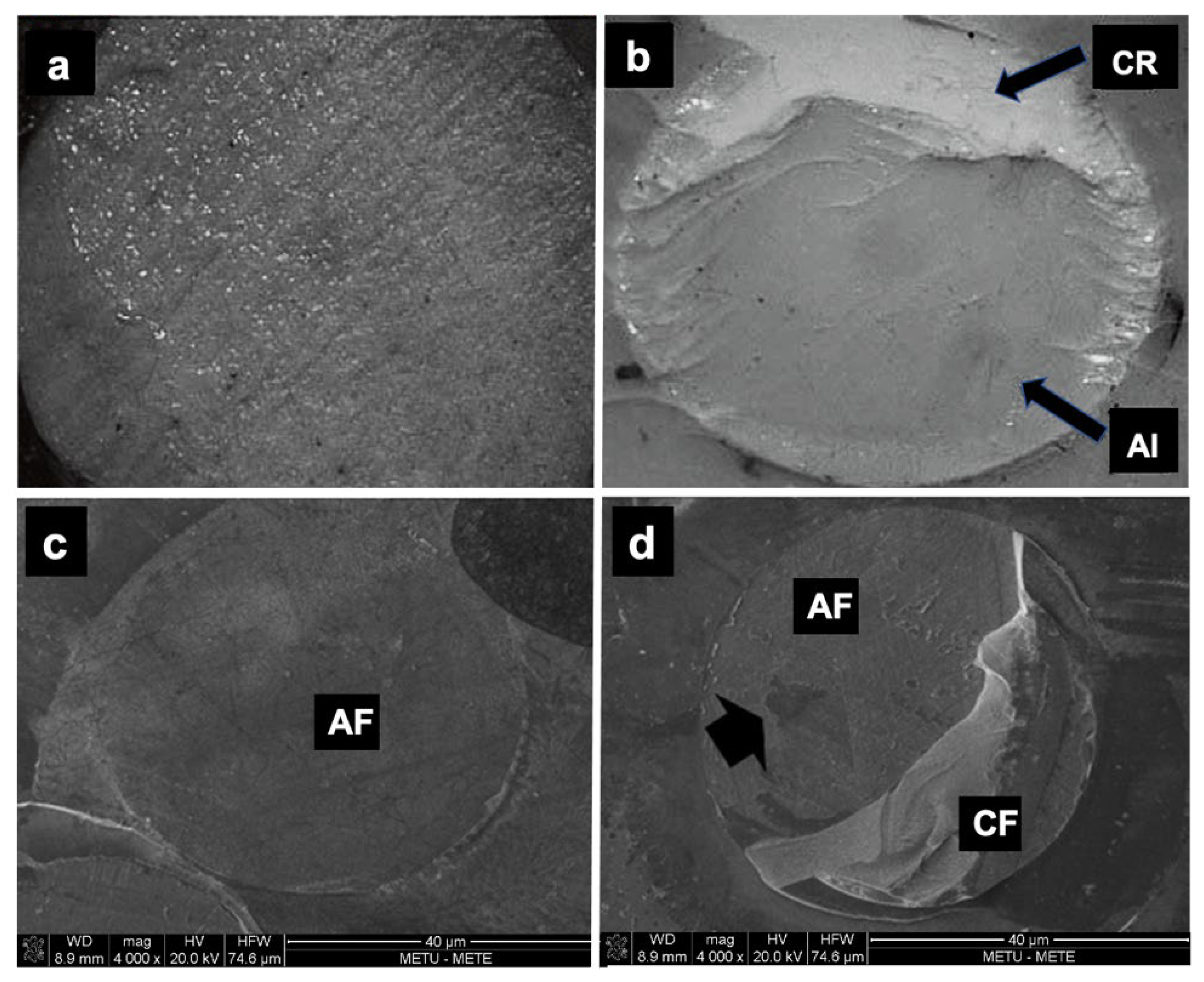
Microscopic assessment of the fractured surfaces and failure types of each CR for enamel and dentin are presented in Figure 2 and Figure 3. There were no significant differences among different CRs and the three types of failures (p = 0.079 and 0.541, respectively). The most common type of failure encountered was adhesive, followed by mixed failure. Significantly more adhesive failures were seen in enamel compared with dentin (p < 0.001). No cohesive failure was observed in any of the groups for both enamel and dentin specimens (Figure 4).
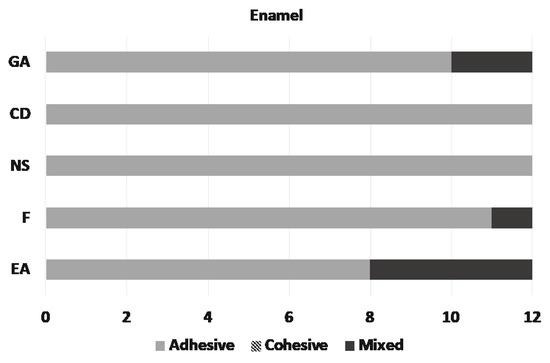
Figure 2.
Distribution of the failure types on enamel.
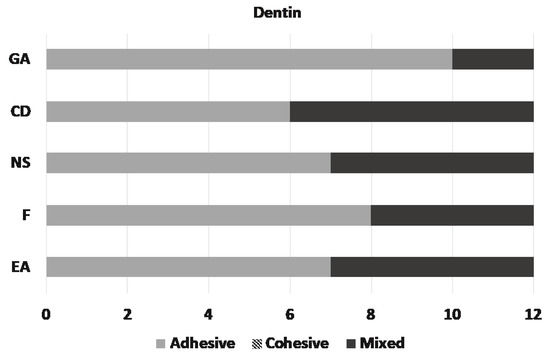
Figure 3.
Distribution of the failure types on dentin.
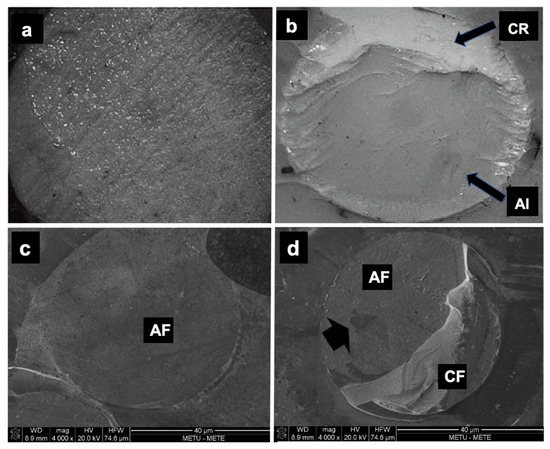
Figure 4.
The light microscopy images of failure modes (40×): (a) adhesive failure; (b) mixed failure. CR: composite resin; AI: adhesive interface. The scanning electron microscope images (×100 magnification) of failure modes: (c) adhesive failure; (d) mixed failure. AF: adhesive failure; CF: cohesive failure.
4. Discussion
Under clinical conditions, operators may find it difficult to choose one of the commercially available CR alternatives and may be tempted by the different characteristics of CRs. Surface gloss is a feature similar to natural enamel and should be copied in order to achieve natural tooth form and aesthetics. The marketing strategies of the newly introduced universal CR; GA include highlighting its natural fluorescence, invisible filling and long-lasting gloss properties [20].
Surface gloss is a more clinically detectable feature for clinicians and patients. Gloss indicates the smoothness of the restoration surface [21]. The high gloss of the material is a positive feature, but long-term gloss retention is more important and difficult. The long-term condition can be assessed by water storage/thermal cycle or by exposing to coloring foods such as coffee [10,22]. Therefore, in the present study, the gloss measurements were repeated after the CR specimens were subjected to darkening by coffee solution. The American Dental Association (ADA) has defined 40–60 GUs as a typically desired gloss [23]. In this study, all the CRs exhibited the GUs. However, significant differences were found among the gloss of the tested CRs. Therefore, the first hypothesis was rejected. Moreover, GA showed superior gloss values compared with other universal CRs either immediately after polishing or after darkening.
The present results are consistent with previous studies reporting that CRs with different organic matrix and filler sizes exhibit different properties [24,25]. All CRs tested in this study have nano-sized particles, but the larger particle size (200 nm) of EA compared with other tested CRs may have led to lower gloss values. On the other hand, GA showed the highest GU. This may be due to the filling technology, high filler load and uniform nano-filler distribution stated by the manufacturer [20].
In the literature color changes have been reported when CRs are exposed to coffee solution [24,26,27,28], and the current study also investigated the effect of coffee on the surface gloss of CRs. Dou et al. [29] have reported that many yellow dyes with low polarity in the content of coffee could diffuse into the organic matrix of the restorative material. Moreover, it has been reported that coffee promotes optical changes on the surfaces of CRs [22]. These findings are consistent with the results of this study, as the glosses of all the tested CRs were decreased after immersion in coffee solution compared with baseline measurements.
Radiopacity is one of the five necessities a dental material must meet according to the ADA [30,31]. CR, having a sufficient radiopacity, allows the clinician to distinguish secondary caries, detect open edges, overhangs, and proximity to dental pulp on radiographs [32]. All five universal CRs tested provided higher radiopacity values than dentin (1.96 mm Al) and 1-mm thick Al, and therefore met the ISO 4049 standard [33]. This finding is a positive feature for the tested materials and means that they cannot be misinterpreted as dental caries on radiography. On the other hand, the radiopacity of EA (69.19 ± 4.02, 3.63 mm Al) and the radiopacity of enamel (3.29 mm Al) tissue were similar. Some previous studies have reported that the radiopacity of restorative materials should be slightly higher than enamel or equal to enamel in order for clinicians to recognize secondary caries [34,35].
It has been reported that the radiopacity of a CR material can exceed the radiopacity of human enamel if the filler loading is raised to 70% or above and the amount of radiopaque oxide in the filler particles is 20% [36,37]. In a study evaluating the radiolucency of direct aesthetic restorative materials, it was reported that the ratio of the monomer that gives the radiolucent image in the CR material also affects the radiopacity [38]. The thicknesses of the specimens in this study were similar for all CRs, but measurements of mean gray values presented variable radiopacity values. Therefore, the second null hypothesis was also rejected. The detected radiopacity differences may be related to the inorganic phases of the evaluated CRs. The most radiopaque CRs were CD and NS (6.65 mm Al, 6.48 mm Al, respectively). When the tested materials were evaluated in terms of filler content percentages, CD (64%) had the lowest filler load by weight but showed the highest radiopacity and was similar to NS with a filler content of 78–80%. On the other hand, EA (82%) with the highest filler loading by weight showed lower radiopacity compared with other tested CRs (F, 76,5%; GA, 82%).
Previous studies have reported that high atomic number fillers increase the radiopacity of CRs by increasing the x-ray adsorption capacity [36,39,40]. Yasa et al. [41] have stated that the radiopacity of a material tends to increase with the presence of high atomic numbered elements in the filler particles. It was observed that the universal CRs used in this study included ytterbium, barium and zirconium with atomic numbers of 70, 56 and 40, respectively. In line with the findings of the study, the fact that the filler ratio is not directly proportional to the radiopacity values may be related to the atomic number of the filler type used.
In the present study, besides the gloss and radiopacity, the bonding capacity of the tested CRs to enamel and dentin with their respective adhesive systems were also evaluated. All universal adhesive systems were applied in self-etch mode in accordance with the manufacturers’ instructions and the third null hypothesis—that there would be no differences among the CRs in terms of SBS to enamel and dentin—was also rejected. The differences in the composition of the adhesives may be the reason for their different bond strength performances.
Two systematic review and meta-analysis studies [16,42] have reported that bond strength was advanced by the use of mild universal adhesives with prior acid etching of enamel; however, this effect was not found to be significant for dentin according to the in vitro literature. The findings of this study also support this report, as the tested CRs used with universal adhesives showed higher SBS to dentin substrate compared with enamel in self-etch mode. One of the controversial issues of universal adhesives is that the increase in surface area of the enamel substrate is lower than that caused by the application of phosphoric acid. This effect is related to the pH of the adhesive [43,44]. The pH of the GPB (pH: 1.5) used with the GA was lower than that of the other adhesives used with the other CRs tested and the bond strength to enamel was the highest, followed by SBU used with F (pH: 2.7) and then TUB used with EA (pH: 2.2) (p > 0.05). Additionally, GPB contains 4-MET and 10-MDTP as functional monomers in addition to 10-methacryloyloxidecyl dihydrogen phosphate (10-MDP). Nagakane et al. [45] have reported that 4-MET creates strong chemical bonds due to the calcium content of the enamel. GPB also contains acetone as a solvent, with its high volatility and hygroscopic nature [46], which can facilitate the permeability of resin monomers. On the other hand, SBU and TUB, which showed similar enamel bond strengths, contain acetone and ethanol, respectively. Therefore, it may not be accurate to interpret the bonding values of CRs by evaluating only the pH value of the adhesive and the solvents in its content. As a matter of fact, according to the results of this study, GBU used with CD (pH: 1.8) showed statistically lower bond strength with PBU used with NS (pH: 2.5). Although there were no significant differences among CRs in terms of failure types, observation of solely adhesive failures also supported the notion of lower bonding values of CD and NS.
The smaller crystals and plaque-like structure of dentin hydroxyapatite compared with enamel is considered more accessible for chemical bonding [47]. Hiraishi et al. [48] have suggested that 10-MDP has a relatively constant interaction with collagen due to hydrophobic interactions between the collagen surface. Four of the adhesives used in the present study contain MDP, however, the purity and amount of each ingredient in each adhesive may differ. In a recent study examining dentin bond interfaces with SEM analysis [49], the self-etch modes of GPB, SBU, and PBU were also evaluated. The adhesive layer thickness of PBU (2–3 μm) was found to be less than that of GPB (8–10 μm) and SBU (8–10 μm). In the present study, the tested CR, NS showed statistically lower bond strength for both enamel and dentin compared with other CRs. Although there is still no consensus on the ideal adhesive layer thickness, some studies [50,51] have suggested that a thicker adhesive layer may be better in terms of durability. The findings regarding CR and NS may be related to the adhesive used.
Studies evaluating the bond strength of adhesives generally test a number of adhesives with a single type of CR. However, in this in vitro study, adhesives and universal CRs of the same brand were matched. This may have influenced the findings of this study. Additionally, long-term water storage or thermal cycle is also one of the limitations of this study in order to evaluate the bonding stability. Although the SBS test set up has been the most commonly employed laboratory technique [52], there are also tests where the bonding is evaluated more realistically, such as microtensile bond strength. The present report evaluated surface gloss, radiopacity, SBS, and failures of contemporary universal composite resins. Future research with the same materials is needed in order to test other mechanical properties such as flexural strength and hardness in order to complete overview of the materials tested [53,54]. Moreover, long-term clinical observations of the tested either the newly introduced or available commercial CRs in service would provide information on the in vivo outcome.
5. Conclusions
Within the limitations of this in vitro study, it can be concluded that:
- The universal CRs tested exhibited different gloss, radiolucency and SBS properties.
- Coffee adversely affected the gloss of all tested materials.
- The highest gloss was recorded for G-aenial ACHORD.
- Charisma Diamond and Neo Spectra showed the highest radiopacity.
- The bond strengths of the materials to enamel and dentin varied. SBS of Charisma Diamond and Neo Spectra to enamel, and Neo Spectra to dentin were weaker compared to the other materials tested. So,
- Clinicians should keep in mind that universal CRs can be superior to each other with their different properties.
Author Contributions
Conceptualization, I.M. and S.G.; Methodology, C.A. and B.T.; Validation, U.K.V.; Formal analysis, U.K.V.; Investigation, C.A.; Resources, S.G.; Data curation, I.M. and S.G.; Writing–original draft, C.A.; Writing–review & editing, U.K.V., B.T., I.M. and S.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Non-Interventional Clinical Researches Ethics Board of Hacettepe University (2020/20-86).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Peutzfeldt, A. Resin composites in dentistry: The monomer systems. Eur. J. Oral Sci. 1997, 105, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Heintze, S.D.; Rousson, V. Clinical effectiveness of direct class II restorations—A meta-analysis. J. Adhes. Dent. 2012, 14, 407–431. [Google Scholar]
- Baldissera, R.A.; Correa, M.B.; Schuch, H.S.; Collares, K.; Nascimento, G.G.; Jardim, P.S.; Moraes, R.R.; Opdam, N.J.M.; Demarco, F.F. Are there universal restorative composites for anterior and posterior teeth? J. Dent. 2013, 41, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- de Abreu, J.L.B.; Sampaio, C.S.; Benalcazar Jalkh, E.B.; Hirata, R. Analysis of the color matching of universal resin composites in anterior restorations. J. Esthet. Restor. Dent. 2021, 33, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Akgul, S.; Gundogdu, C.; Bala, O. Effects of storage time and restoration depth on instrumental color adjustment potential of universal resin composites. J. Oral Sci. 2022, 64, 49–52. [Google Scholar] [CrossRef]
- Hickel, R.; Roulet, J.F.; Bayne, S.; Heintze, S.D.; Mjor, I.A.; Peters, M.; Rousson, V.; Randall, R.; Schmalz, G.; Tyas, M.; et al. Recommendations for conducting controlled clinical studies of dental restorative materials. Science Committee Project 2/98—FDI World Dental Federation study design (Part I) and criteria for evaluation (Part II) of direct and indirect restorations including onlays and partial crowns. J. Adhes. Dent. 2007, 9 (Suppl. 1), 121–147. [Google Scholar]
- Bouschlicher, M.R.; Cobb, D.S.; Boyer, D.B. Radiopacity of compomers, flowable and conventional resin composites for posterior restorations. Oper. Dent. 1999, 24, 20–25. [Google Scholar]
- Derradji, M.; Wang, J.; Liu, W. High performance ceramic-based phthalonitrile micro and nanocomposites. Mater. Lett. 2016, 182, 380–385. [Google Scholar] [CrossRef]
- Derradji, M.; Jun, W.; Wenbin, L. Phthalonitrile Resins and Composites: Properties and Applications, 1st ed.; William Andrew: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Takamizawa, T.; Ishii, R.; Tamura, T.; Yokoyama, M.; Hirokane, E.; Tsujimoto, A.; Miyazaki, M.; Kitahara, N. Handling properties and surface characteristics of universal resin composites. Dent. Mater. 2021, 37, 1390–1401. [Google Scholar] [CrossRef]
- Gurgan, S.; Koc Vural, U.; Miletic, I. Comparison of mechanical and optical properties of a newly marketed universal composite resin with contemporary universal composite resins: An in vitro study. Microsc. Res. Tech. 2022, 85, 1171–1179. [Google Scholar] [CrossRef]
- Ahmed, M.H.; De Munck, J.; Van Landuyt, K.; Peumans, M.; Yoshihara, K.; Van Meerbeek, B. Do Universal Adhesives Benefit from an Extra Bonding Layer? J. Adhes Dent. 2019, 21, 117–132. [Google Scholar] [PubMed]
- Anastasiadis, K.; Verdelis, K.; Eliades, G. The effect of universal adhesives on dentine collagen. Dent. Mater. 2021, 37, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Follak, A.C.; Ilha, B.D.; Oling, J.; Savian, T.; Rocha, R.O.; Soares, F.Z.M. Clinical behavior of universal adhesives in non-carious cervical lesions: A randomized clinical trial. J. Dent. 2021, 113, 103747. [Google Scholar] [CrossRef] [PubMed]
- Nagarkar, S.; Theis-Mahon, N.; Perdigao, J. Universal dental adhesives: Current status, laboratory testing, and clinical performance. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 2121–2131. [Google Scholar] [CrossRef]
- Cuevas-Suarez, C.E.; da Rosa, W.L.O.; Lund, R.G.; da Silva, A.F.; Piva, E. Bonding Performance of Universal Adhesives: An Updated Systematic Review and Meta-Analysis. J. Adhes. Dent. 2019, 21, 7–26. [Google Scholar]
- Gonulol, N.; Yilmaz, F. The effects of finishing and polishing techniques on surface roughness and color stability of nanocomposites. J. Dent. 2012, 40, 64–70. [Google Scholar] [CrossRef]
- Consani, R.L.; Folli, B.L.; Nogueira, M.C.; Correr, A.B.; Mesquita, M.F. Effect of Polymerization Cycles on Gloss, Roughness, Hardness and Impact Strength of Acrylic Resins. Braz. Dent. J. 2016, 27, 176–180. [Google Scholar] [CrossRef]
- Scheidel, D.D.; Takamizawa, T.; Bakmeier, W.W.; Erickson, R.L.; Tsujimoto, A.; Miyazaki, M. Effect of frequency on the fatigue strength of dentin bonds. J. Oral Sci. 2016, 58, 539–546. [Google Scholar] [CrossRef]
- G-aenial ACHORD, GC Corp Technical Manual. Available online: https://europe.gc.dental/en-GB/products/gaenialachord (accessed on 18 December 2022).
- Silikas, N.; Kavvadia, K.; Eliades, G.; Watts, D. Surface characterization of modern resin composites: A multitechnique approach. Am. J. Dent. 2005, 18, 95–100. [Google Scholar]
- Ozera, E.H.; Pascon, F.M.; Correr, A.B.; Puppin-Rontani, R.M.; Castilho, A.R.; Correr-Sobrinho, L.; Paula, A.B. Color Stability and Gloss of Esthetic Restorative Materials after Chemical Challenges. Braz. Dent. J. 2019, 30, 52–57. [Google Scholar] [CrossRef]
- Association, A.D. Product Review: Polishing Systems; American Dental Association: Chicago, IL, USA, 2010. [Google Scholar]
- Oliveira, G.U.; Mondelli, R.F.; Charantola Rodrigues, M.; Franco, E.B.; Ishikiriama, S.K.; Wang, L. Impact of filler size and distribution on roughness and wear of composite resin after simulated toothbrushing. J. Appl. Oral. Sci. 2012, 20, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Lu, H.; Oguri, M.; Powers, J.M. Changes in gloss after simulated generalized wear of composite resins. J. Prosthet. Dent. 2005, 94, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Fontes, S.T.; Fernandez, M.R.; de Moura, C.M.; Meireles, S.S. Color stability of a nanofill composite: Effect of different immersion media. J. Appl. Oral Sci. 2009, 17, 388–391. [Google Scholar] [CrossRef]
- Koroglu, A.; Sahin, O.; Dede, D.O.; Yilmaz, B. Effect of different surface treatment methods on the surface roughness and color stability of interim prosthodontic materials. J. Prosthet. Dent. 2016, 115, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.B.; Gordan, V.V.; Barrett, A.A.; Shen, C. The effect of surface finishing and storage solutions on the color stability of resin-based composites. J. Am. Dent. Assoc. 2004, 135, 587–594. [Google Scholar] [CrossRef]
- Dou, R.; Derby, B. Formation of coffee stains on porous surfaces. Langmuir 2012, 28, 5331–5338. [Google Scholar] [CrossRef]
- Ergucu, Z.; Turkun, L.S.; Onem, E.; Guneri, P. Comparative radiopacity of six flowable resin composites. Oper. Dent. 2010, 35, 436–440. [Google Scholar] [CrossRef]
- Council on Dental Materials, Instruments, and Equipment. Obstacles to the development of a standard for posterior composite resins. J. Am. Dent. Assoc. 1989, 118, 649–651. [Google Scholar] [CrossRef] [PubMed]
- Hara, A.T.; Serra, M.C.; Haiter-Neto, F.; Rodrigues, A.L., Jr. Radiopacity of esthetic restorative materials compared with human tooth structure. Am. J. Dent. 2001, 14, 383–386. [Google Scholar]
- ISO 4049; Dentistry—Polymer Based Restorative Materials. 4th ed. International Organization for Standardization: Geneva, Switzerland, 2009.
- Altintas, S.H.; Yildirim, T.; Kayipmaz, S.; Usumez, A. Evaluation of the radiopacity of luting cements by digital radiography. J. Prosthodont. 2013, 22, 282–286. [Google Scholar] [CrossRef]
- Espelid, I.; Tveit, A.B.; Erickson, R.L.; Keck, S.C.; Glasspoole, E.A. Radiopacity of restorations and detection of secondary caries. Dent. Mater. 1991, 7, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Toyooka, H.; Taira, M.; Wakasa, K.; Yamaki, M.; Fujita, M.; Wada, T. Radiopacity of 12 visible-light-cured dental composite resins. J. Oral Rehabil. 1993, 20, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Watts, D.C. Radiopacity vs. composition of some barium and strontium glass composites. J. Dent. 1987, 15, 38–43. [Google Scholar] [CrossRef]
- Turgut, M.D.; Attar, N.; Onen, A. Radiopacity of direct esthetic restorative materials. Oper. Dent. 2003, 28, 508–514. [Google Scholar] [PubMed]
- Amirouche, A.; Mouzali, M.; Watts, D.C. Radiopacity evaluation of Bis-GMA/TEGDMA/opaque mineral filler dental composites. J. Appl. Polym. Sci. 2007, 104, 1632–1639. [Google Scholar] [CrossRef]
- Hotta, M.; Yamamoto, K. Comparative radiopacity of bonding agents. J. Adhes. Dent. 2009, 11, 207–212. [Google Scholar]
- Yasa, B.; Kucukyilmaz, E.; Yasa, E.; Ertas, E.T. Comparative study of radiopacity of resin-based and glass ionomer-based bulk-fill restoratives using digital radiography. J. Oral Sci. 2015, 57, 79–85. [Google Scholar] [CrossRef]
- Rosa, W.L.; Piva, E.; Silva, A.F. Bond strength of universal adhesives: A systematic review and meta-analysis. J. Dent. 2015, 43, 765–776. [Google Scholar] [CrossRef]
- Li, N.; Nikaido, T.; Alireza, S.; Takagaki, T.; Chen, J.H.; Tagami, J. Phosphoric acid-etching promotes bond strength and formation of acid-base resistant zone on enamel. Oper. Dent. 2013, 38, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Van Landuyt, K.L.; Kanumilli, P.; De Munck, J.; Peumans, M.; Lambrechts, P.; Van Meerbeek, B. Bond strength of a mild self-etch adhesive with and without prior acid-etching. J. Dent. 2006, 34, 77–85. [Google Scholar] [CrossRef]
- Nagakane, K.; Yoshida, Y.; Hirata, I.; Fukuda, R.; Nakayama, Y.; Shirai, K.; Ogawa, T.; Suzuki, K.; Van Meerbeek, B.; Okazaki, M. Analysis of chemical interaction of 4-MET with hydroxyapatite using XPS. Dent. Mater. J. 2006, 25, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; De Munck, J.; Shirai, K.; Hikita, K.; Inoue, S.; Sano, H.; Lambrechts, P.; Van Meerbeek, B. Effect of air-drying and solvent evaporation on the strength of HEMA-rich versus HEMA-free one-step adhesives. Dent. Mater. 2008, 24, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Yaguchi, T. Layering mechanism of MDP-Ca salt produced in demineralization of enamel and dentin apatite. Dent. Mater. 2017, 33, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Hiraishi, N.; Tochio, N.; Kigawa, T.; Otsuki, M.; Tagami, J. Monomer-collagen interactions studied by saturation transfer difference NMR. J. Dent. Res. 2013, 92, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Takamizawa, T.; Imai, A.; Hirokane, E.; Tsujimoto, A.; Barkmeier, W.W.; Erickson, R.L.; Latta, M.A.; Miyazaki, M. SEM observation of novel characteristic of the dentin bond interfaces of universal adhesives. Dent. Mater. 2019, 35, 1791–1804. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, S.; Takamizawa, T.; Barkmeier, W.W.; Tsujimoto, A.; Imai, A.; Watanabe, H.; Erickson, R.L.; Latta, M.A.; Nakatsuka, T.; Miyazaki, M. Effect of double-layer application on bond quality of adhesive systems. J. Mech. Behav. Biomed. Mater. 2018, 77, 501–509. [Google Scholar] [CrossRef]
- Taschner, M.; Kummerling, M.; Lohbauer, U.; Breschi, L.; Petschelt, A.; Frankenberger, R. Effect of double-layer application on dentin bond durability of one-step self-etch adhesives. Oper. Dent. 2014, 39, 416–426. [Google Scholar] [CrossRef]
- Versluis, A.; Tantbirojn, D.; Douglas, W.H. Why do shear bond tests pull out dentin? J. Dent. Res. 1997, 76, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Cacciafesta, V.; Sfondrini, M.F.; Lena, A.; Scribante, A.; Vallittu, P.K.; Lassila, L.V. Flexural strengths of fiber-reinforced composites polymerized with conventional light-curing and additional postcuring. Am. J. Orthod. Dentofacial Orthop. 2007, 132, 524–527. [Google Scholar] [CrossRef]
- Pieniak, D.; Walczak, A.; Walczak, M.; Przystupa, K.; Niewczas, A.M. Hardness and wear resistance of dental biomedical nanomaterials in a humid environment with non-stationary temperatures. Materials 2020, 13, 1255. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).